Piperlongumine Inhibits Titanium Particles-Induced Osteolysis, Osteoclast Formation, and RANKL-Induced Signaling Pathways
Abstract
:1. Introduction
2. Results
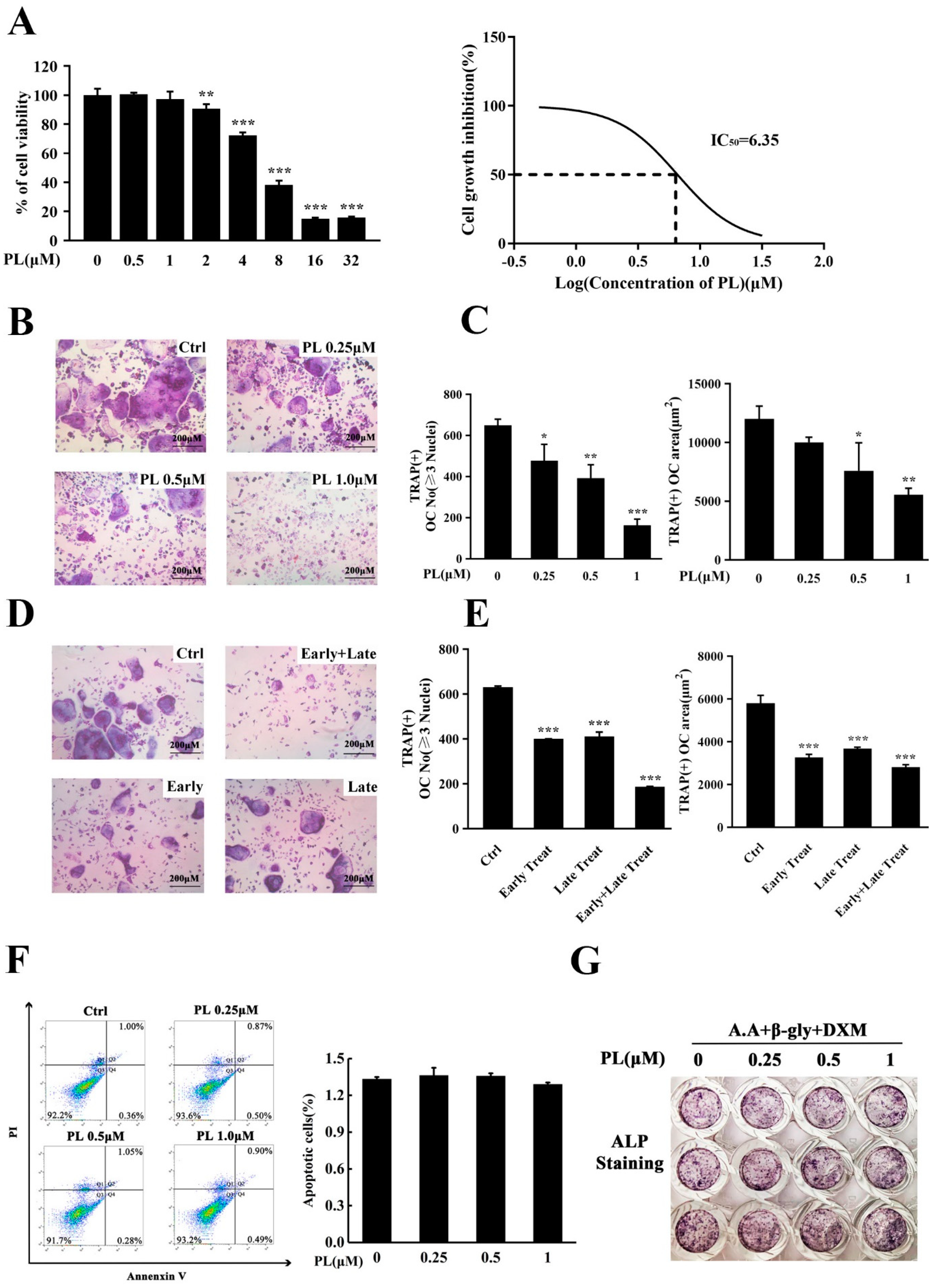
2.1. PL Treatment Suppressed RANKL-Induced Osteoclastogenesis In Vitro
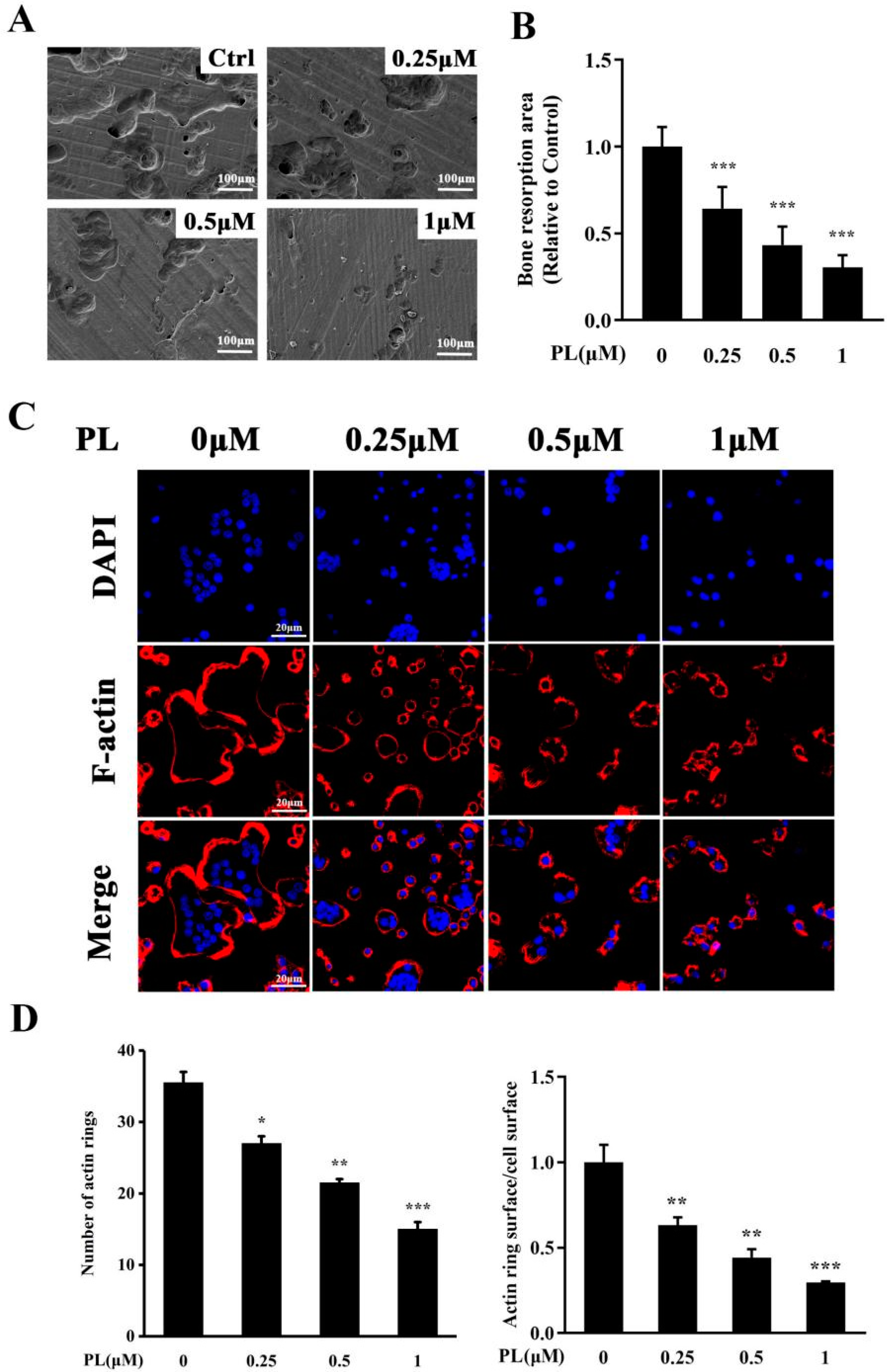
2.2. PL Inhibited the Formation of F-Actin Ring and Osteoclastic Resorption Function
2.3. PL Treatment Inhibited Osteoclastic-Related Genes Expression
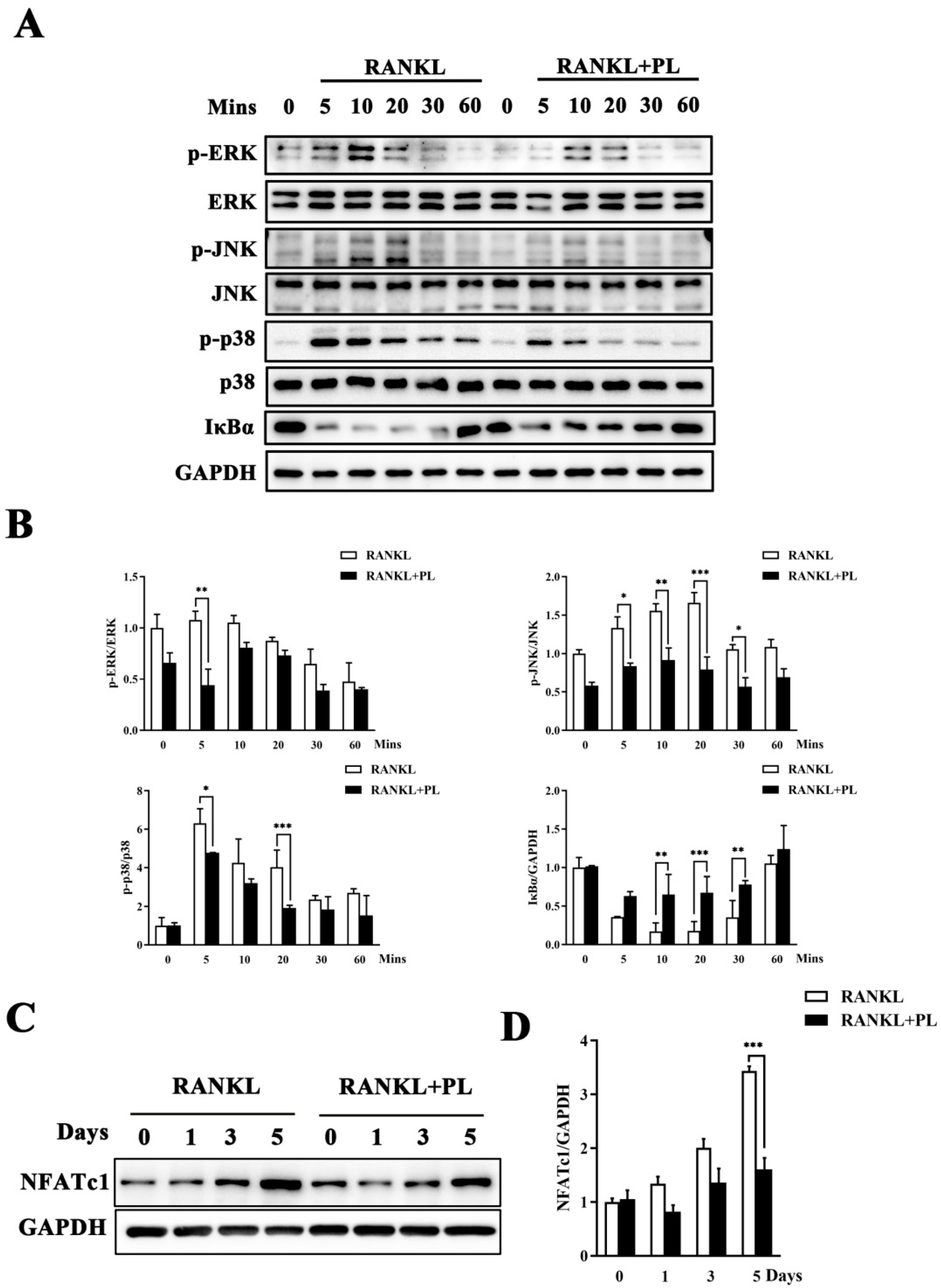
2.4. PL Suppressed RANKL-Stimulated Activations of MAPK and NF-kB Signaling Pathways
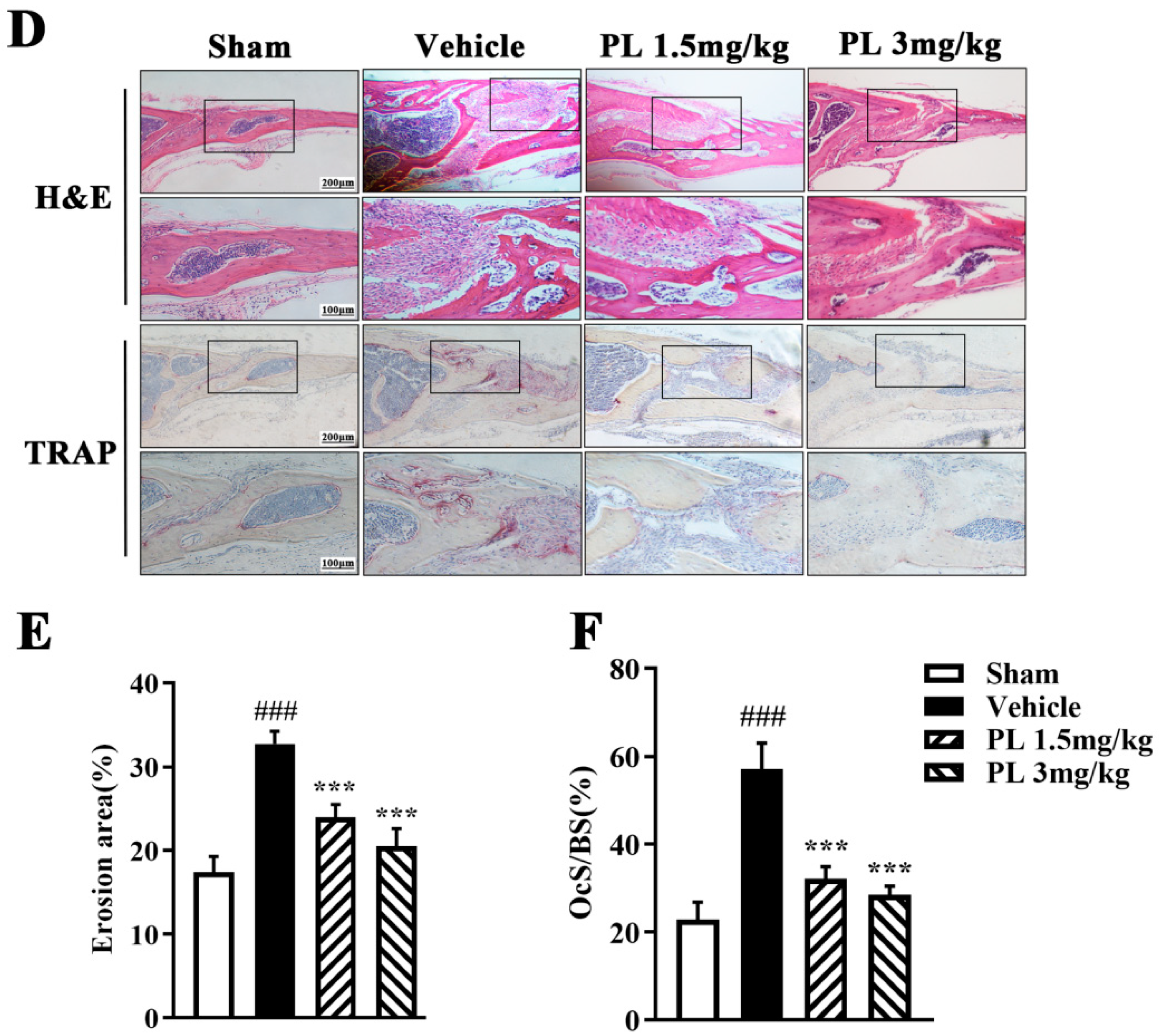
2.5. PL Protected against Ti Particle-Induced Calvarial Osteolysis
3. Discussion
4. Materials and Methods
4.1. Media and Reagents
4.2. Cell Culture and Osteoclast Differentiation
4.3. Cell Viability Assay
4.4. Apoptosis Assay
4.5. In Vitro Osteoblast Differentiation
4.6. Bone Resorption Assay
4.7. F-Actin Ring-Formation Assay
4.8. Western Blot Analysis
4.9. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (X)
4.10. Ti Particle-Induced Calvarial Osteolysis Mouse Model
4.11. Micro-CT Scanning
4.12. Histological Staining and Histomorphometric Analysis
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMM | bone marrow-derived macrophage |
| CTSK | cathepsin K |
| CTR | calcitonin receptor |
| EDTA | ethylenediamine tetraacetic acid |
| ERK | extracellular signal-regulated kinase |
| FBS | fetal bovine serum |
| FITC | Fluorescein isothiocyanate |
| GAPDH | glyceraldehyde phosphate dehydrogenase |
| H&E | hematoxylin and eosin |
| IκBα | the inhibitor of NF-κB |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen activated protein kinase |
