Deacetylasperulosidic Acid Ameliorates Pruritus, Immune Imbalance, and Skin Barrier Dysfunction in 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis NC/Nga Mice
Abstract
:1. Introduction
2. Results
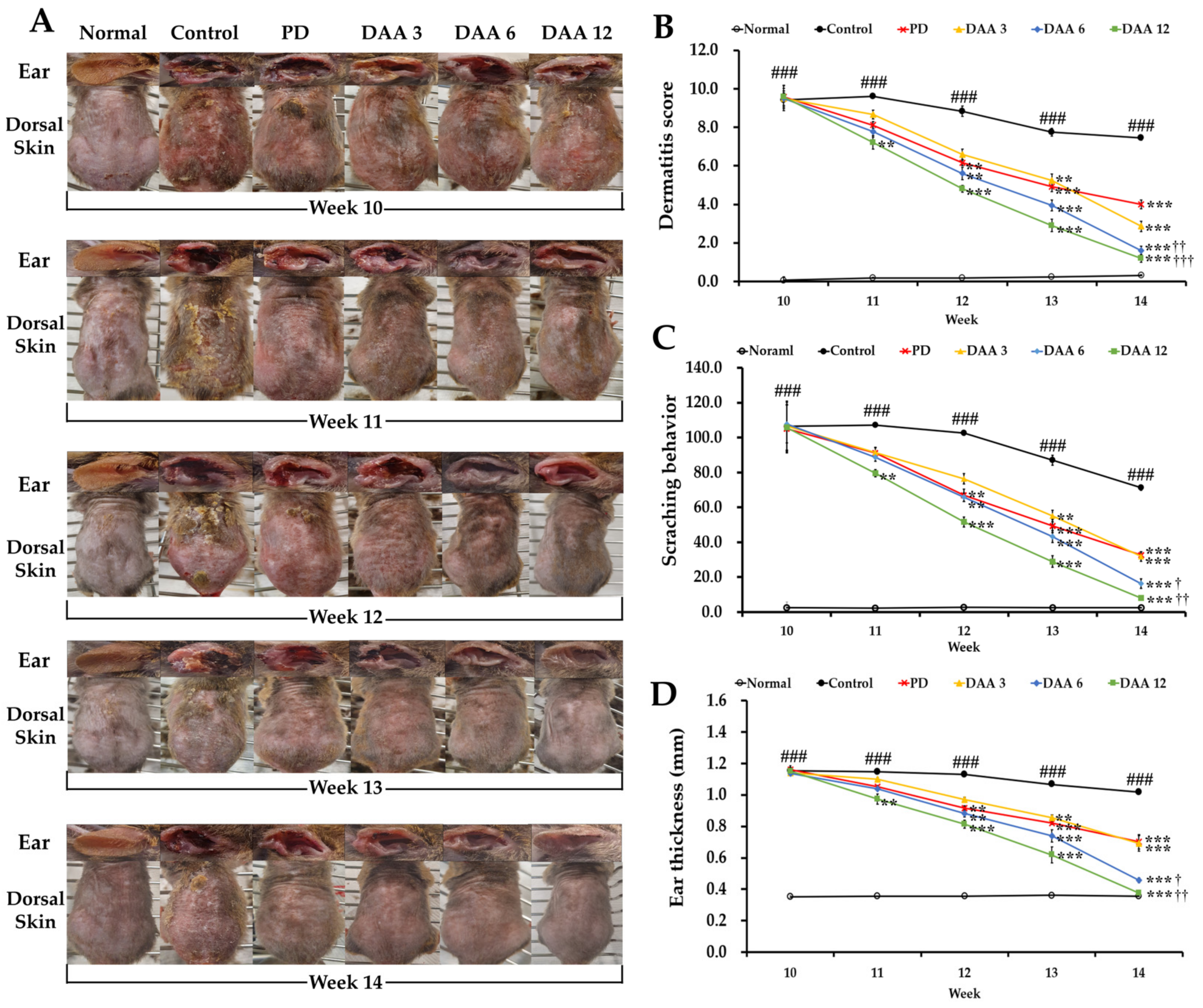
2.1. Deacetylasperulosidic Acid (DAA) Attenuates 2,4-Dinitrochlorobenzene (DNCB)-Induced Atopic Dermatitis (AD)-like Symptoms, Ear Thickness, and Scratching Behavior
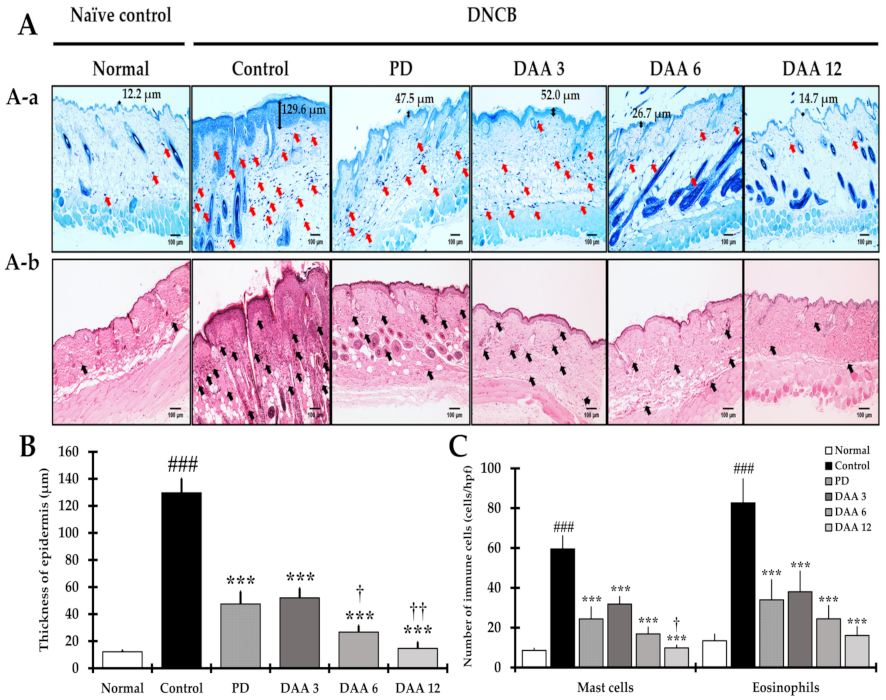
2.2. DAA Reduces the Thickening of the Epidermis, along with Eosinophil and Mast Cell Infiltration
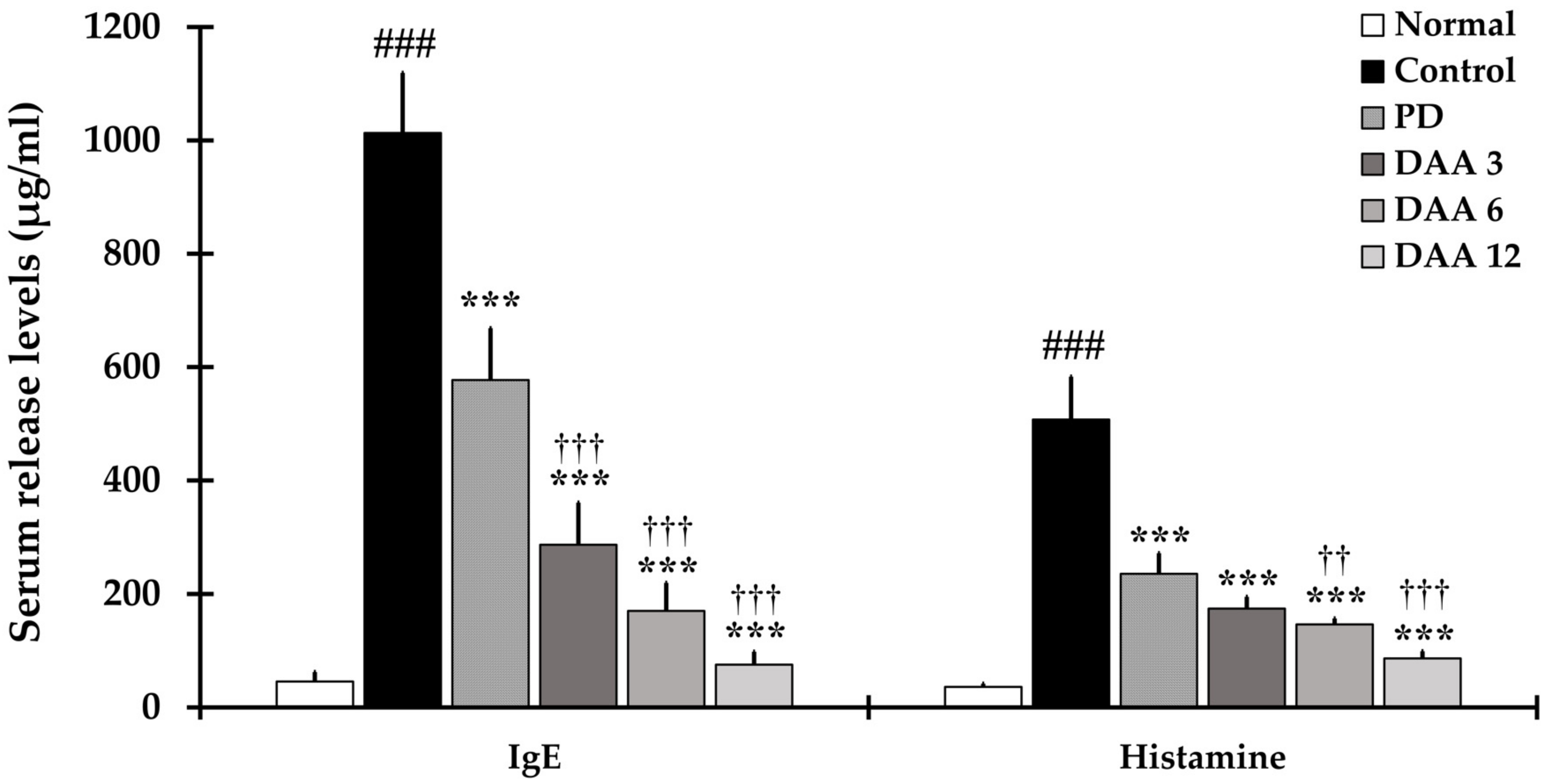
2.3. DAA Reduces Immunoglobulin E (IgE) and Histamine Levels in the Serum of NC/Nga Mice
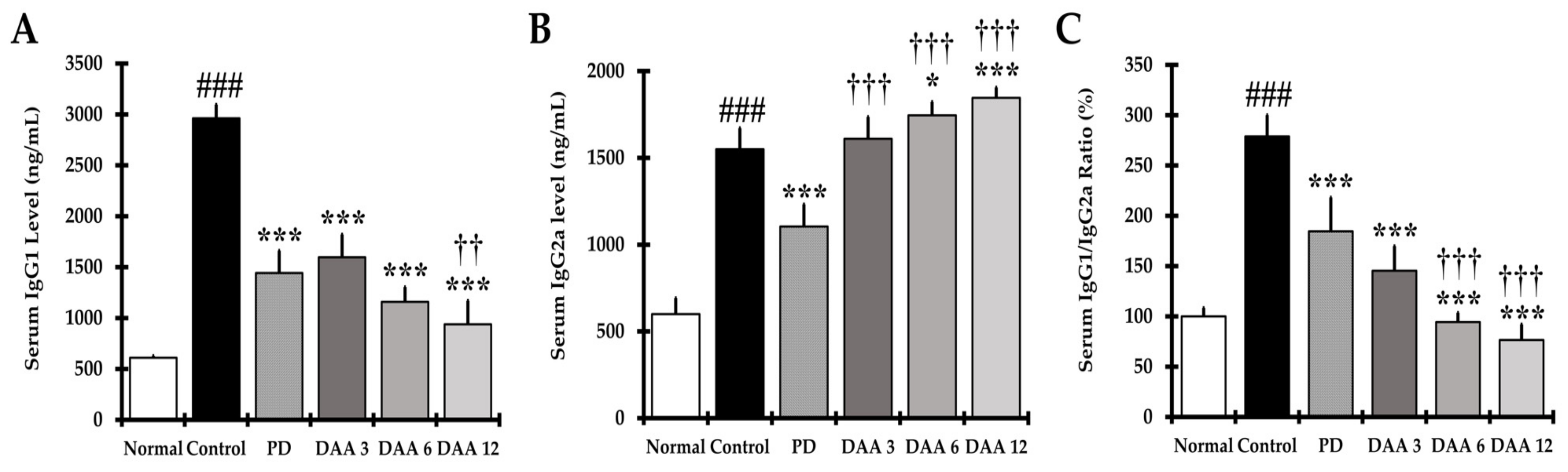
2.4. DAA Restores the IgG1/IgG2a Balance in the Serum
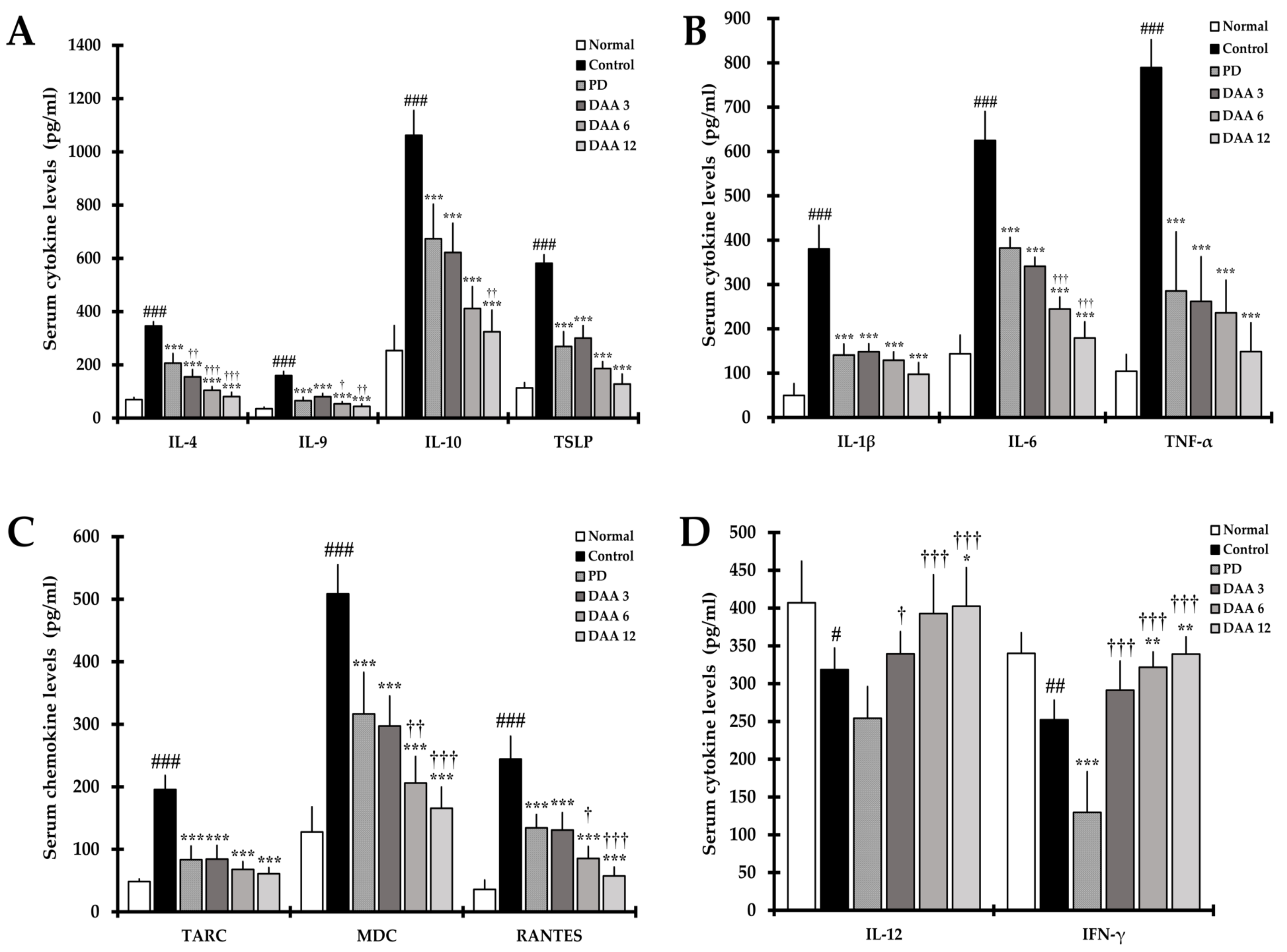
2.5. DAA Regulates Serum Levels of AD-Related Cytokines and Chemokines
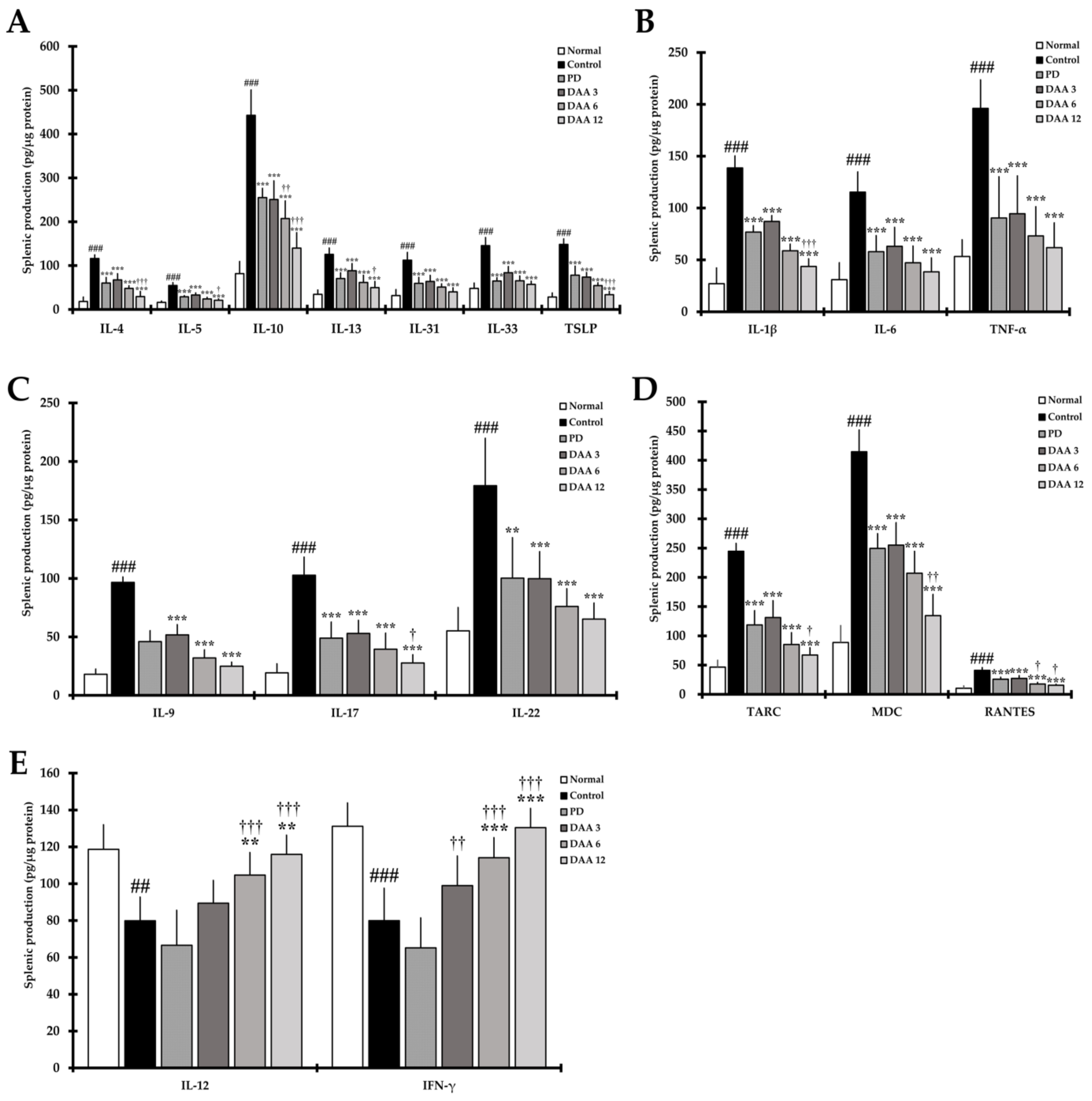
2.6. DAA Regulates the Secretion Levels of AD-Related Cytokines in Splenocytes
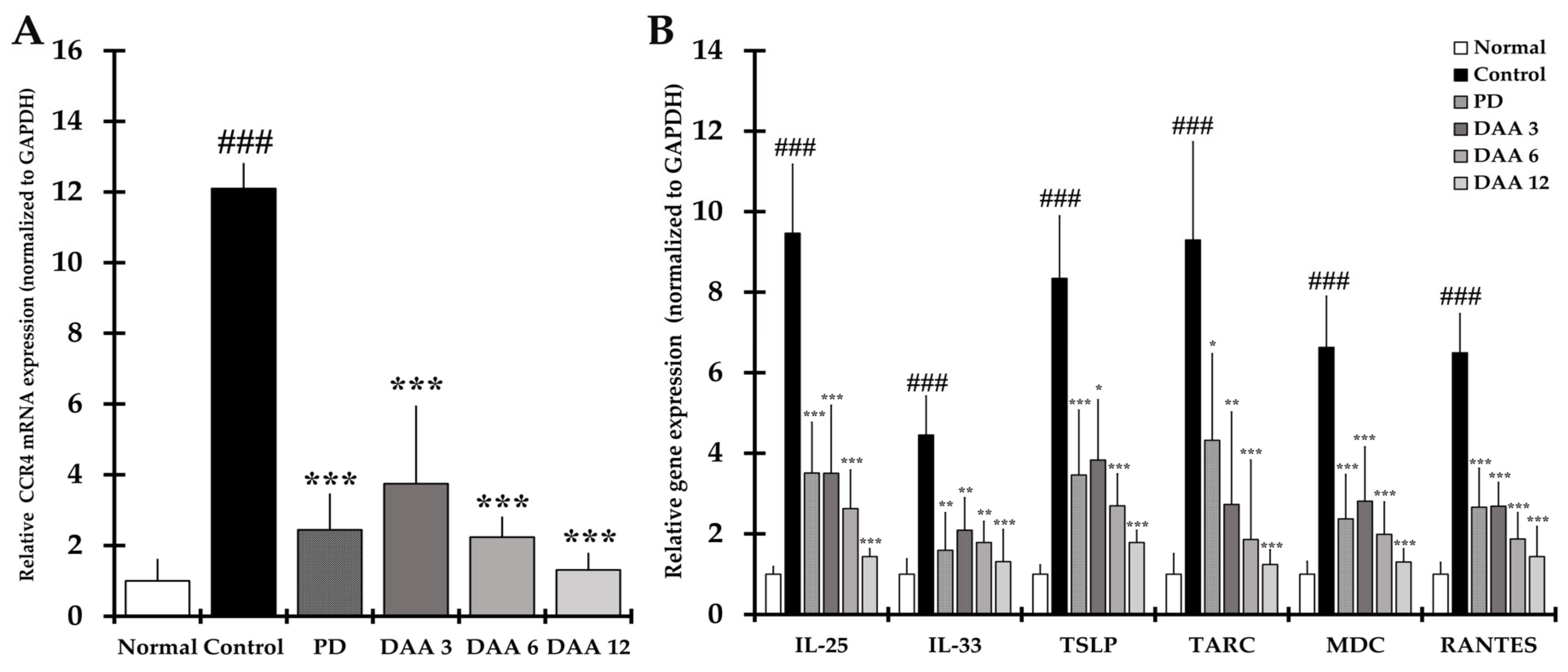
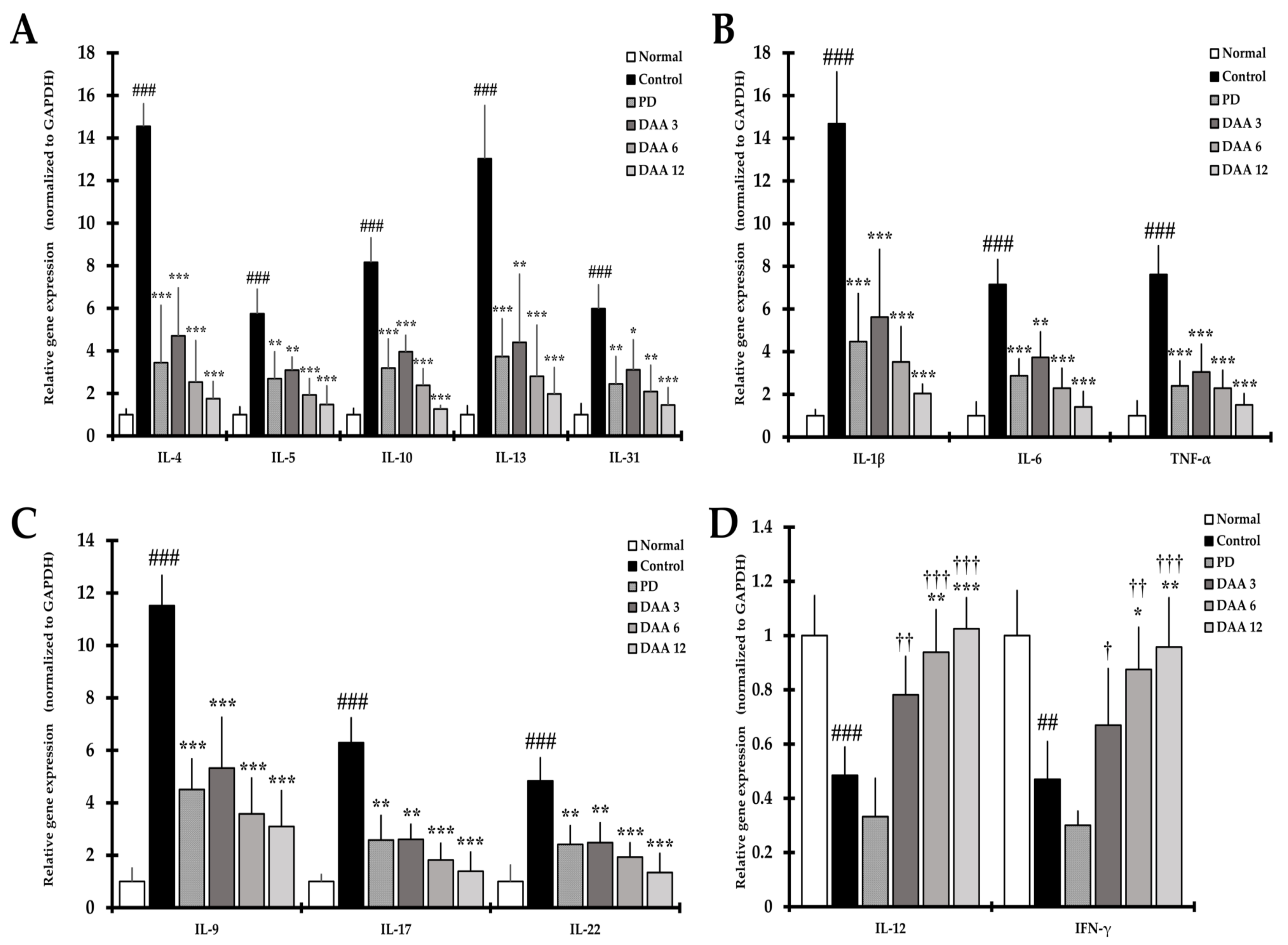
2.7. DAA Inhibits the Gene Expression of Th2 Cell Activation-Related Cytokines, Chemokines and CCR4 in the Dorsal Skin of Mice
2.8. DAA Regulates the Gene Expression of AD-Related Cytokines and Chemokines in the Dorsal Skin of NC/Nga Mice
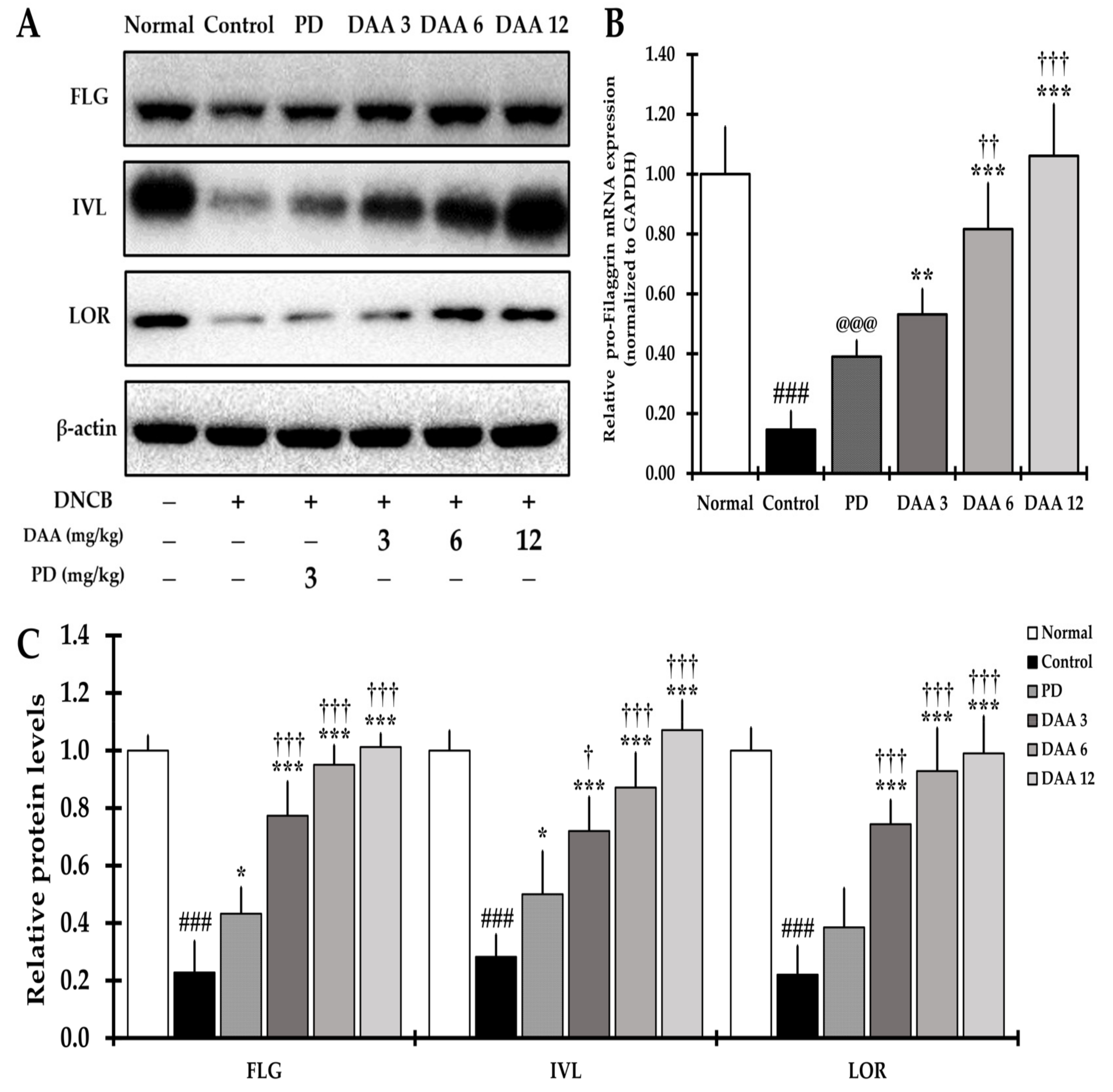
2.9. DAA Alleviates the Defects of the Skin Barrier Function
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
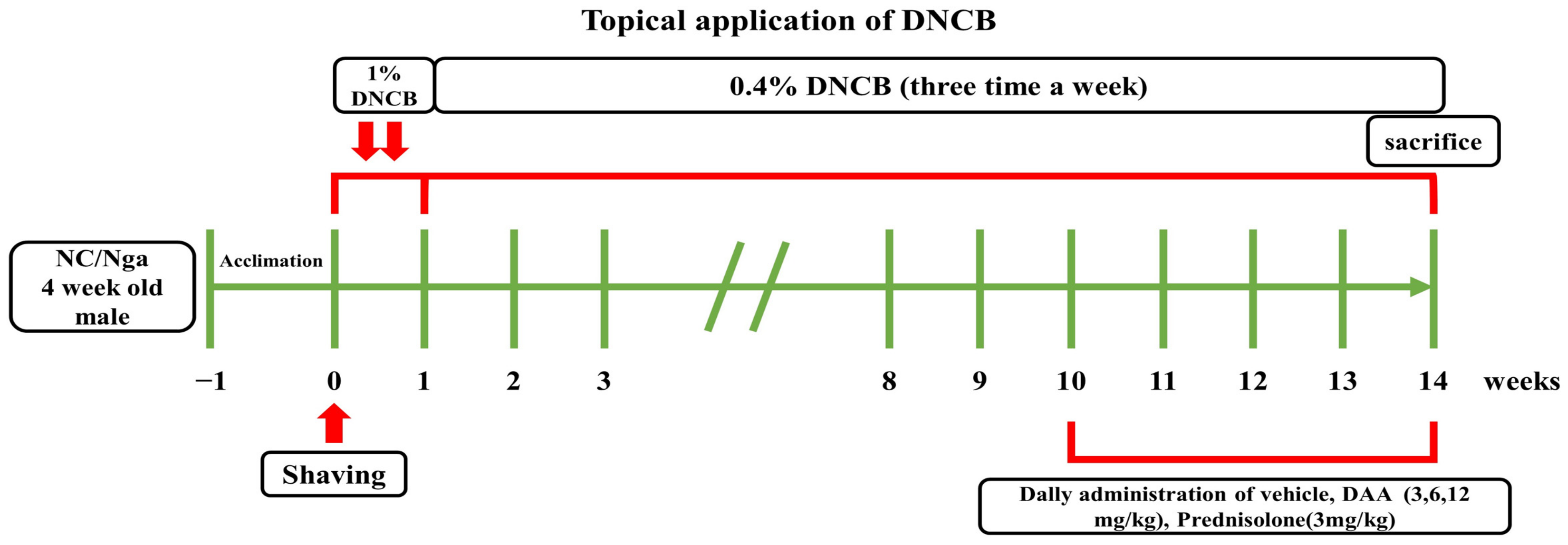
4.3. Application of AD-Like Skin Lesions and Treatment of DAA
4.4. Dermatitis Score and Ear Thickness
4.5. Scratching Behavior
4.6. Histological Analysis
4.7. Serum Immunoglobulin and Histamine Assay
4.8. Serum Cytokines and Chemokines Assay
4.9. Splenocyte Supernatant Isolation and Cytokine and Chemokine Analysis
4.10. RNA Extraction and Quantitative Real Time-Polymerase Chain Reaction (RT-qPCR)
4.11. Protein Extraction and Western Blotting
