New Insights into the Efficacy of Aspalathin and Other Related Phytochemicals in Type 2 Diabetes—A Review
Abstract
:1. Introduction
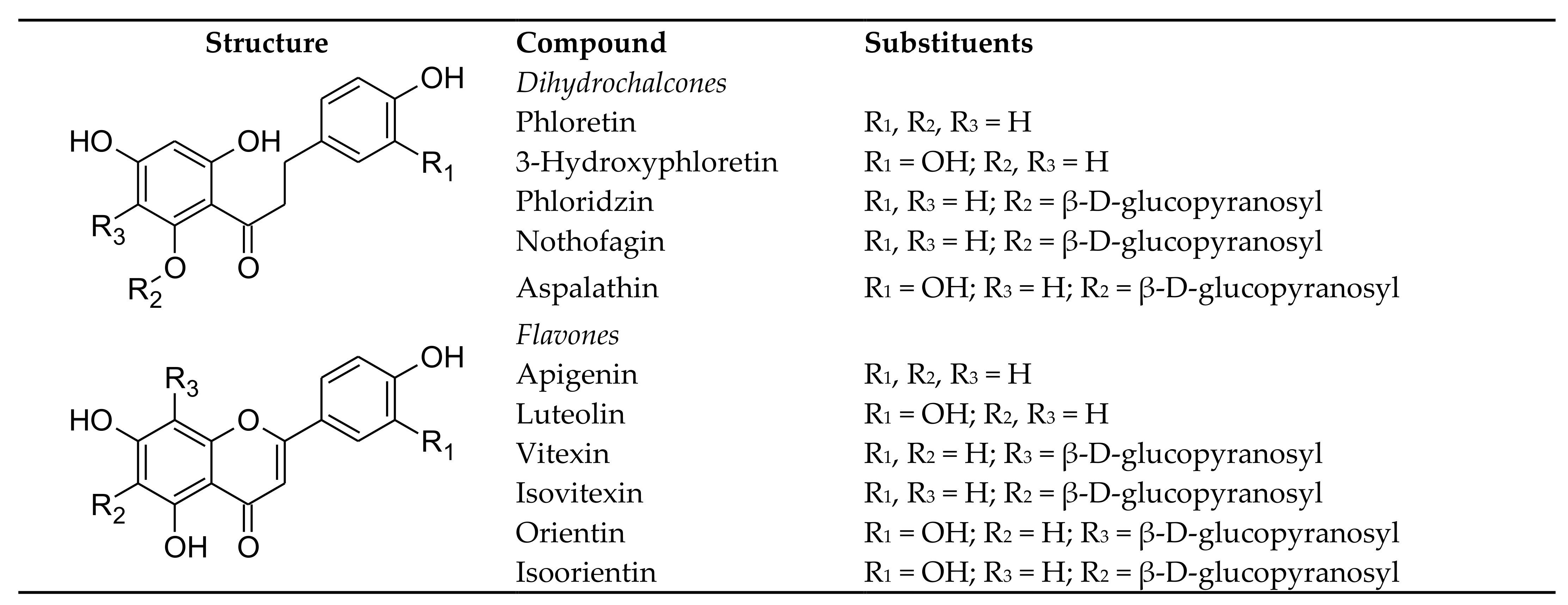
2. Aspalathin and Related Compounds—Structures, Sources, Stability, Bioaccessibility and Bioavailability
2.1. Dietary Sources
2.2. Compound Stability during Processing and Product Storage
2.3. Bioaccessibility and Bioavailability
2.3.1. Aspalathin, Nothofagin and Their Flavones
2.3.2. Phloridzin and Phloretin
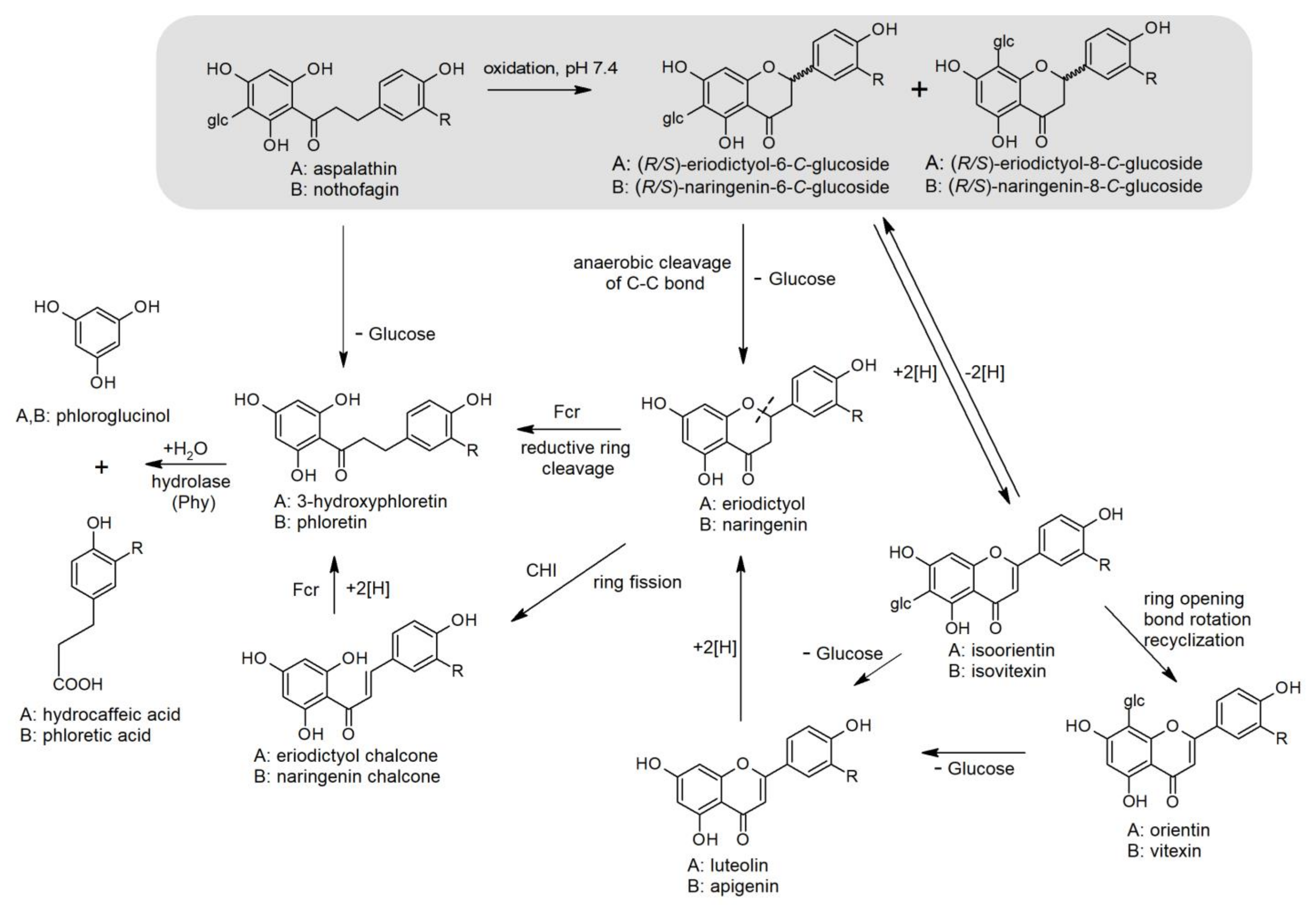
2.3.3. Microbial Biotransformation and Catabolism
3. The Therapeutic Potential of Aspalathin and Related Compounds in Targets for Diabetes from Recent Literature
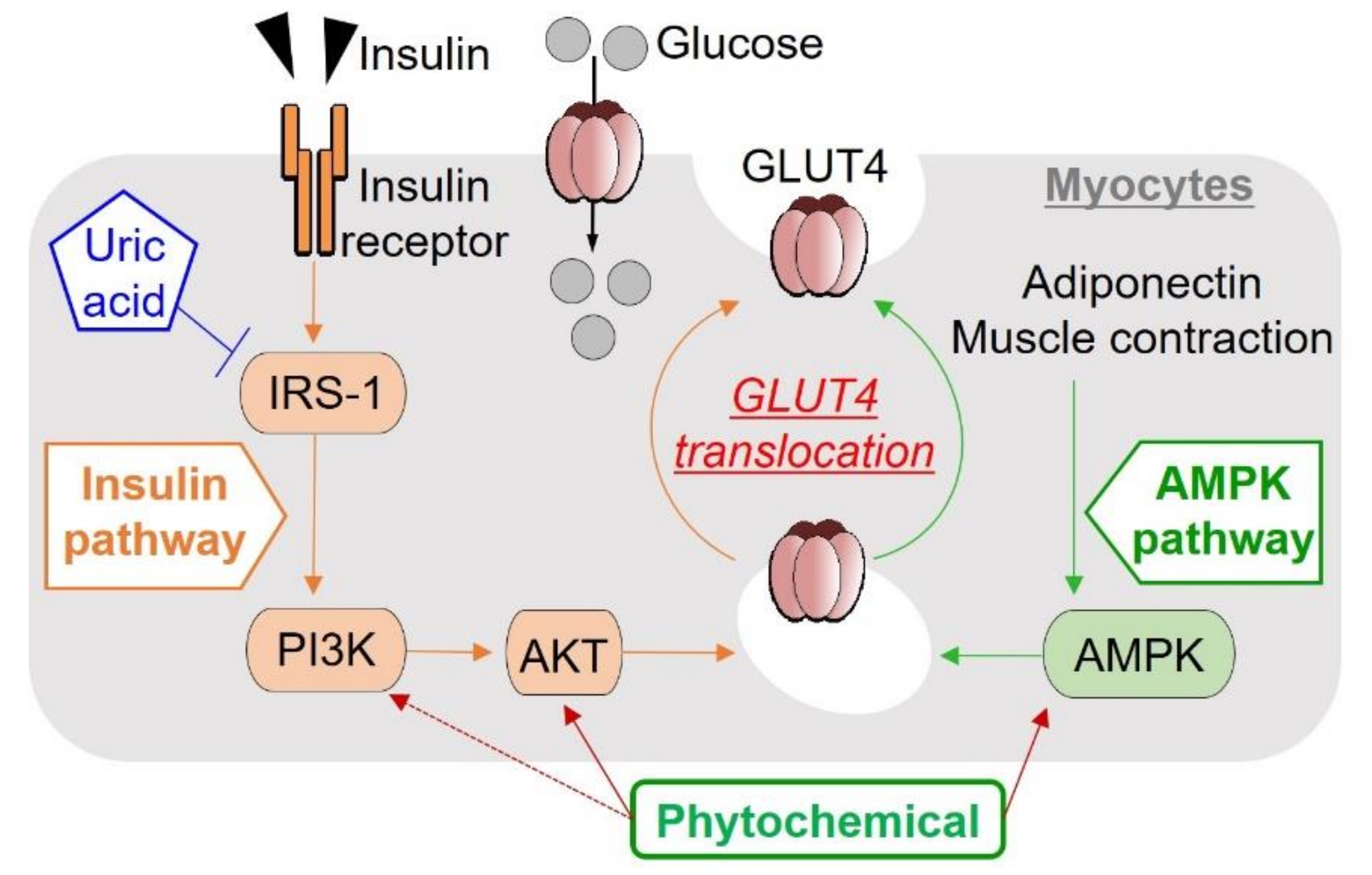
3.1. Insulin Resistance and Hyperuricemia
3.2. Muscle Metabolism
3.3. Molecular Therapeutic Targets for Glucose Homeostasis, Insulin Signaling and Pancreatic β-Cell Protection
3.4. Targeting Gut Microbiota to Reduce Inflammation and Oxidative Stress
3.5. Mitochondrial Dysfuction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| AMPK | 5′-Adenosine monophosphate-activated protein kinase |
| ASP | Aspalathin |
| AUC0-t | Area under the curve |
| BCAAs | Branched-chain amino acids |
| BW | Body mass |
| Cmax | Maximum plasma concentration |
| DAG | Diacylglycerol |
| Ddit3 | DNA damage-inducible transcript 3 |
| DW | Dry weight |
| Fcr | Flavanone- and flavanonol-cleaving reductase |
| FOXO1 | Forkhead box protein O1 |
| GCN5 | Non-repressed protein 5 |
| GLUT2/4 | Glucose transporter 2/4 |
| Hmox1 | Heme oxygenase 1 |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| IL-1β | Interleukin 1 beta |
| IL-10 | Interleukin 10 |
| IMP | Inosine-5′-monophosphate |
| IPGTT | Intraperitoneal glucose tolerance test |
| IR | Insulin resistance |
| LPH | Lactase-phloridzin hydrolase |
| LPS | Lipopolysaccharide(s) |
| MRP2 | Multidrug resistance protein 2 |
| NFκB | Nuclear factor-κB |
| NLRP3 | Nod-like receptor family pyrin domain containing 3 |
| NOT | Nothofagin |
| Nrf1 | Nuclear respiratory factor 1 |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| PKC | Protein kinase C |
| PDX1 | Pancreatic and duodenal homeobox 1 |
| PGC 1α | Peroxisome proliferator-activated receptor γ co-activator 1α |
| P-gp | P-glycoprotein |
| PTPs | Protein tyrosine phosphatases |
| PTP-MEG2 | Megakaryocyte protein tyrosine phosphatase 2 |
| ROS | Reactive oxygen species |
| SGLT1/2 | Sodium-glucose co-transporter-1/2 |
| Sirt1 | Sirtuin 1 |
| SIRT6 | Sirtuin 6 |
| SCFA | Short-chain fatty acid |
| t½ | Elimination half-life |
| tmax | Time at maximum absorption |
| Tfam | Mitochondrial transcription factor |
| TNF-α | Tumour necrosis factor-α |
| T2D | Type 2 diabetes |
| Txnip | Thioredoxin interacting protein |
| Ucp2 | Uncoupling protein 2 |
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org (accessed on 17 October 2021).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besançon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and Regional Estimates and Projections of Diabetes-Related Health Expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhu, S.; Yang, W.; Huang, Q.; Ho, C.-T. The Biological Fate and Bioefficacy of Citrus Flavonoids: Bioavailability, Biotransformation, and Delivery Systems. Food Funct. 2021, 12, 3307–3323. [Google Scholar] [CrossRef]
- Stander, M.A.; Van Wyk, B.-E.; Taylor, M.J.C.; Long, H.S. Analysis of Phenolic Compounds in Rooibos Tea (Aspalathus linearis) with a Comparison of Flavonoid-Based Compounds in Natural Populations of Plants from Different Regions. J. Agric. Food Chem. 2017, 65, 10270–10281. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Vinyagam, R.; Rajamanickam, V.; Sankaran, V.; Venkatesan, S.; David, E. Pharmacological Aspects and Potential Use of Phloretin: A Systemic Review. Mini Rev. Med. Chem. 2019, 19, 1060–1067. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The Bioavailability, Extraction, Biosynthesis and Distribution of Natural Dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Muller, C.J.F.; Malherbe, C.J.; Chellan, N.; Yagasaki, K.; Miura, Y.; Joubert, E. Potential of Rooibos, Its Major C-Glucosyl Flavonoids, and Z-2-(β-D-Glucopyranosyloxy)-3-Phenylpropenoic Acid in Prevention of Metabolic Syndrome. Crit. Rev. Food Sci. Nutr. 2018, 58, 227–246. [Google Scholar] [CrossRef]
- Johnson, R.; de Beer, D.; Dludla, P.; Ferreira, D.; Muller, C.; Joubert, E. Aspalathin from Rooibos (Aspalathus linearis): A Bioactive C-Glucosyl Dihydrochalcone with Potential to Target the Metabolic Syndrome. Planta Med. 2018, 84, 568–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Johnson, R. Hyperglycemia-Induced Oxidative Stress and Heart Disease-Cardioprotective Effects of Rooibos Flavonoids and Phenylpyruvic Acid-2-O-β-D-Glucoside. Nutr. Metab. 2017, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Braune, A.; Blaut, M. Bacterial Species Involved in the Conversion of Dietary Flavonoids in the Human Gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joubert, E.; de Beer, D. Rooibos (Aspalathus linearis) beyond the Farm Gate: From Herbal Tea to Potential Phytopharmaceutical. S. Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Joubert, E.; de Beer, D. Antioxidants of Rooibos Beverages. In Processing and Impact on Antioxidants in Beverages; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–144. ISBN 978-0-12-404738-9. [Google Scholar]