| M-CSF | macrophage colony-stimulating factor |
| NFATc1 | nuclear factor of activated T-cells cytoplasmic 1 |
| NF-κB | nuclear factor-κB |
| PBS | phosphate buffered saline |
| PCR | polymerase chain reaction |
| PI | propidium iodide |
| RANKL | receptor activator of nuclear factor-κB ligand |
| TRAP | tartrate resistant acid phosphatase |
References
- Wang, Q.; Ge, G.; Liang, X.; Bai, J.; Wang, W.; Zhang, W.; Zheng, K.; Yang, S.; Wei, M.; Yang, H.; et al. Punicalagin ameliorates wear-particle-induced inflammatory bone destruction by bi-directional regulation of osteoblastic formation and osteoclastic resorption. Biomater. Sci. 2020, 8, 5157–5171. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, Z.; Yuan, G.; Fang, Z.; Lin, X.; Pu, R.; Kang, Y.; Li, L.; Shao, S.; Ding, J.; et al. Vx-11e protects against titanium-particle-induced osteolysis and osteoclastogenesis by supressing ERK activity. Biochem. Biophys. Res. Commun. 2019, 514, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Gao, H.; Hou, X. Magnesium lithospermate B inhibits titanium particles-induced osteoclast formation by c-fos and inhibiting NFATc1 expression. Connect. Tissue Res. 2019, 60, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Sun, Q.; Chen, G.; Sun, S.; Ma, X.; Qiu, H.; Liu, X.; Xu, L.; Liu, M. Jatrorrhizine Hydrochloride Suppresses RANKL-Induced Osteoclastogenesis and Protects against Wear Particle-Induced Osteolysis. Int. J. Mol. Sci. 2018, 19, 3698. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Gu, Y.; Wang, Y.; Bai, J.; Xiong, L.; Tao, Y.; Xue, Y.; Xu, Y.; Yang, H.; Ye, H.; et al. Inhibitory effect of acetyl-11-keto-β-boswellic acid on titanium particle-induced bone loss by abrogating osteoclast formation and downregulating the ERK signaling pathway. Int. Immunopharmacol. 