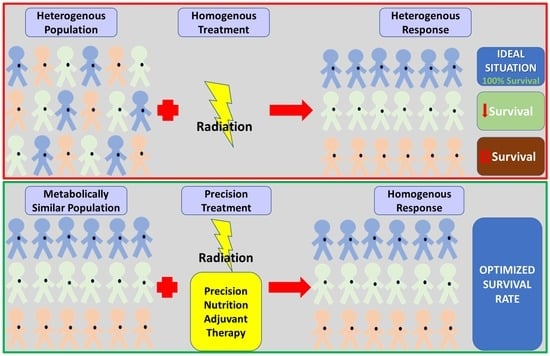
Personalized Nutrition as a Key Contributor to Improving Radiation Response in Breast Cancer
Abstract
1. Introduction
2. Breast Cancer and Radiation Response in the African American Population
2.1. Molecular Disparity in African American Breast Cancer Patients
2.2. IGF-1R and Radiation Response
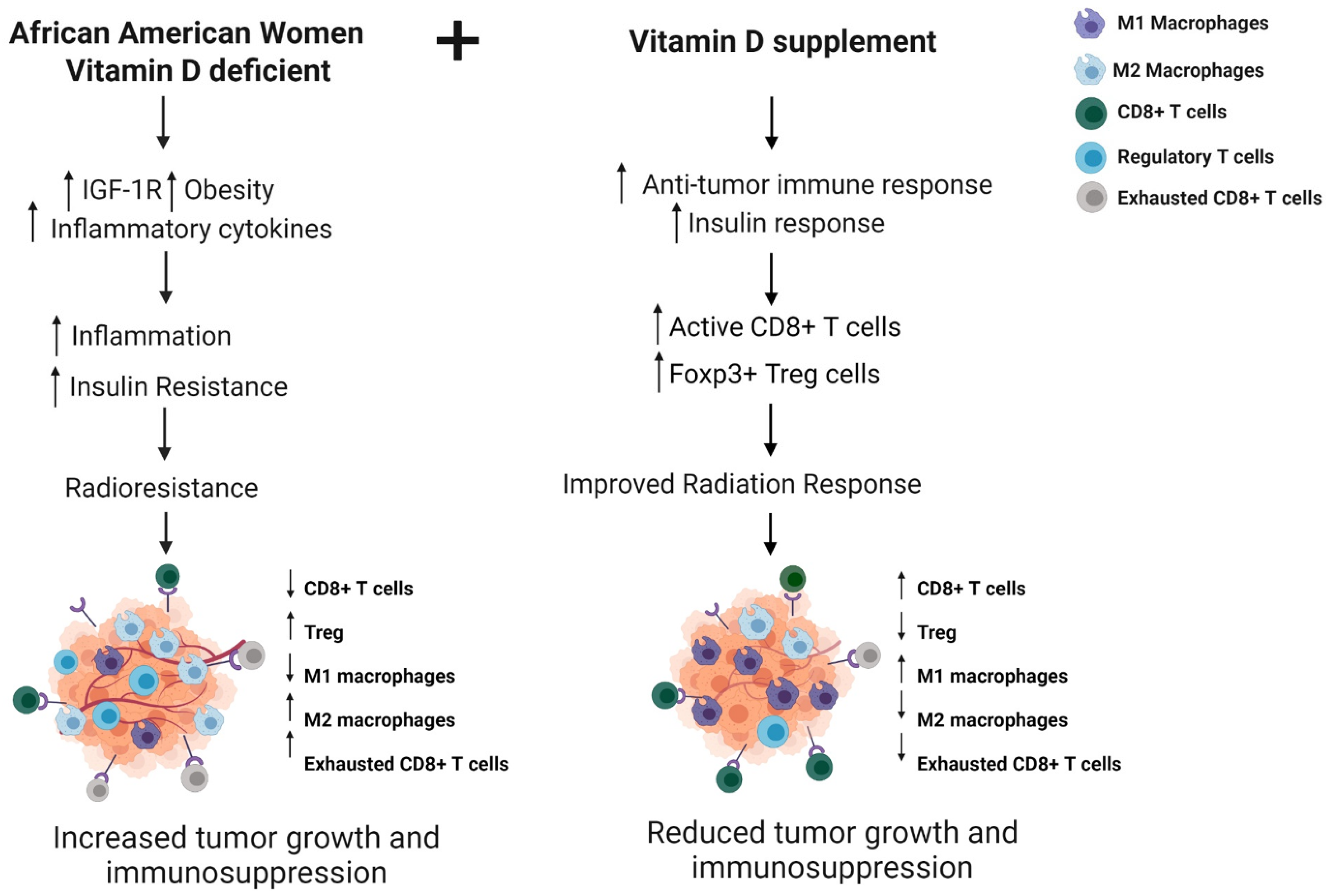
2.3. Vitamin D Supplementation and IGF-1R to Improve Radiation Response
2.4. Vitamin D Deficiency in AA Patients
2.5. Vitamin D and Breast Cancer
2.6. Immune Response and Radiation Response
2.7. Vitamin D Supplementation to Improve Radiation Response in African American Women
3. Obesity, Metabolic Syndrome, and RT
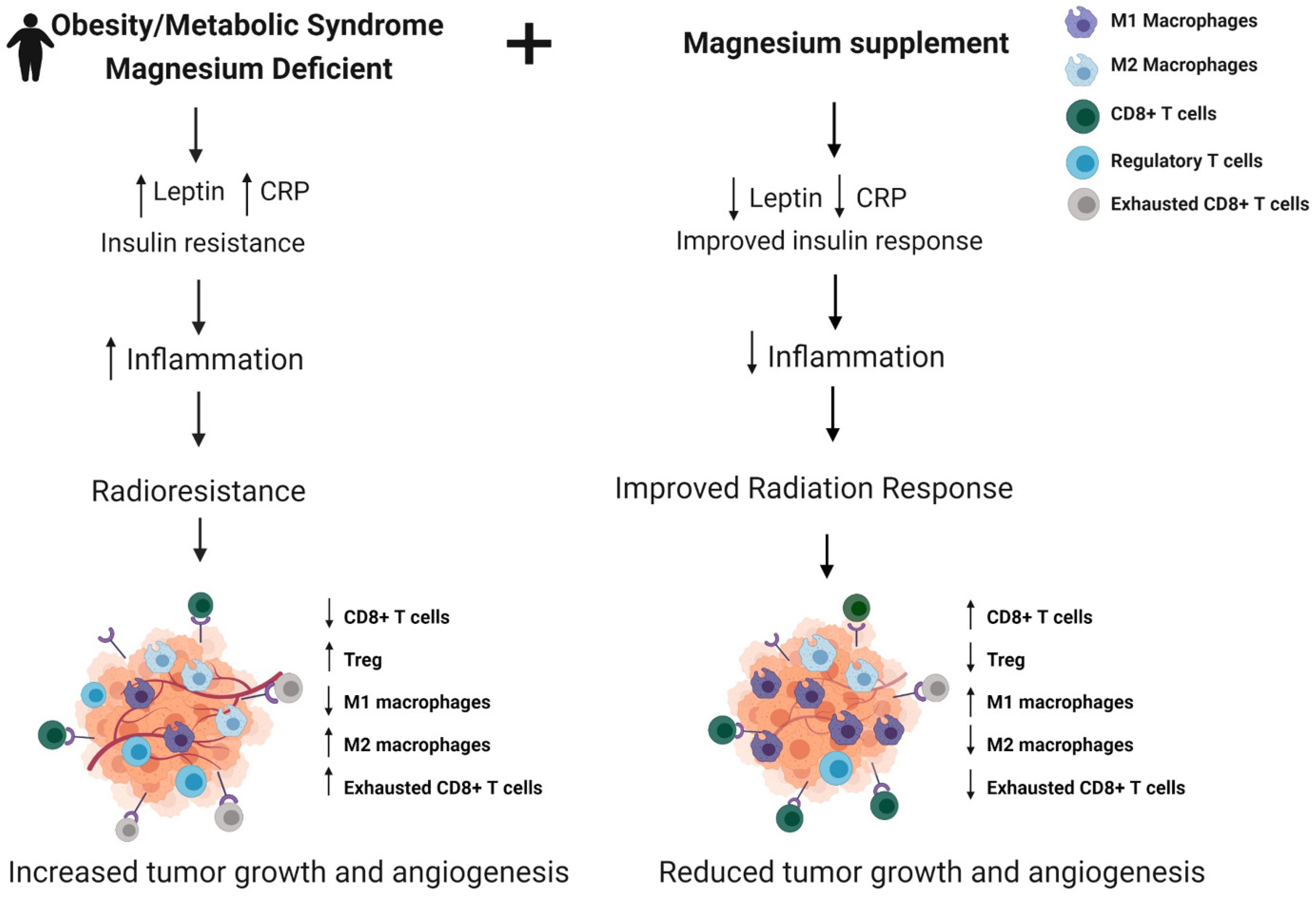
3.1. Obesity/Metabolic Syndrome and Radiation Response and Toxicity
3.2. Magnesium in Obesity/Metabolic Syndrome and Breast Cancer
3.3. Magnesium Supplementation to Improve Radiation Response
4. Breast Cancer and Radiation Response in Aged Population
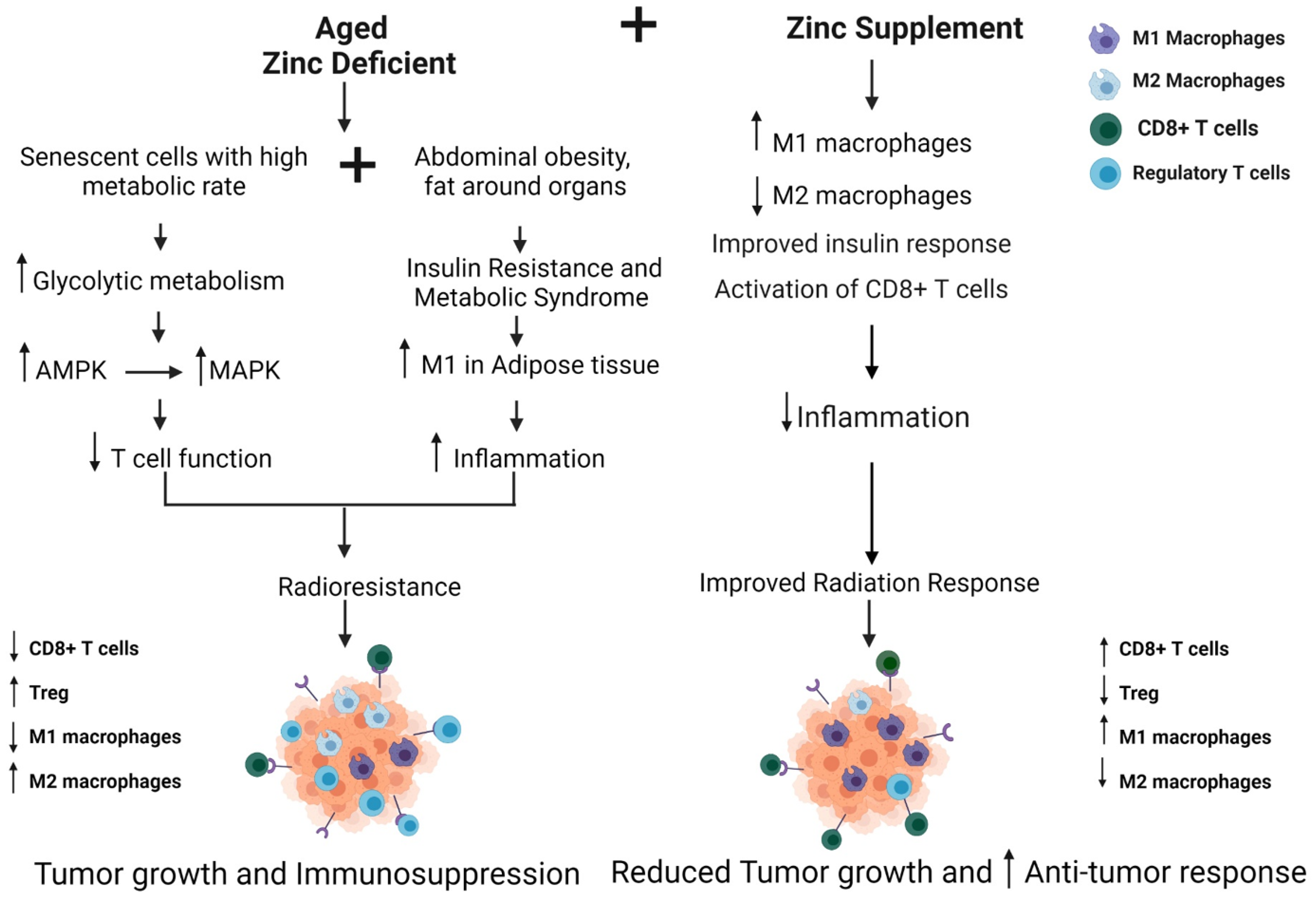
4.1. Aging and Breast Cancer: Influence of Immune Dysfunction
4.2. Radiation Therapy Outcomes and Toxicity in Older Patients
4.3. Zinc Supplementation to Improve Radiation Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balaji, K.; Subramanian, B.; Yadav, P.; Radha, C.A.; Ramasubramanian, V. Radiation therapy for breast cancer: Literature review. Med. Dosim. 2016, 41, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef]
- Aristei, C.; Perrucci, E.; Alì, E.; Marazzi, F.; Masiello, V.; Saldi, S.; Ingrosso, G. Personalization in Modern Radiation Oncology: Methods, Results and Pitfalls. Personalized Interventions and Breast Cancer. Front. Oncol. 2021, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Torres-Roca, J.F. A molecular assay of tumor radiosensitivity: A roadmap towards biology-based personalized radiation therapy. Pers. Med. 2012, 9, 547–557. [Google Scholar] [CrossRef]
- McCall, N.S.; Simone, B.A.; Mehta, M.; Zhan, T.; Ko, K.; Nowak-Choi, K.; Rese, A.; Venkataraman, C.; Andrews, D.W.; Anne’, P.R.; et al. Onco-metabolism: Defining the prognostic significance of obesity and diabetes in women with brain metastases from breast cancer. Breast Cancer Res. Treat. 2018, 172, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Sail, K.; Franzini, L.; Lairson, D.; Du, X. Differences in treatment and survival among African-American and Caucasian women with early stage operable breast cancer. Ethn. Health 2012, 17, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Coghill, A.E.; Suneja, G.; Rositch, A.F.; Shiels, M.S.; Engels, E.A. HIV Infection, Cancer Treatment Regimens, and Cancer Outcomes Among Elderly Adults in the United States. JAMA Oncol. 2019, 5, e191742. [Google Scholar] [CrossRef]
