Metabolic Therapy of Heart Failure: Is There a Future for B Vitamins?
Abstract
1. Introduction
2. The Heart: An Oxidative Tissue
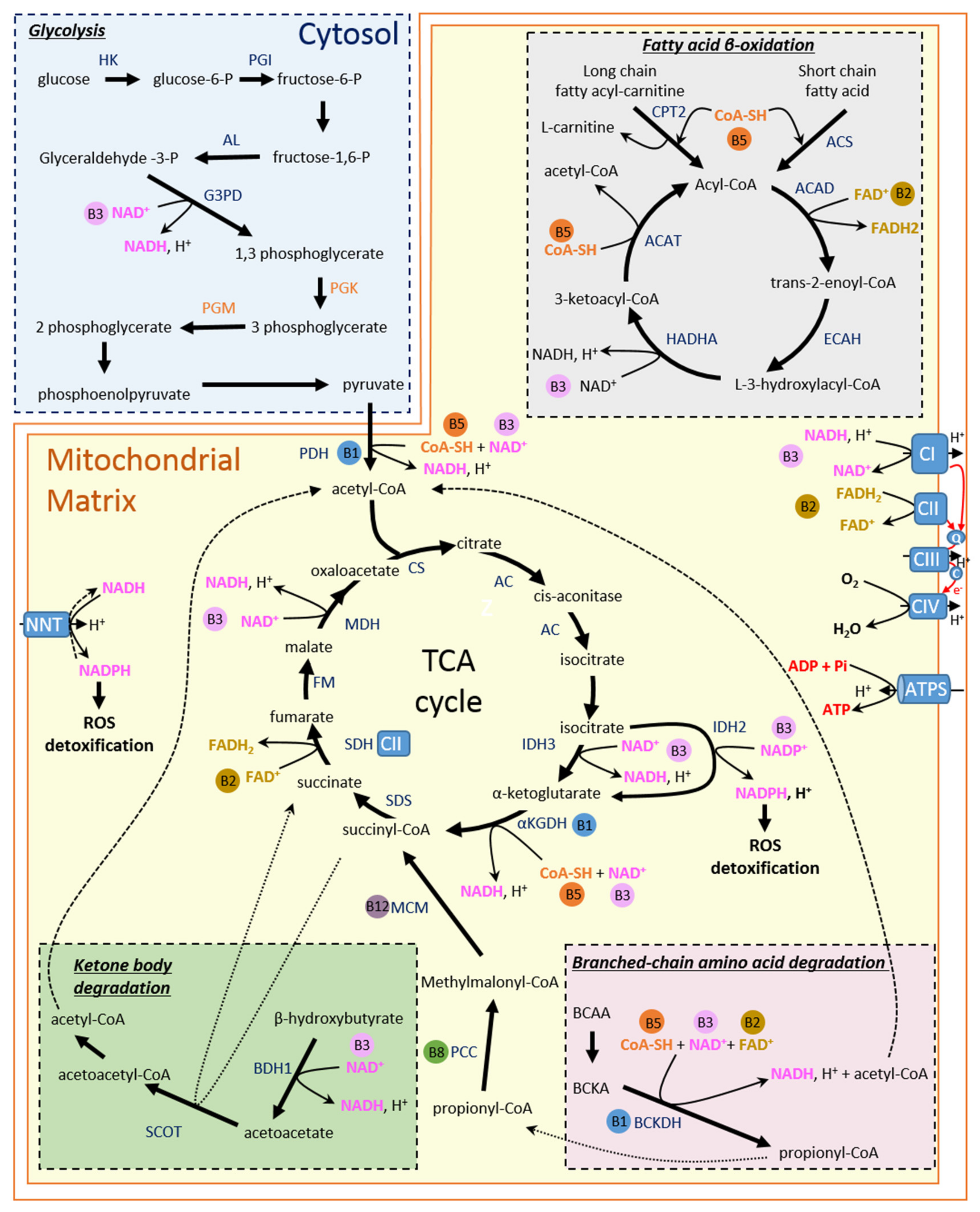
3. Energy Metabolism in Healthy Heart
3.1. Fatty Acid
3.2. Carbohydrates
3.3. Ketone Bodies
3.4. Branched-Chain Amino Acids
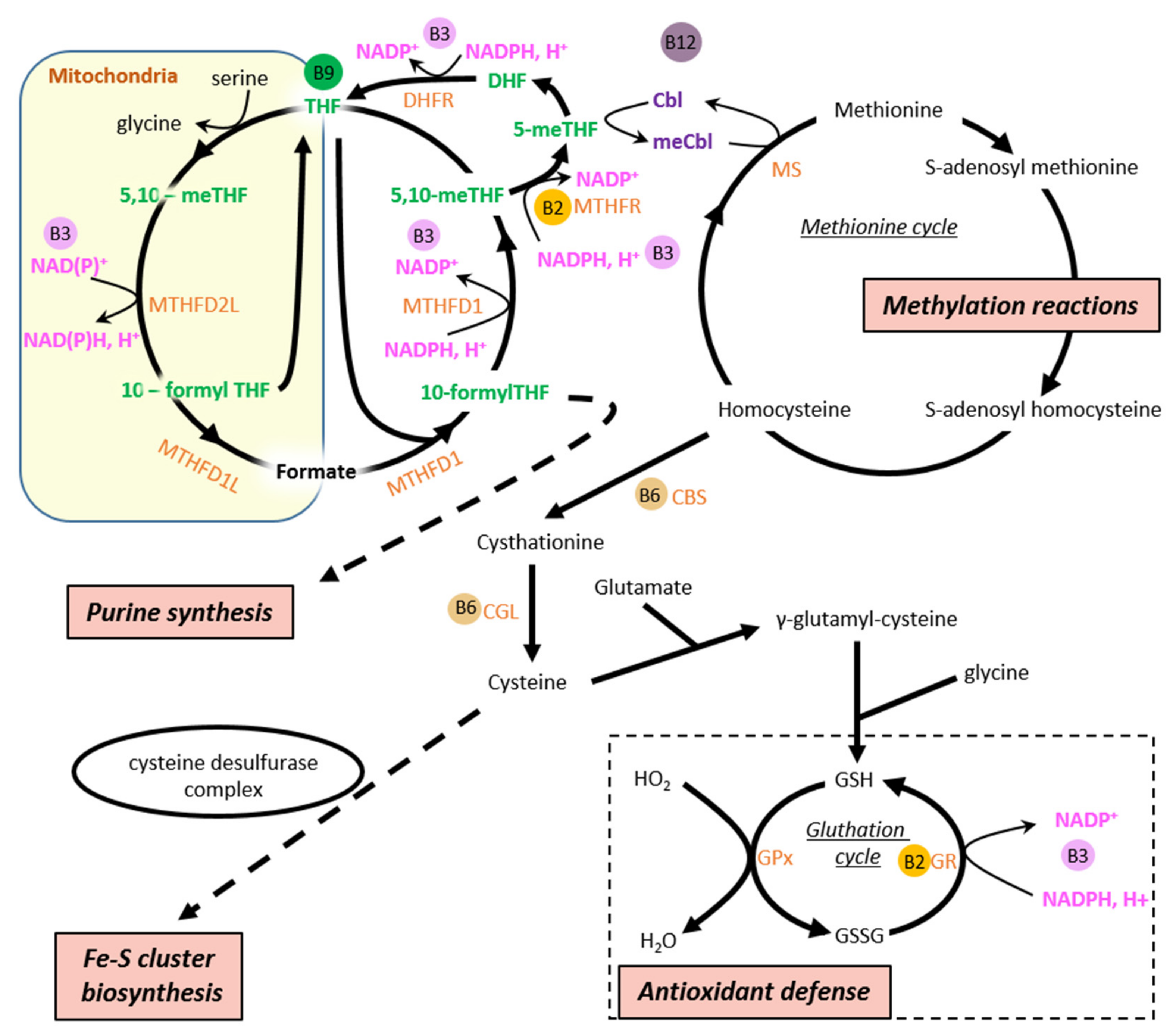
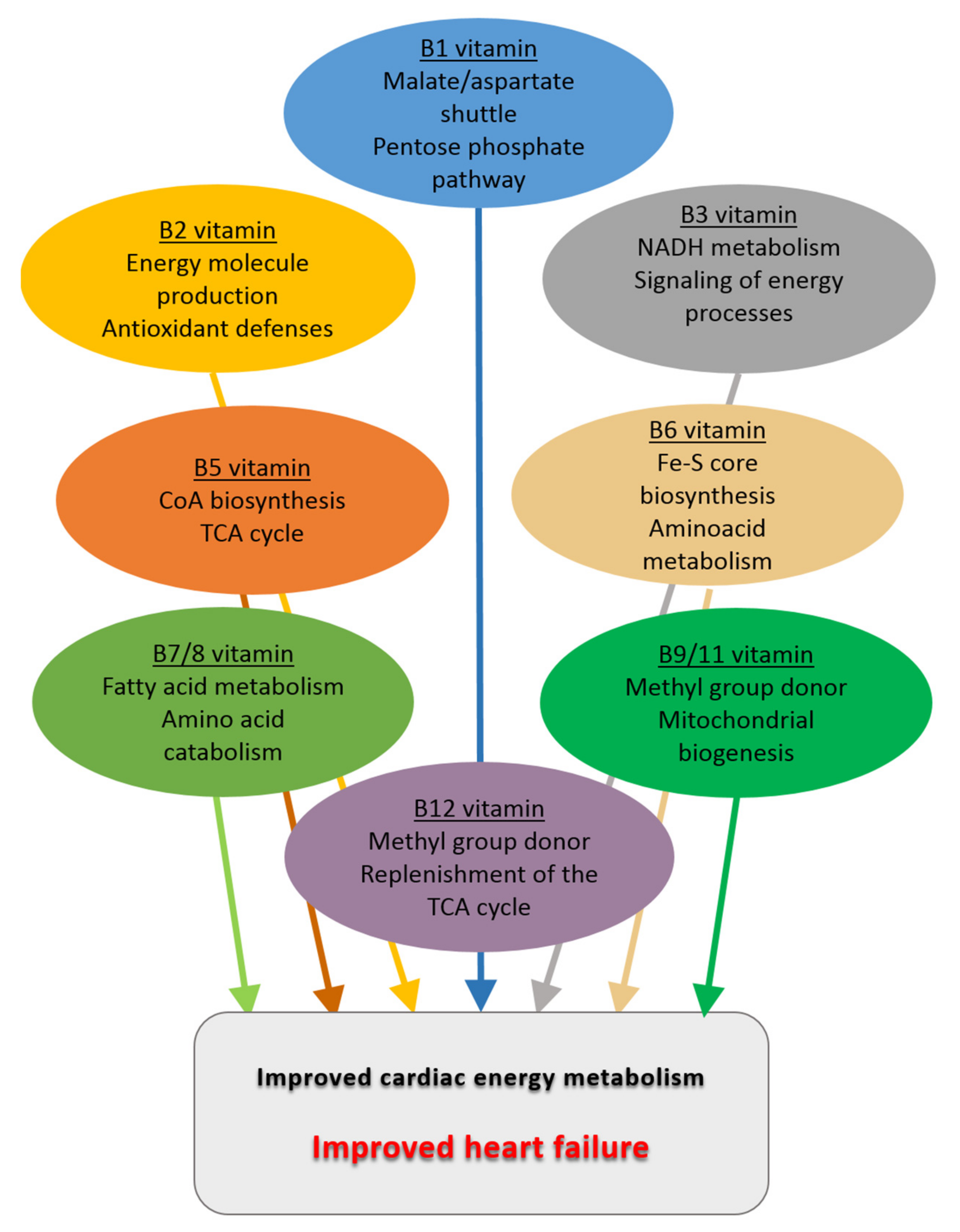
4. Energy Metabolism and B Vitamins
4.1. Vitamin B1
4.2. Vitamin B2
4.3. Vitamin B3
4.4. Vitamin B5
4.5. Vitamin B6
4.6. Vitamin B7/8
4.7. Vitamin B9/11
4.8. Vitamin B12
5. Alterations of Energy Metabolism in Heart Failure
6. B Vitamins in Heart Failure
6.1. Vitamin B1
6.2. Vitamin B2
6.3. Vitamin B3
6.4. Vitamin B5
6.5. Vitamin B7/8
6.6. Vitamin B6, Vitamin B9 and Vitamin B12
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.; Roalfe, A.K.; Adoki, I.; Hobbs, F.D.R.; Taylor, C.J. Survival of patients with chronic heart failure in the community: A systematic review and meta-analysis. Eur. J. Heart Fail. 2019, 21, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V.; Joubert, F. Bioenergetics of the failing heart. Biochim. Biophys. Acta 2011, 1813, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Seyfarth, T.; Sandstede, J.; Landschutz, W.; Lipke, C.; Kostler, H.; von Kienlin, M.; Harre, K.; Hahn, D.; Neubauer, S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. J. Am. Coll. Cardiol. 2002, 40, 1267–1274. [Google Scholar] [CrossRef]
- Shen, W.; Asai, K.; Uechi, M.; Mathier, M.A.; Shannon, R.P.; Vatner, S.F.; Ingwall, J.S. Progressive loss of myocardial ATP due to a loss of total purines during the development of heart failure in dogs: A compensatory role for the parallel loss of creatine. Circulation 1999, 100, 2113–2118. [Google Scholar] [CrossRef]
- Liao, R.; Nascimben, L.; Friedrich, J.; Gwathmey, J.K.; Ingwall, J.S. Decreased energy reserve in an animal model of dilated cardiomyopathy. Relationship to contractile performance. Circ. Res. 1996, 78, 893–902. [Google Scholar] [CrossRef]
- De Jong, K.A.; Lopaschuk, G.D. Complex Energy Metabolic Changes in Heart Failure with Preserved Ejection Fraction and Heart Failure with Reduced Ejection Fraction. Can. J. Cardiol. 2017, 33, 860–871. [Google Scholar] [CrossRef]
- Heggermont, W.A.; Papageorgiou, A.P.; Heymans, S.; van Bilsen, M. Metabolic support for the heart: Complementary therapy for heart failure? Eur. J. Heart Fail. 2016, 18, 1420–1429. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Lykstad, J.; Sharma, S. Biochemistry, Water Soluble Vitamins. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nishimoto, A.; Usery, J.; Winton, J.C.; Twilla, J. High-dose Parenteral Thiamine in Treatment of Wernicke’s Encephalopathy: Case Series and Review of the Literature. In Vivo 2017, 31, 121–124. [Google Scholar] [CrossRef]
- Boina Abdallah, A.; Ogier de Baulny, H.; Kozyraki, R.; Passemard, S.; Fenneteau, O.; Lebon, S.; Rigal, O.; Mesples, B.; Yacouben, K.; Giraudier, S.; et al. How can cobalamin injections be spaced in long-term therapy for inborn errors of vitamin B(12) absorption? Mol. Genet. Metab. 2012, 107, 66–71. [Google Scholar] [CrossRef]
- Georgiopoulos, G.; Chrysohoou, C.; Vogiatzi, G.; Magkas, N.; Bournelis, I.; Bampali, S.; Gruson, D.; Tousoulis, D. Vitamins in Heart Failure: Friend or Enemy? Curr. Pharm. Des. 2017, 23, 3731–3742. [Google Scholar] [CrossRef]
- Ahmed, M.; Azizi-Namini, P.; Yan, A.T.; Keith, M. Thiamin deficiency and heart failure: The current knowledge and gaps in literature. Heart Fail. Rev. 2015, 20, 1–11. [Google Scholar] [CrossRef]
- Keith, M.; Quach, S.; Ahmed, M.; Azizi-Namini, P.; Al-Hesayen, A.; Azevedo, E.; James, R.; Leong-Poi, H.; Ong, G.; Desjardins, S.; et al. Thiamin supplementation does not improve left ventricular ejection fraction in ambulatory heart failure patients: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1287–1295. [Google Scholar] [CrossRef]
- Goel, A.; Kattoor, A.J.; Mehta, J.L. Thiamin therapy for chronic heart failure: Is there any future for this vitamin? Am. J. Clin. Nutr. 2019, 110, 1270–1271. [Google Scholar] [CrossRef]
- Jiang, W.L.; Gu, H.B.; Zhang, Y.F.; Xia, Q.Q.; Qi, J.; Chen, J.C. Vitamin D Supplementation in the Treatment of Chronic Heart Failure: A Meta-analysis of Randomized Controlled Trials. Clin. Cardiol. 2016, 39, 56–61. [Google Scholar] [CrossRef]
- Witham, M.D.; Crighton, L.J.; Gillespie, N.D.; Struthers, A.D.; McMurdo, M.E. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: A randomized controlled trial. Circ. Heart Fail. 2010, 3, 195–201. [Google Scholar] [CrossRef]
- Dalbeni, A.; Scaturro, G.; Degan, M.; Minuz, P.; Delva, P. Effects of six months of vitamin D supplementation in patients with heart failure: A randomized double-blind controlled trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 861–868. [Google Scholar] [CrossRef]
- Majeed Babar, M.Z.; Haider, S.S.; Mustafa, G. Effects of Vitamin D supplementation on physical activity of patients with Heart Failure. Pak. J. Med. Sci. 2016, 32, 1430–1433. [Google Scholar] [CrossRef]
- Janssen, J.J.E.; Grefte, S.; Keijer, J.; de Boer, V.C.J. Mito-Nuclear Communication by Mitochondrial Metabolites and Its Regulation by B-Vitamins. Front. Physiol. 2019, 10, 78. [Google Scholar] [CrossRef]
- Lee, J.H.; Jarreau, T.; Prasad, A.; Lavie, C.; O’Keefe, J.; Ventura, H. Nutritional assessment in heart failure patients. Congest. Heart Fail. 2011, 17, 199–203. [Google Scholar] [CrossRef]
- Azizi-Namini, P.; Ahmed, M.; Yan, A.T.; Keith, M. The role of B vitamins in the management of heart failure. Nutr. Clin. Pract. 2012, 27, 363–374. [Google Scholar] [CrossRef]
- Neubauer, S. The failing heart--an engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Metabolic remodelling in heart failure. Nat. Rev. Cardiol. 2018, 15, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Geraets, I.M.E.; Glatz, J.F.C.; Luiken, J.; Nabben, M. Pivotal role of membrane substrate transporters on the metabolic alterations in the pressure-overloaded heart. Cardiovasc. Res. 2019, 115, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, F.; Alshuweishi, Y.; Salt, I.P. Regulation of nutrient uptake by AMP-activated protein kinase. Cell. Signal. 2020, 76, 109807. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, A.L.; Jordan, J.; Dworak, M.; Merkel, T.; Burnstock, G. Myocardial metabolism in heart failure: Purinergic signalling and other metabolic concepts. Pharmacol. Ther. 2019, 194, 132–144. [Google Scholar] [CrossRef]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Mitchell, P. Keilin’s respiratory chain concept and its chemiosmotic consequences. Science 1979, 206, 1148–1159. [Google Scholar] [CrossRef]
- Jonckheere, A.I.; Smeitink, J.A.; Rodenburg, R.J. Mitochondrial ATP synthase: Architecture, function and pathology. J. Inherit. Metab. Dis. 2012, 35, 211–225. [Google Scholar] [CrossRef]
- Chance, B.; Williams, G.R. The respiratory chain and oxidative phosphorylation. Adv. Enzymol. Relat. Subj. Biochem. 1956, 17, 65–134. [Google Scholar]
