Application of Graphene in Tissue Engineering of the Nervous System
Abstract
1. Introduction
2. Environment of Tissue Regeneration
3. Graphene Characteristics
4. Neural Interface
Graphene: A Neural Interface
5. Two-Dimensional Graphene-Based Scaffolds
GO and rGO
6. Role of Scaffold Dimensionality on Cell Behavior
| Compared Characteristics | 2D Cell Culture | 3D Cell Culture | References |
|---|---|---|---|
| Cell shape |
|
| [90] |
| Communication |
|
| [81] |
| Visibility (analysis of the obtained results) |
|
| [91] |
| Cell differentiation |
|
| [92] |
| Mimicking in vivo conditions |
|
| [93] |
| Ability to receive substances from the medium and study of the therapeutic effect of drugs |
|
| [93] |
| The length of the cell culture and the ability to reproduce the culture conditions |
|
| [94] |
| The cost and difficulty of carrying out cell culture |
|
| [95] |
| Apoptosis |
|
| [96] |
| Proliferation |
|
| [97] |
| Cell junction |
|
| [98] |
7. 3D Graphene-Based Scaffolds
7.1. Graphene Foam
7.2. Hydrogels
7.3. Bioprinting
7.4. Graphene Fiber
8. Biodegradation of GBNs
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 2D-GF | two-dimensional graphene foam |
| 3D | three-dimensional |
| 3D-GF | three-dimensional graphene foam |
| ADSCs | Adipose derived stem cells |
| AMGXs | graphene-based polyacrylamide hydrogels |
| BBB | blood–brain barrier |
| BrdU | 5-bromo-2′-deoxyuridine |
| CCK-8 | Cell Counting Kit-8 |
| CFGO | choline-functionalized injectable GO |
| CNS | central nervous system |
| CNTs | carbon nanotubes |
| DA | dopaminergic neurons |
| DRG | rat dorsal root ganglion neurons |
| ECM | extracellular matrix |
| EHD | electrohydrodynamic jet |
| ELISA | enzyme-linked immunosorbent assay |
| ESCs | embryonic stem cell |
| FESEM | field emission scanning electron microscopy |
| GBNs | graphene-based nanomaterials |
| GFAP | glial fibrillary acidic protein |
| GNPs | printed graphene nanoplatelets |
| GO | graphene oxide |
| GOF | graphene oxide foam |
| HBVP | human brain vascular pericyte cells |
| hESC | human embryonic stem cell |
| hMSCs | human Mesenchymal Stem Cells |
| hNSCs | human neural stem cell |
| LBLC | layer-by-layer casting |
| MG | multi-layered graphene |
| MSCs | mesenchymal stem cells |
| MTT | cell proliferation assay |
| Nestin | neuroepithelial stem cell protein |
| NPG | nanoparticles of graphene |
| NSCs | neural stem cells |
| PAM | polyacrylamide |
| PC12 | rat pheochromocytoma |
| PCL | polycaprolactone |
| PCR | polymerase chain reaction |
| PDA | polydopamine |
| PDMS | Polydimethylsiloxane |
| PET | polyethylene terephthalate |
| PLGA | poly lactic-co-glycolic acid |
| PNS | peripheral nervous system |
| qPCR | quantitative polymerase chain reaction |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| RGCs | retinal ganglion cells |
| RGD | arginylglycylaspartic acid |
| rGO | reduced graphene oxide |
| RT-PCR | real-time polymerase chain reaction |
| SC | Schwann cell |
| SEM | scanning electron microscopy |
| SG | single-layered graphene |
| Sh-sy5y | human neuroblastoma cells |
| TCPs | culture plates |
| TH | L-theanine |
| TRG | thermally reduced graphene |
| Tuj-1 | neuron-specific class III beta-tubulin |
References
- Akhavan, O. Graphene scaffolds in progressive nanotechnology/stem cell-based tissue engineering of the nervous system. J. Mater. Chem. B 2016, 4, 3169–3190. [Google Scholar] [CrossRef] [PubMed]
- Madurani, K.A.; Suprapto, S.; Machrita, N.I.; Bahar, S.L.; Illiya, W.; Kurniawan, F. Progress in Graphene Synthesis and its Application: History, Challenge and the Future Outlook for Research and Industry. ECS J. Solid State Sci. Technol. 2020, 9, 093013. [Google Scholar] [CrossRef]
- Wei, J.; Vo, T.; Inam, F. Epoxy/graphene nanocomposites—Processing and properties: A review. RSC Adv. 2015, 5, 73510–73524. [Google Scholar] [CrossRef]
- Rhee, K.Y. Electronic and Thermal Properties of Graphene. Nanomaterials 2020, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-Based Nanomaterials for Tissue Engineering. Adv. Health Mater. 2012, 2, 244–260. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef]
- Kalia, V.C.; Ray, S.; Patel, S.K.; Singh, M.; Singh, G.P. Biotechnological Applications of Polyhydroxyalkanoates. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 1–420. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef]