- Chaudhary, N.; Kumar, V.; Sangwan, P.; Pant, N.C.; Saxena, A.; Joshi, S.; Yadav, A.N. 3.36—Personalized Nutrition and -Omics, in Comprehensive Foodomics; Cifuentes, A., Ed.; Elsevier: Oxford, UK, 2021; pp. 495–507. [Google Scholar]
- Kviatcovsky, D.; Zheng, D.; Elinav, E. Gut microbiome and its potential link to personalized nutrition. Curr. Opin. Physiol. 2021, 22, 100439. [Google Scholar] [CrossRef]
- Ames, B.N.; Wakimoto, P. Are vitamin and mineral deficiencies a major cancer risk? Nat. Rev. Cancer 2002, 2, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Portolés, C.; Fernández, L.P.; Ramirez de Molina, A. Precision Nutrition for Targeting Lipid Metabolism in Colorectal Cancer. Nutrients 2017, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Reglero, C.; Reglero, G. Precision Nutrition and Cancer Relapse Prevention: A Systematic Literature Review. Nutrients 2019, 11, 2799. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Ganesan, K.; Jayachandran, M.; Xu, B. Diet-Derived Phytochemicals Targeting Colon Cancer Stem Cells and Microbiota in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 3976. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Fedewa, S.A.; Sauer, A.G.; Kramer, J.L.; Smith, R.A.; Jemal, A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J. Clin. 2015, 66, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Arciero, C.A.; Yang, J.; Peng, L.; Ward, K.C.; O’Regan, R.; Sahin, A.A.; Li, X. African American patients with breast cancer have worse prognosis than white patients in certain subtypes and stages. Breast Cancer Res. Treat. 2017, 166, 743–755. [Google Scholar] [CrossRef]
- Newman, G.; Gonzalez-Perez, R.R. Leptin–cytokine crosstalk in breast cancer. Mol. Cell. Endocrinol. 2013, 382, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, T. Consequences of Abdominal Adiposity within the Metabolic Syndrome Paradigm in Black People of African Ancestry. J. Clin. Med. 2014, 3, 897–912. [Google Scholar] [CrossRef]
- Singh, S.K.; Tan, Q.; Brito, C.; De León, M. Insulin-like growth factors I and II receptors in the breast cancer survival disparity among African–American women. Growth Horm. IGF Res. 2010, 20, 245–254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farabaugh, S.M.; Boone, D.; Lee, A.V. Role of IGF1R in Breast Cancer Subtypes, Stemness, and Lineage Differentiation. Front. Endocrinol. 2015, 6, 59. [Google Scholar] [CrossRef]
