Implantation of Various Cell-Free Matrixes Does Not Contribute to the Restoration of Hyaline Cartilage within Full-Thickness Focal Defects
Abstract
:1. Introduction
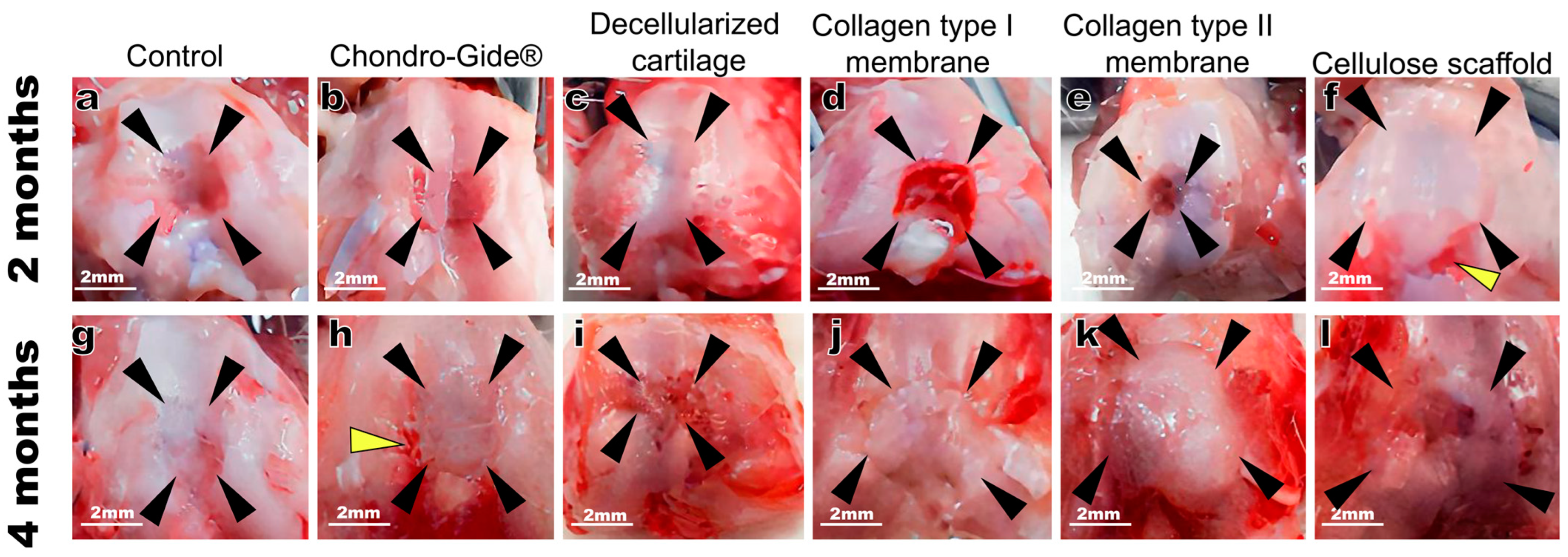
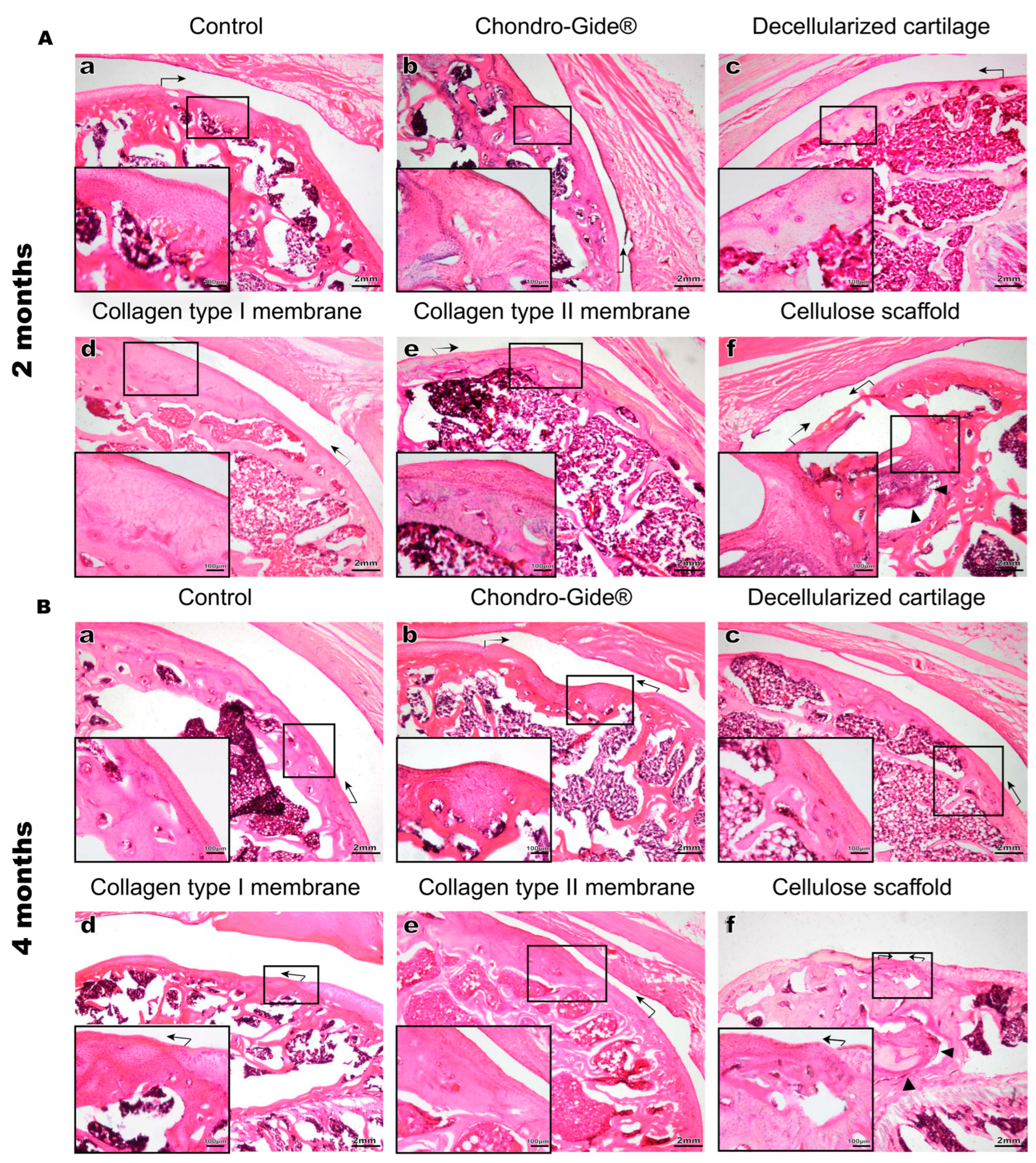
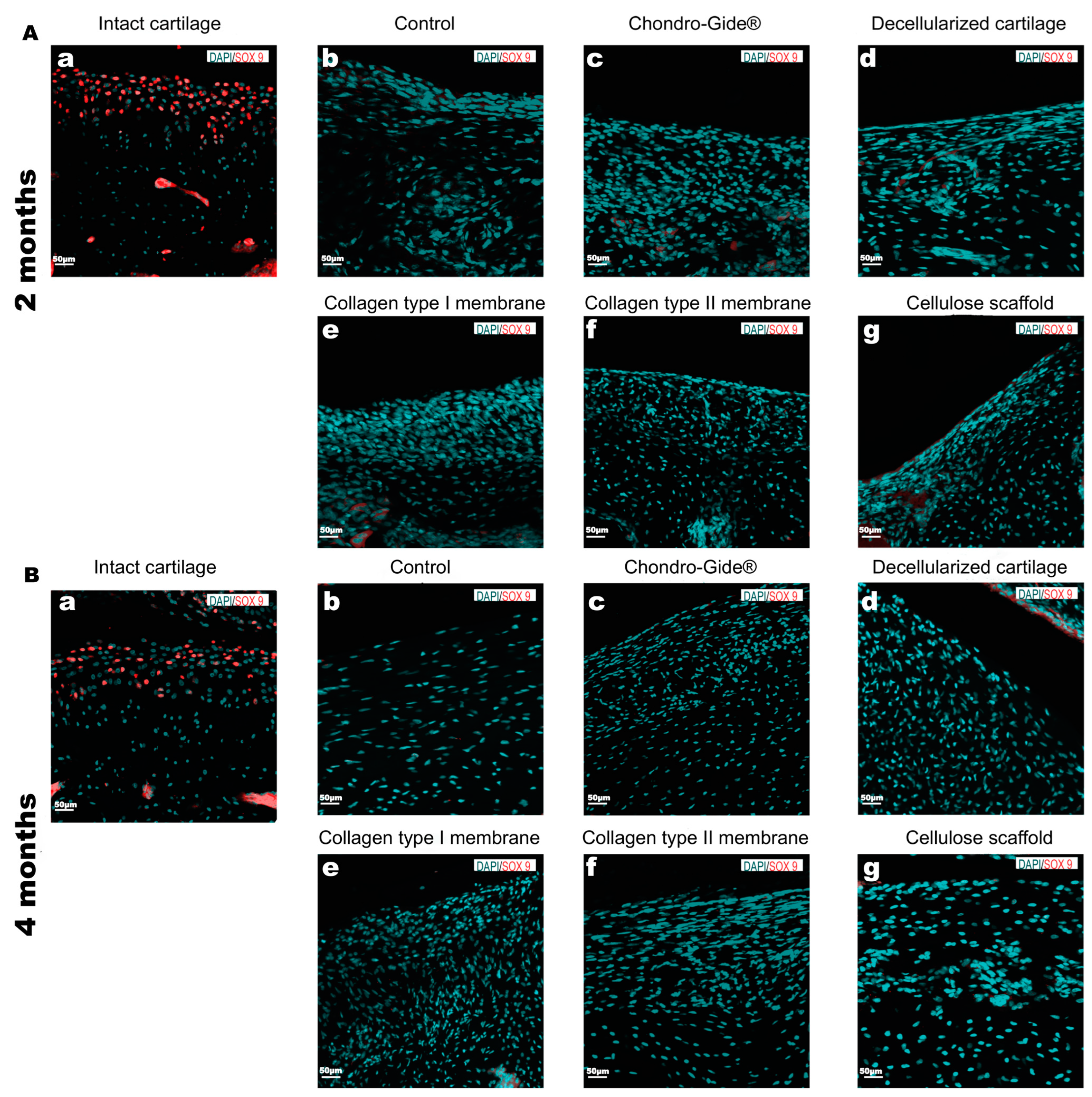
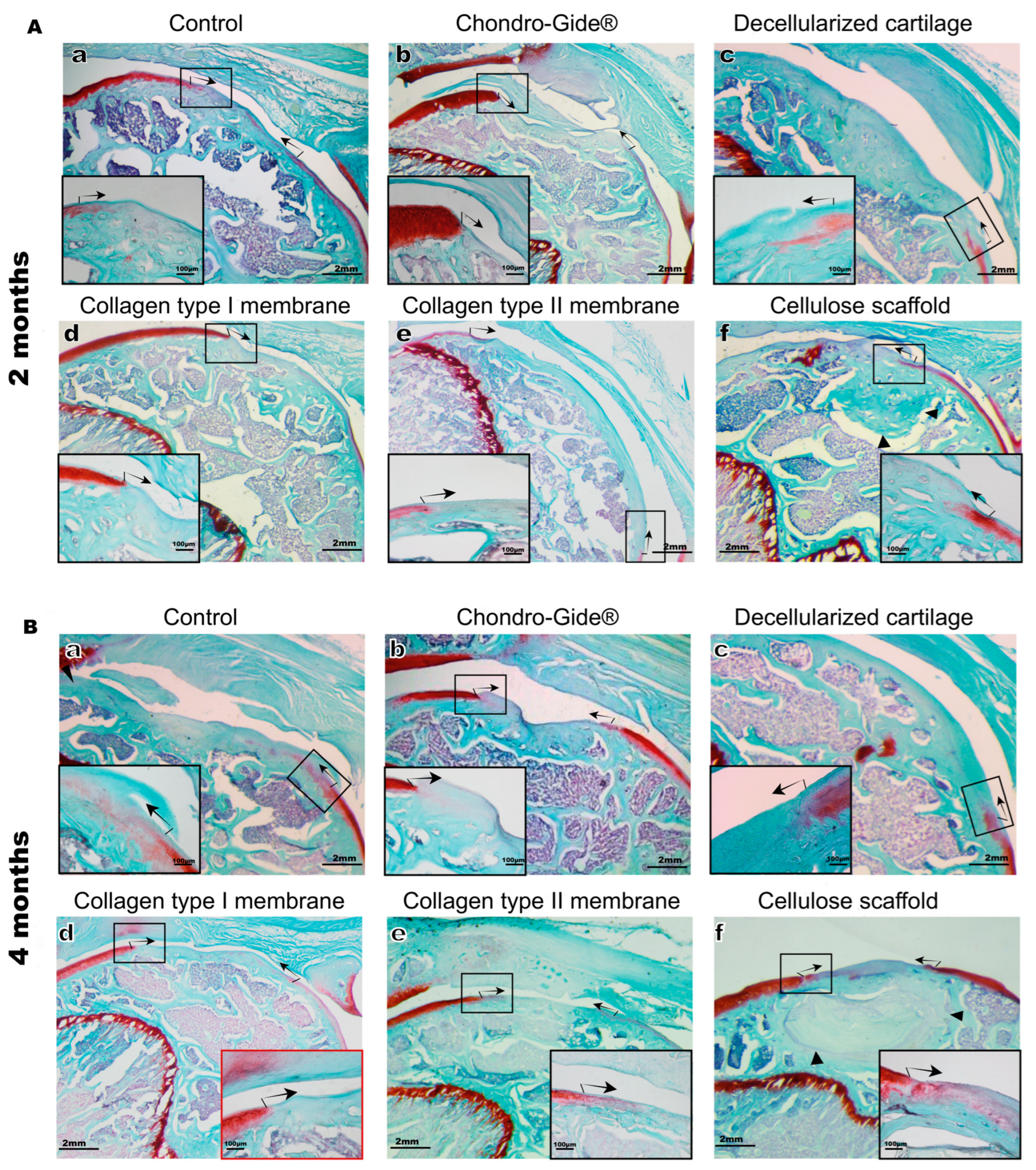
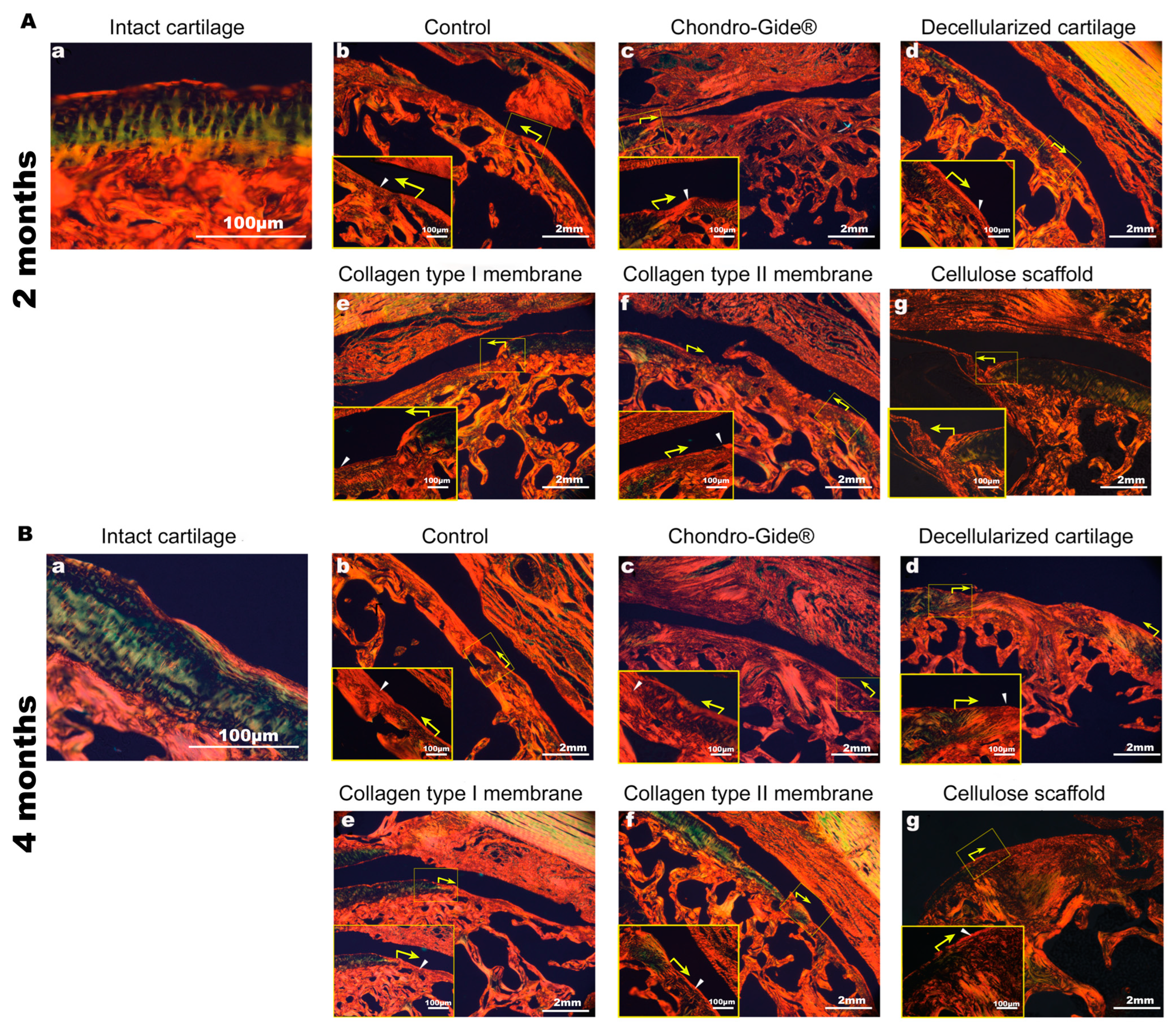
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Scaffolds
4.3. Manufacturing of Type I Collagen Membrane (Membrane I)
4.4. Manufacturing of Type II Collagen Membrane (Membrane II)
4.5. Cartilage Decellularization
4.6. Cellulose Scaffold
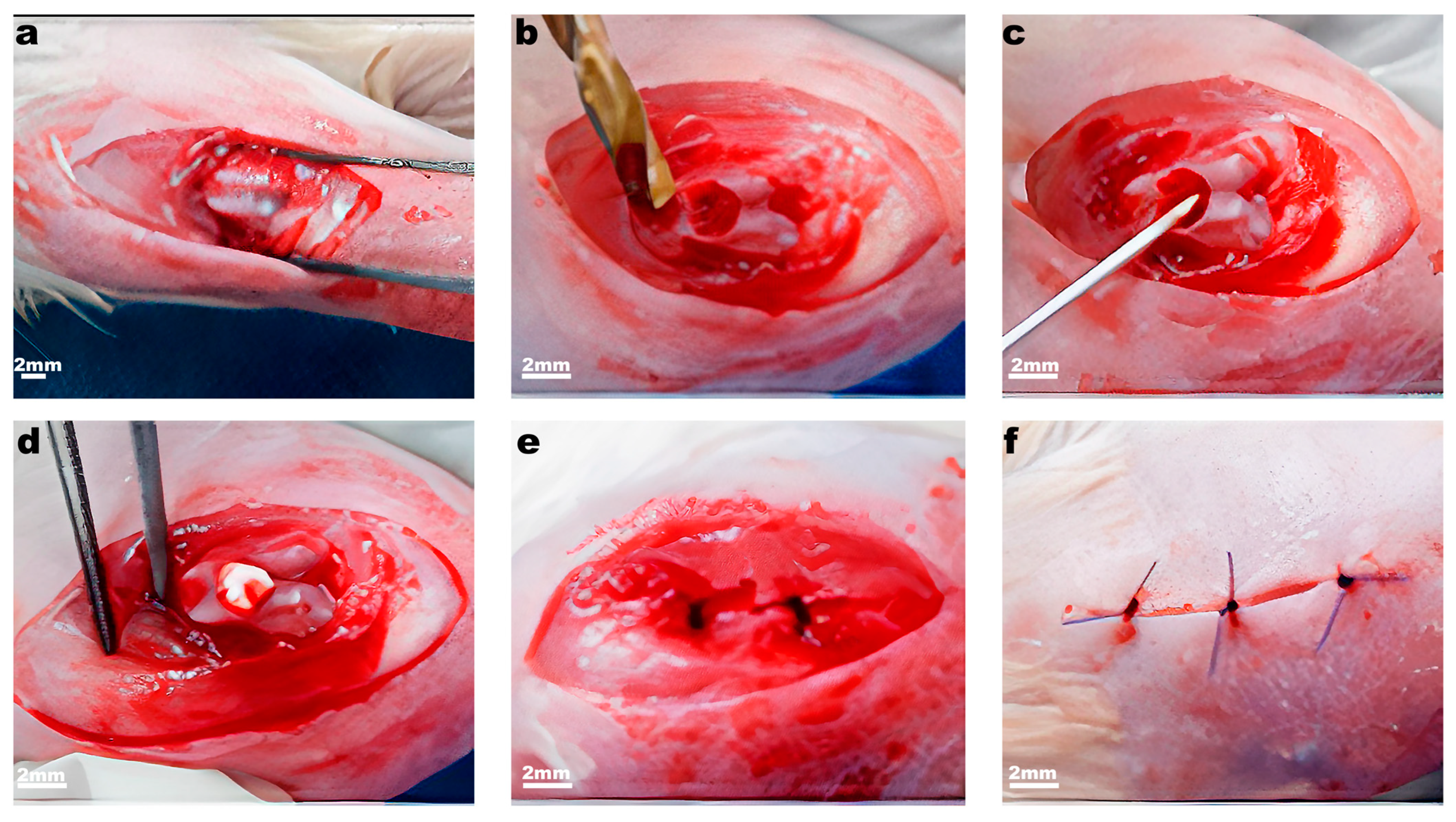
4.7. Experimental Model: Knee Joint Articular Cartilage Full-Thickness Defect
4.8. Tissue Preparation, Histological Staining, and Microscopy
4.9. Immunostaining
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gaut, C.; Sugaya, K. Critical review on the physical and mechanical factors involved in tissue engineering of cartilage. Regen. Med. 2015, 10, 665–679. [Google Scholar] [CrossRef] [Green Version]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinskiy, A.G. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005, 54, 465–480. [Google Scholar]