- Modo, M. Bioscaffold-Induced Brain Tissue Regeneration. Front. Neurosci. 2019, 13, 1156. [Google Scholar] [CrossRef]
- Curcio, M.; Bradke, F. Axon Regeneration in the Central Nervous System: Facing the Challenges from the Inside. Annu. Rev. Cell Dev. Biol. 2018, 34, 495–521. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- Daneshmandi, L.; Barajaa, M.; Rad, A.T.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Adv. Health Mater. 2021, 10, e2001414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Yang, P. Effects of Graphene-Based Materials on the Behavior of Neural Stem Cells. J. Nanomater. 2020, 2020, 2519105. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanal. 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Li, Y.; Li, J.; Deng, Z. Noncovalent DNA decorations of graphene oxide and reduced graphene oxide toward water-soluble metal–carbon hybrid nanostructuresviaself-assembly. J. Mater. Chem. 2010, 20, 900–906. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W.; Zhang, H.; Zou, M.; Chen, Y.; Zhao, Y. Programmable wettability on photocontrolled graphene film. Sci. Adv. 2018, 4, eaat7392. [Google Scholar] [CrossRef]
- Barnes, J.M.; Przybyla, L.; Weaver, V.M. Tissue mechanics regulate brain development, homeostasis and disease. J. Cell Sci. 2017, 130, 71–82. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Kundu, S.C.; Reis, R.L.; Correlo, V.M. Electric Phenomenon: A Disregarded Tool in Tissue Engineering and Regenerative Medicine. Trends Biotechnol. 2020, 38, 24–49. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-graphene in biomedicine: Theranostic applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef]
- Tonelli, F.M.; Goulart, V.A.; Gomes, K.N.; Ladeira, M.S.; Santos, A.K.; Lorençon, E.; O Ladeira, L.; Resende, R.R. Graphene-based nanomaterials: Biological and medical applications and toxicity. Nanomedicine 2015, 10, 2423–2450. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Lao, J.; He, H.; Cheng, T.; Wang, M.; Wang, S.; Huang, F. Multifunctional graphene quantum dots for simultaneous targeted cellular imaging and drug delivery. Colloids Surf. B Biointerfaces 2014, 122, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Bramini, M.; Alberini, G.; Colombo, E.; Chiacchiaretta, M.; DiFrancesco, M.L.; Maya-Vetencourt, J.F.; Maragliano, L.; Benfenati, F.; Cesca, F. Interfacing Graphene-Based Materials with Neural Cells. Front. Syst. Neurosci. 2018, 12, 12. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Accounts Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Plachá, D.; Jampilek, J. Graphenic Materials for Biomedical Applications. Nanomaterials 2019, 9, 1758. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.C.; Patiño, J.; García-Rama, C.; Ferrer, M.L.; Fierro, J.L.G.; Tamayo, A.; Collazos-Castro, J.E.; del Monte, F.; Gutierrez, M.C. 3D free-standing porous scaffolds made of graphene oxide as substrates for neural cell growth. J. Mater. Chem. B 2014, 2, 5698–5706. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, T.; Xu, M.; Gao, F.; Yang, J.; Yang, Z.; Le, W. Graphene oxide promotes the differentiation of mouse embryonic stem cells to dopamine neurons. Nanomedicine 2014, 9, 2445–2455. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, Y.; Zhang, L.; Gao, M.; Kong, Y.; Yang, Y. Preparation of graphene oxide/polyacrylamide composite hydrogel and its effect on Schwann cells attachment and proliferation. Colloids Surf. B Biointerfaces 2016, 143, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Pang, L.; Chen, Y.; Zhang, Y.; Zhang, R.; Lu, B.; Wang, J. Effects of surface charges of graphene oxide on neuronal outgrowth and branching. Analyst 2014, 139, 105–115. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shirazian, S.A.; Rahighi, R. Rolled graphene oxide foams as three-dimensional scaffolds for growth of neural fibers using electrical stimulation of stem cells. Carbon 2016, 97, 71–77. [Google Scholar] [CrossRef]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, X.; Guo, W.; Fu, C.; Pan, S. Theanine-Modified Graphene Oxide Composite Films for Neural Stem Cells Proliferation and Differentiation. J. Nanomater. 2020, 2020, 3068173. [Google Scholar] [CrossRef]