- Brown, G.C. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem. J. 1992, 284 Pt 1, 1–13. [Google Scholar] [CrossRef]
- Balaban, R.S. Cardiac energy metabolism homeostasis: Role of cytosolic calcium. J. Mol. Cell. Cardiol. 2002, 34, 1259–1271. [Google Scholar] [CrossRef]
- Hansford, R.G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev. Physiol. Biochem. Pharmacol 1985, 102, 1–72. [Google Scholar]
- Territo, P.R.; Mootha, V.K.; French, S.A.; Balaban, R.S. Ca2+ activation of heart mitochondrial oxidative phosphorylation: Role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 2000, 278, C423–C435. [Google Scholar] [CrossRef]
- Van der Vusse, G.J.; van Bilsen, M.; Glatz, J.F. Cardiac fatty acid uptake and transport in health and disease. Cardiovasc. Res. 2000, 45, 279–293. [Google Scholar] [CrossRef]
- Calvani, M.; Reda, E.; Arrigoni-Martelli, E. Regulation by carnitine of myocardial fatty acid and carbohydrate metabolism under normal and pathological conditions. Basic Res. Cardiol. 2000, 95, 75–83. [Google Scholar] [CrossRef]
- Eaton, S. Control of mitochondrial beta-oxidation flux. Prog. Lipid Res. 2002, 41, 197–239. [Google Scholar] [CrossRef]
- Sheeran, F.L.; Angerosa, J.; Liaw, N.Y.; Cheung, M.M.; Pepe, S. Adaptations in Protein Expression and Regulated Activity of Pyruvate Dehydrogenase Multienzyme Complex in Human Systolic Heart Failure. Oxidative Med. Cell. Longev. 2019, 2019, 4532592. [Google Scholar] [CrossRef]
- Bonen, A.; Heynen, M.; Hatta, H. Distribution of monocarboxylate transporters MCT1-MCT8 in rat tissues and human skeletal muscle. Appl. Physiol. Nutr. Metab. 2006, 31, 31–39. [Google Scholar] [CrossRef]
- Abdul Kadir, A.; Clarke, K.; Evans, R.D. Cardiac ketone body metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165739. [Google Scholar] [CrossRef] [PubMed]
- Forsey, R.G.; Reid, K.; Brosnan, J.T. Competition between fatty acids and carbohydrate or ketone bodies as metabolic fuels for the isolated perfused heart. Can. J. Physiol. Pharmacol. 1987, 65, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kerbey, A.L.; Randle, P.J.; Cooper, R.H.; Whitehouse, S.; Pask, H.T.; Denton, R.M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: Role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem. J. 1976, 154, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F. System L: Heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflug. Arch. Eur. J. Physiol. 2003, 445, 529–533. [Google Scholar] [CrossRef]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Lu, G.; Sun, H.; She, P.; Youn, J.Y.; Warburton, S.; Ping, P.; Vondriska, T.M.; Cai, H.; Lynch, C.J.; Wang, Y. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J. Clin. Investig. 2009, 119, 1678–1687. [Google Scholar] [CrossRef]
- Lu, G.; Ren, S.; Korge, P.; Choi, J.; Dong, Y.; Weiss, J.; Koehler, C.; Chen, J.N.; Wang, Y. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007, 21, 784–796. [Google Scholar] [CrossRef]
- Funk, C. On the chemical nature of the substance which cures polyneuritis in birds induced by a diet of polished rice. J. Physiol. 1911, 43, 395–400. [Google Scholar] [CrossRef]
- Liu, X.; Bisswanger, H. Interaction of thiamin diphosphate with phosphorylated and dephosphorylated mammalian pyruvate dehydrogenase complex. Biol. Chem. 2005, 386, 11–18. [Google Scholar] [CrossRef]
- Shi, Q.; Karuppagounder, S.S.; Xu, H.; Pechman, D.; Chen, H.; Gibson, G.E. Responses of the mitochondrial alpha-ketoglutarate dehydrogenase complex to thiamine deficiency may contribute to regional selective vulnerability. Neurochem. Int. 2007, 50, 921–931. [Google Scholar] [CrossRef]
- Li, J.; Wynn, R.M.; Machius, M.; Chuang, J.L.; Karthikeyan, S.; Tomchick, D.R.; Chuang, D.T. Cross-talk between thiamin diphosphate binding and phosphorylation loop conformation in human branched-chain alpha-keto acid decarboxylase/dehydrogenase. J. Biol. Chem. 2004, 279, 32968–32978. [Google Scholar] [CrossRef]
- Bettendorff, L.; Wins, P.; Lesourd, M. Subcellular localization and compartmentation of thiamine derivatives in rat brain. Biochim. Biophys. Acta 1994, 1222, 1–6. [Google Scholar] [CrossRef]
- Mkrtchyan, G.; Aleshin, V.; Parkhomenko, Y.; Kaehne, T.; Di Salvo, M.L.; Parroni, A.; Contestabile, R.; Vovk, A.; Bettendorff, L.; Bunik, V. Molecular mechanisms of the non-coenzyme action of thiamin in brain: Biochemical, structural and pathway analysis. Sci. Rep. 2015, 5, 12583. [Google Scholar] [CrossRef]
- Subramanya, S.B.; Subramanian, V.S.; Said, H.M. Chronic alcohol consumption and intestinal thiamin absorption: Effects on physiological and molecular parameters of the uptake process. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G23–G31. [Google Scholar] [CrossRef]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef]
- Barile, M.; Passarella, S.; Quagliariello, E. Thiamine pyrophosphate uptake into isolated rat liver mitochondria. Arch. Biochem. Biophys. 1990, 280, 352–357. [Google Scholar] [CrossRef]
- Kim, J.J.; Miura, R. Acyl-CoA dehydrogenases and acyl-CoA oxidases. Structural basis for mechanistic similarities and differences. Eur. J. Biochem. 2004, 271, 483–493. [Google Scholar] [CrossRef]
- Henriques, B.J.; Katrine Jentoft Olsen, R.; Gomes, C.M.; Bross, P. Electron transfer flavoprotein and its role in mitochondrial energy metabolism in health and disease. Gene 2021, 776, 145407. [Google Scholar] [CrossRef]
- Kim, H.J.; Winge, D.R. Emerging concepts in the flavinylation of succinate dehydrogenase. Biochim. Biophys. Acta 2013, 1827, 627–636. [Google Scholar] [CrossRef]
- Schulz, G.E.; Schirmer, R.H.; Pai, E.F. FAD-binding site of glutathione reductase. J. Mol. Biol. 1982, 160, 287–308. [Google Scholar] [CrossRef]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef]
- Jaeger, B.; Bosch, A.M. Clinical presentation and outcome of riboflavin transporter deficiency: Mini review after five years of experience. J. Inherit. Metab. Dis. 2016, 39, 559–564. [Google Scholar] [CrossRef]
- Goldberger, J. The etiology of Pellagra. Public Health Rep. 1914, 29, 1683–1686. [Google Scholar] [CrossRef]
- Elvehjem, C.; Madden, R.; Strong, F.; Woolley, D. Relation of nicotinic acid and nicotinic acid amide to canine black tongue. J. Am. Chem. Soc. 1937, 59, 1767. [Google Scholar] [CrossRef]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.A.; Imai, S.I. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef]
- Grozio, A.; Mills, K.F.; Yoshino, J.; Bruzzone, S.; Sociali, G.; Tokizane, K.; Lei, H.C.; Cunningham, R.; Sasaki, Y.; Migaud, M.E.; et al. Slc12a8 is a nicotinamide mononucleotide transporter. Nat. Metab. 2019, 1, 47–57. [Google Scholar] [CrossRef]
- Schmidt, M.S.; Brenner, C. Absence of evidence that Slc12a8 encodes a nicotinamide mononucleotide transporter. Nat. Metab. 2019, 1, 660–661. [Google Scholar] [CrossRef]
- Zhang, R. MNADK, a Long-Awaited Human Mitochondrion-Localized NAD Kinase. J. Cell. Physiol. 2015, 230, 1697–1701. [Google Scholar] [CrossRef]