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thaçi, D.; Simpson, E.L.; Beck, L.A.; Bieber, T.; Blauvelt, A.; Papp, K.; Soong, W.; Worm, M.; Szepietowski, J.C.; Sofen, H.; et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: A randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016, 387, 40–52. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, J.S.; Cho, D.H.; Park, H.J. Molecular Mechanisms of Cutaneous Inflammatory Disorder: Atopic Dermatitis. Int. J. Mol. Sci. 2016, 17, 1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puar, N.; Chovatiya, R.; Paller, A.S. New treatments in atopic dermatitis. Ann. Allergy Asthma Immunol. 2021, 126, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Cevikbas, F.; Steinhoff, M. IL-33: A novel danger signal system in atopic dermatitis. J. Investig. Dermatol. 2012, 132, 1326–1329. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Choi, M.J.; Bak, D.H.; Lee, B.C.; Ko, E.J.; Ahn, G.R.; Ahn, S.W.; Kim, M.J.; Na, J.; Kim, B.J. Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice. Sci. Rep. 2018, 8, 11895. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Seong, G.S.; Choung, S.Y. Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function. Nutrients 2020, 12, 249. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.-S.; Lee, S.-J.; Choung, S.-Y. Lithospermum erythrorhizon Alleviates Atopic Dermatitis-like Skin Lesions by Restoring Immune Balance and Skin Barrier Function in 2.4-Dinitrochlorobenzene-Induced NC/Nga Mice. Nutrients 2021, 13, 3209. [Google Scholar] [CrossRef]
- Mizawa, M.; Makino, T.; Hikiami, H.; Shimada, Y.; Shimizu, T. Effectiveness of keishibukuryogan on chronic-stage lichenification associated with atopic dermatitis. ISRN Dermatol. 2012, 2012, 158598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehgal, V.N.; Khurana, A.; Mendiratta, V.; Saxena, D.; Srivastava, G.; Aggarwal, A.K.; Chatterjee, K.J.I.j.o.d. Atopic dermatitis: Clinical connotations, especially a focus on concomitant atopic undertones in immunocompromised/susceptible genetic and metabolic disorders. Indian J. Dermatol. 2016, 61, 241. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Skin Barrier Function via Competition between AHR Axis versus IL-13/IL-4JAKSTAT6/STAT3 Axis: Pathogenic and Therapeutic Implications in Atopic Dermatitis. J. Clin. Med. 2020, 9, 3741. [Google Scholar] [CrossRef] [PubMed]
- Giustizieri, M.L.; Mascia, F.; Frezzolini, A.; De Pita, O.; Chinni, L.M.; Giannetti, A.; Girolomoni, G.; Pastore, S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J. Allergy Clin. Immunol. 2001, 107, 871–877. [Google Scholar] [CrossRef]