- Guo, W.; Qiu, J.; Liu, J.; Liu, H. Graphene microfiber as a scaffold for regulation of neural stem cells differentiation. Sci. Rep. 2017, 7, 5678. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Flash photo stimulation of human neural stem cells on graphene/TiO2 heterojunction for differentiation into neurons. Nanoscale 2013, 5, 10316–10326. [Google Scholar] [CrossRef]
- VijayaVenkataRaman, S.; Thaharah, S.; Zhang, S.; Lu, W.F.; Fuh, J.Y.H. 3D-Printed PCL/rGO Conductive Scaffolds for Peripheral Nerve Injury Repair. Artif. Organs 2019, 43, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wu, D.; Kuddannaya, S.; Zhang, Y.; Wang, Z. Fabrication, Characterization, and Biocompatibility of Polymer Cored Reduced Graphene Oxide Nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 5170–5177. [Google Scholar] [CrossRef] [PubMed]
- D’Abaco, G.M.; Mattei, C.; Nasr, B.; Hudson, E.J.; Alshawaf, A.J.; Chana, G.; Everall, I.P.; Nayagam, B.; Dottori, M.; Skafidas, E. Graphene foam as a biocompatible scaffold for culturing human neurons. R. Soc. Open Sci. 2018, 5, 171364. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, N.; Thakur, V.; Chattopadhyay, M.; Joddar, B. The Efficacy of Graphene Foams for Culturing Mesenchymal Stem Cells and Their Differentiation into Dopaminergic Neurons. Stem Cells Int. 2018, 2018, 3410168. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Jiang, Z.; Li, N.; Liu, P.; Liu, L.; Tang, M.; Cheng, G. Anti-inflammatory effects of three-dimensional graphene foams cultured with microglial cells. Biomaterials 2014, 35, 6930–6940. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.; Jung, W. Highly enhanced compatibility of human brain vascular pericyte cells on monolayer graphene. Bioeng. 2016, 8, 85–91. [Google Scholar] [CrossRef][Green Version]
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Ebrahimian-Hosseinabadi, M.; Kharazi, A.Z. Chitosan/graphene and poly(D, L-lactic-co-glycolic acid)/graphene nano-composites for nerve tissue engineering. Tissue Eng. Regen. Med. 2016, 13, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Convertino, D.; Luin, S.; Marchetti, L.; Coletti, C. Peripheral Neuron Survival and Outgrowth on Graphene. Front. Neurosci. 2018, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Kim, Y.J.; Jeon, C.S.; Lim, K.T.; Seonwoo, H.; Cho, S.-P.; Chung, T.D.; Choung, P.-H.; Choung, Y.-H.; et al. Monolayer Graphene-Directed Growth and Neuronal Differentiation of Mesenchymal Stem Cells. J. Biomed. Nanotechnol. 2015, 11, 2024–2033. [Google Scholar] [CrossRef]
- Heo, C.; Yoo, J.; Lee, S.; Jo, A.; Jung, S.; Yoo, H.; Lee, Y.H.; Suh, M. The control of neural cell-to-cell interactions through non-contact electrical field stimulation using graphene electrodes. Biomaterials 2011, 32, 19–27. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced Differentiation of Human Neural Stem Cells into Neurons on Graphene. Adv. Mater. 2011, 23, H263–H267. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Song, Q.; Su, R.; Zhang, Q.; Kong, T.; Liu, L.; Jin, G.; Tang, M.; Cheng, G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomateials 2011, 32, 9374–9382. [Google Scholar] [CrossRef]
- Tang, M.; Song, Q.; Li, N.; Jiang, Z.; Huang, R.; Cheng, G. Enhancement of electrical signaling in neural networks on graphene films. Biomaterials 2013, 34, 6402–6411. [Google Scholar] [CrossRef]
- Martín, C.; Merino, S.; González-Domínguez, J.M.; Rauti, R.; Ballerini, L.; Prato, M.; Vázquez, E. Graphene Improves the Biocompatibility of Polyacrylamide Hydrogels: 3D Polymeric Scaffolds for Neuronal Growth. Sci. Rep. 2017, 7, 10942. [Google Scholar] [CrossRef]
- Assaf, K.; Leal, C.V.; Derami, M.S.; Duek, E.A.D.R.; Ceragioli, H.; De Oliveira, A.L.R. Sciatic nerve repair using poly(ε-caprolactone) tubular prosthesis associated with nanoparticles of carbon and graphene. Brain Behav. 2017, 7, e00755. [Google Scholar] [CrossRef]