- Lee, M.H.; Malloy, C.R.; Corbin, I.R.; Li, J.; Jin, E.S. Assessing the pentose phosphate pathway using [2, 3-(13) C2 ]glucose. NMR Biomed. 2019, 32, e4096. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, J.A.; Francisco, A.; Passos, L.A.; Figueira, T.R.; Castilho, R.F. The Contribution of Nicotinamide Nucleotide Transhydrogenase to Peroxide Detoxification Is Dependent on the Respiratory State and Counterbalanced by Other Sources of NADPH in Liver Mitochondria. J. Biol. Chem. 2016, 291, 20173–20187. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.G.; von Hardenberg, A.; Hohl, M.; Loffler, J.R.; Kohlhaas, M.; Becker, J.; Reil, J.C.; Kazakov, A.; Bonnekoh, J.; Stadelmaier, M.; et al. Reversal of Mitochondrial Transhydrogenase Causes Oxidative Stress in Heart Failure. Cell Metab. 2015, 22, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Mericskay, M. Nicotinamide adenine dinucleotide homeostasis and signalling in heart disease: Pathophysiological implications and therapeutic potential. Arch. Cardiovasc. Dis. 2016, 109, 207–215. [Google Scholar] [CrossRef]
- Matsushima, S.; Sadoshima, J. The role of sirtuins in cardiac disease. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1375–H1389. [Google Scholar] [CrossRef]
- Sanz, M.N.; Grimbert, L.; Moulin, M.; Gressette, M.; Rucker-Martin, C.; Lemaire, C.; Mericskay, M.; Veksler, V.; Ventura-Clapier, R.; Garnier, A.; et al. Inducible Cardiac-Specific Deletion of Sirt1 in Male Mice Reveals Progressive Cardiac Dysfunction and Sensitization of the Heart to Pressure Overload. Int. J. Mol. Sci. 2019, 20, 505. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Baum, C.L.; Selhub, J.; Rosenberg, I.H. The hydrolysis of nicotinamide adenine nucleotide by brush border membranes of rat intestine. Biochem. J. 1982, 204, 203–207. [Google Scholar] [CrossRef]
- Gross, C.J.; Henderson, L.M. Digestion and absorption of NAD by the small intestine of the rat. J. Nutr. 1983, 113, 412–420. [Google Scholar] [CrossRef]
- Shats, I.; Williams, J.G.; Liu, J.; Makarov, M.V.; Wu, X.; Lih, F.B.; Deterding, L.J.; Lim, C.; Xu, X.; Randall, T.A.; et al. Bacteria Boost Mammalian Host NAD Metabolism by Engaging the Deamidated Biosynthesis Pathway. Cell Metab. 2020, 31, 564–579.e567. [Google Scholar] [CrossRef]
- Kropotov, A.; Kulikova, V.; Nerinovski, K.; Yakimov, A.; Svetlova, M.; Solovjeva, L.; Sudnitsyna, J.; Migaud, M.E.; Khodorkovskiy, M.; Ziegler, M.; et al. Equilibrative Nucleoside Transporters Mediate the Import of Nicotinamide Riboside and Nicotinic Acid Riboside into Human Cells. Int. J. Mol. Sci. 2021, 22, 1391. [Google Scholar] [CrossRef]
- Vaur, P.; Brugg, B.; Mericskay, M.; Li, Z.; Schmidt, M.S.; Vivien, D.; Orset, C.; Jacotot, E.; Brenner, C.; Duplus, E. Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 5440–5452. [Google Scholar] [CrossRef]
- Mathialagan, S.; Bi, Y.A.; Costales, C.; Kalgutkar, A.S.; Rodrigues, A.D.; Varma, M.V.S. Nicotinic acid transport into human liver involves organic anion transporter 2 (SLC22A7). Biochem. Pharmacol. 2020, 174, 113829. [Google Scholar] [CrossRef]
- Fletcher, R.S.; Ratajczak, J.; Doig, C.L.; Oakey, L.A.; Callingham, R.; Da Silva Xavier, G.; Garten, A.; Elhassan, Y.S.; Redpath, P.; Migaud, M.E.; et al. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol. Metab. 2017, 6, 819–832. [Google Scholar] [CrossRef]
- Kulikova, V.; Shabalin, K.; Nerinovski, K.; Yakimov, A.; Svetlova, M.; Solovjeva, L.; Kropotov, A.; Khodorkovskiy, M.; Migaud, M.E.; Ziegler, M.; et al. Degradation of Extracellular NAD+ Intermediates in Cultures of Human HEK293 Cells. Metabolites 2019, 9, 293. [Google Scholar] [CrossRef]
- Martinez, D.L.; Tsuchiya, Y.; Gout, I. Coenzyme A biosynthetic machinery in mammalian cells. Biochem. Soc. Trans. 2014, 42, 1112–1117. [Google Scholar] [CrossRef]
- Prasad, P.D.; Wang, H.; Huang, W.; Fei, Y.J.; Leibach, F.H.; Devoe, L.D.; Ganapathy, V. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch. Biochem. Biophys. 1999, 366, 95–106. [Google Scholar] [CrossRef]
- Naquet, P.; Kerr, E.W.; Vickers, S.D.; Leonardi, R. Regulation of coenzyme A levels by degradation: The ‘Ins and Outs’. Prog. Lipid. Res. 2020, 78, 101028. [Google Scholar] [CrossRef]
- Patra, S.; Barondeau, D.P. Mechanism of activation of the human cysteine desulfurase complex by frataxin. Proc. Natl. Acad. Sci. USA 2019, 116, 19421–19430. [Google Scholar] [CrossRef]
- Le Breton, N.; Wright, J.J.; Jones, A.J.Y.; Salvadori, E.; Bridges, H.R.; Hirst, J.; Roessler, M.M. Using Hyperfine Electron Paramagnetic Resonance Spectroscopy to Define the Proton-Coupled Electron Transfer Reaction at Fe-S Cluster N2 in Respiratory Complex I. J. Am. Chem. Soc. 2017, 139, 16319–16326. [Google Scholar] [CrossRef]
- Markevich, N.I.; Markevich, L.N.; Hoek, J.B. Computational Modeling Analysis of Generation of Reactive Oxygen Species by Mitochondrial Assembled and Disintegrated Complex II. Front. Physiol. 2020, 11, 557721. [Google Scholar] [CrossRef] [PubMed]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of B vitamins on the one-carbon transfer pathways. Chem. Biol. Interact. 2006, 163, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Szebenyi, D.M.; Anguera, M.C.; Thiel, D.J.; Stover, P.J. Lack of catalytic activity of a murine mRNA cytoplasmic serine hydroxymethyltransferase splice variant: Evidence against alternative splicing as a regulatory mechanism. Biochemistry 2001, 40, 4932–4939. [Google Scholar] [CrossRef]
- Zhu, W.; Lin, A.; Banerjee, R. Kinetic properties of polymorphic variants and pathogenic mutants in human cystathionine gamma-lyase. Biochemistry 2008, 47, 6226–6232. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Stojanovski, B.M.; Hunter, G.A.; Na, I.; Uversky, V.N.; Jiang, R.H.Y.; Ferreira, G.C. 5-Aminolevulinate synthase catalysis: The catcher in heme biosynthesis. Mol. Genet. Metab. 2019, 128, 178–189. [Google Scholar] [CrossRef]
- Albersen, M.; Bosma, M.; Knoers, N.V.; de Ruiter, B.H.; Diekman, E.F.; de Ruijter, J.; Visser, W.F.; de Koning, T.J.; Verhoeven-Duif, N.M. The intestine plays a substantial role in human vitamin B6 metabolism: A Caco-2 cell model. PLoS ONE 2013, 8, e54113. [Google Scholar] [CrossRef]
- Mehansho, H.; Hamm, M.W.; Henderson, L.M. Transport and metabolism of pyridoxal and pyridoxal phosphate in the small intestine of the rat. J. Nutr. 1979, 109, 1542–1551. [Google Scholar] [CrossRef]
- Said, H.M.; Ortiz, A.; Ma, T.Y. A carrier-mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco-2 cells: Regulation by a PKA-mediated pathway. Am. J. Physiol. Cell Physiol. 2003, 285, C1219–C1225. [Google Scholar] [CrossRef]
- Said, Z.M.; Subramanian, V.S.; Vaziri, N.D.; Said, H.M. Pyridoxine uptake by colonocytes: A specific and regulated carrier-mediated process. Am. J. Physiol. Cell Physiol. 2008, 294, C1192–C1197. [Google Scholar] [CrossRef]
- Tong, L. Striking Diversity in Holoenzyme Architecture and Extensive Conformational Variability in Biotin-Dependent Carboxylases. Adv. Protein Chem. Struct. Biol. 2017, 109, 161–194. [Google Scholar] [CrossRef]
- Kusunoki, J.; Kanatani, A.; Moller, D.E. Modulation of fatty acid metabolism as a potential approach to the treatment of obesity and the metabolic syndrome. Endocrine 2006, 29, 91–100. [Google Scholar] [CrossRef]