- Dunn, S.E.; Kari, F.W.; French, J.; Leininger, J.R.; Travlos, G.; Wilson, R.; Barrett, J.C. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997, 57. [Google Scholar]
- Haluska, P.; Shaw, H.M.; Batzel, G.N.; Yin, D.; Molina, J.R.; Molife, L.R.; Yap, T.A.; Roberts, M.L.; Sharma, A.; Gualberto, A.; et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin. Cancer Res. 2007, 13, 5834–5840. [Google Scholar] [CrossRef]
- Ruggeri, B.A.; Klurfeld, D.; Kritchevsky, D.; Furlanetto, R.W. Caloric restriction and 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth in rats: Alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer Res. 1989, 49, 4130–4134. [Google Scholar] [PubMed]
- Jin, L.; Lim, M.; Zhao, S.; Sano, Y.; Simone, B.A.; Savage, J.E.; Wickstrom, E.; Camphausen, K.; Pestell, R.G.; Simone, N. The metastatic potential of triple-negative breast cancer is decreased via caloric restriction-mediated reduction of the miR-17~92 cluster. Breast Cancer Res. Treat. 2014, 146, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Simone, B.; Palazzo, J.; Savage, J.E.; Sano, Y.; Dan, T.; Jin, L.; Champ, C.; Zhao, S.; Lim, M.; et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle 2013, 12, 1955–1963. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Sarantopoulos, J.; Patnaik, A.; Papadopoulos, K.; Lin, C.-C.; Rodon, J.; Murphy, B.; Roth, B.; McCaffery, I.; Gorski, K.S.; et al. Phase I, Pharmacokinetic, and Pharmacodynamic Study of AMG 479, a Fully Human Monoclonal Antibody to Insulin-Like Growth Factor Receptor 1. J. Clin. Oncol. 2009, 27, 5800–5807. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Pfeiffer, R.M.; Falk, R.T.; Horne, H.N.; Xiang, J.; Pollak, M.; Brinton, L.A.; Storniolo, A.M.V.; Sherman, M.E.; Gierach, G.L.; et al. Serum insulin-like growth factor (IGF)-I and IGF binding protein-3 in relation to terminal duct lobular unit involution of the normal breast in Caucasian and African American women: The Susan G. Komen Tissue Bank. Int. J. Cancer 2018, 143, 496–507. [Google Scholar] [CrossRef]
- Vadgama, J.V.; Wu, Y.; Datta, G.; Khan, H.; Chillar, R. Plasma Insulin-Like Growth Factor-I and Serum IGF-Binding Protein 3 Can Be Associated with the Progression of Breast Cancer, and Predict the Risk of Recurrence and the Probability of Survival in African-American and Hispanic Women. Oncology 1999, 57, 330–340. [Google Scholar] [CrossRef]
- Kaleko, M.; Rutter, W.J.; Miller, A.D. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol. Cell. Biol. 1990, 10. [Google Scholar] [CrossRef]