- Cepelak, I.; Dodig, S.; Pavic, I. Filaggrin and atopic march. Biochem. Med. 2019, 29, 020501. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Chantry, D.; Raport, C.J.; Wood, C.L.; Nishimura, M.; Godiska, R.; Yoshie, O.; Gray, P.W. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 1998, 273, 1764–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeki, H.; Tamaki, K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J. Dermatol. Sci. 2006, 43, 75–84. [Google Scholar] [CrossRef]
- Matsuo, K.; Nagakubo, D.; Komori, Y.; Fujisato, S.; Takeda, N.; Kitamatsu, M.; Nishiwaki, K.; Quan, Y.S.; Kamiyama, F.; Oiso, N.; et al. CCR4 Is Critically Involved in Skin Allergic Inflammation of BALB/c Mice. J. Investig. Dermatol. 2018, 138, 1764–1773. [Google Scholar] [CrossRef] [Green Version]
- Aust, G.; Simchen, C.; Heider, U.; Hmeidan, F.A.; Blumenauer, V.; Spanel-Borowski, K.J.M.h.r. Eosinophils in the human corpus luteum: The role of RANTES and eotaxin in eosinophil attraction into periovulatory structures. Mol. Hum. Reprod. 2000, 6, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Roan, F.; Ziegler, S.F. The atopic march: Current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol. Rev. 2017, 278, 116–130. [Google Scholar] [CrossRef]
- Poulsen, L.K.; Hummelshoj, L. Triggers of IgE class switching and allergy development. Ann. Med. 2007, 39, 440–456. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Hammerberg, B.; Olivry, T.; Orton, S.M.J.V.d. Skin mast cell histamine release following stem cell factor and high-affinity immunoglobulin E receptor cross-linking in dogs with atopic dermatitis. Vet. Dermatol. 2001, 12, 339–346. [Google Scholar] [CrossRef]
- Noben-Trauth, N.; Hu-Li, J.; Paul, W.E. Conventional, naive CD4+ T cells provide an initial source of IL-4 during Th2 differentiation. J. Immunol. 2000, 165, 3620–3625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.; Braathen, L.; Simon, H.U.J.A. Eosinophils and atopic dermatitis. Allergy 2004, 59, 561–570. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef]
- Furue, M. T helper type 2 signatures in atopic dermatitis. J. Cutan. Immunol. Allergy 2018, 1, 93–99. [Google Scholar] [CrossRef]
