Interrogating the Behaviour of a Styryl Dye Interacting with a Mesoscopic 2D-MOF and Its Luminescent Vapochromic Sensing
Abstract
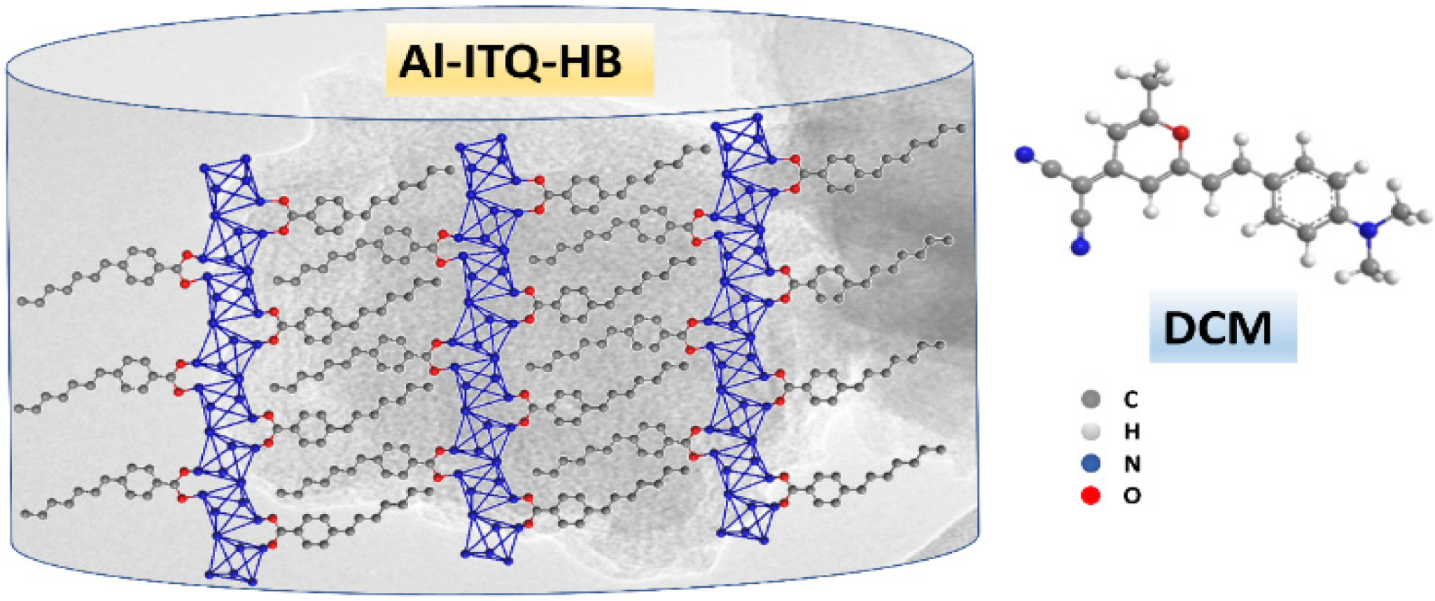
:1. Introduction
2. Result and Discussion
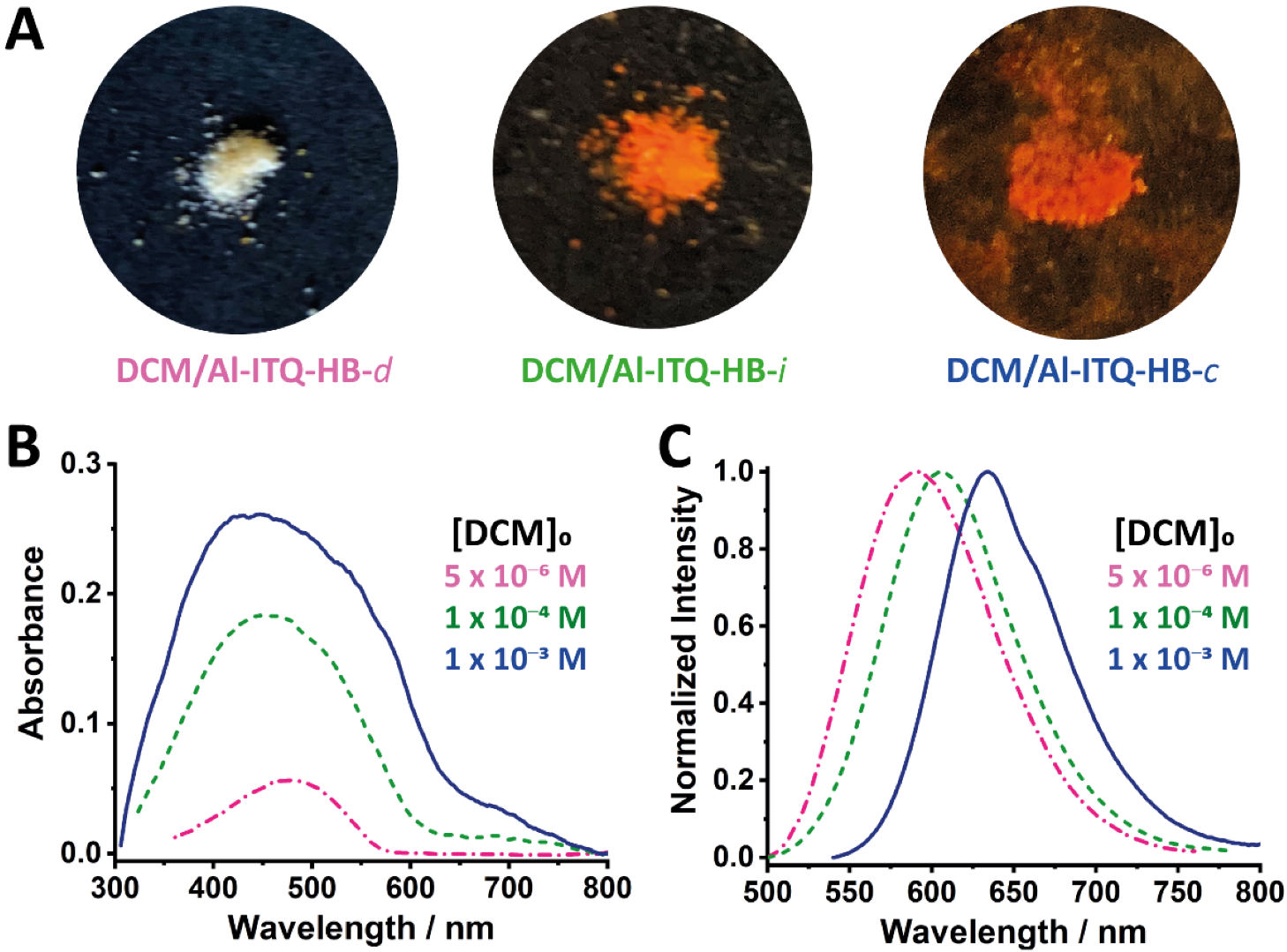
2.1. Steady-State Observations
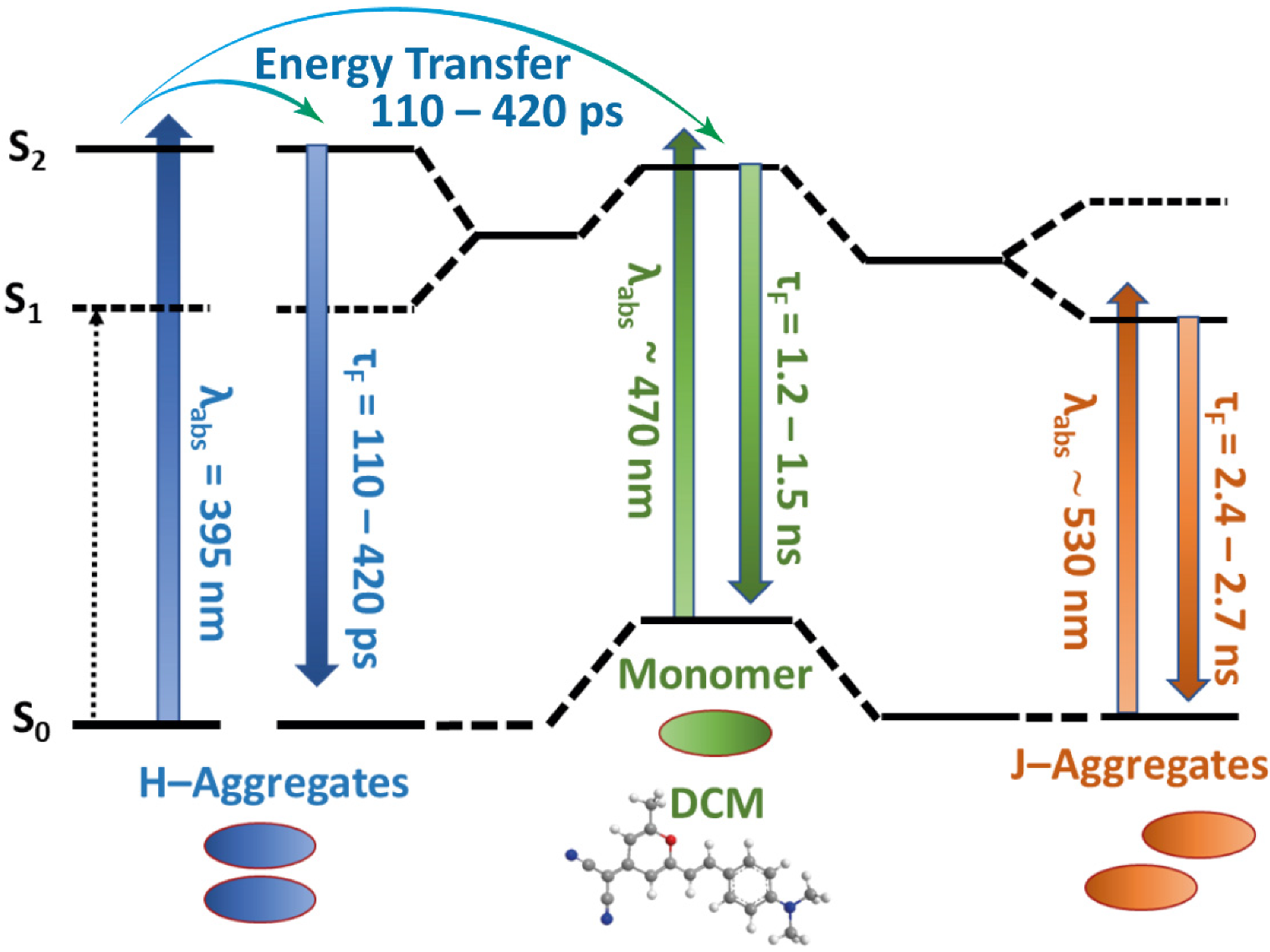
2.2. Time-Resolved Emission Measurements
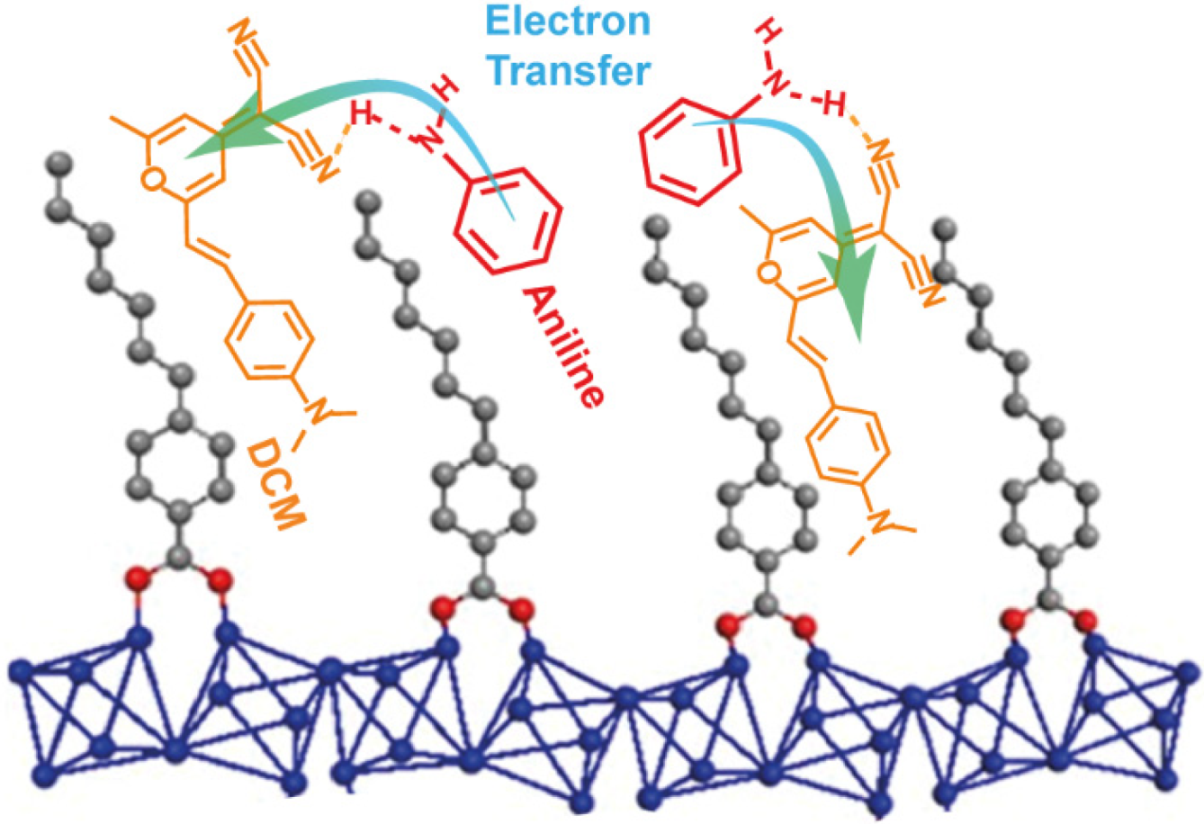
2.3. Luminescence Vapochromic Sensing
3. Materials and Methods
3.1. Synthesis and Characterization of the Materials
3.2. Experimental Procedures
3.2.1. Luminescent Vapochromic Experiments
3.2.2. Steady-State Spectroscopic and Time-Resolved Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DCM | trans-4-(Dicyanomethylene)-2-Methyl-6-(4-Dimethylaminostyryl)-4H-Pyran |
| 2D | Two-Dimensional |
| MOF | Metal Organic Frameworks |
| UV | Ultraviolet |
| ps | Picosecond |
| EnT | Energy Transfer |
| CT | Charge Transfer |
| SBU | Secondary Building Unit |
| 3D | Three-Dimensional |
| HB | 4-Heptylbenzoic Acid |
| HSA | Human Serum Albumin |
| PVK | Polyvinyl Carbazole |
| ICT | Intramolecular Charge Transfer |
| D | Donor |
| A | Acceptor |
| LE | Locally Excited |
| TICT | Twisted Intramolecular Charge Transfer |
| ns | Nanosecond |
| fs | Femtosecond |
| MeOH | Methanol |
| DMSO | Dimethyl Sulfoxide |
| MCM41 | Mobil Catalytic Materials of number 41 |
| NDC | Naphthalene Dicarboxylic Acid |
| FWHM | Full Width at Half Maximum |
| 1D | One-Dimensional |
| TCSPC | Time-Correlated Single-Photon Counting |
| TRES | Time-Resolved Emission Spectra |
| AN | Aniline |
| MAN | Methylaniline |
| DMA | Dimethylaniline |
| BAM | Benzylamine |
| TOL | Toluene |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| DFT | Density Functional Theory |
| ET | Electron Transfer |
| PXRD | Power X-ray Diffraction |
| TGA | Thermogravimetric Analysis |
| DTA | Differential Thermal Analysis |
| TEM | Transmission Electron Microscopy |
| CP/MAS NMR 13C | Cross-Polarization Magic-Angle Spinning Carbon-13 Nuclear Magnetic Resonance |
| IRF | Instrument Response Function |
References
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yang, H. Exploration of Zr–Metal–Organic Framework as Efficient Photocatalyst for Hydrogen Production. Nanoscale Res. Lett. 2017, 12, 539. [Google Scholar] [CrossRef]