- Defteralı, Ç.; Verdejo, R.; Majeed, S.; Boschetti-De-Fierro, A.; Méndez-Gómez, H.R.; Díaz-Guerra, E.; Fierro, D.; Buhr, K.; Abetz, C.; Martínez-Murillo, R.; et al. In Vitro Evaluation of Biocompatibility of Uncoated Thermally Reduced Graphene and Carbon Nanotube-Loaded PVDF Membranes with Adult Neural Stem Cell-Derived Neurons and Glia. Front. Bioeng. Biotechnol. 2016, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Dybowska-Sarapuk, L.; Sosnowicz, W.; Krzeminski, J.; Grzeczkowicz, A.; Granicka, L.H.; Kotela, A.; Jakubowska, M. Printed Graphene Layer as a Base for Cell Electrostimulation—Preliminary Results. Int. J. Mol. Sci. 2020, 21, 7865. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A.; Zhang, Y.; Risner, M.L.; Li, D.; Xu, Y.; Sappington, R.M. Impact of Graphene on the Efficacy of Neuron Culture Substrates. Adv. Health Mater. 2018, 7, 1701290. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Differentiation of human neural stem cells into neural networks on graphene nanogrids. J. Mater. Chem. B 2013, 1, 6291–6301. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Lee, J.H.; Kang, S.H.; Hwang, E.Y.; Hwang, Y.-S.; Lee, M.H.; Han, D.-W.; Park, J.-C. Enhanced Neural Cell Adhesion and Neurite Outgrowth on Graphene-Based Biomimetic Substrates. BioMed Res. Int. 2014, 2014, 212149. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, W.C.; Manga, K.K.; Ang, P.K.; Lu, J.; Liu, Y.P.; Lim, C.T.; Loh, K.P. Fluorinated Graphene for Promoting Neuro-Induction of Stem Cells. Adv. Mater. 2012, 24, 4285–4290. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Foffani, G.; Pesenti, A.; Bianchi, A.; Chiesa, V.; Baselli, G.; Caputo, E.; Tamma, F.; Rampini, P.; Egidi, M.; et al. Movement-related modulation of neural activity in human basal ganglia and its L -DOPA dependency: Recordings from deep brain stimulation electrodes in patients with Parkinson’s disease. Neurol. Sci. 2002, 23, s101–s102. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-W.; Schendel, A.A.; Mikael, S.; Brodnick, S.K.; Richner, T.; Ness, J.P.; Hayat, M.R.; Atry, F.; Frye, S.T.; Pashaie, R.; et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 5258. [Google Scholar] [CrossRef]
- Woods, G.A.; Rommelfanger, N.J.; Hong, G. Bioinspired Materials for In Vivo Bioelectronic Neural Interfaces. Matter 2020, 3, 1087–1113. [Google Scholar] [CrossRef]
- Gutruf, P.; Yin, R.T.; Lee, K.B.; Ausra, J.; Brennan, J.A.; Qiao, Y.; Xie, Z.; Peralta, R.; Talarico, O.; Murillo, A.; et al. Wireless, battery-free, fully implantable multimodal and multisite pacemakers for applications in small animal models. Nat. Commun. 2019, 10, 5742. [Google Scholar] [CrossRef] [PubMed]
- Chew, D.J.; Zhu, L.; Delivopoulos, E.; Minev, I.R.; Musick, K.M.; Mosse, C.A.; Craggs, M.; Donaldson, N.; Lacour, S.P.; McMahon, S.B.; et al. A Microchannel Neuroprosthesis for Bladder Control after Spinal Cord Injury in Rat. Sci. Transl. Med. 2013, 5, 210ra155. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Jang, M.J.; Joo, S.; Sun, W.; Nam, Y. Surface-printed microdot array chips for the quantification of axonal collateral branching of a single neuron in vitro. Lab A Chip 2014, 14, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Breukers, R.D.; Gilmore, K.J.; Kita, M.; Wagner, K.K.; Higgins, M.J.; Moulton, S.E.; Clark, G.M.; Officer, D.L.; Kapsa, R.M.I.; Wallace, G.G. Creating conductive structures for cell growth: Growth and alignment of myogenic cell types on polythiophenes. J. Biomed. Mater. Res. Part A 2010, 95, 256–268. [Google Scholar] [CrossRef]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically Strong, Electrically Conductive, and Biocompatible Graphene Paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Bramini, M.; Sacchetti, S.; Armirotti, A.; Rocchi, A.; Vázquez, E.; Castellanos, V.L.; Bandiera, T.; Cesca, F.; Benfenati, F. Graphene Oxide Nanosheets Disrupt Lipid Composition, Ca2+ Homeostasis, and Synaptic Transmission in Primary Cortical Neurons. ACS Nano 2016, 10, 7154–7171. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Abouei, E.; Hatamie, S.; Ghasemi, E. Accelerated differentiation of neural stem cells into neurons on ginseng-reduced graphene oxide sheets. Carbon 2013, 66, 395–406. [Google Scholar] [CrossRef]
- Lu, Y.; Lyu, H.; Richardson, A.; Lucas, T.H.; Kuzum, D. Flexible Neural Electrode Array Based-on Porous Graphene for Cortical Microstimulation and Sensing. Sci. Rep. 2016, 6, 33526. [Google Scholar] [CrossRef] [PubMed]
- Kuzum, D.; Takano, H.; Shim, E.; Reed, J.C.; Juul, H.; Richardson, A.G.; De Vries, J.; Bink, H.; Dichter, M.A.; Lucas, T.H.; et al. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 2014, 5, 5259. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef]
- Li, L.; Clevers, H. Coexistence of Quiescent and Active Adult Stem Cells in Mammals. Science 2010, 327, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Ho, M.S.; Zorec, R.; Parpura, V. Neuroglia in Neurodegenerative Diseases. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2019. [Google Scholar]