- Gittler, J.K.; Shemer, A.; Suarez-Farinas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.A.; Schlapbach, C. TH9 cells in skin disorders. Semin. Immunopathol. 2017, 39, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Soroosh, P.; Doherty, T.A. Th9 and allergic disease. Immunology 2009, 127, 450–458. [Google Scholar] [CrossRef]
- Ubel, C.; Graser, A.; Koch, S.; Rieker, R.J.; Lehr, H.A.; Muller, M.; Finotto, S. Role of Tyk-2 in Th9 and Th17 cells in allergic asthma. Sci. Rep. 2014, 4, 5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Xue, H.B.; Guan, X.H.; Shu, C.M.; Zhang, J.H.; Yu, J. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin. Exp. Immunol. 2014, 175, 25–31. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Gadina, M.; Siegel, R.M. Cytokines and cytokine receptors. In Clinical Immunology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 127–155.e121. [Google Scholar]
- Wang, Z.Y.; Sato, H.; Kusam, S.; Sehra, S.; Toney, L.M.; Dent, A.L. Regulation of IL-10 gene expression in Th2 cells by Jun proteins. J. Immunol. 2005, 174, 2098–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnowicki, T.; Krueger, J.G.; Guttman-Yassky, E. Skin barrier and immune dysregulation in atopic dermatitis: An evolving story with important clinical implications. J. Allergy Clin. Immunol. Pract. 2014, 2, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H. The role of IL-22 and Th22 cells in human skin diseases. J. Allergy Clin. Immunol. Pract. 2013, 72, 3–8. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Zaniboni, M.C.; Samorano, L.P.; Orfali, R.L.; Aoki, V. Skin barrier in atopic dermatitis: Beyond filaggrin. An. Bras. Dermatol. 2016, 91, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Leung, D.Y. Epidermal barrier in atopic dermatitis. Allergy Asthma Immunol. Res. 2012, 4, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.I.J.J.o.c.s. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cork, M.J.; Danby, S.G.; Vasilopoulos, Y.; Hadgraft, J.; Lane, M.E.; Moustafa, M.; Guy, R.H.; Macgowan, A.L.; Tazi-Ahnini, R.; Ward, S.J. Epidermal barrier dysfunction in atopic dermatitis. J. Investig. Dermatol. 2009, 129, 1892–1908. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D.J.C.i. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanel, K.H.; Cornelissen, C.; Luscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waljee, A.K.; Rogers, M.A.; Lin, P.; Singal, A.G.; Stein, J.D.; Marks, R.M.; Ayanian, J.Z.; Nallamothu, B.K. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ 2017, 357, j1415. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, Y.; Takeuchi, H.; Sakuma, S.; Sengoku, T.; Takakura, S. Characterization of a 2,4-dinitrochlorobenzene-induced chronic dermatitis model in rats. Skin Pharmacol. Physiol. 2009, 22, 240–247. [Google Scholar] [CrossRef]
- Oh, J.S.; Seong, G.S.; Kim, Y.D.; Choung, S.Y. Effects of Deacetylasperulosidic Acid on Atopic Dermatitis through Modulating Immune Balance and Skin Barrier Function in HaCaT, HMC-1, and EOL-1 Cells. Molecules 2021, 26, 3298. [Google Scholar] [CrossRef]
- Jang, S.; Ohn, J.; Kim, J.W.; Kang, S.M.; Jeon, D.; Heo, C.Y.; Lee, Y.S.; Kwon, O.; Kim, K.H. Caffeoyl-Pro-His amide relieve DNCB-Induced Atopic Dermatitis-Like phenotypes in BALB/c mice. Sci. Rep. 2020, 10, 8417. [Google Scholar] [CrossRef]
- Renert-Yuval, Y.; Guttman-Yassky, E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann. Allergy Asthma Immunol. 2020, 124, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef]
- Schmid-Grendelmeier, P.; Altznauer, F.; Fischer, B.; Bizer, C.; Straumann, A.; Menz, G.; Blaser, K.; Wuthrich, B.; Simon, H.U. Eosinophils express functional IL-13 in eosinophilic inflammatory diseases. J. Immunol. 2002, 169, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Bao, K.; Reinhardt, R.L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; He, C.; Nair, L.; Yeung, J.; Egwuagu, C.E. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015, 75, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.Y.M.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef]
- Han, E.J.; Fernando, I.P.S.; Kim, H.S.; Jeon, Y.J.; Madusanka, D.M.D.; Dias, M.; Jee, Y.; Ahn, G. Oral Administration of Sargassum horneri Improves the HDM/DNCB-Induced Atopic Dermatitis in NC/Nga Mice. Nutrients 2020, 12, 2482. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guan, L.; Tang, L.; Liu, S.; Zhou, Y.; Chen, C.; He, Z.; Xu, L. T Helper 9 Cells: A New Player in Immune-Related Diseases. DNA Cell Biol. 2019, 38, 1040–1047. [Google Scholar] [CrossRef] [Green Version]