- Klein, N.; Herzog, C.; Sabo, M.; Senkovska, I.; Getzschmann, J.; Paasch, S.; Lohe, M.R.; Brunner, E.; Kaskel, S. Monitoring adsorption-induced switching by 129Xe NMR spectroscopy in a new metal–organic framework Ni2(2,6-ndc)2(dabco). Phys. Chem. Chem. Phys. 2010, 12, 11778–11784. [Google Scholar] [CrossRef]
- Liu, J.; Wöll, C. Surface-supported metal–organic framework thin films: Fabrication methods, applications, and challenges. Chem. Soc. Rev. 2017, 46, 5730–5770. [Google Scholar] [CrossRef] [PubMed]
- Dolgopolova, E.A.; Rice, A.M.; Martin, C.R.; Shustova, N.B. Photochemistry and photophysics of MOFs: Steps towards MOF-based sensing enhancements. Chem. Soc. Rev. 2018, 47, 4710–4728. [Google Scholar] [CrossRef]
- Kaur, H.; Sundriyal, S.; Pachauri, V.; Ingebrandt, S.; Kim, K.-H.; Sharma, A.L.; Deep, A. Luminescent metal-organic frameworks and their composites: Potential future materials for organic light emitting displays. Coord. Chem. Rev. 2019, 401, 213077. [Google Scholar] [CrossRef]
- di Nunzio, M.R.; Caballero-Mancebo, E.; Cohen, B.; Douhal, A. Photodynamical behaviour of MOFs and related composites: Relevance to emerging photon-based science and applications. J. Photochem. Photobiol. C 2020, 44, 100355. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 13384–13397. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chi, C.; Zhang, K.; Qian, Y.; Gupta, K.M.; Kang, Z.; Jiang, J.; Zhao, D. Reversed thermo-switchable molecular sieving membranes composed of two-dimensional metal-organic nanosheets for gas separation. Nat. Commun. 2017, 8, 14460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.-Y.; Li, W.-J.; Guo, A.; Chang, L.; Li, Y.; Ruan, W.-J. Zn(ii) porphyrin based nano-/microscale metal–organic frameworks: Morphology dependent sensitization and photocatalytic oxathiolane deprotection. RSC Adv. 2016, 6, 26199–26202. [Google Scholar] [CrossRef]
- Ghorbanloo, M.; Safarifard, V.; Morsali, A. Heterogeneous catalysis with a coordination modulation synthesized MOF: Morphology-dependent catalytic activity. New J. Chem. 2017, 41, 3957–3965. [Google Scholar] [CrossRef]