- Taunk, N.K.; Goyal, S.; Moran, M.S.; Yang, Q.; Parikh, R.; Haffty, B.G. Prognostic significance of IGF-1R expression in patients treated with breast-conserving surgery and radiation therapy. Radiother. Oncol. 2010, 96, 204–208. [Google Scholar] [CrossRef]
- Yanochko, G.M.; Eckhart, W. Type I insulin-like growth factor receptor over-expression induces proliferation and anti-apoptotic signaling in a three-dimensional culture model of breast epithelial cells. Breast Cancer Res. 2006, 8, R18. [Google Scholar] [CrossRef]
- Valenciano, A.; Henríquez-Hernández, L.A.; Moreno, M.; Lloret, M.; Lara, P.C. Role of IGF-1 Receptor in Radiation Response. Transl. Oncol. 2012, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Teoh, N.C.; Dan, Y.Y.; Swisshelm, K.; Lehman, S.; Wright, J.H.; Haque, J.; Gu, Y.; Fausto, N. Defective DNA strand break repair causes chromosomal instability and accelerates liver carcinogenesis in mice. Hepatology 2008, 47, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Abdou, Y.; Attwood, K.; Cheng, T.-Y.D.; Yao, S.; Bandera, E.V.; Zirpoli, G.R.; Ondracek, R.P.; Stein, L.; Bshara, W.; Khoury, T.; et al. Racial differences in CD8+ T cell infiltration in breast tumors from Black and White women. Breast Cancer Res. 2020, 22, 62. [Google Scholar] [CrossRef]
- Zavala, V.A.; Bracci, P.M.; Carethers, J.M.; Carvajal-Carmona, L.; Coggins, N.B.; Cruz-Correa, M.R.; Davis, M.; de Smith, A.J.; Dutil, J.; Figueiredo, J.C.; et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 2020, 124, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Stenström, J.; Hedenfalk, I.; Hagerling, C. Regulatory T lymphocyte infiltration in metastatic breast cancer—an independent prognostic factor that changes with tumor progression. Breast Cancer Res. 2021, 23, 27. [Google Scholar] [CrossRef]
- Komatsu, N.; Hori, S. Full restoration of peripheral Foxp3+ regulatory T cell pool by radioresistant host cells in scurfy bone marrow chimeras. Proc. Natl. Acad. Sci. USA 2007, 104, 8959–8964. [Google Scholar] [CrossRef]
- Bos, P.D.; Plitas, G.; Rudra, D.; Lee, S.Y.; Rudensky, A.Y. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J. Exp. Med. 2013, 210, 2435–2466. [Google Scholar] [CrossRef]
- Abdou, Y.; Elkhanany, A.; Attwood, K.; Ji, W.; Takabe, K.; Opyrchal, M. Primary and secondary breast angiosarcoma: Single center report and a meta-analysis. Breast Cancer Res. Treat. 2019, 178, 523–533. [Google Scholar] [CrossRef]