2021, 94, 107459. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.W.; Kim, H.Y.; Lee, S.Y.; Lee, W.S.; Hong, K.W.; Kim, C.D. Suppression of RANKL-induced osteoclast differentiation by cilostazol via SIRT1-induced RANK inhibition. Biochim. Biophys. Acta 2015, 10 Pt A, 2137–2144. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Xiao, L.; Ge, G.; Zhong, M.; Zhu, J.; Qin, J.; Feng, C.; Zhang, W.; Bai, J.; Zhu, X.; et al. Puerarin inhibits titanium particle-induced osteolysis and RANKL-induced osteoclastogenesis via suppression of the NF-κB signaling pathway. J. Cell. Mol. Med. 2020, 24, 11972–11983. [Google Scholar] [CrossRef]
- Cardemil, C.; Omar, O.M.; Norlindh, B.; Wexell, C.L.; Thomsen, P. The effects of a systemic single dose of zoledronic acid on post-implantation bone remodelling and inflammation in an ovariectomised rat model. Biomaterials 2013, 34, 1546–1561. [Google Scholar] [CrossRef]
- McClung, M.; Harris, S.T.; Miller, P.D.; Bauer, D.C.; Davison, K.S.; Dian, L.; Hanley, D.A.; Kendler, D.L.; Yuen, C.K.; Lewiecki, E.M. Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug holiday. Am. J. Med. 2013, 126, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Yamori, M.; Yamamoto, K.; Saito, K.; Asai, K.; Sumi, E.; Goto, K.; Takahashi, K.; Nakayama, T.; Bessho, K. Risk of osteomyelitis of the jaw induced by oral bisphosphonates in patients taking medications for osteoporosis: A hospital-based cohort study in Japan. Bone 2012, 51, 882–887. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S210–S243. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Biswal, B.K. Piperlongumine, a potent anticancer phytotherapeutic: Perspectives on contemporary status and future possibilities as an anticancer agent. Pharmacol. Res. 2020, 156, 104772. [Google Scholar] [CrossRef]
- Thatikonda, S.; Pooladanda, V.; Sigalapalli, D.K.; Godugu, C. Piperlongumine regulates epigenetic modulation and alleviates psoriasis-like skin inflammation via inhibition of hyperproliferation and inflammation. Cell Death Dis. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrique, T.; Zanon, C.F.; Girol, A.P.; Stefanini, A.C.B.; Contessoto, N.S.A.; da Silveira, N.J.F.; Bezerra, D.P.; Silveira, E.R.; Barbosa-Filho, J.M.; Cornélio, M.L.; et al. Biological and physical approaches on the role of piplartine (piperlongumine) in cancer. Sci. Rep. 2020, 10, 22283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, C.; Yuan, M.; Huang, C.; Chen, L.; Su, T.; Liao, Z.; Gan, L. Piperlongumine produces antidepressant-like effects in rats exposed to chronic unpredictable stress. Behav. Pharmacol. 2019, 30, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Na, H.H.; Jin, H.O.; Kim, K.C. Increased Expression of FosB through Reactive Oxygen Species Accumulation Functions as Pro-Apoptotic Protein in Piperlongumine Treated MCF7 Breast Cancer Cells. Mol. Cells 2019, 42, 884–892. [Google Scholar]
- Yao, Y.; Sun, Y.; Shi, M.; Xia, D.; Zhao, K.; Zeng, L.; Yao, R.; Zhang, Y.; Li, Z.; Niu, M.; et al. Piperlongumine induces apoptosis and reduces bortezomib resistance by inhibiting STAT3 in multiple myeloma cells. Oncotarget 2016, 7, 