- Dong, R.; Pfeffermann, M.; Liang, H.; Zheng, Z.; Zhu, X.; Zhang, J.; Feng, X. Large-Area, Free-Standing, Two-Dimensional Supramolecular Polymer Single-Layer Sheets for Highly Efficient Electrocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2015, 54, 12058–12063. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lin, Z.; Peng, F.; Wang, W.; Huang, R.; Wang, C.; Yan, J.; Liang, J.; Zhang, Z.; Zhang, T.; et al. Self-Supporting Metal–Organic Layers as Single-Site Solid Catalysts. Angew. Chem. Int. Ed. 2016, 55, 4962–4966. [Google Scholar] [CrossRef] [PubMed]
- Beldon, P.J.; Tominaka, S.; Singh, P.; Saha Dasgupta, T.; Bithell, E.G.; Cheetham, A.K. Layered structures and nanosheets of pyrimidinethiolate coordination polymers. Chem. Commun. 2014, 50, 3955–3957. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.M.; Navarro, I.; Díaz, U.; Primo, J.; Corma, A. Single-Layered Hybrid Materials Based on 1D Associated Metalorganic Nanoribbons for Controlled Release of Pheromones. Angew. Chem. Int. Ed. 2016, 55, 11026–11030. [Google Scholar] [CrossRef] [Green Version]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration. Chem. Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vaesen, S.; Vishnuvarthan, M.; Ragon, F.; Serre, C.; Vimont, A.; Daturi, M.; De Weireld, G.; Maurin, G. Probing the adsorption performance of the hybrid porous MIL-68(Al): A synergic combination of experimental and modelling tools. J. Mater. Chem. 2012, 22, 10210–10220. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [Green Version]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. 2D Metal–Organic Frameworks as Multifunctional Materials in Heterogeneous Catalysis and Electro/Photocatalysis. Adv. Mater. 2019, 31, 1900617. [Google Scholar] [CrossRef] [PubMed]
- García-García, P.; Moreno, J.M.; Díaz, U.; Bruix, M.; Corma, A. Organic–inorganic supramolecular solid catalyst boosts organic reactions in water. Nat. Commun. 2016, 7, 10835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, P.R. Laser dye DCM, its spectral properties, synthesis and comparison with other dyes in the red. Opt. Commun. 1979, 29, 331–333. [Google Scholar] [CrossRef]
- Meyer, M.; Mialocq, J.C.; Perly, B. Photoinduced intramolecular charge transfer and trans-cis isomerization of the DCM styrene dye: Picosecond and nanosecond laser spectroscopy, high-performance liquid chromatography, and nuclear magnetic resonance studies. J. Phys. Chem. 1990, 94, 98–104. [Google Scholar] [CrossRef]