- Koru-Sengul, T.; Santander, A.M.; Miao, F.; Sanchez, L.G.; Jorda, M.; Glück, S.; Ince, T.A.; Nadji, M.; Chen, Z.; Penichet, M.L.; et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast Cancer Res. Treat. 2016, 158, 113–126. [Google Scholar] [CrossRef]
- Schnellhardt, S.; Erber, R.; Büttner-Herold, M.; Rosahl, M.-C.; Ott, O.J.; Strnad, V.; Beckmann, M.W.; King, L.; Hartmann, A.; Fietkau, R.; et al. Accelerated Partial Breast Irradiation: Macrophage Polarisation Shift Classification Identifies High-Risk Tumours in Early Hormone Receptor-Positive Breast Cancer. Cancers 2020, 12, 446. [Google Scholar] [CrossRef]
- Mukhtar, R.A.; Moore, A.P.; Nseyo, O.; Baehner, F.L.; Au, A.; Moore, D.H.; Twomey, P.; Campbell, M.J.; Esserman, L.J. Elevated PCNA+ tumor-associated macrophages in breast cancer are associated with early recurrence and non-Caucasian ethnicity. Breast Cancer Res. Treat. 2011, 130, 635–644. [Google Scholar] [CrossRef]
- O’Meara, T.; Safonov, A.; Casadevall, D.; Qing, T.; Silber, A.; Killelea, B.; Hatzis, C.; Pusztai, L. Immune microenvironment of triple-negative breast cancer in African-American and Caucasian women. Breast Cancer Res. Treat. 2019, 175, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.J.; Riester, M.; Zheng, Y.; Yoshimatsu, T.F.; Sanni, A.; Oluwasola, O.; Veloso, A.; Labrot, E.; Wang, S.; Odetunde, A.; et al. Characterization of Nigerian breast cancer reveals prevalent homologous recombination deficiency and aggressive molecular features. Nat. Commun. 2018, 9, 4181. [Google Scholar] [CrossRef] [PubMed]
- Milas, L. Tumor Bed Effect in Murine Tumors: Relationship to Tumor Take and Tumor Macrophage Content. Radiat. Res. 1990, 123, 232. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-S.; Chen, F.-H.; Wang, C.-C.; Huang, H.-L.; Jung, S.-M.; Wu, C.-J.; Lee, C.-C.; McBride, W.H.; Chiang, C.-S.; Hong, J.-H. Macrophages from Irradiated Tumors Express Higher Levels of iNOS, Arginase-I and COX-2, and Promote Tumor Growth. Int. J. Radiat. Oncol. 2007, 68, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, E.; Presta, I.; Maisano, D.; Donato, A.; Pirrone, C.K.; Cardillo, G.; Corrado, S.D.; Mignogna, C.; Mancuso, T.; Donato, G.; et al. Role of Macrophages in Brain Tumor Growth and Progression. Int. J. Mol. Sci. 2018, 19, 1005. [Google Scholar] [CrossRef]
- Bandera, E.V.; Chandran, U.; Hong, C.-C.; Troester, M.A.; Bethea, T.; Adams-Campbell, L.L.; Haiman, C.A.; Park, S.-Y.; Olshan, A.F.; Ambrosone, C.B.; et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res. Treat. 2015, 150, 655–666. [Google Scholar] [CrossRef]