- Abo Alrob, O.; Lopaschuk, G.D. Role of CoA and acetyl-CoA in regulating cardiac fatty acid and glucose oxidation. Biochem. Soc. Trans. 2014, 42, 1043–1051. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Collado, M.S.; Henke, B.R.; Olson, M.W.; Hoang, S.A.; Hamilton, C.A.; Pourtaheri, T.D.; Chapman, K.A.; Summar, M.M.; Johns, B.A.; et al. A novel small molecule approach for the treatment of propionic and methylmalonic acidemias. Mol. Genet. Metab. 2021, 133, 71–82. [Google Scholar] [CrossRef]
- Cozzolino, C.; Villani, G.R.; Frisso, G.; Scolamiero, E.; Albano, L.; Gallo, G.; Romanelli, R.; Ruoppolo, M. Biochemical and molecular characterization of 3-Methylcrotonylglycinuria in an Italian asymptomatic girl. Genet. Mol. Biol. 2018, 41, 379–385. [Google Scholar] [CrossRef]
- Koury, M.J.; Ponka, P. New insights into erythropoiesis: The roles of folate, vitamin B12, and iron. Annu. Rev. Nutr. 2004, 24, 105–131. [Google Scholar] [CrossRef]
- Choi, S.W.; Mason, J.B. Folate status: Effects on pathways of colorectal carcinogenesis. J. Nutr. 2002, 132, 2413S–2418S. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Gregory, J.F., 3rd; Cuskelly, G.J.; Shane, B.; Toth, J.P.; Baumgartner, T.G.; Stacpoole, P.W. Primed, constant infusion with [2H3]serine allows in vivo kinetic measurement of serine turnover, homocysteine remethylation, and transsulfuration processes in human one-carbon metabolism. Am. J. Clin. Nutr. 2000, 72, 1535–1541. [Google Scholar] [CrossRef]
- Joseph, J.; Loscalzo, J. Methoxistasis: Integrating the roles of homocysteine and folic acid in cardiovascular pathobiology. Nutrients 2013, 5, 3235–3256. [Google Scholar] [CrossRef]
- Teyssier, C.; Ma, H.; Emter, R.; Kralli, A.; Stallcup, M.R. Activation of nuclear receptor coactivator PGC-1alpha by arginine methylation. Genes Dev. 2005, 19, 1466–1473. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Pooya, S.; Lorentz, S.; Gauchotte, G.; Arnold, C.; Gueant, J.L.; Battaglia-Hsu, S.F. Decreased vitamin B12 availability induces ER stress through impaired SIRT1-deacetylation of HSF1. Cell Death Dis. 2013, 4, e553. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Adhikari, S.; Patro, B.S.; Chattopadhyay, S.; Mukherjee, T. Free radical scavenging behavior of folic acid: Evidence for possible antioxidant activity. Free Radic. Biol. Med. 2001, 30, 1390–1399. [Google Scholar] [CrossRef]
- Tucker, E.J.; Hershman, S.G.; Kohrer, C.; Belcher-Timme, C.A.; Patel, J.; Goldberger, O.A.; Christodoulou, J.; Silberstein, J.M.; McKenzie, M.; Ryan, M.T.; et al. Mutations in MTFMT underlie a human disorder of formylation causing impaired mitochondrial translation. Cell Metab. 2011, 14, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.J.; Dainty, J.R.; Finglas, P.M. Folic acid metabolism in human subjects revisited: Potential implications for proposed mandatory folic acid fortification in the UK. Br. J. Nutr. 2007, 98, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.Q.; Najmi, M.; Lin, K.; Aluri, S.; Fiser, A.; Goldman, I.D.; Zhao, R. A proton-coupled folate transporter mutation causing hereditary folate malabsorption locks the protein in an inward-open conformation. J. Biol. Chem. 2020, 295, 15650–15661. [Google Scholar] [CrossRef]
- Krautler, B. Biochemistry of B12-cofactors in human metabolism. Subcell. Biochem. 2012, 56, 323–346. [Google Scholar] [CrossRef]
- Mascarenhas, R.; Ruetz, M.; McDevitt, L.; Koutmos, M.; Banerjee, R. Mobile loop dynamics in adenosyltransferase control binding and reactivity of coenzyme B12. Proc. Natl. Acad. Sci. USA 2020, 117, 30412–30422. [Google Scholar] [CrossRef]
- Quadros, E.V. Advances in the understanding of cobalamin assimilation and metabolism. Br. J. Haematol. 2010, 148, 195–204. [Google Scholar] [CrossRef]
- Kristiansen, M.; Kozyraki, R.; Jacobsen, C.; Nexo, E.; Verroust, P.J.; Moestrup, S.K. Molecular dissection of the intrinsic factor-vitamin B12 receptor, cubilin, discloses regions important for membrane association and ligand binding. J. Biol. Chem. 1999, 274, 20540–20544. [Google Scholar] [CrossRef]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Kolwicz, S.C., Jr.; Purohit, S.; Tian, R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 2013, 113, 603–616. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Energy metabolism in heart failure. J. Physiol. 2004, 555, 1–13. [Google Scholar] [CrossRef]
- Chandler, M.P.; Kerner, J.; Huang, H.; Vazquez, E.; Reszko, A.; Martini, W.Z.; Hoppel, C.L.; Imai, M.; Rastogi, S.; Sabbah, H.N.; et al. Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1538–H1543. [Google Scholar] [CrossRef]
- Doenst, T.; Pytel, G.; Schrepper, A.; Amorim, P.; Farber, G.; Shingu, Y.; Mohr, F.W.; Schwarzer, M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc. Res. 2010, 86, 461–470. [Google Scholar] [CrossRef]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef]
- Sack, M.N.; Rader, T.A.; Park, S.; Bastin, J.; McCune, S.A.; Kelly, D.P. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 1996, 94, 2837–2842. [Google Scholar] [CrossRef]
- Karbowska, J.; Kochan, Z.; Smolenski, R.T. Peroxisome proliferator-activated receptor alpha is downregulated in the failing human heart. Cell Mol. Biol. Lett. 2003, 8, 49–53. [Google Scholar]
- Garnier, A.; Fortin, D.; Delomenie, C.; Momken, I.; Veksler, V.; Ventura-Clapier, R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J. Physiol. 2003, 551, 491–501. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Razeghi, P.; Young, M.E.; Alcorn, J.L.; Moravec, C.S.; Frazier, O.H.; Taegtmeyer, H. Metabolic gene expression in fetal and failing human heart. Circulation 2001, 104, 2923–2931. [Google Scholar] [CrossRef]
- Piquereau, J.; Ventura-Clapier, R. Maturation of Cardiac Energy Metabolism During Perinatal Development. Front. Physiol. 2018, 9, 959. [Google Scholar] [CrossRef]
- Akki, A.; Smith, K.; Seymour, A.M. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol. Cell. Biochem. 2008, 311, 215–224. [Google Scholar] [CrossRef]
- Degens, H.; de Brouwer, K.F.; Gilde, A.J.; Lindhout, M.; Willemsen, P.H.; Janssen, B.J.; van der Vusse, G.J.; van Bilsen, M. Cardiac fatty acid metabolism is preserved in the compensated hypertrophic rat heart. Basic Res. Cardiol. 2006, 101, 17–26. [Google Scholar] [CrossRef]
- Biesemann, N.; Mendler, L.; Wietelmann, A.; Hermann, S.; Schafers, M.; Kruger, M.; Boettger, T.; Borchardt, T.; Braun, T. Myostatin regulates energy homeostasis in the heart and prevents heart failure. Circ. Res. 2014, 115, 296–310. [Google Scholar] [CrossRef]
- Taegtmeyer, H. Metabolism—The lost child of cardiology. J. Am. Coll. Cardiol. 2000, 36, 1386–1388. [Google Scholar] [CrossRef]