- de Beer, D.; Miller, N.; Joubert, E. Production of Dihydrochalcone-Rich Green Rooibos (Aspalathus linearis) Extract Taking into Account Seasonal and Batch-to-Batch Variation in Phenolic Composition of Plant Material. S. Afr. J. Bot. 2017, 110, 138–143. [Google Scholar] [CrossRef]
- Viraragavan, A.; Hlengwa, N.; de Beer, D.; Riedel, S.; Miller, N.; Bowles, S.; Walczak, B.; Muller, C.; Joubert, E. Model Development for Predicting in Vitro Bio-Capacity of Green Rooibos Extract Based on Composition for Application as Screening Tool in Quality Control. Food Funct. 2020, 11, 3084–3094. [Google Scholar] [CrossRef]
- de Cassia Vilhena da Silva, R.; Bolda Mariano, L.N.; Bidinha, E.R.; Bueno de Almeida, C.L.; Cechinel-Filho, V.; Santos Zanuncio, V.S.; Silva, D.B.; Gasparotto Junior, A.; de Souza, P. Ethyl Acetate Fraction from Leandra dasytricha (A. Gray) Cong. Leaves Promotes Vasodilatation and Reduces Blood Pressure in Normotensive and Hypertensive Rats. Evid.-Based Complement. Altern. Med. 2021, 2021, e7203934. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Zimmermann, B.F.; Schulze-Kaysers, N.; Schieber, A. Characterization of Phenolic and Other Polar Compounds in Peel and Flesh of Pink Guava (Psidium guajava L. Cv. ‘Criolla’) by Ultra-High Performance Liquid Chromatography with Diode Array and Mass Spectrometric Detection. Food Res. Int. 2017, 100, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, Z.; Xiang, Y.; Deng, T.; Zhao, X.; Shi, S.; Zheng, Q.; Gao, X.; Li, W. The Effects of Drying Methods on Chemical Profiles and Antioxidant Activities of Two Cultivars of Psidium guajava Fruits. LWT—Food Sci. Technol. 2020, 118, 108723. [Google Scholar] [CrossRef]
- Niederberger, K.E.; Tennant, D.R.; Bellion, P. Dietary Intake of Phloridzin from Natural Occurrence in Foods. Br. J. Nutr. 2020, 123, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Liu, H.-Y.; Luo, M.; Xia, Y.; Yang, X.; Li, H.-Y.; Wu, D.-T.; Sun, Q.; Geng, F.; Li, H.-B.; et al. Sweet Tea (Lithocarpus polystachyus Rehd.) as a New Natural Source of Bioactive Dihydrochalcones with Multiple Health Benefits. Crit. Rev. Food Sci. Nutr. 2020, 1–18. [Google Scholar] [CrossRef]
- Zielinska, D.; Szawara-Nowak, D.; Zielinski, H. Comparison of Spectrophotometric and Electrochemical Methods for the Evaluation of the Antioxidant Capacity of Buckwheat Products after Hydrothermal Treatment. J. Agric. Food Chem. 2007, 55, 6124–6131. [Google Scholar] [CrossRef]
- Li, H.; Cao, D.; Yi, J.; Cao, J.; Jiang, W. Identification of the Flavonoids in Mungbean (Phaseolus radiatus L.) Soup and Their Antioxidant Activities. Food Chem. 2012, 135, 2942–2946. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, Z. Analysis of Selected Phenolic Acids and Flavonoids in Amaranthus cruentus and Chenopodium quinoa Seeds and Sprouts by HPLC. Acta Chromatogr. 2008, 20, 661–672. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora edulis: An Insight into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef]
- da Silveira, T.F.F.; de Souza, T.C.L.; Carvalho, A.V.; Ribeiro, A.B.; Kuhnle, G.G.C.; Godoy, H.T. White Açaí Juice (Euterpe oleracea): Phenolic Composition by LC-ESI-MS/MS, Antioxidant Capacity and Inhibition Effect on the Formation of Colorectal Cancer Related Compounds. J. Funct. Foods 2017, 36, 215–223. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Liu, Q.; Bao, J.; Liu, Q. Identification and Quantification of Polyphenols in Hull, Bran and Endosperm of Common Buckwheat (Fagopyrum esculentum) Seeds. J. Funct. Foods 2017, 38, 363–369. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, P.; Qin, F.; Zhou, Q.; Gao, B.; Huang, H.; Yang, H.; Shi, H.; (Lucy) Yu, L. Chemical Composition and Antioxidative and Anti-Inflammatory Properties of Ten Commercial Mung Bean Samples. LWT—Food Sci. Technol. 2013, 54, 171–178. [Google Scholar] [CrossRef]
- Zeraik, M.L.; Yariwake, J.H. Quantification of Isoorientin and Total Flavonoids in Passiflora edulis Fruit Pulp by HPLC-UV/DAD. Microchem. J. 2010, 96, 86–91. [Google Scholar] [CrossRef]
- Sanchez, B.A.O.; Celestino, S.M.C.; de Abreu Gloria, M.B.; Celestino, I.C.; Lozada, M.I.O.; Júnior, S.D.A.; de Alencar, E.R.; de Lacerda de Oliveira, L. Pasteurization of Passion Fruit Passiflora setacea Pulp to Optimize Bioactive Compounds Retention. Food Chem. X 2020, 6, 100084. [Google Scholar] [CrossRef] [PubMed]
- Human, C.; Danton, O.; de Beer, D.; Maruyama, T.; Alexander, L.; Malherbe, C.; Hamburger, M.; Joubert, E. Identification of a Novel Di-C-Glycosyl Dihydrochalcone and the Thermal Stability of Polyphenols in Model Ready-to-Drink Beverage Solutions with Cyclopia subternata Extract as Functional Ingredient. Food Chem. 2021, 351, 129273. [Google Scholar] [CrossRef]