- Maleki, M.; Zarezadeh, R.; Nouri, M.; Sadigh, A.R.; Pouremamali, F.; Asemi, Z.; Kafil, H.S.; Alemi, F.; Yousefi, B. Graphene Oxide: A Promising Material for Regenerative Medicine and Tissue Engineering. Biomol. Concepts 2020, 11, 182–200. [Google Scholar] [CrossRef] [PubMed]
- Bullo, S.; Buskaran, K.; Baby, R.; Dorniani, D.; Fakurazi, S.; Hussein, M.Z. Dual Drugs Anticancer Nanoformulation using Graphene Oxide-PEG as Nanocarrier for Protocatechuic Acid and Chlorogenic Acid. Pharm. Res. 2019, 36, 91. [Google Scholar] [CrossRef]
- Sainz-Urruela, C.; Vera-López, S.; Andrés, M.P.S.; Díez-Pascual, A.M. Graphene Oxides Derivatives Prepared by an Electrochemical Approach: Correlation between Structure and Properties. Nanomaterials 2020, 10, 2532. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef]
- Feng, Z.-Q.; Yan, K.; Shi, C.; Xu, X.; Wang, T.; Li, R.; Dong, W.; Zheng, J. Neurogenic differentiation of adipose derived stem cells on graphene-based mat. Mater. Sci. Eng. C 2018, 90, 685–692. [Google Scholar] [CrossRef]
- Sánchez-González, S.; Diban, N.; Bianchi, F.; Ye, H.; Urtiaga, A. Evidences of the Effect of GO and rGO in PCL Membranes on the Differentiation and Maturation of Human Neural Progenitor Cells. Macromol. Biosci. 2018, 18, e1800195. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.G.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar] [CrossRef]
- Severino, F.P.U.; Ban, J.; Song, Q.; Tang, M.; Bianconi, G.; Cheng, G.; Torre, V. The role of dimensionality in neuronal network dynamics. Sci. Rep. 2016, 6, 29640. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Das, S.; Singh, S.; Singh, V.; Joung, D.; Dowding, J.M.; Reid, D.; Anderson, J.; Zhai, L.; Khondaker, S.I.; Self, W.T.; et al. Oxygenated Functional Group Density on Graphene Oxide: Its Effect on Cell Toxicity. Part. Part. Syst. Charact. 2013, 30, 148–157. [Google Scholar] [CrossRef]
- McCallion, C.; Burthem, J.; Rees-Unwin, K.; Golovanov, A.; Pluen, A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur. J. Pharm. Biopharm. 2016, 104, 235–250. [Google Scholar] [CrossRef] [PubMed]
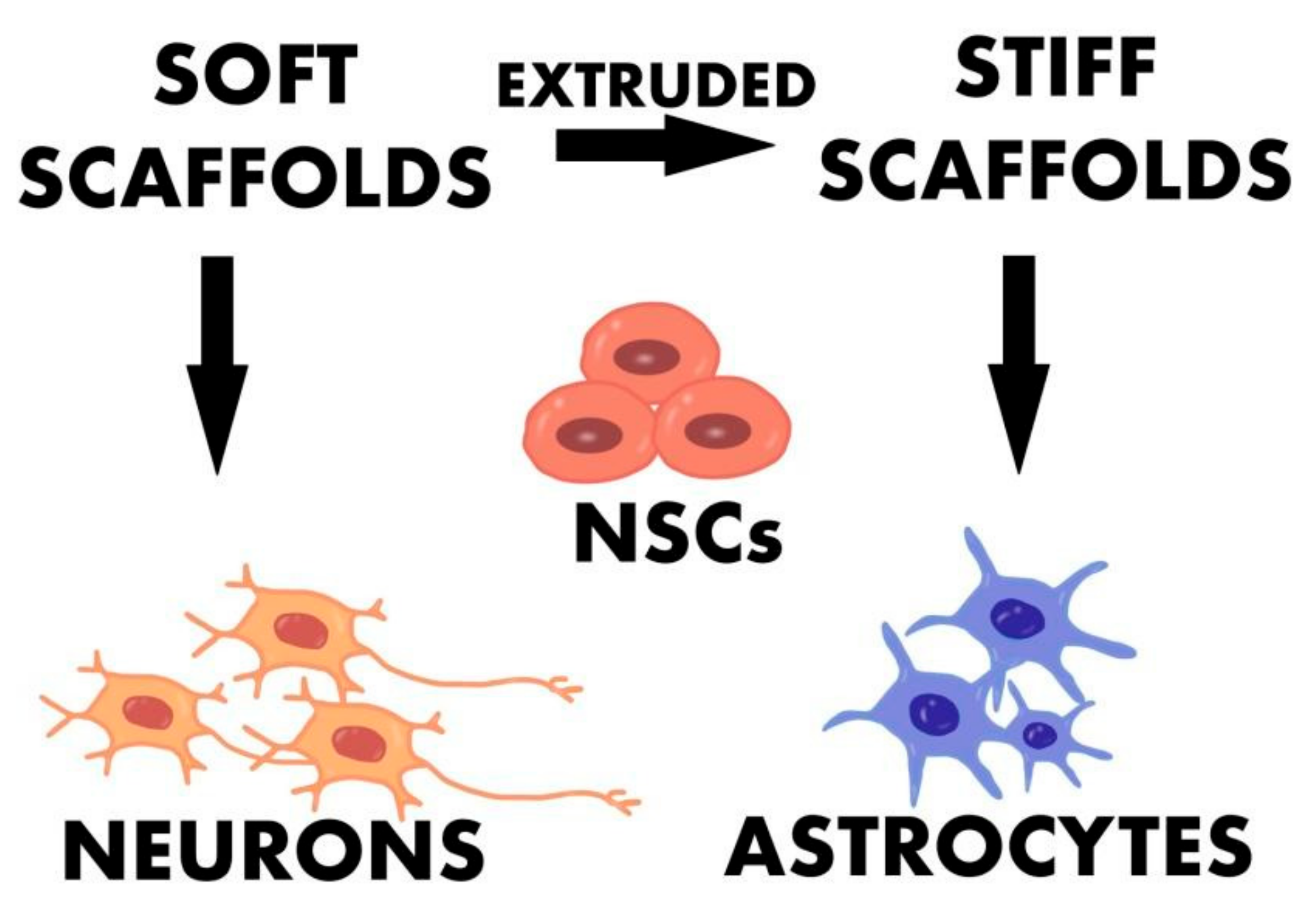
- Ma, Q.; Yang, L.; Jiang, Z.; Song, Q.; Xiao, M.; Zhang, D.; Ma, X.; Wen, T.; Cheng, G. Three-Dimensional Stiff Graphene Scaffold on Neural Stem Cells Behavior. ACS Appl. Mater. Interfaces 2016, 8, 34227–34233. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Processing-morphology-properties relationships. Compos. Part A Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Song, H.S.; Kwon, O.S.; Kim, J.-H.; Conde, J.; Artzi, N. 3D hydrogel scaffold doped with 2D graphene materials for biosensors and bioelectronics. Biosens. Bioelectron. 2017, 89, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Barrilleaux, B.; Phinney, D.G.; Prockop, D.J.; O’Connor, K.C. Review:Ex VivoEngineering of Living Tissues with Adult Stem Cells. Tissue Eng. 2006, 12, 3007–3019. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Rahim, M.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; Diogo, D.M.D.M.; Gaspar, V.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2016, 12, 910–919. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Lelièvre, S.A.; Kwok, T.; Chittiboyina, S. Architecture in 3D cell culture: An essential feature for in vitro toxicology. Toxicol. Vitr. 2017, 45, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Soares, C.P.; Midlej, V.D.V.P.; De Oliveira, M.E.W.; Benchimol, M.; Costa, M.; Mermelstein, C. 2D and 3D-Organized Cardiac Cells Shows Differences in Cellular Morphology, Adhesion Junctions, Presence of Myofibrils and Protein Expression. PLoS ONE 2012, 7, e38147. [Google Scholar] [CrossRef]
- Bei, H.P.; Yang, Y.; Zhang, Q.; Tian, Y.; Luo, X.; Yang, M.; Zhao, X. Graphene-Based Nanocomposites for Neural Tissue Engineering. Molecules 2019, 24, 658. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef]
- Giguère, N.; Nanni, S.B.; Trudeau, L.-E. On Cell Loss and Selective Vulnerability of Neuronal Populations in Parkinson’s Disease. Front. Neurol. 2018, 9, 455. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Pradhan, K.; Das, G.; Khan, J.; Gupta, V.; Barman, S.; Adak, A.; Ghosh, S. Neuro-Regenerative Choline-Functionalized Injectable Graphene Oxide Hydrogel Repairs Focal Brain Injury. ACS Chem. Neurosci. 2018, 10, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-Q.; Wang, T.; Zhao, B.; Li, J.; Jin, L. Soft Graphene Nanofibers Designed for the Acceleration of Nerve Growth and Development. Adv. Mater. 2015, 27, 6462–6468. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef]