- Leong, H.S.; Brownsey, R.W.; Kulpa, J.E.; Allard, M.F. Glycolysis and pyruvate oxidation in cardiac hypertrophy—Why so unbalanced? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 135, 499–513. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- De Sousa, E.; Veksler, V.; Minajeva, A.; Kaasik, A.; Mateo, P.; Mayoux, E.; Hoerter, J.; Bigard, X.; Serrurier, B.; Ventura-Clapier, R. Subcellular creatine kinase alterations. Implications in heart failure. Circ. Res. 1999, 85, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ingwall, J.S.; Weiss, R.G. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ. Res. 2004, 95, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Kuznetsov, A.; Veksler, V.; Boehm, E.; Anflous, K. Functional coupling of creatine kinases in muscles: Species and tissue specificity. Mol. Cell Biochem. 1998, 184, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Bayeva, M.; Ardehali, H. Mitochondrial dysfunction and oxidative damage to sarcomeric proteins. Curr. Hypertens. Rep. 2010, 12, 426–432. [Google Scholar] [CrossRef]
- Gorelik, O.; Almoznino-Sarafian, D.; Feder, I.; Wachsman, O.; Alon, I.; Litvinjuk, V.; Roshovsky, M.; Modai, D.; Cohen, N. Dietary intake of various nutrients in older patients with congestive heart failure. Cardiology 2003, 99, 177–181. [Google Scholar] [CrossRef]
- Arcand, J.; Floras, V.; Ahmed, M.; Al-Hesayen, A.; Ivanov, J.; Allard, J.P.; Newton, G.E. Nutritional inadequacies in patients with stable heart failure. J. Am. Diet. Assoc. 2009, 109, 1909–1913. [Google Scholar] [CrossRef]
- Krim, S.R.; Campbell, P.; Lavie, C.J.; Ventura, H. Micronutrients in chronic heart failure. Curr. Heart Fail. Rep. 2013, 10, 46–53. [Google Scholar] [CrossRef]
- Keith, M.E.; Walsh, N.A.; Darling, P.B.; Hanninen, S.A.; Thirugnanam, S.; Leong-Poi, H.; Barr, A.; Sole, M.J. B-vitamin deficiency in hospitalized patients with heart failure. J. Am. Diet. Assoc. 2009, 109, 1406–1410. [Google Scholar] [CrossRef]
- Mitu, O.; Cirneala, I.A.; Lupsan, A.I.; Iurciuc, M.; Mitu, I.; Dimitriu, D.C.; Costache, A.D.; Petris, A.O.; Costache, I.I. The Effect of Vitamin Supplementation on Subclinical Atherosclerosis in Patients without Manifest Cardiovascular Diseases: Never-ending Hope or Underestimated Effect? Molecules 2020, 25, 1717. [Google Scholar] [CrossRef]
- Jain, A.; Mehta, R.; Al-Ani, M.; Hill, J.A.; Winchester, D.E. Determining the Role of Thiamine Deficiency in Systolic Heart Failure: A Meta-Analysis and Systematic Review. J. Card. Fail. 2015, 21, 1000–1007. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. Thiamine Therapy for Heart Failure: A Promise or Fiction? Cardiovasc. Drugs Ther. 2018, 32, 313–317. [Google Scholar] [CrossRef]
- Seligmann, H.; Halkin, H.; Rauchfleisch, S.; Kaufmann, N.; Motro, M.; Vered, Z.; Ezra, D. Thiamine deficiency in patients with congestive heart failure receiving long-term furosemide therapy: A pilot study. Am. J. Med. 1991, 91, 151–155. [Google Scholar] [CrossRef]
- Zenuk, C.; Healey, J.; Donnelly, J.; Vaillancourt, R.; Almalki, Y.; Smith, S. Thiamine deficiency in congestive heart failure patients receiving long term furosemide therapy. Can. J. Clin. Pharmacol. 2003, 10, 184–188. [Google Scholar]
- Katta, N.; Balla, S.; Alpert, M.A. Does Long-Term Furosemide Therapy Cause Thiamine Deficiency in Patients with Heart Failure? A Focused Review. Am. J. Med. 2016, 129, 753.e7–753.e11. [Google Scholar] [CrossRef]
- Rieck, J.; Halkin, H.; Almog, S.; Seligman, H.; Lubetsky, A.; Olchovsky, D.; Ezra, D. Urinary loss of thiamine is increased by low doses of furosemide in healthy volunteers. J. Lab. Clin. Med. 1999, 134, 238–243. [Google Scholar] [CrossRef]
- Yamada, Y.; Kusakari, Y.; Akaoka, M.; Watanabe, M.; Tanihata, J.; Nishioka, N.; Bochimoto, H.; Akaike, T.; Tachibana, T.; Minamisawa, S. Thiamine treatment preserves cardiac function against ischemia injury via maintaining mitochondrial size and ATP levels. J. Appl. Physiol. 2021, 130, 26–35. [Google Scholar] [CrossRef]
- Katare, R.; Caporali, A.; Emanueli, C.; Madeddu, P. Benfotiamine improves functional recovery of the infarcted heart via activation of pro-survival G6PD/Akt signaling pathway and modulation of neurohormonal response. J. Mol. Cell. Cardiol. 2010, 49, 625–638. [Google Scholar] [CrossRef]
- Ahmed, L.A.; Hassan, O.F.; Galal, O.; Mansour, D.F.; El-Khatib, A. Beneficial effects of benfotiamine, a NADPH oxidase inhibitor, in isoproterenol-induced myocardial infarction in rats. PLoS ONE 2020, 15, e0232413. [Google Scholar] [CrossRef]
- Radonjic, T.; Rankovic, M.; Ravic, M.; Zivkovic, V.; Srejovic, I.; Jeremic, J.; Jeremic, N.; Sretenovic, J.; Matic, S.; Jakovljevic, V.; et al. The Effects of Thiamine Hydrochloride on Cardiac Function, Redox Status and Morphometric Alterations in Doxorubicin-Treated Rats. Cardiovasc. Toxicol 2020, 20, 111–120. [Google Scholar] [CrossRef]
- Katare, R.G.; Caporali, A.; Oikawa, A.; Meloni, M.; Emanueli, C.; Madeddu, P. Vitamin B1 analog benfotiamine prevents diabetes-induced diastolic dysfunction and heart failure through Akt/Pim-1-mediated survival pathway. Circ. Heart Fail. 2010, 3, 294–305. [Google Scholar] [CrossRef]
- Goncalves, A.C.; Moreira, E.J.S.; Portari, G.V. Benfotiamine supplementation prevents oxidative stress in anterior tibialis muscle and heart. J. Integr. Med. 2019, 17, 423–429. [Google Scholar] [CrossRef]
- Kohda, Y.; Umeki, M.; Kono, T.; Terasaki, F.; Matsumura, H.; Tanaka, T. Thiamine ameliorates diabetes-induced inhibition of pyruvate dehydrogenase (PDH) in rat heart mitochondria: Investigating the discrepancy between PDH activity and PDH E1alpha phosphorylation in cardiac fibroblasts exposed to high glucose. J. Pharmacol. Sci. 2010, 113, 343–352. [Google Scholar] [CrossRef]
- Shimon, I.; Almog, S.; Vered, Z.; Seligmann, H.; Shefi, M.; Peleg, E.; Rosenthal, T.; Motro, M.; Halkin, H.; Ezra, D. Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am. J. Med. 1995, 98, 485–490. [Google Scholar] [CrossRef]
- Schoenenberger, A.W.; Schoenenberger-Berzins, R.; der Maur, C.A.; Suter, P.M.; Vergopoulos, A.; Erne, P. Thiamine supplementation in symptomatic chronic heart failure: A randomized, double-blind, placebo-controlled, cross-over pilot study. Clin. Res. Cardiol. 2012, 101, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Pfitzenmeyer, P.; Guilland, J.C.; d’Athis, P.; Petit-Marnier, C.; Gaudet, M. Thiamine status of elderly patients with cardiac failure including the effects of supplementation. Int. J. Vitam. Nutr. Res. 1994, 64, 113–118. [Google Scholar] [PubMed]
- Mousavi, M.; Namazi, S.; Avadi, M.; Amirahmadi, M.; Salehifar, D. Thiamine Supplementation in Patients with Chronic Heart Failure Receiving Optimum Medical Treatment. J. Cardiol. Curr. Res. 2017, 9, 00316. [Google Scholar] [CrossRef]
- Smithline, H.A.; Donnino, M.; Blank, F.S.J.; Barus, R.; Coute, R.A.; Knee, A.B.; Visintainer, P. Supplemental thiamine for the treatment of acute heart failure syndrome: A randomized controlled trial. BMC Complement. Altern. Med. 2019, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.M.; Woodside, J.V.; McGartland, C.; Roberts, M.J.; Nicholls, D.P.; McKeown, P.P. Nutritional intake and oxidative stress in chronic heart failure. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 376–382. [Google Scholar] [CrossRef]