- Marguet, S.; Mialocq, J.C.; Millie, P.; Berthier, G.; Momicchioli, F. Intramolecular charge transfer and trans-cis isomerization of the DCM styrene dye in polar solvents. A CS INDO MRCI study. Chem. Phys. 1992, 160, 265–279. [Google Scholar] [CrossRef]
- Martin, M.M.; Plaza, P.; Meyer, Y.H. Ultrafast intramolecular charge transfer in the merocyanine dye DCM. Chem. Phys. 1995, 192, 367–377. [Google Scholar] [CrossRef]
- Gustavsson, T.; Baldacchino, G.; Mialocq, J.C.; Pommeret, S. A femtosecond fluorescence up-conversion study of the dynamic Stokes shift of the DCM dye molecule in polar and non-polar solvents. Chem. Phys. Lett. 1995, 236, 587–594. [Google Scholar] [CrossRef]
- Pommeret, S.; Gustavsson, T.; Naskrecki, R.; Baldacchino, G.; Mialocq, J.-C. Femtosecond absorption and emission spectroscopy of the DCM laser dye. J. Mol. Liq. 1995, 64, 101–112. [Google Scholar] [CrossRef]
- Maciejewski, A.; Naskrecki, R.; Lorenc, M.; Ziolek, M.; Karolczak, J.; Kubicki, J.; Matysiak, M.; Szymanski, M. Transient absorption experimental set-up with femtosecond time resolution. Femto- and picosecond study of DCM molecule in cyclohexane and methanol solution. J. Mol. Struct. 2000, 555, 1–13. [Google Scholar] [CrossRef]
- Van Tassle, A.J.; Prantil, M.A.; Fleming, G.R. Investigation of the Excited State Structure of DCM via Ultrafast Electronic Pump/Vibrational Probe. J. Phys. Chem. B 2006, 110, 18989–18995. [Google Scholar] [CrossRef]
- Petsalakis, I.D.; Georgiadou, D.G.; Vasilopoulou, M.; Pistolis, G.; Dimotikali, D.; Argitis, P.; Theodorakopoulos, G. Theoretical Investigation on the Effect of Protonation on the Absorption and Emission Spectra of Two Amine-Group-Bearing, Red “Push−Pull” Emitters, 4-Dimethylamino-4′-nitrostilbene and 4-(dicyanomethylene)-2-methyl-6-p-(dimethylamino) styryl-4H-pyran, by DFT and TDDFT Calculations. J. Phys. Chem. A 2010, 114, 5580–5587. [Google Scholar]
- Pal, S.K.; Sukul, D.; Mandal, D.; Sen, S.; Bhattacharyya, K. Solvation dynamics of DCM in micelles. Chem. Phys. Lett. 2000, 327, 91–96. [Google Scholar] [CrossRef]
- Mandal, D.; Sen, S.; Bhattacharyya, K.; Tahara, T. Femtosecond study of solvation dynamics of DCM in micelles. Chem. Phys. Lett. 2002, 359, 77–82. [Google Scholar] [CrossRef]
- Pal, S.K.; Sukul, D.; Mandal, D.; Bhattacharyya, K. Solvation Dynamics of DCM in Lipid. J. Phys. Chem. B 2000, 104, 4529–4531. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.K.; Sukul, D.; Mandal, D.; Sen, S.; Bhattacharyya, K. Solvation Dynamics of DCM in Dipalmitoyl Phosphatidylcholine Lipid. Tetrahedron 2000, 56, 6999–7002. [Google Scholar] [CrossRef]