73497–73508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.; Chen, H.P.; Ma, Q. Piperlongumine alleviates lupus nephritis in MRL-Fas(lpr) mice by regulating the frequency of Th17 and regulatory T cells. Immunol. Lett. 2014, 161, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Borjihan, G.; Zhao, R.; Sun, Z.; Hammond, G.B.; Uryu, T. Antihyperlipidemic compounds from the fruit of Piper longum L. Phytother. Res. 2009, 23, 1194–1196. [Google Scholar] [CrossRef]
- Iwashita, M.; Oka, N.; Ohkubo, S.; Saito, M.; Nakahata, N. Piperlongumine, a constituent of Piper longum L., inhibits rabbit platelet aggregation as a thromboxane A(2) receptor antagonist. Eur. J. Pharmacol. 2007, 570, 38–42. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.A.; Eom, S.Y.; Lee, S.H.; Min, K.R.; Kim, Y. Inhibitory effect of piperlonguminine on melanin production in melanoma B16 cell line by downregulation of tyrosinase expression. Pigment. Cell Res. 2006, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Pumroy, R.A.; Baker, C.; Rodrigues, T.; Guerreiro, A.; Sousa, B.B.; Marques, M.C.; de Almeida, B.P.; Lee, S.; Leites, E.P.; et al. Allosteric Antagonist Modulation of TRPV2 by Piperlongumine Impairs Glioblastoma Progression. ACS Cent. Sci. 2021, 7, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xiao, Y.; Zeng, S.; Zou, Y.; Qiu, Q.; Huang, M.; Zhan, Z.; Liang, L.; Yang, X.; Xu, H. Piperlongumine inhibits the proliferation, migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2018, 67, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, P.; Du, X.; Zhang, Q.; Zhu, Y. Piperlongumine attenuates collagen-induced arthritis via expansion of myeloid-derived suppressor cells and inhibition of the activation of fibroblast-like synoviocytes. Mol. Med. Rep. 2015, 11, 2689–2694. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, G.; Zhang, Q.; Zhao, F.; Yu, X.; Ma, X.; Liu, M. Phillyrin Attenuates Osteoclast Formation and Function and Prevents LPS-Induced Osteolysis in Mice. Front. Pharmacol. 2019, 10, 1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teitelbaum, S.L. The osteoclast and its unique cytoskeleton. Ann. N. Y. Acad. Sci. 2011, 1240, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shang, G.Q.; Xiang, S.; Zhang, H.N.; Wang, Y.Z.; Xu, H. Zoledronic acid and teriparatide have a complementary therapeutic effect on aseptic loosening in a rabbit model. BMC Musculoskelet. Disord. 2021, 22, 580. [Google Scholar] [CrossRef] [PubMed]
- Kurotaki, D.; Yoshida, H.; Tamura, T. Epigenetic and transcriptional regulation of osteoclast differentiation. Bone 2020, 138, 115471. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar]
- Yavropoulou, M.P.; Yovos, J.G. Osteoclastogenesis—Current knowledge and future perspectives. J. Musculoskelet. Neuronal Interact. 2008, 8, 204–216. [Google Scholar] [PubMed]
- Hu, B.; Wu, F.; Shi, Z.; He, B.; Zhao, X.; Wu, H.; Yan, S. Dehydrocostus lactone attenuates osteoclastogenesis and osteoclast-induced bone loss by modulating NF-κB signalling pathway. J. Cell. Mol. Med. 2019, 23, 5762–5770. [Google Scholar] [CrossRef]
- Kwak, S.C.; Cheon, Y.H.; Lee, C.H.; Jun, H.Y.; Yoon, K.H.; Lee, M.S.; Kim, J.Y. Grape Seed Proanthocyanidin Extract Prevents Bone Loss via Regulation of Osteoclast Differentiation, Apoptosis, and Proliferation. Nutrients 2020, 12, 3164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, S.; Liu, P.; Huang, J.; Qiu, J.; Wen, Z.; Yuan, J.; Qiu, H.; Liu, Y.; Liu, Q.; et al. Carnosol suppresses RANKL-induced osteoclastogenesis and attenuates titanium particles-induced osteolysis. J. Cell. Physiol. 