- Dietze, E.C.; Chavez, T.A.; Seewaldt, V.L. Obesity and Triple-Negative Breast Cancer: Disparities, Controversies, and Biology. Am. J. Pathol. 2018, 188, 280–290. [Google Scholar] [CrossRef]
- Xie, S.; Pirianov, G.; Colston, K. Vitamin D analogues suppress IGF-I signalling and promote apoptosis in breast cancer cells. Eur. J. Cancer 1999, 35, 1717–1723. [Google Scholar] [CrossRef]
- Yu, H.; Rohan, T. Role of the insulin-like growth factor family in cancer development and progression. J. Natl. Cancer Inst. 2000, 92, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Arensberg, M.; Richards, J.; Benjamin, J.; Kerr, K.; Hegazi, R. Opportunities for Quality Improvement Programs (QIPs) in the Nutrition Support of Patients with Cancer. Healthcare 2020, 8, 227. [Google Scholar] [CrossRef]
- Yao, S.; Ambrosone, C.B. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J. Steroid Biochem. Mol. Biol. 2013, 136, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hu, W.; Lu, L.; Zhao, Y.; Zhou, Y.; Xiao, Z.; Zhang, L.; Zhang, H.; Li, X.; Li, W.; et al. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm. Sin. B 2018, 9, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Green, A.K.; Hankinson, S.E.; Bertone-Johnson, E.R.; Tamimi, R.M. Mammographic density, plasma vitamin D levels and risk of breast cancer in postmenopausal women. Int. J. Cancer 2010, 127, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Morin, S.O.; Tayeh, B.B.; Goubard, A.; Josselin, E.; Castellano, R.; Fauriat, C.; Guittard, G.; Olive, D.; Nunès, J.A. Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer. Front. Immunol. 2019, 10, 1307. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sayers, T.J.; Colburn, N.H.; Milner, J.A.; Young, H.A. Impact of dietary components on NK and Treg cell function for cancer prevention. Mol. Carcinog. 2015, 54, 669–678. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Sims, J.N.; Miele, L.; Noubissi, F.; Lowe, L.; Fonseca, D.D.; Alo, R.A.; Payton, M.; Tchounwou, P.B. Health and Racial Disparity in Breast Cancer. Adv. Exp. Med. Biol. 2019, 1152, 31–49. [Google Scholar] [CrossRef]
- Huang, R.; Xiang, J.; Zhou, P.-K. Vitamin D, gut microbiota, and radiation-related resistance: A love-hate triangle. J. Exp. Clin. Cancer Res. 2019, 38, 493. [Google Scholar] [CrossRef]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Rohan, T.E.; Manson, J.E.; Li, J.; Ho, G.Y.F.; Xue, X.; Anderson, G.L.; et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 2009, 101, 48–60. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018; CDC National Center for Health Statistics: Hyattsville, MD, USA, 2020; pp. 1–8.