- Pal, S.K.; Mandal, D.; Sukul, D.; Bhattacharyya, K. Solvation dynamics of 4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran (DCM) in a microemulsion. Chem. Phys. Lett. 1999, 312, 178–184. [Google Scholar] [CrossRef]
- Pal, S.K.; Mandal, D.; Sukul, D.; Sen, S.; Bhattacharyya, K. Solvation Dynamics of DCM in Human Serum Albumin. J. Phys. Chem. B 2001, 105, 1438–1441. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Wang, L.; Liu, Y.; Ning, Y.; Zhao, J.; Liu, X.; Wu, S.; He, X.; Lin, J.; Wang, L.; et al. Lasing behavior in DCM-doped PVK microcavity. Synth. Met. 2000, 111–112, 563–565. [Google Scholar] [CrossRef]
- Halder, A.; Sen, P.; Burman, A.D.; Bhattacharyya, K. Solvation Dynamics of DCM in a Polypeptide−Surfactant Aggregate: Gelatin−Sodium Dodecyl Sulfate. Langmuir 2004, 20, 653–657. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Aydin, M.; Xu, W.; Zhu, H.-R.; Akins, D.L. Spectroscopy and dynamics of DCM encapsulated in MCM-41 and Y zeolite mesoporous materials. J. Mol. Struct. 2004, 689, 153–158. [Google Scholar] [CrossRef]
- di Nunzio, M.R.; Perenlei, G.; Douhal, A. Confinement Effect of Micro- and Mesoporous Materials on the Spectroscopy and Dynamics of a Stilbene Derivative Dye. Int. J. Mol. Sci. 2019, 20, 1316. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, M.; Sánchez, F.; Douhal, A. Efficient multicolor and white light emission from Zr-based MOF composites: Spectral and dynamic properties. J. Mater. Chem. C 2015, 3, 11300–11310. [Google Scholar] [CrossRef]
- Meyer, M.; Mialocq, J.C. Ground state and singlet excited state of laser dye DCM: Dipole moments and solvent induced spectral shifts. Opt. Commun. 1987, 64, 264–268. [Google Scholar] [CrossRef]
- Hsing-Kang, Z.; Ren-Lan, M.; Er-pin, N.; Chu, G. Behaviour of the laser dye 4-dicyanomethylene-2-methyl-6-dimethylaminostryryl-4H-pyran in the excited singlet state. J. Photochem. 1985, 29, 397–404. [Google Scholar] [CrossRef]
- Rettig, W.; Majenz, W. Competing adiabatic photoreaction channels in stilbene derivatives. Chem. Phys. Lett. 1989, 154, 335–341. [Google Scholar] [CrossRef]