- Klonowska, J.; Glen, J.; Nowicki, R.J.; Trzeciak, M. New Cytokines in the Pathogenesis of Atopic Dermatitis-New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Sun, L.; Jiang, T.; Zhang, D.; He, D.; Nie, H. TNFalpha promotes Th17 cell differentiation through IL-6 and IL-1beta produced by monocytes in rheumatoid arthritis. J. Immunol. Res. 2014, 2014, 385352. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, Y.; Nakayamada, S.; Kubo, S.; Nakano, K.; Iwata, S.; Miyagawa, I.; Ma, X.; Trimova, G.; Sakata, K.; Tanaka, Y. Th22 Cells Promote Osteoclast Differentiation via Production of IL-22 in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 2901. [Google Scholar] [CrossRef] [Green Version]
- Angkasekwinai, P.; Dong, C. IL-9-producing T cells: Potential players in allergy and cancer. Nat. Rev. Immunol. 2021, 21, 37–48. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Li, J.; Li, S.; Zhang, Y.; Shen, J.; Tan, W.; Wu, C. Detection of IL-9 producing T cells in the PBMCs of allergic asthmatic patients. BMC Immunol. 2017, 18, 38. [Google Scholar] [CrossRef] [Green Version]
- Gröne, A.J.V.i.; Immunopathology. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar] [CrossRef]
- Biedermann, T.; Skabytska, Y.; Kaesler, S.; Volz, T. Regulation of T Cell Immunity in Atopic Dermatitis by Microbes: The Yin and Yang of Cutaneous Inflammation. Front. Immunol. 2015, 6, 353. [Google Scholar] [CrossRef] [PubMed]
- Duhen, T.; Geiger, R.; Jarrossay, D.; Lanzavecchia, A.; Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009, 10, 857–863. [Google Scholar] [CrossRef]
- Kato, Y.; Pawankar, R.; Kimura, Y.; Kawana, S. Increased expression of RANTES, CCR3 and CCR5 in the lesional skin of patients with atopic eczema. Int. Arch. Allergy Immunol. 2006, 139, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Mizoguchi, E.; Oettgen, H.; Bhan, A.K.; Geha, R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Investig. 1999, 103, 1103–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roan, F.; Obata-Ninomiya, K.; Ziegler, S.F. Epithelial cell-derived cytokines: More than just signaling the alarm. J. Clin. Investig. 2019, 129, 1441–1451. [Google Scholar] [CrossRef] [Green Version]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef]