- Khan, I.M.; Gilbert, S.J.; Singhrao, S.K.; Duance, V.C.; Archer, C.W. Cartilage integration: Evaluation of the reasons for failure of integration during cartilage repair: A review. Eur. Cells Mater. 2008, 16, 26–39. [Google Scholar] [CrossRef]
- Biswal, S.; Hastie, T.; Andriacchi, T.P.; Bergman, G.A.; Dillingham, M.F.; Lang, P. Risk factors for progressive cartilage loss in the knee: A longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum. 2002, 46, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Davies-Tuck, M.L.; Wluka, A.E.; Wang, Y.; Teichtahl, A.J.; Jones, G.; Ding, C.; Cicuttini, F.M. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthr. Cartil. 2008, 16, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.D.; Scott, W.N. The role of debridement: Through small portals. J. Arthroplasty 2003, 18 (Suppl. S1), 10–13. [Google Scholar] [CrossRef] [PubMed]
- Steadman, J.R.; Rodkey, W.G.; Rodrigo, J.J. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res. 2001, 39, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous chondrocyte implantation: A systematic review. J. Bone Jt. Surg. Ser. A. 2010, 92, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Bartha, L.; Vajda, A.; Duska, Z.; Rahmeh, H.; Hangody, L. Autologous osteochondral mosaicplasty grafting. J. Orthop. Sports Phys. Ther. 2006, 36, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Alini, M.; Stoddart, M.J.; Evans, C.; Miclau, T.; Steiner, S. Barriers and strategies for the clinical translation of advanced orthopaedic tissue engineering protocols. Eur. Cells Mater. 2014, 27, 17–21. [Google Scholar] [CrossRef]
- Maia, F.R.; Carvalho, M.R.; Oliveira, J.M.; Reis, R.L. Tissue engineering strategies for osteochondral repair. Adv. Exp. Med. Biol. 2018, 1059, 353–371. [Google Scholar] [PubMed]
- Kwan, H.; Chisari, E.; Khan, W.S. Cell-free scaffolds as a monotherapy for focal chondral knee defects. Materials 2020, 13, 306. [Google Scholar] [CrossRef] [Green Version]
- Kalkan, R.; Nwekwo, C.W.; Adali, T. The use of scaffolds in cartilage regeneration. Crit. Rev. Eukaryot. Gene Exp. 2018, 28, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Murin, D.V.; Voloshin, V.P.; Kazantseva, I.A.; Bezuglova, T.; Gaganov, L.E. Collection of Articles of the XI Congress of the Russian Arthroscopic Society Dedicated to the 130th Anniversary of the Birth of Academician N. N. Priorov; Human and Health: Moscow, Russia, 2015; pp. 58–59. [Google Scholar]
- Fontana, A. A novel technique for treating cartilage defects in the hip: A fully arthroscopic approach to using autologous matrix-induced chondrogenesis. Arthrosc. Tech. 2012, 1, e63–e68. [Google Scholar] [CrossRef] [PubMed]
- Afanasyevskaya, E.; Medvedeva, E.; Gazimieva, B.; Kurenkova, A.; Kytko, O.; Panyushkin, P.; Istranov, L.; Istranova, E.; Shekhter, A.; Lychagin, A.; et al. Comparison of chondroplastic properties of the Chondro-Gide® and Chondroteck collagen membranes did not reveal reparative differences in the rat model of full thickness defect of articular cartilage. Pathol. Physiol. Exp. Ther. 2020, 64, 102–109. [Google Scholar]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Gudas, R.; Gudaite, A.; Pocius, A.; Gudiene, A.; Čekanauskas, E.; Monastyreckiene, E.; Basevičius, A. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am. J. Sports Med. 2012, 40, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, M.; He, J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng. Transl. Med. 2021, 6, e10206. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Newton, P.T.; Bouderlique, T.; Sejnohova, M.; Zikmund, T.; Kozhemyakina, E.; Xie, M.; Krivanek, J.; Kaiser, J.; Qian, H.; et al. Superficial cells are self-renewing chondrocyte progenitors, which form the articular cartilage in juvenile mice. FASEB J. 2017, 31, 1067–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumai, T.; Yui, N.; Yatabe, K.; Sasaki, C.; Fujii, R.; Takenaga, M.; Fujiya, H.; Niki, H.; Yudoh, K. A novel, self-assembled artificial cartilage–hydroxyapatite conjugate for combined articular cartilage and subchondral bone repair: Histopathological analysis of cartilage tissue engineering in rat knee joints. Int. J. Nanomed. 2019, 14, 1283–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhollander, A.A.; Liekens, K.; Almqvist, K.F.; Verdonk, R.; Lambrecht, S.; Elewaut, D.; Verbruggen, G.; Verdonk, P.C. A pilot study of the use of an osteochondral scaffold plug for cartilage repair in the knee and how to deal with early clinical failures. Arthroscopy 2012, 28, 225–233. [Google Scholar] [CrossRef]