- Bondarev, S.L.; Knyukshto, V.N.; Stepuro, V.I.; Stupak, A.P.; Turban, A.A. Fluorescence and Electronic Structure of the Laser Dye DCM in Solutions and in Polymethylmethacrylate. J. Appl. Spectrosc. 2004, 71, 194–201. [Google Scholar] [CrossRef]
- Caballero-Mancebo, E.; Cohen, B.; Moreno, J.M.; Corma, A.; Díaz, U.; Douhal, A. Exploring the Photodynamics of a New 2D-MOF Composite: Nile Red@Al–ITQ-HB. ACS Omega 2018, 3, 1600–1608. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Martín, C.; Van der Auweraer, M.; Hofkens, J.; Tan, J.-C. Electroluminescent Guest@MOF Nanoparticles for Thin Film Optoelectronics and Solid-State Lighting. Adv. Opt. Mater. 2020, 8, 2000670. [Google Scholar] [CrossRef]
- Möslein, A.F.; Gutiérrez, M.; Cohen, B.; Tan, J.-C. Near-Field Infrared Nanospectroscopy Reveals Guest Confinement in Metal–Organic Framework Single Crystals. Nano Lett. 2020, 20, 7446–7454. [Google Scholar] [CrossRef] [PubMed]
- Pomogaev, V.A.; Svetlichnyi, V.A.; Pomogaev, A.V.; Svetlichnaya, N.N.; Kopylova, T.N. Theoretic and Experimental Study of Photoprocesses in Substituted 4-Dicyanomethylene-4H-pyrans. High Energ. Chem. 2005, 39, 403–407. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, R.; Cao, Z.; Zhang, Q. Intramolecular charge transfer and photoisomerization of the dcm styrene dye: A theoretical study. J. Theor. Comput. Chem. 2008, 7, 719–736. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, Z.; Tong, J.; Shen, X.Y.; Qin, A.; Sun, J.Z.; Tang, B.Z. The fluorescence properties and aggregation behavior of tetraphenylethene–perylenebisimide dyads. J. Mater. Chem. C 2015, 3, 3559–3568. [Google Scholar] [CrossRef]
- Kasha, M.; Rawls, H.R.; Ashraf El-Bayoumi, M. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef] [Green Version]
- Eisfeld, A.; Briggs, J.S. The J- and H-bands of organic dye aggregates. Chem. Phys. 2006, 324, 376–384. [Google Scholar] [CrossRef]
- Bricks, J.L.; Slominskii, Y.L.; Panas, I.D.; Demchenko, A.P. Fluorescent J-aggregates of cyanine dyes: Basic research and applications review. Methods Appl. Fluoresc. 2017, 6, 012001. [Google Scholar] [CrossRef] [Green Version]
- Hestand, N.J.; Spano, F.C. Expanded Theory of H- and J-Molecular Aggregates: The Effects of Vibronic Coupling and Intermolecular Charge Transfer. Chem. Rev. 2018, 118, 7069–7163. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Mialocq, J.C.; Rougée, M. Fluorescence lifetime measurements of the two isomers of the laser dye DCM. Chem. Phys. Lett. 1988, 150, 484–490. [Google Scholar] [CrossRef]