- Sadilova, E.; Stintzing, F.C.; Kammerer, D.R.; Carle, R. Matrix Dependent Impact of Sugar and Ascorbic Acid Addition on Color and Anthocyanin Stability of Black Carrot, Elderberry and Strawberry Single Strength and from Concentrate Juices upon Thermal Treatment. Food Res. Int. 2009, 42, 1023–1033. [Google Scholar] [CrossRef]
- Capuano, E.; Oliviero, T.; van Boekel, M.A.J.S. Modeling Food Matrix Effects on Chemical Reactivity: Challenges and Perspectives. Crit. Rev. Food Sci. Nutr. 2018, 58, 2814–2828. [Google Scholar] [CrossRef]
- Walters, N.A.; de Villiers, A.; Joubert, E.; de Beer, D. Improved HPLC Method for Rooibos Phenolics Targeting Changes Due to Fermentation. J. Food Compos. Anal. 2017, 55, 20–29. [Google Scholar] [CrossRef]
- de Beer, D.; Tobin, J.; Walczak, B.; van der Rijst, M.; Joubert, E. Phenolic Composition of Rooibos Changes during Simulated Fermentation: Effect of Endogenous Enzymes and Fermentation Temperature on Reaction Kinetics. Food Res. Int. 2019, 121, 185–196. [Google Scholar] [CrossRef]
- Miller, N.; de Beer, D.; Aucamp, M.; Malherbe, C.J.; Joubert, E. Inulin as Microencapsulating Agent Improves Physicochemical Properties of Spray-Dried Aspalathin-Rich Green Rooibos (Aspalathus linearis) Extract with α-Glucosidase Inhibitory Activity. J. Funct. Foods 2018, 48, 400–409. [Google Scholar] [CrossRef]
- Human, C.; de Beer, D.; Muller, M.; van der Rijst, M.; Aucamp, M.; Tredoux, A.; de Villiers, A.; Joubert, E. Shelf-Life Stability of Ready-to-Use Green Rooibos Iced Tea Powder—Assessment of Physical, Chemical, and Sensory Properties. Molecules 2021, 26, 5260. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Duncan, C.E.; Talcott, S.T. Phytochemical Composition and Thermal Stability of Two Commercial Açai Species, Euterpe oleracea and Euterpe precatoria. Food Chem. 2009, 115, 1199–1205. [Google Scholar] [CrossRef]
- de Paepe, D.; Valkenborg, D.; Coudijzer, K.; Noten, B.; Servaes, K.; de Loose, M.; Voorspoels, S.; Diels, L.; van Droogenbroeck, B. Thermal Degradation of Cloudy Apple Juice Phenolic Constituents. Food Chem. 2014, 162, 176–185. [Google Scholar] [CrossRef]
- van der Sluis, A.A.; Dekker, M.; van Boekel, M.A.J.S. Activity and Concentration of Polyphenolic Antioxidants in Apple Juice. 3. Stability during Storage. J. Agric. Food Chem. 2005, 53, 1073–1080. [Google Scholar] [CrossRef]
- Heras-Ramírez, M.E.; Quintero-Ramos, A.; Camacho-Dávila, A.A.; Barnard, J.; Talamás-Abbud, R.; Torres-Muñoz, J.V.; Salas-Muñoz, E. Effect of Blanching and Drying Temperature on Polyphenolic Compound Stability and Antioxidant Capacity of Apple Pomace. Food Bioprocess Technol. 2012, 5, 2201–2210. [Google Scholar] [CrossRef]
- Lavelli, V.; Vantaggi, C. Rate of Antioxidant Degradation and Color Variations in Dehydrated Apples as Related to Water Activity. J. Agric. Food Chem. 2009, 57, 4733–4738. [Google Scholar] [CrossRef]
- Lavelli, V.; Corti, S. Phloridzin and Other Phytochemicals in Apple Pomace: Stability Evaluation upon Dehydration and Storage of Dried Product. Food Chem. 2011, 129, 1578–1583. [Google Scholar] [CrossRef]
- Gulsunoglu, Z.; Karbancioglu-Guler, F.; Raes, K.; Kilic-Akyilmaz, M. Soluble and Insoluble-Bound Phenolics and Antioxidant Activity of Various Industrial Plant Wastes. Int. J. Food Prop. 2019, 22, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Sęczyk, Ł.; Gawlik-Dziki, U.; Świeca, M. Influence of Phenolic-Food Matrix Interactions on In Vitro Bioaccessibility of Selected Phenolic Compounds and Nutrients Digestibility in Fortified White Bean Paste. Antioxidants 2021, 10, 1825. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A Review on Insoluble-Bound Phenolics in Plant-Based Food Matrix and Their Contribution to Human Health with Future Perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Owens, J. Chris Lipinski Discusses Life and Chemistry after the Rule of Five. Drug Discov. Today 2003, 8, 12–16. [Google Scholar] [CrossRef]
- Courts, F.L.; Williamson, G. The Occurrence, Fate and Biological Activities of C-Glycosyl Flavonoids in the Human Diet. Crit. Rev. Food Sci. Nutr. 2015, 55, 1352–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breiter, T.; Laue, C.; Kressel, G.; Gröll, S.; Engelhardt, U.H.; Hahn, A. Bioavailability and Antioxidant Potential of Rooibos Flavonoids in Humans Following the Consumption of Different Rooibos Formulations. Food Chem. 2011, 128, 338–347. [Google Scholar] [CrossRef]
- Courts, F.L.; Williamson, G. The C-Glycosyl Flavonoid, Aspalathin, Is Absorbed, Methylated and Glucuronidated Intact in Humans. Mol. Nutr. Food Res. 2009, 53, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Mullen, W.; Pecorari, M.; Serafini, M.; Crozier, A. Bioavailability of C-Linked Dihydrochalcone and Flavanone Glucosides in Humans Following Ingestion of Unfermented and Fermented Rooibos Teas. J. Agric. Food Chem. 2009, 57, 7104–7111. [Google Scholar] [CrossRef]
- Kreuz, S.; Joubert, E.; Waldmann, K.-H.; Ternes, W. Aspalathin, a Flavonoid in Aspalathus linearis (Rooibos), Is Absorbed by Pig Intestine as a C-Glycoside. Nutr. Res. 2008, 28, 690–701. [Google Scholar] [CrossRef]