- Decker, R.S.; Um, H.; Dyment, N.A.; Cottingham, N.; Enomoto-iwamoto, M.; Kronenberg, M.S.; Maye, P.; Rowe, D.W.; Koyama, E.; Pacifici, M. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev. Biol. 2017, 426, 56–58. [Google Scholar] [CrossRef]
- Currie, J.D.; Kawaguchi, A.; Traspas, R.M.; Schuez, M.; Chara, O.; Tanaka, E.M. Live imaging of axolotl digit regeneration reveals spatiotemporal choreography of diverse connective tissue progenitor pools. Dev. Cell. 2016, 39, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, X.; Zhang, R.; Huang, Y.; Li, Y.; Shan, M.; Zhong, X.; Xing, Y.; Wang, M.; Zhang, Y.; et al. Cartilage Extracellular Matrix Scaffold With Kartogenin-Encapsulated PLGA Microspheres for Cartilage Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, S.; Liang, Y.; Liu, Q. Combination of kartogenin and transforming growth factor-β3 supports synovial fluid-derived mesenchymal stem cell-based cartilage regeneration. Am. J. Transl. Res. 2019, 11, 2056–2069. [Google Scholar]
- Shetty, A.A.; Kim, S.J.; Nakamura, N.; Brittberg, M. Techniques in Cartilage Repair Surgery; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Gromovykh, T.I.; Danilchuk, T.N.; Hanh, P.M. Gluconacetobacter Hansenii gh-1/2008 Bacterial Strain—Bacterial Cellulose Producer. Patent No. RU 2464307 C1, 20 October 2012. [Google Scholar]
- Rittié, L. Method for picrosirius red-polarization detection of collagen fibers in tissue sections. In Fibrosis; Humana Press: New York, NY, USA, 2017; Volume 1627, pp. 395–407. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibragimova, S.I.; Medvedeva, E.V.; Romanova, I.A.; Istranov, L.P.; Istranova, E.V.; Lychagin, A.V.; Nedorubov, A.A.; Timashev, P.S.; Telpukhov, V.I.; Chagin, A.S. Implantation of Various Cell-Free Matrixes Does Not Contribute to the Restoration of Hyaline Cartilage within Full-Thickness Focal Defects. Int. J. Mol. Sci. 2022, 23, 292. https://doi.org/10.3390/ijms23010292