- Li, X.; Sinks, L.E.; Rybtchinski, B.; Wasielewski, M.R. Ultrafast Aggregate-to-Aggregate Energy Transfer within Self-assembled Light-Harvesting Columns of Zinc Phthalocyanine Tetrakis(Perylenediimide). J. Am. Chem. Soc. 2004, 126, 10810–10811. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.; Möslein, A.F.; Tan, J.-C. Facile and Fast Transformation of Nonluminescent to Highly Luminescent Metal–Organic Frameworks: Acetone Sensing for Diabetes Diagnosis and Lead Capture from Polluted Water. ACS Appl. Mater. Interfaces 2021, 13, 7801–7811. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef] [Green Version]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.-P.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Kumpulainen, T.; Lang, B.; Rosspeintner, A.; Vauthey, E. Ultrafast Elementary Photochemical Processes of Organic Molecules in Liquid Solution. Chem. Rev. 2017, 117, 10826–10939. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Navarro, R.; Sánchez, F.; Douhal, A. Photodynamics of Zr-based MOFs: Effect of explosive nitroaromatics. Phys. Chem. Chem. Phys. 2017, 19, 16337–16347. [Google Scholar] [CrossRef] [PubMed]
- Organero, J.A.; Tormo, L.; Douhal, A. Caging ultrafast proton transfer and twisting motion of 1-hydroxy-2-acetonaphthone. Chem. Phys. Lett. 2002, 363, 409–414. [Google Scholar] [CrossRef]



| Sample/Exc = 470 nm | λobs/nm | τ1/ps (±50) | a1 | c1 | τ2/ns (±0.2) | a2 | c2 | τ3/ns (±0.3) | a3 | c3 |
| DCM/ Al-ITQ-HB (c0 = 1 × 10−3 M) | 550 | 420 | 56 | 25 | 1.5 | 41 | 65 | 2.7 | 3 | 10 |
| 575 | 28 | 8 | 54 | 58 | 18 | 34 | ||||
| 600 | 8 | 2 | 49 | 38 | 43 | 60 | ||||
| 630 | –100 | –100 | 29 | 19 | 71 | 81 | ||||
| 660 | –100 | –100 | 12 | 7 | 88 | 93 | ||||
| 700 | –100 | –100 | 5 | 3 | 95 | 97 | ||||
| 720 | –100 | –100 | 5 | 3 | 95 | 97 | ||||
| DCM /Al-ITQ-HB (c0 = 1 × 10−4 M) | 525 | 270 | 37 | 9 | 1.5 | 58 | 77 | 2.7 | 5 | 14 |
| 550 | 19 | 3 | 63 | 64 | 18 | 33 | ||||
| 575 | 7 | 1 | 54 | 43 | 39 | 56 | ||||
| 600 | –100 | –100 | 36 | 24 | 64 | 76 | ||||
| 630 | –100 | –100 | 11 | 6 | 89 | 94 | ||||
| 660 | –100 | –100 | 2 | 1 | 98 | 99 | ||||
| 700 | –100 | –100 | 2 | 1 | 98 | 99 | ||||
| DCM/ Al-ITQ-HB (c0 = 5 × 10−6 M) | 525 | 180 | 32 | 5 | 1.4 | 55 | 67 | 2.5 | 13 | 28 |