- Bhandari, R.; Kelley, G.A.; Hartley, T.A.; Rockett, I.R.H. Metabolic Syndrome Is Associated with Increased Breast Cancer Risk: A Systematic Review with Meta-Analysis. Int. J. Breast Cancer 2014, 2014, 189384. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, J.F.; et al. Overweight, Obesity, and Postmenopausal Invasive Breast Cancer Risk: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Biello, F.; Platini, F.; D’Avanzo, F.; Cattrini, C.; Mennitto, A.; Genestroni, S.; Martini, V.; Marzullo, P.; Aimaretti, G.; Gennari, A. Insulin/IGF Axis in Breast Cancer: Clinical Evidence and Translational Insights. Biomolecules 2021, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Atoum, M.F.; Alzoughool, F.; Al-Hourani, H. Linkage Between Obesity Leptin and Breast Cancer. Breast Cancer Basic Clin. Res. 2020, 14, 1178223419898458. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef]
- Li, Y.R.; Ro, V.; Tchou, J.C. Obesity, Metabolic Syndrome, and Breast Cancer: From Prevention to Intervention. Curr. Surg. Rep. 2018, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Jiang, L.; Guo, W.; Shao, L.; Liu, Y.; Wang, L. The Association between Leptin Level and Breast Cancer: A Meta-Analysis. PLoS ONE 2013, 8, e67349. [Google Scholar] [CrossRef] [PubMed]
- Andò, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, Leptin and Breast Cancer: Epidemiological Evidence and Proposed Mechanisms. Cancers 2019, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Monjazeb, A.M.; Decock, J. The Obesity Paradox in Cancer, Tumor Immunology, and Immunotherapy: Potential Therapeutic Implications in Triple Negative Breast Cancer. Front. Immunol. 2019, 10, 1940. [Google Scholar] [CrossRef] [PubMed]
- Kolb, R.; Zhang, W. Obesity and Breast Cancer: A Case of Inflamed Adipose Tissue. Cancers 2020, 12, 1686. [Google Scholar] [CrossRef]
- Deng, T.; Lyon, C.L.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2021, 4, e213520. [Google Scholar] [CrossRef] [PubMed]
- Jiralerspong, S.; Goodwin, P. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. J. Clin. Oncol. 2016, 34, 4203–4216. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.; Wiggins, C.L.; Nibbe, A.M.; Storlie, C.B.; Prossnitz, E.R.; Royce, M.; Lomo, L.C.; Hill, D.A. Obesity and survival among a cohort of breast cancer patients is partially mediated by tumor characteristics. NPJ Breast Cancer 2019, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Lega, I.C.; Austin, P.C.; Fischer, H.D.; Fung, K.; Krzyzanowska, M.K.; Amir, E.; Lipscombe, L.L. The Impact of Diabetes on Breast Cancer Treatments and Outcomes: A Population-Based Study. Diabetes Care 2018, 41, 755–761. [Google Scholar] [CrossRef]
- Sabol, R.A.; Villela, V.A.; Denys, A.; Freeman, B.T.; Hartono, A.B.; Wise, R.M.; Harrison, M.A.A.; Sandler, M.B.; Hossain, F.; Miele, L.; et al. Obesity-Altered Adipose Stem Cells Promote Radiation Resistance of Estrogen Receptor Positive Breast Cancer through Paracrine Signaling. Int. J. Mol. Sci. 2020, 21, 2722. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Takita, C.; Wright, J.L.; Reis, I.M.; Zhao, W.; Nelson, O.L.; Hu, J.J. Characterization of risk factors for adjuvant radiotherapy-associated pain in a tri-racial/ethnic breast cancer population. Pain 2016, 157, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gil, J.L.; Takita, C.; Wright, J.; Reis, I.M.; Zhao, W.; Lally, B.E.; Hu, J.L. Inflammatory biomarker C-reactive protein and radiotherapy-induced early adverse skin reactions in patients with breast cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1873–1883. [Google Scholar] [CrossRef]
- Fang, P.; Tan, K.S.; Troxel, A.B.; Rengan, R.; Freedman, G.; Lin, L.L. High body mass index is associated with worse quality of life in breast cancer patients receiving radiotherapy. Breast Cancer Res. Treat. 2013, 141, 125–133. [Google Scholar] [CrossRef] [PubMed]
- De Langhe, S.; Mulliez, T.; Veldeman, L.; Remouchamps, V.; Van Greveling, A.; Gilsoul, M.; De Schepper, E.; De Ruyck, K.; De Neve, W.; Thierens, H. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer 2014, 14, 711. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium in Disease Prevention and Overall Health. Adv. Nutr. 2013, 4, 378S–383S. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Low serum magnesium levels and metabolic syndrome. Acta Diabetol. 2002, 39, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Meigs, J.B.; O’Donnell, C.J.; Jacques, P.F.; McKeown, N.M. Higher Magnesium Intake Reduces Risk of Impaired Glucose and Insulin Metabolism and Progression From Prediabetes to Diabetes in Middle-Aged Americans. Diabetes Care 2013, 37, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Mooren, F.C.; Krüger, K.; Völker, K.; Golf, S.W.; Wadepuhl, M.; Kraus, A. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects—A double-blind, placebo-controlled, randomized trial. Diabetes Obes. Metab. 2011, 13, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Cahill, F.; Shahidi, M.; Shea, J.; Wadden, D.; Gulliver, W.; Randell, E.; Vasdev, S.; Sun, G. High Dietary Magnesium Intake Is Associated with Low Insulin Resistance in the Newfoundland Population. PLoS ONE 2013, 8, e58278. [Google Scholar] [CrossRef] [PubMed]