- Bowles, S.; Joubert, E.; de Beer, D.; Louw, J.; Brunschwig, C.; Njoroge, M.; Lawrence, N.; Wiesner, L.; Chibale, K.; Muller, C. Intestinal Transport Characteristics and Metabolism of C-Glucosyl Dihydrochalcone, Aspalathin. Molecules 2017, 22, 554. [Google Scholar] [CrossRef] [Green Version]
- Damiani, E.; Carloni, P.; Rocchetti, G.; Senizza, B.; Tiano, L.; Joubert, E.; de Beer, D.; Lucini, L. Impact of Cold versus Hot Brewing on the Phenolic Profile and Antioxidant Capacity of Rooibos (Aspalathus linearis) Herbal Tea. Antioxidants 2019, 8, 499. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tie, X.; Bao, B.; Wu, X.; Zhang, Y. Metabolism of Flavone C-Glucosides and p-Coumaric Acid from Antioxidant of Bamboo Leaves (AOB) in Rats. Br. J. Nutr. 2007, 97, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Huo, T.; Qin, F.; Lu, X.; Li, F. Determination and Pharmacokinetics of Orientin in Rabbit Plasma by Liquid Chromatography after Intravenous Administration of Orientin and Trollius chinensis Bunge Extract. J. Chromatogr. B 2007, 853, 221–226. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Yuan, Z.; Zhang, L.; Xu, L.; Cui, Y.; Duan, K. Pharmacokinetics and Tissue Distribution Study of Orientin in Rat by Liquid Chromatography. J. Pharm. Biomed. Anal. 2008, 47, 429–434. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Xu, L.; Li, M.; Jing, X.; Zhang, L. Pharmacokinetic Study of Three Active Flavonoid Glycosides in Rat after Intravenous Administration of Trollius ledebourii Extract by Liquid Chromatography. Biomed. Chromatogr. 2008, 22, 1130–1136. [Google Scholar] [CrossRef]
- Sun, Y.; Tsao, R.; Chen, F.; Li, H.; Wang, J.; Peng, H.; Zhang, K.; Deng, Z. The Phytochemical Composition, Metabolites, Bioavailability and in Vivo Antioxidant Activity of Tetrastigma hemsleyanum Leaves in Rats. J. Funct. Foods 2017, 30, 179–193. [Google Scholar] [CrossRef]
- Tremmel, M.; Kiermaier, J.; Heilmann, J. In Vitro Metabolism of Six C-Glycosidic Flavonoids from Passiflora incarnata L. Int. J. Mol. Sci. 2021, 22, 6566. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.-G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by Small Intestinal Epithelial Cell α-Glucosidases Is a Critical Step in the Absorption and Metabolism of Dietary Flavonoid Glycosides in Humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Mi, L.; Shen, X.; Feng, T.; Liu, X.; Wang, Q. Study on Pharmacokinetics, Tissue Distribution, and Excretion of Phloretin and Its Prodrug 2′,4′,6′,4-Tetra-O-Acetylphloretin in Rats Using LC–MS/MS. Acta Chromatogr. 2019, 31, 63–70. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fan, Y.; Wang, M.; Wang, J.; Cheng, J.X.; Zou, J.B.; Zhang, X.F.; Shi, Y.J.; Guo, D.Y. Studies on Pharmacokinetic Properties and Absorption Mechanism of Phloretin: In Vivo and in Vitro. Biomed. Pharmacother. 2020, 132, 110809. [Google Scholar] [CrossRef]
- Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. Bioavailability of Phloretin and Phloridzin in Rats. J. Nutr. 2001, 131, 3227–3230. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Gao, Z.; Wang, A.; Jia, L.; Zhang, X.; Fang, M.; Yi, K.; Li, Q.; Hu, H. Comparative Oral and Intravenous Pharmacokinetics of Phlorizin in Rats Having Type 2 Diabetes and in Normal Rats Based on Phase II Metabolism. Food Funct. 2019, 10, 1582–1594. [Google Scholar] [CrossRef]
- Stracke, B.A.; Rüfer, C.E.; Bub, A.; Seifert, S.; Weibel, F.P.; Kunz, C.; Watzl, B. No Effect of the Farming System (Organic/Conventional) on the Bioavailability of Apple (Malus domestica Bork., Cultivar Golden Delicious) Polyphenols in Healthy Men: A Comparative Study. Eur. J. Nutr. 2010, 49, 301–310. [Google Scholar] [CrossRef]
- Mullen, W.; Borges, G.; Lean, M.E.J.; Roberts, S.A.; Crozier, A. Identification of Metabolites in Human Plasma and Urine after Consumption of a Polyphenol-Rich Juice Drink. J. Agric. Food Chem. 2010, 58, 2586–2595. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Braune, A.; Gütschow, M.; Blaut, M. An NADH-Dependent Reductase from Eubacterium ramulus Catalyzes the Stereospecific Heteroring Cleavage of Flavanones and Flavanonols. Appl. Environ. Microbiol. 2019, 85, e01233-19. [Google Scholar] [CrossRef] [Green Version]
- Maurer, J.M.; Schellekens, R.C.A.; van Rieke, H.M.; Wanke, C.; Iordanov, V.; Stellaard, F.; Wutzke, K.D.; Dijkstra, G.; van der Zee, M.; Woerdenbag, H.J.; et al. Gastrointestinal PH and Transit Time Profiling in Healthy Volunteers Using the IntelliCap System Confirms Ileo-Colonic Release of ColoPulse Tablets. PLoS ONE 2015, 10, e0129076. [Google Scholar] [CrossRef]
- Wei, B.; Wang, Y.-K.; Qiu, W.-H.; Wang, S.-J.; Wu, Y.-H.; Xu, X.-W.; Wang, H. Discovery and Mechanism of Intestinal Bacteria in Enzymatic Cleavage of C–C Glycosidic Bonds. Appl. Microbiol. Biotechnol. 2020, 104, 1883–1890. [Google Scholar] [CrossRef]
- Braune, A.; Blaut, M. Intestinal Bacterium Eubacterium cellulosolvens. Deglycosylates Flavonoid C- and O-Glucosides. Appl. Environ. Microbiol. 2012, 78, 8151–8153. [Google Scholar] [CrossRef] [Green Version]