| 550 | 15 | 2 | 56 | 51 | 29 | 47 | ||||
| 575 | 7 | 1 | 46 | 35 | 47 | 64 | ||||
| 600 | –100 | –100 | 34 | 21 | 66 | 79 | ||||
| 630 | –100 | –100 | 18 | 10 | 82 | 90 | ||||
| 660 | –100 | –100 | 11 | 4 | 89 | 96 | ||||
| 700 | –100 | –100 | 11 | 4 | 89 | 96 | ||||
| Sample/Exc = 371 nm | λobs/nm | τ1/ps (±50) | a1 | c1 | τ2/ns (±0.2) | a2 | c2 | τ3/ns (±0.3) | a3 | c3 |
| DCM/ Al-ITQ-HB (c0 = 1 × 10−3 M) | 550 | 170 | 53 | 12 | 1.2 | 41 | 66 | 2.6 | 6 | 22 |
| 575 | 30 | 4 | 49 | 51 | 21 | 45 | ||||
| 700 | –100 | –100 | 2 | 1 | 98 | 99 | ||||
| 720 | –100 | –100 | 1 | 1 | 99 | 99 | ||||
| DCM/ Al-ITQ-HB (c0 = 1 × 10−4 M) | 525 | 160 | 49 | 9 | 1.3 | 43 | 65 | 2.6 | 8 | 26 |
| 550 | 31 | 4 | 47 | 49 | 22 | 47 | ||||
| 660 | –100 | –100 | 1 | 1 | 99 | 99 | ||||
| 700 | –100 | –100 | 1 | 1 | 99 | 99 | ||||
| DCM/ Al-ITQ-HB (c0 = 5 × 10−6 M) | 525 | 110 | 40 | 4 | 1.2 | 40 | 45 | 2.6 | 20 | 51 |
| 550 | 20 | 2 | 43 | 34 | 37 | 64 | ||||
| 630 | 2 | 1 | 11 | 5 | 87 | 94 | ||||
| 660 | –100 | –100 | 8 | 4 | 92 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Nunzio, M.R.; Gutiérrez, M.; Moreno, J.M.; Corma, A.; Díaz, U.; Douhal, A. Interrogating the Behaviour of a Styryl Dye Interacting with a Mesoscopic 2D-MOF and Its Luminescent Vapochromic Sensing. Int. J. Mol. Sci. 2022, 23, 330. https://doi.org/10.3390/ijms23010330
di Nunzio MR, Gutiérrez M, Moreno JM, Corma A, Díaz U, Douhal A. Interrogating the Behaviour of a Styryl Dye Interacting with a Mesoscopic 2D-MOF and Its Luminescent Vapochromic Sensing. International Journal of Molecular Sciences. 2022; 23(1):330. https://doi.org/10.3390/ijms23010330
Chicago/Turabian Styledi Nunzio, Maria Rosaria, Mario Gutiérrez, José María Moreno, Avelino Corma, Urbano Díaz, and Abderrazzak Douhal. 2022. "Interrogating the Behaviour of a Styryl Dye Interacting with a Mesoscopic 2D-MOF and Its Luminescent Vapochromic Sensing" International Journal of Molecular Sciences 23, no. 1: 330. https://doi.org/10.3390/ijms23010330
APA Styledi Nunzio, M. R., Gutiérrez, M., Moreno, J. M., Corma, A., Díaz, U., & Douhal, A. (2022). Interrogating the Behaviour of a Styryl Dye Interacting with a Mesoscopic 2D-MOF and Its Luminescent Vapochromic Sensing. International Journal of Molecular Sciences, 23(1), 330. https://doi.org/10.3390/ijms23010330