- Kurapati, R.; Mukherjee, S.P.; Martín, C.; Bepete, G.; Vázquez, E.; Pénicaud, A.; Fadeel, B.; Bianco, A. Degradation of Single-Layer and Few-Layer Graphene by Neutrophil Myeloperoxidase. Angew. Chem. Int. Ed. 2018, 57, 11722–11727. [Google Scholar] [CrossRef]
- Saei, A.; Yazdani, M.; Lohse, S.E.; Bakhtiary, Z.; Serpooshan, V.; Ghavami, M.; Asadian, M.; Mashaghi, S.; Dreaden, E.; Mashaghi, A.; et al. Nanoparticle Surface Functionality Dictates Cellular and Systemic Toxicity. Chem. Mater. 2017, 29, 6578–6595. [Google Scholar] [CrossRef]
- Girish, C.M.; Sasidharan, A.; Gowd, G.S.; Nair, S.; Koyakutty, M. Confocal Raman Imaging Study Showing Macrophage Mediated Biodegradation of Graphene In Vivo. Adv. Health Mater. 2013, 2, 1489–1500. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Gliga, A.R.; Lazzaretto, B.; Brandner, B.; Fielden, M.; Vogt, C.; Newman, L.; Rodrigues, A.F.; Shao, W.; Fournier, P.M.; et al. Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale 2018, 10, 1180–1188. [Google Scholar] [CrossRef]
- Arnold, A.M.; Holt, B.D.; Daneshmandi, L.; Laurencin, C.T.; Sydlik, S.A. Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Dimiev, A.; Ceriotti, G.; Behabtu, N.; Zakhidov, D.; Pasquali, M.; Saito, R.; Tour, J.M. Direct Real-Time Monitoring of Stage Transitions in Graphite Intercalation Compounds. ACS Nano 2013, 7, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Lalwani, G.; Rusakova, I.; Sitharaman, B. Degradation of Graphene by Hydrogen Peroxide. Part. Part. Syst. Charact. 2014, 31, 745–750. [Google Scholar] [CrossRef]
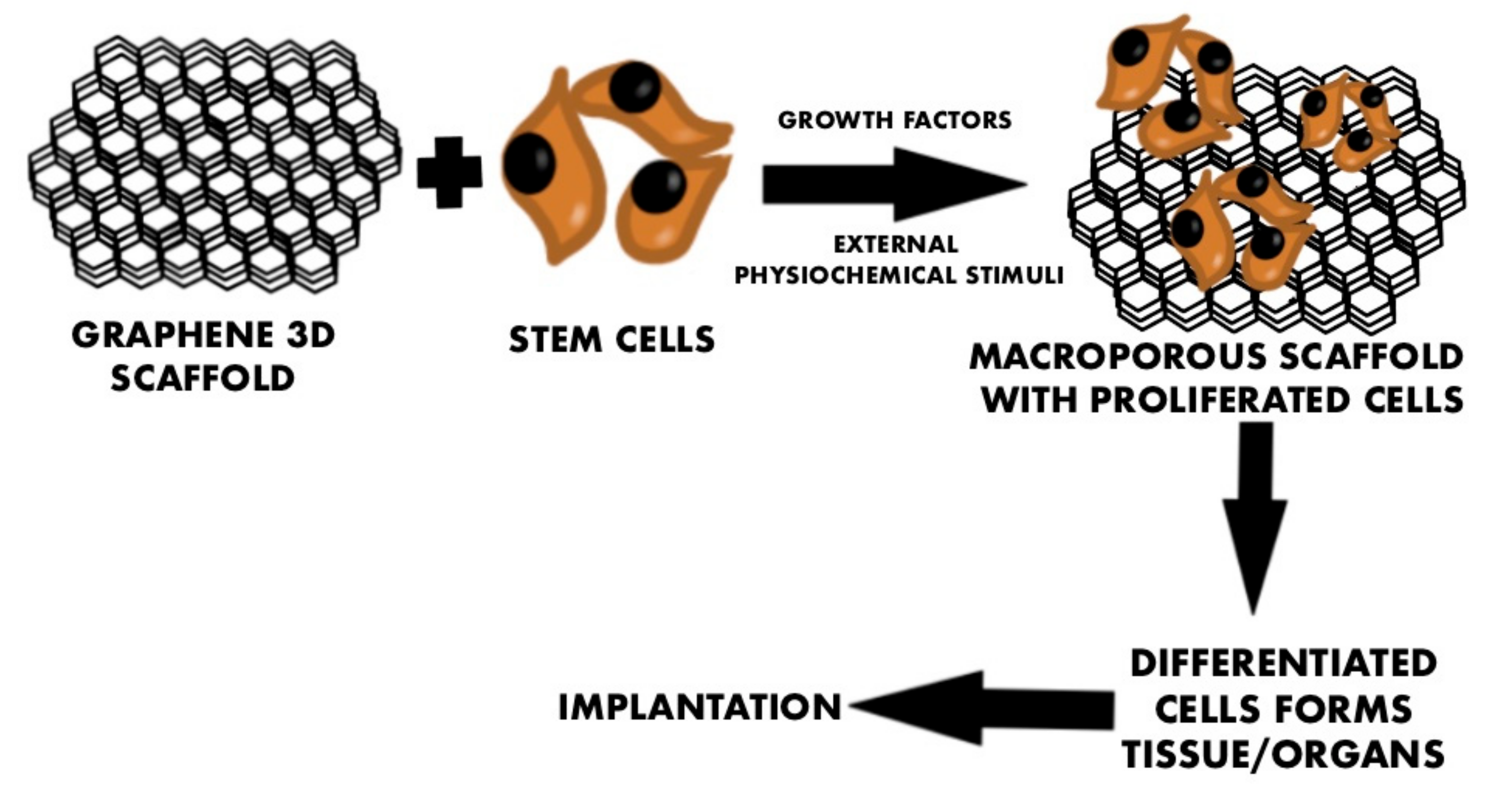
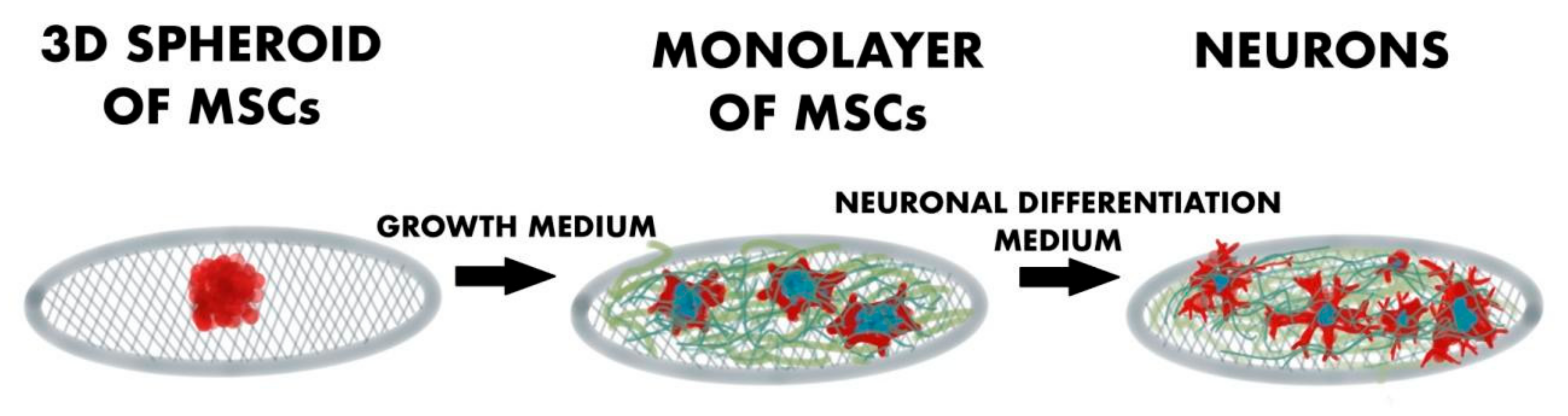
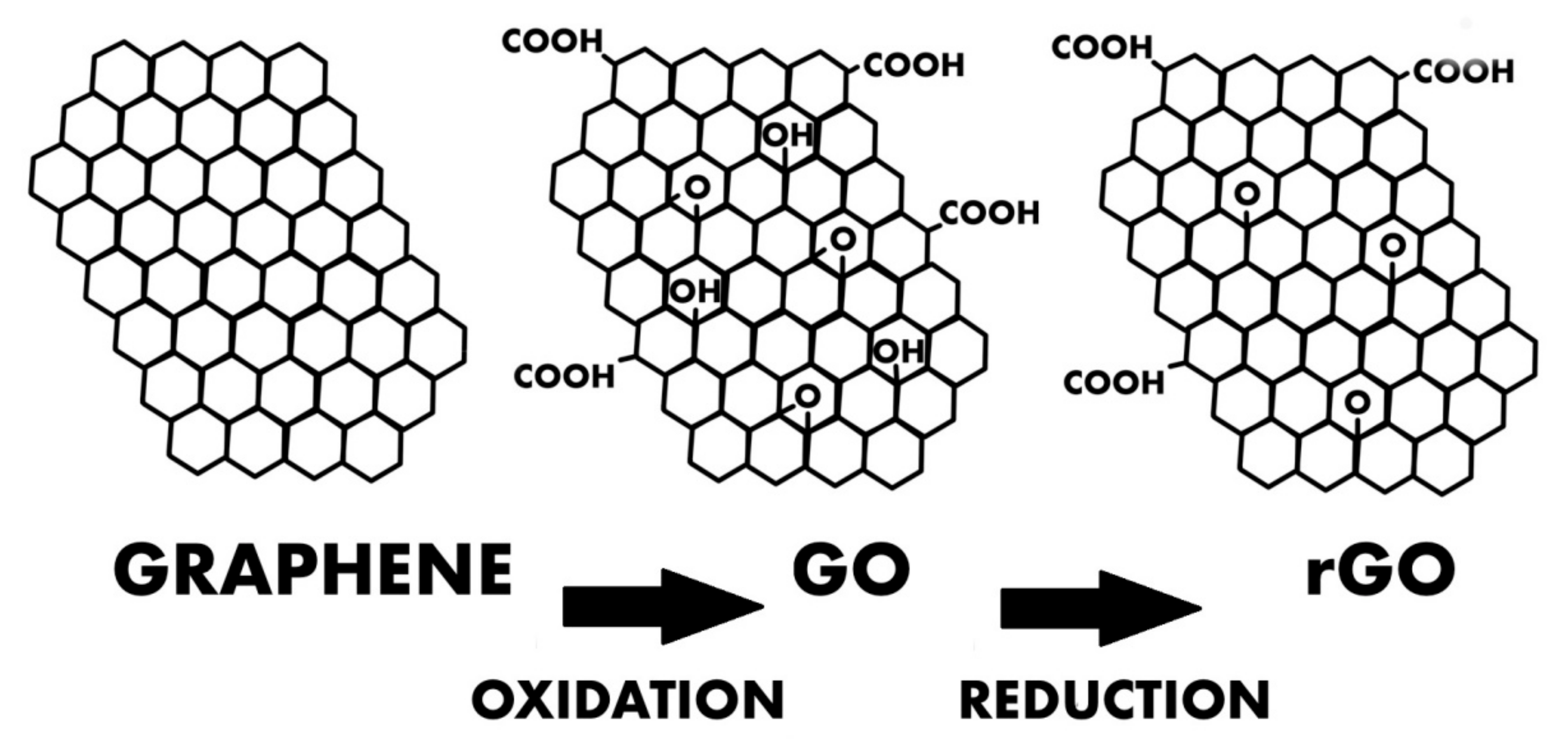
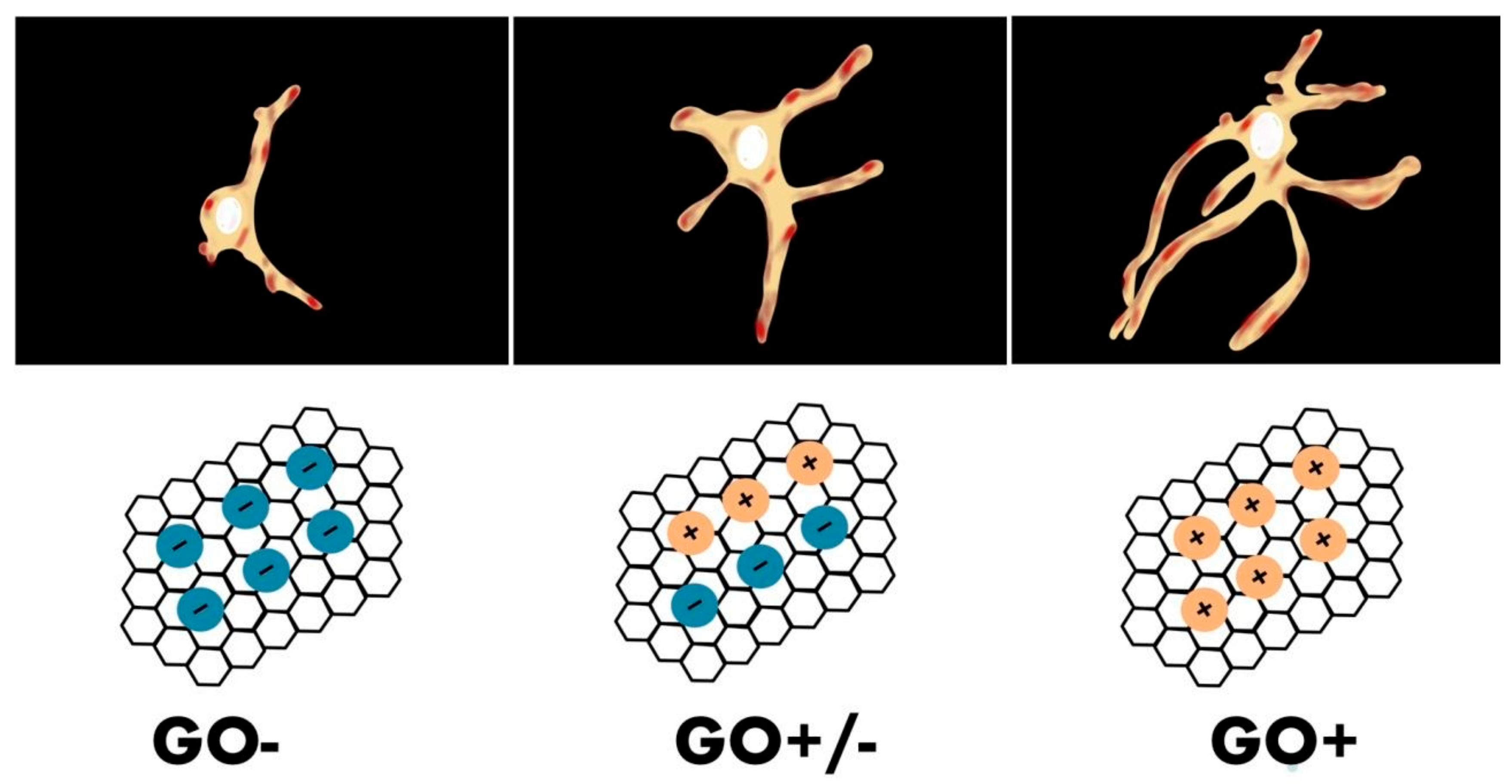
| Types of GBNs Used | Types of Cells Used | Study Performed In Vitro/In Vivo | Year of Publication | Reported Origin of the Graphene | The Most Important Results and Conclusions | Evaluation Methods | References |
|---|---|---|---|---|---|---|---|
| GO | Rat ENPCs | in vitro | 2014 | - | 14 days were enough to observe differentiated nerve cells | Live/Dead® Viability Kit, | [26] |
| GO, CNTs, graphene | Mouse ESCs | in vitro | 2014 | Shandong Tianyuan Co. Ltd. (China) | GO allows for the efficient differentiation of ESCs into dopamine neurons | Immunofluorescence Staining, Real Time PCR (RT-PCR) | [27] |
| GO | SC | in vitro | 2016 | - | High concentration of GO is not optimal for the proliferation of SC | CCK-8 Assay, Immunofluorescence Staining, Microscopic Analysis | [28] |
| GO | Primary Rat Hippocampal Neurons | in vitro | 2014 | Nanoon (Hebei, China) | Positively charged scaffold (GO-NH2) characterized by the best neuronal proliferation | SEM Imaging, Immunochemistry Staining, Fluorescence Imaging | [29] |
| GO | hNSC | in vitro | 2015 | - | Cell proliferation on GOFs was significantly higher than in the control sample where cells were sown on the commonly used in tissue engineering PDMS | Fluorescence Imaging, SEM Imaging | [30] |
| GO | Mouse MSCs and PC12 | in vitro | 2015 | Sigma Aldrich (USA) | The GO / PCL scaffold allowed for better proliferation and differentiation of mMSCs and PC12-L | Cell Morphologies Using FESEM, CCK-8 Assay, qRT-PCR | [31] |
| GO | NSCs | in vitro | 2020 | Chengdu Organic Chemicals Co., Ltd., China | NSC cells on the scaffold containing graphene had the highest rate of spreading | Survival Assays, MTT Assay, RT-PCR | [32] |
| Types of GBNs Used | Types of Cells Used | Study Performed In Vitro/In Vivo | Year of Publication | Reported Origin of the Graphene | The Most Important Results and Conclusions | Evaluation Methods | References |
|---|---|---|---|---|---|---|---|
| rGO microfiber | Neural Stem Cells (NSCs) | in vitro | 2017 | - | rGO microfibers may constitute suitable conditions for the cell culture of nerve cells | Immunofluorescence Staining, Fluorescent Calcium Imaging, Quantitative Polymerase Chain Reaction (qPCR) | [33] |