- Braune, A.; Blaut, M. Deglycosylation of Puerarin and Other Aromatic C-Glucosides by a Newly Isolated Human Intestinal Bacterium: A Newly Isolated C-Glucoside-Cleaving Bacterium. Environ. Microbiol. 2011, 13, 482–494. [Google Scholar] [CrossRef]
- Zheng, S.; Geng, D.; Liu, S.; Wang, Q.; Liu, S.; Wang, R. A Newly Isolated Human Intestinal Bacterium Strain Capable of Deglycosylating Flavone C-Glycosides and Its Functional Properties. Microb. Cell Fact. 2019, 18, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goris, T.; Cuadrat, R.R.C.; Braune, A. Flavonoid-Modifying Capabilities of the Human Gut Microbiome—An In Silico Study. Nutrients 2021, 13, 2688. [Google Scholar] [CrossRef] [PubMed]
- Herles, C.; Braune, A.; Blaut, M. First Bacterial Chalcone Isomerase Isolated from Eubacterium ramulus. Arch. Microbiol. 2004, 181, 428–434. [Google Scholar] [CrossRef]
- Gall, M.; Thomsen, M.; Peters, C.; Pavlidis, I.; Jonczyk, P.; Grünert, P.; Beutel, S.; Scheper, T.; Gross, E.; Backes, M.; et al. Enzymatic Conversion of Flavonoids Using Bacterial Chalcone Isomerase and Enoate Reductase. Angew. Chem. Int. Ed. Engl. 2014, 53, 1439–1442. [Google Scholar] [CrossRef]
- Schoefer, L.; Braune, A.; Blaut, M. Cloning and Expression of a Phloretin Hydrolase Gene from Eubacterium ramulus and Characterization of the Recombinant Enzyme. Appl. Environ. Microbiol. 2004, 70, 6131–6137. [Google Scholar] [CrossRef] [Green Version]
- Braune, A.; Engst, W.; Blaut, M. Degradation of Neohesperidin Dihydrochalcone by Human Intestinal Bacteria. J. Agric. Food Chem. 2005, 53, 1782–1790. [Google Scholar] [CrossRef]
- Yang, G.; Hong, S.; Yang, P.; Sun, Y.; Wang, Y.; Zhang, P.; Jiang, W.; Gu, Y. Discovery of an Ene-Reductase for Initiating Flavone and Flavonol Catabolism in Gut Bacteria. Nat. Commun. 2021, 12, 790. [Google Scholar] [CrossRef]
- Braune, A.; Gütschow, M.; Engst, W.; Blaut, M. Degradation of Quercetin and Luteolin by Eubacterium ramulus. Appl. Environ. Microbiol. 2001, 67, 5558–5567. [Google Scholar] [CrossRef] [Green Version]
- Schoefer, L.; Mohan, R.; Schwiertz, A.; Braune, A.; Blaut, M. Anaerobic Degradation of Flavonoids by Clostridium orbiscindens. Appl. Environ. Microbiol. 2003, 69, 5849–5854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys)Function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Bruns, I.; Sauer, B.; Burger, M.C.; Eriksson, J.; Hofmann, U.; Braun, Y.; Harter, P.N.; Luger, A.-L.; Ronellenfitsch, M.W.; Steinbach, J.P.; et al. Disruption of Peroxisome Proliferator–Activated Receptor γ Coactivator (PGC)-1α Reverts Key Features of the Neoplastic Phenotype of Glioma Cells. J. Biol. Chem. 2019, 294, 3037–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Pina, A.F.; Borges, D.O.; Meneses, M.J.; Branco, P.; Birne, R.; Vilasi, A.; Macedo, M.P. Insulin: Trigger and Target of Renal Functions. Front. Cell Developm. Biol. 2020, 8, 519. [Google Scholar] [CrossRef]
- Gill, A.; Kukreja, S.; Malhotra, N.; Chhabra, N. Correlation of the Serum Insulin and the Serum Uric Acid Levels with the Glycated Haemoglobin Levels in the Patients of Type 2 Diabetes Mellitus. J. Clin. Diagn. Res. 2013, 7, 1295–1297. [Google Scholar] [CrossRef]
- Bardin, T.; Richette, P. Definition of Hyperuricemia and Gouty Conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, Y.; Huang, T.; Zhang, Y.; Li, Z.; Luo, C.; Luo, Y.; Yuan, H.; Hisatome, I.; Yamamoto, T.; et al. High Uric Acid Directly Inhibits Insulin Signalling and Induces Insulin Resistance. Biochem. Biophys. Res. Commun. 2014, 447, 707–714. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Hyperuricemia-Induced Endothelial Insulin Resistance: The Nitric Oxide Connection. Pflugers Arch. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Hirano, Y.; Nishio, M.; Furuya, Y.; Nakamura, H.; Watanabe, T. Xanthine Oxidase Inhibitory Activity and Hypouricemic Effect of Aspalathin from Unfermented Rooibos. J. Food Sci. 2013, 78, H1935–H1939. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.-I.; Yoshizawa, F.; Yagasaki, K. Hyperuricemia in Type 2 Diabetic Model KK-Ay/Ta Mice: A Potent Animal Model with Positive Correlation between Insulin Resistance and Plasma High Uric Acid Levels. BMC Res. Notes 2017, 10, 577. [Google Scholar] [CrossRef] [Green Version]
- Kondo, S.; Adachi, S.; Yoshizawa, F.; Yagasaki, K. Antidiabetic Effect of Taxifolin in Cultured L6 Myotubes and Type 2 Diabetic Model KK-Ay/Ta Mice with Hyperglycemia and Hyperuricemia. Curr. Issues Mol. Biol. 2021, 43, 1293–1306. [Google Scholar] [CrossRef]
- Adachi, S.; Nihei, K.; Ishihara, Y.; Yoshizawa, F.; Yagasaki, K. Anti-Hyperuricemic Effect of Taxifolin in Cultured Hepatocytes and Model Mice. Cytotechnology 2017, 69, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sánchez, F.D.; Vargas-Abonce, V.P.; Guerrero-Castillo, A.P.; Santos-Villavicencio, M.D.L.; Eseiza-Acevedo, J.; Meza-Arana, C.E.; Gulias-Herrero, A.; Gómez-Sámano, M.Á. Serum Uric Acid Concentration is Associated with Insulin Resistance and Impaired Insulin Secretion in Adults at Risk for Type 2 Diabetes. Prim. Care Diabetes 2021, 15, 293–299. [Google Scholar] [CrossRef]