- McKeag, N.A.; McKinley, M.C.; Harbinson, M.T.; McGinty, A.; Neville, C.E.; Woodside, J.V.; McKeown, P.P. Dietary Micronutrient Intake and Micronutrient Status in Patients With Chronic Stable Heart Failure: An Observational Study. J. Cardiovasc. Nurs. 2017, 32, 148–155. [Google Scholar] [CrossRef]
- Witte, K.K.; Nikitin, N.P.; Parker, A.C.; von Haehling, S.; Volk, H.D.; Anker, S.D.; Clark, A.L.; Cleland, J.G. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur. Heart J. 2005, 26, 2238–2244. [Google Scholar] [CrossRef]
- Wang, P.; Fan, F.; Li, X.; Sun, X.; Ma, L.; Wu, J.; Shen, C.; Zhu, H.; Dong, Z.; Wang, C.; et al. Riboflavin attenuates myocardial injury via LSD1-mediated crosstalk between phospholipid metabolism and histone methylation in mice with experimental myocardial infarction. J. Mol. Cell. Cardiol. 2018, 115, 115–129. [Google Scholar] [CrossRef]
- Lucas, T.G.; Henriques, B.J.; Rodrigues, J.V.; Bross, P.; Gregersen, N.; Gomes, C.M. Cofactors and metabolites as potential stabilizers of mitochondrial acyl-CoA dehydrogenases. Biochim. Biophys. Acta 2011, 1812, 1658–1663. [Google Scholar] [CrossRef]
- Ma, Z.; Qin, X.; Zhong, X.; Liao, Y.; Su, Y.; Liu, X.; Liu, P.; Lu, J.; Zhou, S. Flavine adenine dinucleotide inhibits pathological cardiac hypertrophy and fibrosis through activating short chain acyl-CoA dehydrogenase. Biochem. Pharm. 2020, 178, 114100. [Google Scholar] [CrossRef]
- Wang, G.; Li, W.; Lu, X.; Zhao, X. Riboflavin alleviates cardiac failure in Type I diabetic cardiomyopathy. Heart Int. 2011, 6, e21. [Google Scholar] [CrossRef]
- Karamanlidis, G.; Lee, C.F.; Garcia-Menendez, L.; Kolwicz, S.C., Jr.; Suthammarak, W.; Gong, G.; Sedensky, M.M.; Morgan, P.G.; Wang, W.; Tian, R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 2013, 18, 239–250. [Google Scholar] [CrossRef]
- Lee, C.F.; Chavez, J.D.; Garcia-Menendez, L.; Choi, Y.; Roe, N.D.; Chiao, Y.A.; Edgar, J.S.; Goo, Y.A.; Goodlett, D.R.; Bruce, J.E.; et al. Normalization of NAD+ Redox Balance as a Therapy for Heart Failure. Circulation 2016, 134, 883–894. [Google Scholar] [CrossRef]
- Di Lisa, F.; Menabo, R.; Canton, M.; Barile, M.; Bernardi, P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J. Biol. Chem. 2001, 276, 2571–2575. [Google Scholar] [CrossRef]
- Diguet, N.; Trammell, S.A.J.; Tannous, C.; Deloux, R.; Piquereau, J.; Mougenot, N.; Gouge, A.; Gressette, M.; Manoury, B.; Blanc, J.; et al. Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy. Circulation 2017, 137, 2256–2273. [Google Scholar] [CrossRef]
- Vignier, N.; Chatzifrangkeskou, M.; Morales Rodriguez, B.; Mericskay, M.; Mougenot, N.; Wahbi, K.; Bonne, G.; Muchir, A. Rescue of biosynthesis of nicotinamide adenine dinucleotide protects the heart in cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet. 2018, 27, 3870–3880. [Google Scholar] [CrossRef]
- Chiang, S.; Braidy, N.; Maleki, S.; Lal, S.; Richardson, D.R.; Huang, M.L. Mechanisms of impaired mitochondrial homeostasis and NAD+ metabolism in a model of mitochondrial heart disease exhibiting redox active iron accumulation. Redox Biol. 2021, 46, 102038. [Google Scholar] [CrossRef]
- Abdellatif, M.; Trummer-Herbst, V.; Koser, F.; Durand, S.; Adao, R.; Vasques-Novoa, F.; Freundt, J.K.; Voglhuber, J.; Pricolo, M.R.; Kasa, M.; et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef]
- Tong, D.; Schiattarella, G.G.; Jiang, N.; Altamirano, F.; Szweda, P.A.; Elnwasany, A.; Lee, D.I.; Yoo, H.; Kass, D.A.; Szweda, L.I.; et al. NAD+ Repletion Reverses Heart Failure with Preserved Ejection Fraction. Circ. Res. 2021, 128, 1629–1641. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.L.; Martin, O.J.; Lai, L.; Riley, N.M.; Richards, A.L.; Vega, R.B.; Leone, T.C.; Pagliarini, D.J.; Muoio, D.M.; Bedi, K.C., Jr.; et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.P.; Yamamoto, T.; Oka, S.; Sadoshima, J. The function of nicotinamide phosphoribosyltransferase in the heart. DNA Repair 2014, 23, 64–68. [Google Scholar] [CrossRef]
- Tannous, C.; Booz, G.W.; Altara, R.; Muhieddine, D.H.; Mericskay, M.; Refaat, M.M.; Zouein, F.A. Nicotinamide adenine dinucleotide: Biosynthesis, consumption and therapeutic role in cardiac diseases. Acta Physiol. 2021, 231, e13551. [Google Scholar] [CrossRef] [PubMed]
- Tannous, C.; Deloux, R.; Karoui, A.; Mougenot, N.; Burkin, D.; Blanc, J.; Coletti, D.; Lavery, G.; Li, Z.; Mericskay, M. NMRK2 Gene Is Upregulated in Dilated Cardiomyopathy and Required for Cardiac Function and NAD Levels during Aging. Int. J. Mol. Sci. 2021, 22, 3534. [Google Scholar] [CrossRef]
- Walker, M.A.; Tian, R. Raising NAD in Heart Failure: Time to Translate? Circulation 2018, 137, 2274–2277. [Google Scholar] [CrossRef] [PubMed]
- Akar, F.G.; Young, L.H. NAD Repletion Therapy: A Silver Bullet for HFpEF? Circ. Res. 2021, 128, 1642–1645. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, D.D.; Qiu, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’Brien, K.D.; Tian, R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020, 130, 6054–6063. [Google Scholar] [CrossRef]
- Ostman-Smith, I.; Brown, G.; Johnson, A.; Land, J.M. Dilated cardiomyopathy due to type II X-linked 3-methylglutaconic aciduria: Successful treatment with pantothenic acid. Br. Heart J. 1994, 72, 349–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kose, A.; Parlakpinar, H.; Ozhan, O.; Ermis, N.; Yildiz, A.; Vardi, N.; Cigremis, Y. Therapeutic effects of dexpanthenol on the cardiovascular and respiratory systems following cecal ligation and puncture-induced sepsis in rats. Biotech. Histochem. 2020, 95, 428–437. [Google Scholar] [CrossRef]
- Kalkan, F.; Parlakpinar, H.; Disli, O.M.; Tanriverdi, L.H.; Ozhan, O.; Polat, A.; Cetin, A.; Vardi, N.; Otlu, Y.O.; Acet, A. Protective and therapeutic effects of dexpanthenol on isoproterenol-induced cardiac damage in rats. J. Cell. Biochem. 2018, 119, 7479–7489. [Google Scholar] [CrossRef]
- Demirci, B.; Demir, O.; Dost, T.; Birincioglu, M. Protective effect of vitamin B5 (dexpanthenol) on cardiovascular damage induced by streptozocin in rats. Bratisl Lek Listy 2014, 115, 190–196. [Google Scholar] [CrossRef]
- Velazquez-Arellano, A.; Hernandez-Esquivel Mde, L.; Sanchez, R.M.; Ortega-Cuellar, D.; Rodriguez-Fuentes, N.; Cano, S.; Leon-Del-Rio, A.; Carvajal, K. Functional and metabolic implications of biotin deficiency for the rat heart. Mol. Genet. Metab. 2008, 95, 213–219. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Hyperhomocysteinemia in health and disease: Where we are now, and where do we go from here ? Clin. Chem. Lab. Med. 2012, 50, 2075–2080. [Google Scholar] [CrossRef]
- Marti-Carvajal, A.J.; Sola, I.; Lathyris, D.; Karakitsiou, D.E.; Simancas-Racines, D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2009, 4, CD006612. [Google Scholar] [CrossRef]
- Qureshi, S.S.; Gupta, J.K.; Goyal, A.; Narayan Yadav, H. A novel approach in the management of hyperhomocysteinemia. Med. Hypotheses 2019, 129, 109245. [Google Scholar] [CrossRef]