- Ikeda, K.; Nakajima, H.; Suzuki, K.; Kagami, S.; Hirose, K.; Suto, A.; Saito, Y.; Iwamoto, I. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood 2003, 101, 3594–3596. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Wu, Q.; Jang, Y.P.; Choung, S.Y. Pinus densiflora bark extract ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice by regulating Th1/Th2 balance and skin barrier function. Phytother. Res. 2018, 32, 1135–1143. [Google Scholar] [CrossRef]
- Kim, I.S.; Kim, M.J.; Shin, D.H.; Son, K.H.; Park, H.Y.; Lee, J.S. Arazyme inhibits cytokine expression and upregulates skin barrier protein expression. Mol. Med. Rep. 2013, 8, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttman-Yassky, E.; Ungar, B.; Malik, K.; Dickstein, D.; Suprun, M.; Estrada, Y.D.; Xu, H.; Peng, X.; Oliva, M.; Todd, D.; et al. Molecular signatures order the potency of topically applied anti-inflammatory drugs in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2017, 140, 1032–1042.e1013. [Google Scholar] [CrossRef] [Green Version]
- Suto, H.; Matsuda, H.; Mitsuishi, K.; Hira, K.; Uchida, T.; Unno, T.; Ogawa, H.; Ra, C. NC/Nga Mice: A Mouse Model for Atopic Dermatitis. Int. Arch. Allergy Immunol. 1999, 120 (Suppl. S1), 70–75. [Google Scholar] [CrossRef]
- Takano, N.; Arai, I.; Kurachi, M. Analysis of the spontaneous scratching behavior by NC/Nga mice: A possible approach to evaluate antipruritics for subjects with atopic dermatitis. Eur. J. Pharmacol. 2003, 471, 223–228. [Google Scholar] [CrossRef]
| Gene | Forward | Reverse |
|---|---|---|
| IL-1β (m) | TGT GTT TTC CTC CTT GCC TCT GAT | TGC TGC CTA ATG TCC CCT TGA AT |
| IL-4 (m) | ACG GAG ATG GAT GTG CCA AAC | AGC ACC TTG GAA GCC CTA CAG A |
| IL-5 (m) | TCA GCT GTG TCT GGG CCA CT | TT ATG AGT AGG GAC AGG AAG CCT CA |
| IL-6 (m) | CCA CTT CAC AAG TCG GAG GCT TA | GCA AGT GCA TCA TCG TTG TTC ATA C |
| IL-9 (m) | GGG CAT CAG AGA CAC CAA T | GGA CGG AGA GAC ACA AGC A |
| IL-10 (m) | GAC CAG CTG GAC AAC ATA CTG CTA A | GAT AAG GCT TGG CAA CCC AAG TAA |
| IL-12 (m) | TGA ACT GGC GTT GGA AGC | GCG GGT CTG GTT TGA TGA |