| rGO | hNSCs | in vitro | 2013 | - | GO-TiO2 scaffold electrostimulation allowed not only to increase the proliferation of hNSCs but also allowed for neuronal differentiation | Immunofluorescence Staining | [34] |
| rGO | PC12 | in vitro | 2018 | Sigma-Aldrich Pte Ltd., Singapore | Cell proliferation and differentiation were higher in PCL / rGO scaffolds than in scaffolds without rGO | SEM Imaging, Prestoblue Assay, RT-PCR, Fluorescence Microscopy Imaging | [35] |
| rGO nanofibers | hMSCs | in vitro | 2016 | - | From day 5 of culture, the cells on the graphene scaffold showed better proliferation | SEM Imaging, Confocal Microscopy Imaging | [36] |
| Types of GBNs Used | Types of Cells Used | Study Performed In Vitro/In Vivo | Year of Publication | Reported Origin of the Graphene | The Most Important Results and Conclusions | Evaluation Methods | References |
|---|---|---|---|---|---|---|---|
| graphene foam | Human Embryonic Stem Cell (hESC) | in vitro | 2018 | Graphene Laboratories, Inc. (Graphene Foam Calverton, NY, USA) | Porous structure of the graphene foam allows for cell penetration onto the scaffold | Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR), Immunofluorescence Staining, SEM Imaging, Helium Ion Microscopy Imaging | [37] |
| graphene foam | Mesenchymal Stem Cells (MSCs) | in vitro | 2018 | Graphene Supermarket (Calverton, NY) | Graphene foam allows the differentiation of MSCs into selected cells of the nervous system | Flow Cytometry Analysis | [38] |
| graphene foam, graphene film | Mouse NSCs | in vitro | 2013 | - | The graphene scaffold allows good interaction between the scaffold and cells, which is essential for good cell differentiation | SEM Imaging, Cell Viability Assay, Immunofluorescence Staining, Western Blotting | [39] |
| graphene foam | NSCs | in vitro | 2014 | - | The study suggests that only 3D graphene foam has antimicrobial properties, while 2D scaffolding does not | Flow Cytometry Analysis, Enzyme-Linked Immunosorbent Assay (ELISA), Western Blotting, Microscopic Analysis, MTT Assay, Immunohistological Staining | [40] |
| Types of GBNs Used | Types of Cells Used | Study Performed In Vitro/In Vivo | Year of Publication | Reported Origin of the Graphene | The Most Important Results and Conclusions | Evaluation Methods | References |
|---|---|---|---|---|---|---|---|
| SG | Human Brain Vascular Pericyte (HBVP) Cells | in vitro | 2016 | - | A significantly higher amount of HBVP cells was observed on the scaffold containing graphene | Optical Microscopy Imaging | [41] |
| SG AND MG | Rat Schwann Cell (rat SC) | in vivo, in vitro | 2018 | Suzhou Tanfeng Graphene Technology Co., Ltd. (China) | The SG and MG scaffolds allow for the regeneration of damaged peripheral nerves | CCK-8 Assay, SEM Imaging, Immunofluorescence Staining | [42] |
| SG | Rat Pheochromocytoma (PC12) | in vitro | 2016 | Neutrino (Iran) | Promising use of the SG and chitin scaffold for the proliferation of nerve cells | MTT Assay | [43] |
| SG | PC12 and Rat Dorsal Root Ganglion (DRG) primary neurons | in vitro | 2018 | - | The scaffold containing graphene allowed extending the length of the neurons by 27% compared to the control sample | Viability Assays, Optical Microscopy Imaging | [44] |
| SG and MG | Rat SC | in vivo | 2018 | Suzhou Tanfeng Graphene Technology Co., Ltd. | Cultures on PDA/RGD-SG/PCL and PDA/RGD-MG/PCL showed results similar to autograft | CCK-8 Assay, SEM Imaging, Immunofluorescence Staining | [42] |
| SG | hMSCs | in vitro | 2015 | - | High quality single-layer graphene (SG) allowed obtaining a spheroid on a 2D scaffold, which lasted 7 days | Western Blotting, Nissle Staining, qRT-PCR Fluorescent Calcium Imaging | [45] |
| SG | Human Neuroblastoma Cells (Sh-sy5y Cells) | in vitro | 2011 | - | The best effects were observed with stimulation using a weak electric field | Immunofluorescence Staining Optical and Fluorescence Microscopic Imaging | [46] |
| Types of GBNs Used | Types of Cells Used | Study Performed In Vitro/In Vivo | Year of Publication | Reported Origin of the Graphene | The Most Important Results and Conclusions | Evaluation Methods | References |
|---|---|---|---|---|---|---|---|
| graphene film | Human NSCs (hNSCs) | in vitro | 2011 | - | Due to its unique properties, graphene allows the differentiation of hNSCs mainly into neurons, not glia | Immunofluorescence Staining, Microarray Experiments | [47] |