- Dhanya, R.; Arya, A.D.; Nisha, P.; Jayamurthy, P. Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line. Front. Pharmacol. 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and Its Metabolite Isorhamnetin Promote Glucose Uptake through Different Signalling Pathways in Myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Williamson, G. Quercetin Lowers Plasma Uric Acid in Pre-Hyperuricaemic Males: A Randomised, Double-Blinded, Placebo-Controlled, Cross-over Trial. Br. J. Nutr. 2016, 115, 800–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, S.-I.; Kondo, S.; Sato, Y.; Yoshizawa, F.; Yagasaki, K. Anti-Hyperuricemic Effect of Isorhamnetin in Cultured Hepatocytes and Model Mice: Structure-Activity Relationships of Methylquercetins as Inhibitors of Uric Acid Production. Cytotechnology 2019, 71, 181–192. [Google Scholar] [CrossRef]
- Komatsu, W.; Kishi, H.; Yagasaki, K.; Ohhira, S. Urolithin A Attenuates Pro-Inflammatory Mediator Production by Suppressing PI3-K/Akt/NF-ΚB and JNK/AP-1 Signaling Pathways in Lipopolysaccharide-Stimulated RAW264 Macrophages: Possible Involvement of NADPH Oxidase-Derived Reactive Oxygen Species. Eur. J. Pharmacol. 2018, 833, 411–424. [Google Scholar] [CrossRef]
- Adachi, S.; Sasaki, K.; Kondo, S.; Komatsu, W.; Yoshizawa, F.; Isoda, H.; Yagasaki, K. Antihyperuricemic Effect of Urolithin A in Cultured Hepatocytes and Model Mice. Molecules 2020, 25, 5136. [Google Scholar] [CrossRef]
- Adachi, S.-I.; Oyama, M.; Kondo, S.; Yagasaki, K. Comparative Effects of Quercetin, Luteolin, Apigenin and Their Related Polyphenols on Uric Acid Production in Cultured Hepatocytes and Suppression of Purine Bodies-Induced Hyperuricemia by Rutin in Mice. Cytotechnology 2021, 73, 343–351. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9221. [Google Scholar] [CrossRef]
- Ghasemi, A. Uric Acid-Induced Pancreatic β-Cell Dysfunction. BMC Endocr. Disord. 2021, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin Signalling and the Regulation of Glucose and Lipid Metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Qu, Z.; Zhou, S.; Li, P.; Liu, C.; Yuan, B.; Zhang, S.; Liu, A. Natural Products and Skeletal Muscle Health. J. Nutr. Biochem. 2021, 93, 108619. [Google Scholar] [CrossRef] [PubMed]
- Kawano, A.; Nakamura, H.; Hata, S.; Minakawa, M.; Miura, Y.; Yagasaki, K. Hypoglycemic Effect of Aspalathin, a Rooibos Tea Component from Aspalathus linearis, in Type 2 Diabetic Model db/db Mice. Phytomedicine 2009, 16, 437–443. [Google Scholar] [CrossRef]
- Son, M.J.; Minakawa, M.; Miura, Y.; Yagasaki, K. Aspalathin Improves Hyperglycemia and Glucose Intolerance in Obese Diabetic ob/ob Mice. Eur. J. Nutr. 2013, 52, 1607–1619. [Google Scholar] [CrossRef]
- Yagasaki, K. Phytochemicals, Their Intestinal Metabolites, and Skeletal Muscle Function. In Nutrition and Skeletal Muscle; Walrand, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 421–438. ISBN 978-0-12-810410-1. [Google Scholar]
- Kamakura, R.; Son, M.J.; de Beer, D.; Joubert, E.; Miura, Y.; Yagasaki, K. Antidiabetic Effect of Green Rooibos (Aspalathus linearis) Extract in Cultured Cells and Type 2 Diabetic Model KK-Ay Mice. Cytotechnology 2015, 67, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sociali, G.; Magnone, M.; Ravera, S.; Damonte, P.; Vigliarolo, T.; Von Holtey, M.; Vellone, V.G.; Millo, E.; Caffa, I.; Cea, M.; et al. Pharmacological Sirt6 Inhibition Improves Glucose Tolerance in a Type 2 Diabetes Mouse Model. FASEB J. 2017, 31, 3138–3149. [Google Scholar] [CrossRef] [Green Version]
- Mutlu, B.; Puigserver, P. GCN5 Acetyltransferase in Cellular Energetic and Metabolic Processes. BBA-Gene Regul. Mech. 2021, 1864, 194626. [Google Scholar] [CrossRef]
- Kuang, J.; Chen, L.; Tang, Q.; Zhang, J.; Li, Y.; He, J. The Role of SIRT6 in Obesity and Diabetes. Front. Physiol. 2018, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Rahnasto-Rilla, M.; Kokkola, T.; Jarho, E.; Lahtela-Kakkonen, M.; Moaddel, R. N-Acylethanolamines Bind to SIRT6. ChemBioChem 2016, 17, 77–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heger, V.; Tyni, J.; Hunyadi, A.; Horáková, L.; Lahtela-Kakkonen, M.; Rahnasto-Rilla, M. Quercetin Based Derivatives as Sirtuin Inhibitors. Biomed. Pharmacother. 2019, 111, 1326–1333. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Sousa, J.L.C.; Carvalho, F.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. Inhibition of Protein Tyrosine Phosphatase 1B by Flavonoids: A Structure—Activity Relationship Study. Food Chem. Toxicol. 2018, 111, 474–481. [Google Scholar] [CrossRef]
- Tiwari, N. Therapeutic Targets for Diabetes Mellitus: An Update. Clin. Pharmacol. Biopharm. 2014, 3, 1. [Google Scholar] [CrossRef]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-Glycosylation on Anti-Diabetic, Anti-Alzheimer’s Disease and Anti-Inflammatory Potential of Apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Viraragavan, A. Assessment of Chemical Markers as Surrogates for Efficacy and Safety of Rooibos Extracts. Master’s Thesis, University of Zululand, KwaDlangezwa, South Africa, 2017. [Google Scholar]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, Y.M.; Park, H.J.; Sohn, H.S.; Jung, H.A. The Effects of C-Glycosylation of Luteolin on Its Antioxidant, Anti-Alzheimer’s Disease, Anti-Diabetic, and Anti-Inflammatory Activities. Arch. Pharm. Res. 2014, 37, 1354–1363. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Yu, J.S.; Hwang, J.Y.; So, H.M.; Seo, S.O.; Kim, J.K.; Jang, T.S.; Chung, S.J.; Kim, K.H. Phloridzin Acts as an Inhibitor of Protein-Tyrosine Phosphatase MEG2 Relevant to Insulin Resistance. Molecules 2021, 26, 1612. [Google Scholar] [CrossRef] [PubMed]