| IL-13 (m) | CAA TTG CAA TGC CAT CTA CAG GAC | CGA AAC AGT TGC TTT GTG TAG CTG A |
| IL-17 (m) | AAG GCA GCA GCG ATC ATC C | GGA ACG GTT GAG GTA GTC TGA G |
| IL-22 (m) | CAG CTC CTG TCA CAT CAG CGG T | AGG TCC AGT TCC CCA ATC GCC T |
| IL-25 (m) | CTC AAC AGC AGG GCC ACT C | GTC TGT AGG CTG ACG CAG TGT G |
| IL-31 (m) | ATA CAG CTG CCG TGT TTC AG | AGC CAT CTT ATC ACC CAA GAA |
| IL-33 (m) | GAT GAG ATG TCT CGG CTG CTT G | AGC CGT TAC GGA TAT GGT GGT C |
| IFN-γ (m) | CGG CAC AGT CAT TGA AAG CCT A | GGC ACC ACT AGT TGG TTG TCT TTG |
| TNF-α (m) | TAC TGA ACT TCG GGG TGA TTG GTC | CAG CCT TGT CCC TTG AAG AGA ACC |
| TSLP (m) | TGC AAG TAC TAG TAC GGA TGG GGC | GGA CTT CTT GTG CCA TTT CCT GAG |
| CCR4 (m) | TCT ACA GCG GCA TCT TCT TCA T | CAG TAC GTG TGG TGG TGC TCT G |
| Pro-Filaggrin (m) | GAA TCC ATA TTT ACA GCA AAG CAC CTT G | GGT ATG TCC AAT GTG ATT GCA CGA TTG |
| GAPDH (m) | ACT TTG TCA AGC TCA TTT CC | TGC AGC GAA CTT TAT TGA TG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-S.; Seong, G.-S.; Kim, Y.-D.; Choung, S.-Y. Deacetylasperulosidic Acid Ameliorates Pruritus, Immune Imbalance, and Skin Barrier Dysfunction in 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis NC/Nga Mice. Int. J. Mol. Sci. 2022, 23, 226. https://doi.org/10.3390/ijms23010226
Oh J-S, Seong G-S, Kim Y-D, Choung S-Y. Deacetylasperulosidic Acid Ameliorates Pruritus, Immune Imbalance, and Skin Barrier Dysfunction in 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis NC/Nga Mice. International Journal of Molecular Sciences. 2022; 23(1):226. https://doi.org/10.3390/ijms23010226
Chicago/Turabian StyleOh, Jin-Su, Geum-Su Seong, Yong-Deok Kim, and Se-Young Choung. 2022. "Deacetylasperulosidic Acid Ameliorates Pruritus, Immune Imbalance, and Skin Barrier Dysfunction in 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis NC/Nga Mice" International Journal of Molecular Sciences 23, no. 1: 226. https://doi.org/10.3390/ijms23010226
APA StyleOh, J.-S., Seong, G.-S., Kim, Y.-D., & Choung, S.-Y. (2022). Deacetylasperulosidic Acid Ameliorates Pruritus, Immune Imbalance, and Skin Barrier Dysfunction in 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis NC/Nga Mice. International Journal of Molecular Sciences, 23(1), 226. https://doi.org/10.3390/ijms23010226