| graphene film | Mouse Hippocampal Cells | in vitro | 2011 | - | Graphene is a good environment for the development of mouse hippocampal cells; it also allows their neuronal differentiation | Analyzed Via Phase Contrast Microscopy | [48] |
| graphene film | Mouse NSCs | in vitro | 2013 | - | Graphene film allows the differentiation of cells that are able to communicate with other cells | Immunofluorescence Staining | [49] |
| Types of GBNs Used | Types of Cells Used | Study Performed In Vitro/In Vivo | Year of Publication | Reported Origin of the Graphene | The Most Important Results and Conclusions | Evaluation Methods | References |
|---|---|---|---|---|---|---|---|
| AMGXs | Primary Rat Hippocampal Neurons | in vitro | 2017 | Bay Carbon Inc. | Cell networks between cultured cells were observed only on graphene-containing scaffolds | Immunofluorescence Staining, Fluorescent Calcium Imaging | [50] |
| NPG | - | in vivo | 2017 | - | The most myelinated axons were observed on scaffolds containing graphene | Differential Scanning Calorimetry, Fracture Surfaces of The Membranes, Dynamic Mechanical Analysis | [51] |
| TRG | Mouse NSCs | in vitro | 2016 | - | Scaffold containing TRG allows for appropriate proliferation and adherence of mouse NSC | Immunofluorescence Staining, Morphological Analysis of Neurons and Oligodendrocytes, Cell Death Assay | [52] |
| GNPs | Mammalian NE-4C NSC | in vitro | 2020 | - | The obtained ink creates suitable conditions for the cell culture of nerve cells | Scanning Electron Microscopy (SEM) Imaging | [53] |
| graphene | Retinal Ganglion Cells (RGCs) | in vitro | 2018 | - | Despite the lack of significant influence of the use of graphene on cell proliferation, the possible use of graphene as an electrode has been confirmed | Cell Survival Assay, Receptor-Mediated Endocytosis Assay Neurite Outgrowth Assay, Ion Channel Activity Assay | [54] |
| graphene nanogrids | hNSCs | in vitro | 2013 | - | Graphene nanogrids, due to their unique properties, allow for neuronal differentiation | Immunofluorescence Staining, Fluorescence Imaging, | [55] |
| CNTs | PC12 | in vitro | 2014 | - | CNTs showed the best results in cell proliferation of all the materials tested | Immunofluorescence Staining | [56] |
| Fluorinated graphene | MSCs | in vitro | 2012 | - | Fluorinated graphene improves the proliferation of MSCs | Immunofluorescence Staining | [57] |
| Characteristics | Advantages | Limitations | References | |
|---|---|---|---|---|
| Graphene |
|
|
| [47,82] |
| GO |
|
|
| [83,84] |
| rGO |
|
|
| [75,76] |
| Characteristics | Advantages | Limitations | References | |
|---|---|---|---|---|
| Foams |
|
|
| [85] |
| Fibers |
|
|
| [86] |
| Hydrogels |
|
|
| [87] |
| Bioprinting Products |
|
|
| [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ławkowska, K.; Pokrywczyńska, M.; Koper, K.; Kluth, L.A.; Drewa, T.; Adamowicz, J., on behalf of the Trauma and Reconstructive Urology Working Party of the European Association of Urology Young Academic Urologists. Application of Graphene in Tissue Engineering of the Nervous System. Int. J. Mol. Sci. 2022, 23, 33. https://doi.org/10.3390/ijms23010033
Ławkowska K, Pokrywczyńska M, Koper K, Kluth LA, Drewa T, Adamowicz J on behalf of the Trauma and Reconstructive Urology Working Party of the European Association of Urology Young Academic Urologists. Application of Graphene in Tissue Engineering of the Nervous System. International Journal of Molecular Sciences. 2022; 23(1):33. https://doi.org/10.3390/ijms23010033
Chicago/Turabian StyleŁawkowska, Karolina, Marta Pokrywczyńska, Krzysztof Koper, Luis Alex Kluth, Tomasz Drewa, and Jan Adamowicz on behalf of the Trauma and Reconstructive Urology Working Party of the European Association of Urology Young Academic Urologists. 2022. "Application of Graphene in Tissue Engineering of the Nervous System" International Journal of Molecular Sciences 23, no. 1: 33. https://doi.org/10.3390/ijms23010033
APA StyleŁawkowska, K., Pokrywczyńska, M., Koper, K., Kluth, L. A., Drewa, T., & Adamowicz, J., on behalf of the Trauma and Reconstructive Urology Working Party of the European Association of Urology Young Academic Urologists. (2022). Application of Graphene in Tissue Engineering of the Nervous System. International Journal of Molecular Sciences, 23(1), 33. https://doi.org/10.3390/ijms23010033