- Dominguez Rieg, J.A.; Rieg, T. What Does Sodium-Glucose Co-Transporter 1 Inhibition Add: Prospects for Dual Inhibition. Diabetes Obes. Metab. 2019, 21 (Suppl. S2), 43–52. [Google Scholar] [CrossRef] [Green Version]
- Dudash, J.; Zhang, X.; Zeck, R.E.; Johnson, S.G.; Cox, G.G.; Conway, B.R.; Rybczynski, P.J.; Demarest, K.T. Glycosylated Dihydrochalcones as Potent and Selective Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 5121–5125. [Google Scholar] [CrossRef]
- Ho, L.-T.; Kulkarni, S.S.; Lee, J.-C. Development of Sodium-Dependent Glucose Co-Transporter 2 Inhibitors as Potential Anti-Diabetic Therapeutics. Curr. Top. Med. Chem. 2011, 11, 1476–1512. [Google Scholar] [CrossRef]
- Jesus, A.R.; Vila-Viçosa, D.; Machuqueiro, M.; Marques, A.P.; Dore, T.M.; Rauter, A.P. Targeting Type 2 Diabetes with C-Glucosyl Dihydrochalcones as Selective Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: Synthesis and Biological Evaluation. J. Med. Chem. 2017, 60, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, H.; Meng, F. In Silico Modeling of Aspalathin and Nothofagin against SGLT2. J. Theor. Comput. Chem. 2015, 14, 1550056. [Google Scholar] [CrossRef]
- Moens, C.; Bensellam, M.; Himpe, E.; Muller, C.J.F.; Jonas, J.; Bouwens, L. Aspalathin Protects Insulin-Producing β Cells against Glucotoxicity and Oxidative Stress-Induced Cell Death. Mol. Nutr. Food Res. 2020, 64, 1901009. [Google Scholar] [CrossRef] [PubMed]
- Wondafrash, D.Z.; Nire’a, A.T.; Tafere, G.G.; Desta, D.M.; Berhe, D.A.; Zewdie, K.A. Thioredoxin-Interacting Protein as a Novel Potential Therapeutic Target in Diabetes Mellitus and Its Underlying Complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, K.; Zhang, S.; Wu, Z.; Zhu, D.; Chen, F.; Lei, Z.-N.; Liu, W.; Xiao, C.; Chen, Z.-S. Reconstruction of Intestinal Microecology of Type 2 Diabetes by Fecal Microbiota Transplantation: Why and How. Bosn. J. Basic Med. Sci. 2021. [Google Scholar] [CrossRef]
- Li, W.-Z.; Stirling, K.; Yang, J.-J.; Zhang, L. Gut Microbiota and Diabetes: From Correlation to Causality and Mechanism. World J. Diabetes 2020, 11, 293–308. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut Microbiome and Type 2 Diabetes: Where We Are and Where to Go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P.-F. Diabetes and Gut Microbiota. World J. Diabetes 2021, 12, 1693–1703. [Google Scholar] [CrossRef]
- Guo, G.L.; Xie, W. Metformin Action through the Microbiome and Bile Acids. Nat. Med. 2018, 24, 1789–1790. [Google Scholar] [CrossRef]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- Yuan, L.; Li, X.; He, S.; Gao, C.; Wang, C.; Shao, Y. Effects of Natural Flavonoid Isoorientin on Growth Performance and Gut Microbiota of Mice. J. Agric. Food Chem. 2018, 66, 9777–9784. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis Gut Microbiota Associated with Inflammation and Impaired Mucosal Immune Function in Intestine of Humans with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2015, 5, 8096. [Google Scholar] [CrossRef]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 Cells in Human Disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef]
- He, S.; Li, X.; Li, C.; Deng, H.; Shao, Y.; Yuan, L. Isoorientin Attenuates Benzo[a]Pyrene-Induced Colonic Injury and Gut Microbiota Disorders in Mice. Food Res. Int. 2019, 126, 108599. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and Antimicrobial Activity of Phloretin and Its Glycosilated Derivatives Present in Apple and Kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef]
- Chang, W.-T.; Huang, W.-C.; Liou, C.-J. Evaluation of the Anti-Inflammatory Effects of Phloretin and Phlorizin in Lipopolysaccharide-Stimulated Mouse Macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; An, Y.; Ren, J.; Yan, D.; Cui, J.; Li, D.; Li, M.; Wang, M.; Zhong, G. Phloretin Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice by Regulating the Gut Microbiota. Pharmacol. Res. 2019, 150, 104489. [Google Scholar] [CrossRef]