2021, 236, 1950–1966. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, K.; Fang, X.; Lu, F.; Zhang, W.; Song, X.; Chen, L.; Sun, J.; Chen, H. Inhibition of Lipopolysaccharide-Induced Inflammatory Bone Loss by Saikosaponin D is Associated with Regulation of the RANKL/RANK Pathway. Drug Des. Dev. Ther. 2021, 15, 4741–4757. [Google Scholar] [CrossRef]
- Nagy, V.; Penninger, J.M. The RANKL-RANK Story. Gerontology 2015, 61, 534–542. [Google Scholar] [CrossRef] [PubMed]
- David, J.P.; Sabapathy, K.; Hoffmann, O.; Idarraga, M.H.; Wagner, E.F. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J. Cell Sci. 2002, 115 Pt 22, 4317–4325. [Google Scholar] [CrossRef] [Green Version]
- Gingery, A.; Bradley, E.; Shaw, A.; Oursler, M.J. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J. Cell. Biochem. 2003, 89, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Udagawa, N.; Itoh, K.; Suda, K.; Murase, Y.; Nishihara, T.; Suda, T.; Takahashi, N. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology 2002, 143, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Han, S.I.; Kim, H.H.; Lee, Z.H. TAK1-dependent activation of AP-1 and c-Jun N-terminal kinase by receptor activator of NF-kappaB. J. Biochem. Mol. Biol. 2002, 35, 371–376. [Google Scholar] [PubMed] [Green Version]