- Strauss, E.; Supinski, W.; Radziemski, A.; Oszkinis, G.; Pawlak, A.L.; Gluszek, J. Is hyperhomocysteinemia a causal factor for heart failure? The impact of the functional variants of MTHFR and PON1 on ischemic and non-ischemic etiology. Int. J. Cardiol. 2017, 228, 37–44. [Google Scholar] [CrossRef]
- Vizzardi, E.; Bonadei, I.; Zanini, G.; Frattini, S.; Fiorina, C.; Raddino, R.; Dei Cas, L. Homocysteine and heart failure: An overview. Recent Pat. Cardiovasc. Drug Discov. 2009, 4, 15–21. [Google Scholar] [CrossRef]
- Herrmann, M.; Muller, S.; Kindermann, I.; Gunther, L.; Konig, J.; Bohm, M.; Herrmann, W. Plasma B vitamins and their relation to the severity of chronic heart failure. Am. J. Clin. Nutr. 2007, 85, 117–123. [Google Scholar] [CrossRef]
- Agoston-Coldea, L.; Mocan, T.; Gatfosse, M.; Lupu, S.; Dumitrascu, D.L. Plasma homocysteine and the severity of heart failure in patients with previous myocardial infarction. Cardiol. J. 2011, 18, 55–62. [Google Scholar] [PubMed]
- Polytarchou, K.; Dimitroglou, Y.; Varvarousis, D.; Christodoulis, N.; Psachoulia, C.; Pantziou, C.; Mourouzis, I.; Pantos, C.; Manolis, A.S. Methylmalonic acid and vitamin B12 in patients with heart failure. Hellenic J. Cardiol. 2020, 61, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Herzlich, B.C.; Lichstein, E.; Schulhoff, N.; Weinstock, M.; Pagala, M.; Ravindran, K.; Namba, T.; Nieto, F.J.; Stabler, S.P.; Allen, R.H.; et al. Relationship among homocyst(e)ine, vitamin B-12 and cardiac disease in the elderly: Association between vitamin B-12 deficiency and decreased left ventricular ejection fraction. J. Nutr. 1996, 126, 1249S–1253S. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.L.; Jeejeebhoy, K.N.; Sole, M.J. The management of conditioned nutritional requirements in heart failure. Heart Fail. Rev. 2006, 11, 75–82. [Google Scholar] [CrossRef]
- Van der Wal, H.H.; Comin-Colet, J.; Klip, I.T.; Enjuanes, C.; Grote Beverborg, N.; Voors, A.A.; Banasiak, W.; van Veldhuisen, D.J.; Bruguera, J.; Ponikowski, P.; et al. Vitamin B12 and folate deficiency in chronic heart failure. Heart 2015, 101, 302–310. [Google Scholar] [CrossRef]
- Aksoy, N.; Aksoy, M.; Cakmak, M.; Gergerlioglu, H.S.; Davutoglu, V.; Soydinc, S.; Meram, I. Increased homocysteine in heart failure: A result of renal impairment? Clin. Chem. Lab. Med. 2006, 44, 1324–1329. [Google Scholar] [CrossRef]
- Ipcioglu, O.M.; Ozcan, O.; Gultepe, M.; Ates, A.; Basoglu, C.; Cakir, E. Reduced urinary excretion of homocysteine could be the reason of elevated plasma homocysteine in patients with psychiatric illnesses. Clin. Biochem. 2008, 41, 831–835. [Google Scholar] [CrossRef]
- Stuhlinger, M.C.; Tsao, P.S.; Her, J.H.; Kimoto, M.; Balint, R.F.; Cooke, J.P. Homocysteine impairs the nitric oxide synthase pathway: Role of asymmetric dimethylarginine. Circulation 2001, 104, 2569–2575. [Google Scholar] [CrossRef]
- Tyagi, N.; Ovechkin, A.V.; Lominadze, D.; Moshal, K.S.; Tyagi, S.C. Mitochondrial mechanism of microvascular endothelial cells apoptosis in hyperhomocysteinemia. J. Cell. Biochem. 2006, 98, 1150–1162. [Google Scholar] [CrossRef]
- Munjal, C.; Tyagi, N.; Lominadze, D.; Tyagi, S.C. Matrix metalloproteinase-9 in homocysteine-induced intestinal microvascular endothelial paracellular and transcellular permeability. J. Cell. Biochem. 2012, 113, 1159–1169. [Google Scholar] [CrossRef]
- Majors, A.; Ehrhart, L.A.; Pezacka, E.H. Homocysteine as a risk factor for vascular disease. Enhanced collagen production and accumulation by smooth muscle cells. Arter. Thromb. Vasc. Biol. 1997, 17, 2074–2081. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, H.; Liu, N.; Chen, J.; Gu, Y.; Chen, J.; Yang, K. Role of Hyperhomocysteinemia and Hyperuricemia in Pathogenesis of Atherosclerosis. J. Stroke Cerebrovasc. Dis. 2017, 26, 2695–2699. [Google Scholar] [CrossRef]
- Joseph, J.; Washington, A.; Joseph, L.; Koehler, L.; Fink, L.M.; Hauer-Jensen, M.; Kennedy, R.H. Hyperhomocysteinemia leads to adverse cardiac remodeling in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2567–H2574. [Google Scholar] [CrossRef]
- Joseph, J.; Joseph, L.; Shekhawat, N.S.; Devi, S.; Wang, J.; Melchert, R.B.; Hauer-Jensen, M.; Kennedy, R.H. Hyperhomocysteinemia leads to pathological ventricular hypertrophy in normotensive rats. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H679–H686. [Google Scholar] [CrossRef]
- Kennedy, R.H.; Owings, R.; Shekhawat, N.; Joseph, J. Acute negative inotropic effects of homocysteine are mediated via the endothelium. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H812–H817. [Google Scholar] [CrossRef][Green Version]
- Kennedy, R.H.; Owings, R.; Joseph, J.; Melchert, R.B.; Hauer-Jensen, M.; Boerma, M. Acute dilatory and negative inotropic effects of homocysteine are inhibited by an adenosine blocker. Clin. Exp. Pharmacol. Physiol. 2006, 33, 340–344. [Google Scholar] [CrossRef]
- Clarke, R.; Halsey, J.; Lewington, S.; Lonn, E.; Armitage, J.; Manson, J.E.; Bonaa, K.H.; Spence, J.D.; Nygard, O.; Jamison, R.; et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch. Intern. Med. 2010, 170, 1622–1631. [Google Scholar] [CrossRef]
- Ebbing, M.; Bonaa, K.H.; Arnesen, E.; Ueland, P.M.; Nordrehaug, J.E.; Rasmussen, K.; Njolstad, I.; Nilsen, D.W.; Refsum, H.; Tverdal, A.; et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J. Intern. Med. 2010, 268, 367–382. [Google Scholar] [CrossRef]
- Towfighi, A.; Arshi, B.; Markovic, D.; Ovbiagele, B. Homocysteine-lowering therapy and risk of recurrent stroke, myocardial infarction and death: The impact of age in the VISP trial. Cerebrovasc. Dis. 2014, 37, 263–267. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.; Zheng, Y.; Muka, T.; Troup, J.; Hu, F.B. Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Wang, Y.; Li, L.; Liao, Y.; Zhang, Y.; Yu, D. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine 2019, 98, e17095. [Google Scholar] [CrossRef] [PubMed]
- Qipshidze, N.; Tyagi, N.; Sen, U.; Givvimani, S.; Metreveli, N.; Lominadze, D.; Tyagi, S.C. Folic acid mitigated cardiac dysfunction by normalizing the levels of tissue inhibitor of metalloproteinase and homocysteine-metabolizing enzymes postmyocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1484–H1493. [Google Scholar] [CrossRef] [PubMed]
- Octavia, Y.; Kararigas, G.; de Boer, M.; Chrifi, I.; Kietadisorn, R.; Swinnen, M.; Duimel, H.; Verheyen, F.K.; Brandt, M.M.; Fliegner, D.; et al. Folic acid reduces doxorubicin-induced cardiomyopathy by modulating endothelial nitric oxide synthase. J. Cell. Mol. Med. 2017, 21, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Panda, B.P.; Kohli, K.; Fahim, M.; Dubey, K. Folic acid ameliorates celecoxib cardiotoxicity in a doxorubicin heart failure rat model. Pharm. Biol. 2017, 55, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, R.; Ouyang, S.; Ma, F.; Liu, Z.; Wu, J. Folic acid prevents cardiac dysfunction and reduces myocardial fibrosis in a mouse model of high-fat diet-induced obesity. Nutr. Metab. 2017, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhou, X.; Chen, P.; Lin, J.F. Folic acid attenuates remodeling and dysfunction in the aging heart through the ER stress pathway. Life Sci. 2021, 264, 118718. [Google Scholar] [CrossRef]