Ibragimova SI, Medvedeva EV, Romanova IA, Istranov LP, Istranova EV, Lychagin AV, Nedorubov AA, Timashev PS, Telpukhov VI, Chagin AS. Implantation of Various Cell-Free Matrixes Does Not Contribute to the Restoration of Hyaline Cartilage within Full-Thickness Focal Defects. International Journal of Molecular Sciences. 2022; 23(1):292. https://doi.org/10.3390/ijms23010292
Chicago/Turabian StyleIbragimova, Shabnam I., Ekaterina V. Medvedeva, Irina A. Romanova, Leonid P. Istranov, Elena V. Istranova, Aleksey V. Lychagin, Andrey A. Nedorubov, Peter S. Timashev, Vladimir I. Telpukhov, and Andrei S. Chagin. 2022. "Implantation of Various Cell-Free Matrixes Does Not Contribute to the Restoration of Hyaline Cartilage within Full-Thickness Focal Defects" International Journal of Molecular Sciences 23, no. 1: 292. https://doi.org/10.3390/ijms23010292
APA StyleIbragimova, S. I., Medvedeva, E. V., Romanova, I. A., Istranov, L. P., Istranova, E. V., Lychagin, A. V., Nedorubov, A. A., Timashev, P. S., Telpukhov, V. I., & Chagin, A. S. (2022). Implantation of Various Cell-Free Matrixes Does Not Contribute to the Restoration of Hyaline Cartilage within Full-Thickness Focal Defects. International Journal of Molecular Sciences, 23(1), 292. https://doi.org/10.3390/ijms23010292