- Soysa, N.S.; Alles, N.; Shimokawa, H.; Jimi, E.; Aoki, K.; Ohya, K. Inhibition of the classical NF-kappaB pathway prevents osteoclast bone-resorbing activity. J. Bone Miner. Metab. 2009, 27, 131–139. [Google Scholar] [CrossRef]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xu, L.; Ma, X.; Xu, J.; Wang, J.; Xian, M.; Zhou, X.; Wang, M.; Wang, F.; Qin, A.; et al. MAGED1 is a negative regulator of bone remodeling in mice. Am. J. Pathol. 2015, 185, 2653–2667. [Google Scholar] [CrossRef] [PubMed]
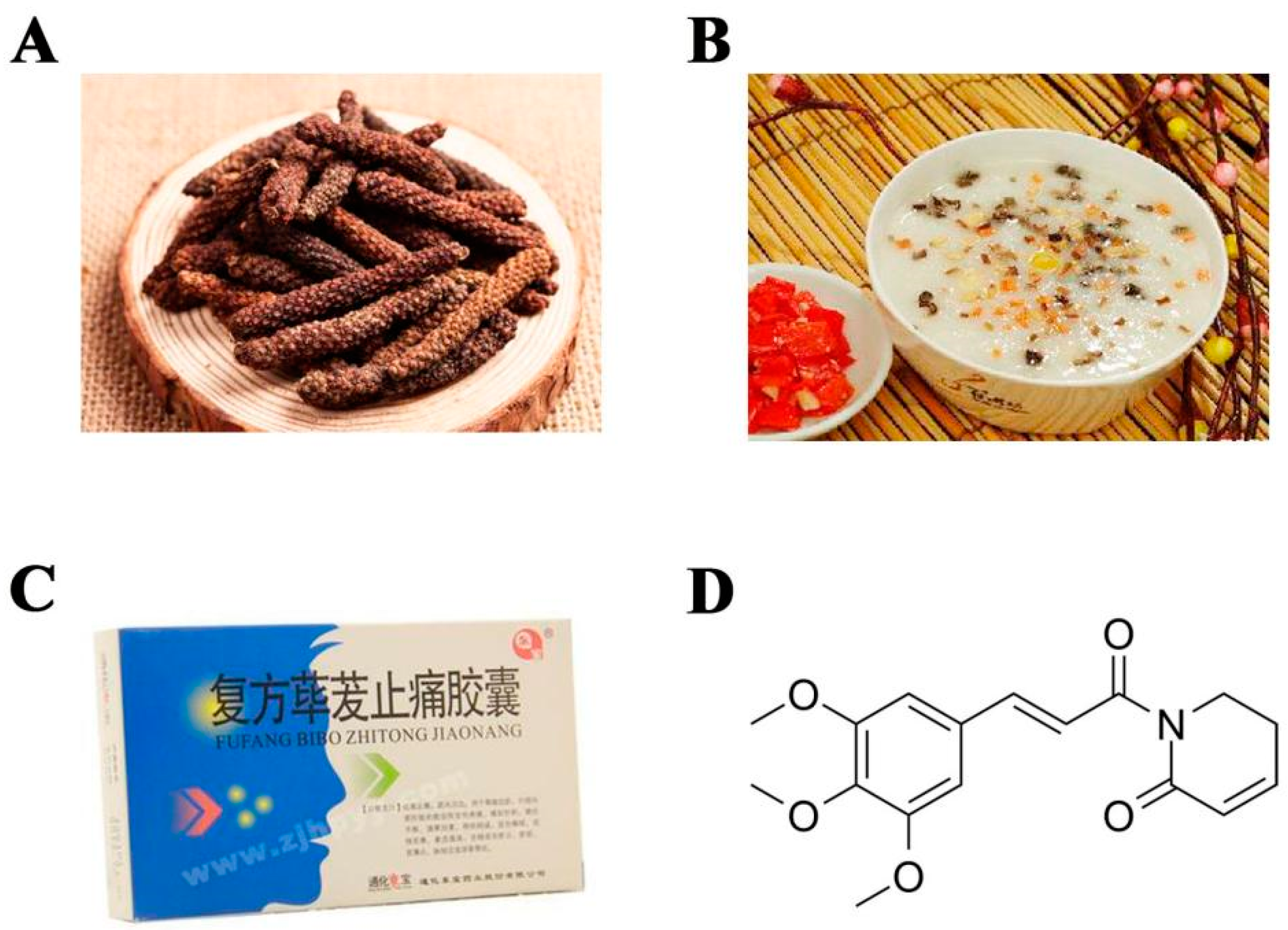


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Diao, L.; Zhang, Y.; Yang, X.; Zhou, J.; Mao, Y.; Shi, X.; Zhao, F.; Liu, M. Piperlongumine Inhibits Titanium Particles-Induced Osteolysis, Osteoclast Formation, and RANKL-Induced Signaling Pathways. Int. J. Mol. Sci. 2022, 23, 2868. https://doi.org/10.3390/ijms23052868
Liu X, Diao L, Zhang Y, Yang X, Zhou J, Mao Y, Shi X, Zhao F, Liu M. Piperlongumine Inhibits Titanium Particles-Induced Osteolysis, Osteoclast Formation, and RANKL-Induced Signaling Pathways. International Journal of Molecular Sciences. 2022; 23(5):2868. https://doi.org/10.3390/ijms23052868
Chicago/Turabian StyleLiu, Xuan, Li Diao, Yudie Zhang, Xue Yang, Junnan Zhou, Yuhang Mao, Xiaotian Shi, Fuli Zhao, and Mei Liu. 2022. "Piperlongumine Inhibits Titanium Particles-Induced Osteolysis, Osteoclast Formation, and RANKL-Induced Signaling Pathways" International Journal of Molecular Sciences 23, no. 5: 2868. https://doi.org/10.3390/ijms23052868
APA StyleLiu, X., Diao, L., Zhang, Y., Yang, X., Zhou, J., Mao, Y., Shi, X., Zhao, F., & Liu, M. (2022). Piperlongumine Inhibits Titanium Particles-Induced Osteolysis, Osteoclast Formation, and RANKL-Induced Signaling Pathways. International Journal of Molecular Sciences, 23(5), 2868. https://doi.org/10.3390/ijms23052868