- Lamberts, R.R.; Caldenhoven, E.; Lansink, M.; Witte, G.; Vaessen, R.J.; St Cyr, J.A.; Stienen, G.J. Preservation of diastolic function in monocrotaline-induced right ventricular hypertrophy in rats. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1869–H1876. [Google Scholar] [CrossRef]
- Jakovljevic Uzelac, J.; Djukic, T.; Radic, T.; Mutavdzin, S.; Stankovic, S.; Rakocevic, J.K.; Labudovic Borovic, M.; Milic, N.; Simic, T.; Savic-Radojevic, A.; et al. Folic acid affects cardiometabolic, oxidative stress, and immunohistochemical parameters in monocrotaline-induced rat heart failure. Can. J. Physiol. Pharmacol. 2020, 98, 708–716. [Google Scholar] [CrossRef]
- Ruiz, M.; Courilleau, D.; Jullian, J.C.; Fortin, D.; Ventura-Clapier, R.; Blondeau, J.P.; Garnier, A. A cardiac-specific robotized cellular assay identified families of human ligands as inducers of PGC-1alpha expression and mitochondrial biogenesis. PLoS ONE 2012, 7, e46753. [Google Scholar] [CrossRef]
- Hagar, H.H. Folic acid and vitamin B(12) supplementation attenuates isoprenaline-induced myocardial infarction in experimental hyperhomocysteinemic rats. Pharmacol. Res. 2002, 46, 213–219. [Google Scholar] [CrossRef]
- Piquereau, J.; Moulin, M.; Zurlo, G.; Mateo, P.; Gressette, M.; Paul, J.L.; Lemaire, C.; Ventura-Clapier, R.; Veksler, V.; Garnier, A. Cobalamin and folate protect mitochondrial and contractile functions in a murine model of cardiac pressure overload. J. Mol. Cell. Cardiol. 2016, 102, 33–44. [Google Scholar] [CrossRef]
- Miner, S.E.; Cole, D.E.; Evrovski, J.; Forrest, Q.; Hutchison, S.; Holmes, K.; Ross, H.J. Pyridoxine improves endothelial function in cardiac transplant recipients. J. Heart Lung Transplant. 2001, 20, 964–969. [Google Scholar] [CrossRef]
- Balmain, B.N.; Jay, O.; Morris, N.R.; Stewart, G.M.; Shiino, K.; McFarland, A.J.; Jayasinghe, R.; Chan, J.; Sabapathy, S. Folic acid supplementation improves vascular endothelial function, yet not skin blood flow during exercise in the heat, in patients with heart failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R810–R819. [Google Scholar] [CrossRef]
- Pillai, V.B.; Sundaresan, N.R.; Kim, G.; Gupta, M.; Rajamohan, S.B.; Pillai, J.B.; Samant, S.; Ravindra, P.V.; Isbatan, A.; Gupta, M.P. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J. Biol. Chem. 2010, 285, 3133–3144. [Google Scholar] [CrossRef]
| Vitamin | Specie | Effects | Studies |
|---|---|---|---|
| B1 | Mice | Positive effects on cardiac function: | |
| - myocardial infarction | [158] | ||
| - diabetes-induced cardiac dysfunction | [161] | ||
| Rat | Positive effects on cardiac function: | ||
| - ischemic injury | [157] | ||
| - myocardial infarction | [159] | ||
| - doxorubicin cardiotoxicity | [160] | ||
| - diabetes-induced cardiac dysfunction | [163] | ||
| Human | Supplementation (100 to 300 mg/day) in HF patients: | ||
| - increase in left ventricular ejection fraction | [153,164,165] | ||
| - better functional capacity (NYHA class) | [153] | ||
| - no change in walking time | [165] | ||
| - no benefit on cardiac function (FE) | [15,167] | ||
| - no improvement in the quality of life | [15,166,167,168] | ||
| B2 | Mice | Supplementation with riboflavin: reduction of myocardial ischemic injury | [172] |
| Rat | FAD treatment decreases cardiac hypertrophy and fibrosis in SHR rats | [174] | |
| Supplementation with riboflavin: protect heart function (type1 diabetes) | [175] | ||
| Human | Supplementation with a cocktail of vitamins and minerals, including riboflavin: improvement of ventricular function | [171] | |
| B3 | Mice | Supplementation with NMN | |
| - preserves of cardiac mitochondrial function in complex-I deficient mice exhibiting accelerated HF in response to chronic stress | [176] | ||
| - delays the development of HF in mice with mitochondrial dysfunction | [177] | ||
| Supplementation with NR | |||
| - preserves cardiac function in Srf mutation induced-DCM | [179] | ||
| - preserves cardiac function in Lmna mutation induced-DCM | [180] | ||
| - improves cardiac mitochondrial function and ameliorates HFpEF phenotype | [183] | ||
| Supplementation with nicotinamide improves diastolic dysfunction induced by aging | [182] | ||
| Exogenous NAD blocks cardiac hypertrophy response | [235] | ||
| B5 | Rat | Supplementation with nicotinamide improves diastolic dysfunction induced by hypertension or cardiometabolic syndrome | [182] |
| Human | oral NR administration: improvement of PBMC respiration and reduced proinflammatory cytokine gene expression in patients with HF | [190] | |
| Rat | Treatment with dexpanthenol, a vitamin B5 precursor: | ||
| - protection of the heart during sepsis | [192] | ||
| - protection heart from isoproterenol-induced damage | [193] | ||
| - beneficial effects on endothelial function (type 1 diabetes) | [194] | ||
| B6/B9/B12 | Mice | Folate supplementation protects cardiac function: | |
| - myocardial infarction | [223] | ||
| - doxorubicin cardiotoxicity | [224] | ||
| - high-fat diet-induced obesity | [226] | ||
| Supplementation with folate and cobalamin: Preservation of left ventricular ejection fraction (pressure overload induced-HF) | [232] | ||
| Rat | Folate supplementation protects cardiac function: celecoxib cardiotoxicity | [225] | |
| Folate supplementation protects diastolic function and prevents fibrosis: monocrotaline-induced hypertrophy | [228,229] | ||
| Supplementation with folate and cobalamin: reduction of cardiac damage (isoproterenol-induced infarction) | [231] | ||
| Human | Supplementation with B6/B9/B12: decrease in risk of stroke and myocardial infarction in patients older than 69 | [220] | |
| Supplementation with folate: | |||
| - decrease in risk of stroke | [221,222] | ||
| - decrease in risk of CVD in patients without preexisting CVD | [222] | ||
| Pyridoxine: improvement of endothelial function (cardiac transplant recipients) | [233] | ||
| Folate: improvement of endothelial function (HF patients) | [234] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piquereau, J.; Boitard, S.E.; Ventura-Clapier, R.; Mericskay, M. Metabolic Therapy of Heart Failure: Is There a Future for B Vitamins? Int. J. Mol. Sci. 2022, 23, 30. https://doi.org/10.3390/ijms23010030
Piquereau J, Boitard SE, Ventura-Clapier R, Mericskay M. Metabolic Therapy of Heart Failure: Is There a Future for B Vitamins? International Journal of Molecular Sciences. 2022; 23(1):30. https://doi.org/10.3390/ijms23010030
Chicago/Turabian StylePiquereau, Jérôme, Solène E. Boitard, Renée Ventura-Clapier, and Mathias Mericskay. 2022. "Metabolic Therapy of Heart Failure: Is There a Future for B Vitamins?" International Journal of Molecular Sciences 23, no. 1: 30. https://doi.org/10.3390/ijms23010030
APA StylePiquereau, J., Boitard, S. E., Ventura-Clapier, R., & Mericskay, M. (2022). Metabolic Therapy of Heart Failure: Is There a Future for B Vitamins? International Journal of Molecular Sciences, 23(1), 30. https://doi.org/10.3390/ijms23010030