- Ninfali, P.; Dominici, S.; Angelino, D.; Gennari, L.; Buondelmonte, C.; Giorgi, L. An Enzyme-Linked Immunosorbent Assay for the Measurement of Plasma Flavonoids in Mice Fed Apigenin-C-Glycoside. J. Sci. Food Agric. 2013, 93, 3087–3093. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.I.G.; Rios-Santos, F.; Balogun, S.O.; de Oliveira Martins, D.T. Vitexin Reduces Neutrophil Migration to Inflammatory Focus by Down-Regulating pro-Inflammatory Mediators via Inhibition of P38, ERK1/2 and JNK Pathway. Phytomedicine 2016, 23, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, T.; Zhang, Y.; Huang, K.; Yang, S.; Lv, H.; Chen, Y.; Zhang, C.; Guan, X. Vitexin Alleviates High-Fat Diet Induced Brain Oxidative Stress and Inflammation via Anti-Oxidant, Anti-Inflammatory and Gut Microbiota Modulating Properties. Free Radic. Biol. Med. 2021, 171, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; de Vos, W.M.; Belzer, C. Action and Function of Akkermansia muciniphila in Microbiome Ecology, Health and Disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Mangwana, N. The in Vitro Faecal Evaluation of Prebiotic Effects of Rooibos Phenolic Compounds on the Gut Microbiota of Vervet Monkeys (Chlorocebus Pygerythrus). Master’s Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2020. [Google Scholar]
- Mthembu, S.X.H.; Muller, C.J.F.; Dludla, P.V.; Madoroba, E.; Kappo, A.P.; Mazibuko-Mbeje, S.E. Rooibos Flavonoids, Aspalathin, Isoorientin, and Orientin Ameliorate Antimycin A-Induced Mitochondrial Dysfunction by Improving Mitochondrial Bioenergetics in Cultured Skeletal Muscle Cells. Molecules 2021, 26, 6289. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Johnson, R.; Joubert, E.; Louw, J.; Ziqubu, K.; Tiano, L.; Silvestri, S.; Orlando, P.; Opoku, A.R.; et al. Aspalathin, a Natural Product with the Potential to Reverse Hepatic Insulin Resistance by Improving Energy Metabolism and Mitochondrial Respiration. PLoS ONE 2019, 14, e0216172. [Google Scholar] [CrossRef] [Green Version]
- Dludla, P.V.; Johnson, R.; Mazibuko-Mbeje, S.E.; Muller, C.J.F.; Louw, J.; Joubert, E.; Orlando, P.; Silvestri, S.; Chellan, N.; Nkambule, B.B.; et al. Fermented Rooibos Extract Attenuates Hyperglycemia-Induced Myocardial Oxidative Damage by Improving Mitochondrial Energetics and Intracellular Antioxidant Capacity. S. Afr. J. Bot. 2020, 131, 143–150. [Google Scholar] [CrossRef]
| Compound | Infusion (mg/L) 1 | Extract (g/kg) 2 | ||
|---|---|---|---|---|
| Fermented (n = 114) 3 | Green (n= 29) 4 | Fermented (n= 74) 3 | Green (n = 10) 4 | |
| Aspalathin | 5.8 (nd 5–15.7) 6 | 179 (76–255) | 5.8 (1.6–15) | 95 (54–116) |
| Nothofagin | 1.0 (nd–2.8) | 15 (7–25) | 0.7 (0.3–1.8) | 7.7 (3.6–12) |
| Orientin | 11 (10–14) | 15 (7.6–21) | 7.9 (4.4–9.0) | 8.6 (7.4–9.7) |
| Isoorientin | 15 (7.4–21) | 15 (7.6–21) | 8.3 (4.7–10.3 | 9.1 (7.2–10.6) |
| Vitexin | 2.3 (1.3–3.3) | 2.4 (1.2–3.3) | nq 5 | 1.6 (1.2–1.8) |
| Isovitexin | 2.4 (1.4–3.3) | 3.0 (1.6–4.4) | nq | 1.9 (1.5–2.2) |
| Dosage Form | ASP (mg) 1 | NOT (mg) 1 | Compound and Metabolites 2 | ASP Excretion in Urine | Ref. | |
|---|---|---|---|---|---|---|
| Plasma | Urine | |||||
| Green rooibos infusion (300 mL; 14 g/L, added to boiling water and infused for 10 min) | 91 | nq 3 | nd 4 | Methylated ASP; methylated and glucuronidated ASP | Max. conc. reached <2 h after ingestion; 0.74% excreted during 0–24 h | [49] |
| Green rooibos ‘ready-to-drink’ beverage (500 mL) | 41 | 7 | nd | Glucuronidated ASP (2); methylated and glucuronidated ASP (3); methylated and sulphated ASP; sulphated ASP; NOT & metabolites not detected | Most excreted <5 h after ingestion; 0.22% excreted during 0–24 h | [50] |
| Fermented rooibos ‘ready-to-drink’ beverage (500 mL) | 3.6 | 0.8 | nd | methylated and glucuronidated ASP (3); methylated and sulphated ASP; sulphated ASP; NOT & metabolites not detected | 0.09% excreted during 0–24 h | [50] |
| Green rooibos infusion (20 g/L; 10 min infused in freshly-boiled water) | 287 | 34 | ASP | ASP; glucuronidated ASP; methylated ASP; methylated and glucuronidated ASP (3); methylated and sulphated ASP; sulphated ASP; glucuronidated 3-hydroxyphoretin; NOT; glucuronidated NOT; glucuronidated phloretin | 0.17% recovery rate at tmax | [48] |
| Isolated fraction 5 of green rooibos, reconstituted in 500 mL water to similar phenolic content as green rooibos infusion | ASP | As for infusion, except for glucuronidated nothofagin | 0.10% recovery rate at tmax | [48] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muller, C.J.F.; Joubert, E.; Chellan, N.; Miura, Y.; Yagasaki, K. New Insights into the Efficacy of Aspalathin and Other Related Phytochemicals in Type 2 Diabetes—A Review. Int. J. Mol. Sci. 2022, 23, 356. https://doi.org/10.3390/ijms23010356
Muller CJF, Joubert E, Chellan N, Miura Y, Yagasaki K. New Insights into the Efficacy of Aspalathin and Other Related Phytochemicals in Type 2 Diabetes—A Review. International Journal of Molecular Sciences. 2022; 23(1):356. https://doi.org/10.3390/ijms23010356
Chicago/Turabian StyleMuller, Christo J. F., Elizabeth Joubert, Nireshni Chellan, Yutaka Miura, and Kazumi Yagasaki. 2022. "New Insights into the Efficacy of Aspalathin and Other Related Phytochemicals in Type 2 Diabetes—A Review" International Journal of Molecular Sciences 23, no. 1: 356. https://doi.org/10.3390/ijms23010356
APA StyleMuller, C. J. F., Joubert, E., Chellan, N., Miura, Y., & Yagasaki, K. (2022). New Insights into the Efficacy of Aspalathin and Other Related Phytochemicals in Type 2 Diabetes—A Review. International Journal of Molecular Sciences, 23(1), 356. https://doi.org/10.3390/ijms23010356