- Cortés, Y.E.; Moses, L. Magnesium disturbances in critically ill patients. Compend. (Yardley PA) 2007, 29, 420. [Google Scholar]
- Son, E.-W.; Lee, S.-R.; Choi, H.-S.; Koo, H.-J.; Huh, J.-E.; Kim, M.-H.; Pyo, S. Effects of supplementation with higher levels of manganese and magnesium on immune function. Arch. Pharmacal Res. 2007, 30, 743–749. [Google Scholar] [CrossRef]
- Huang, W.-Q.; Long, W.-Q.; Mo, X.-F.; Zhang, N.; Luo, H.; Lin, F.-Y.; Huang, J.; Zhang, C.-X. Direct and indirect associations between dietary magnesium intake and breast cancer risk. Sci. Rep. 2019, 9, 5764. [Google Scholar] [CrossRef]
- Anastassopoulou, J.; Theophanides, T. Magnesium-DNA interactions and the possible relation of magnesium to carcinogenesis. Irradiation and free radicals. Crit. Rev. Oncol. Hematol. 2002, 42, 79–91. [Google Scholar] [CrossRef]
- Wolf, F.; Maier, J.A.M.; Nasulewicz-Goldeman, A.; Feillet-Coudray, C.; Simonacci, M.; Mazur, A.; Cittadini, A. Magnesium and neoplasia: From carcinogenesis to tumor growth and progression or treatment. Arch. Biochem. Biophys. 2006, 458, 24–32. [Google Scholar] [CrossRef]
- Mendes, P.M.V.; Bezerra, D.L.C.; dos Santos, L.R.; de Oliveira Santos, R.; de Sousa Melo, S.R.; Morais, J.B.S.; Severo, J.S.; Vieira, C.; do Nascimento Marreiro, D. Magnesium in Breast Cancer: What Is Its Influence on the Progression of This Disease? Biol. Trace Elem. Res. 2018, 184, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Darbari, D.S.; Ballas, S.K.; Clauw, D.J. Thinking beyond sickling to better understand pain in sickle cell disease. Eur. J. Haematol. 2014, 93, 89–95. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Morcillo, M.A.; García-Cano, J.; Arias-González, L.; García-Gil, E.; Artacho-Cordón, F.; Ríos-Arrabal, S.; Valero, M.L.; Cimas, F.J.; Serrano-Oviedo, L.; Villas, M.V.; et al. Abrogation of the p38 MAPK alpha signaling pathway does not promote radioresistance but its activity is required for 5-Fluorouracil-associated radiosensitivity. Cancer Lett. 2013, 335, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Thornton, T.M.; Rincon, M. Non-Classical P38 Map Kinase Functions: Cell Cycle Checkpoints and Survival. Int. J. Biol. Sci. 2009, 5, 44–52. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Henley, J. Age and cancer risk: A potentially modifiable relationship. Am. J. Prev. Med. 2014, 46 (Suppl. 1), S7–S15. [Google Scholar] [CrossRef]
- NCI. Study Forecasts New Breast Cancer Cases by 2030. 2021. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2015/breast-forecast (accessed on 23 December 2021).
- Haynes, L. Aging of the Immune System: Research Challenges to Enhance the Health Span of Older Adults. Front. Aging 2020, 1, 2. [Google Scholar] [CrossRef]
- Johnson, S.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Aging of the Immune System. Mechanisms and Therapeutic Targets. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 5), S422–S428. [Google Scholar] [CrossRef] [PubMed]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef]
- Catana, C.S.; Calin, G.A.; Berindan-Neagoe, I. Inflamma-miRs in Aging and Breast Cancer: Are They Reliable Players? Front. Med. 2015, 2, 85. [Google Scholar] [CrossRef] [PubMed]
- Berben, L.; Floris, G.; Kenis, C.; Dalmasso, B.; Smeets, A.; Vos, H.; Neven, P.; Martinez, A.A.; Laenen, A.; Wildiers, H.; et al. Age-related remodelling of the blood immunological portrait and the local tumor immune response in patients with luminal breast cancer. Clin. Transl. Immunol. 2020, 9, e1184. [Google Scholar] [CrossRef] [PubMed]
- Deleidi, M.; Jäggle, M.; Rubino, G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front. Neurosci. 2015, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Tesarova, P. Breast cancer in the elderly-Should it be treated differently? Rep. Pract. Oncol. Radiother. 2012, 18, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kocik, J.; Pajączek, M.; Kryczka, T. Worse survival in breast cancer in elderly may not be due to underutilization of medical procedures as observed upon changing healthcare system in Poland. BMC Cancer 2019, 19, 749. [Google Scholar] [CrossRef]
- Cubanski, J.; Koma, W. How Many Seniors Live in Poverty? 2018. Available online: https://files.kff.org/attachment/Issue-Brief-How-Many-Seniors-Live-in-Poverty (accessed on 1 December 2021).
- Kruger, J.; Kohl, H.W., III; Miles, I.J. Prevalence of regular physical activity among adults--United States, 2001 and 2005. MMWR Morb. Mortal Wkly. Rep. 2007, 56, 1209–1212. [Google Scholar]
- Connolly, B.S.; Barnett, C.; Vogt, K.N.; Li, T.; Stone, J.; Boyd, N.F. A Meta-Analysis of Published Literature on Waist-to-Hip Ratio and Risk of Breast Cancer. Nutr. Cancer 2002, 44, 127–138. [Google Scholar] [CrossRef]
- Denkinger, M.M.D.; Hasch, M.; Gerstmayer, A.; Kreienberg, R.; Nikolaus, T.; Hancke, K. Predicting fatigue in older breast cancer patients receiving radiotherapy. Z. Gerontol. Geriatr. 2015, 48, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Haase, H.; Rink, L. The immune system and the impact of zinc during aging. Immun. Ageing 2009, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, J.; Xie, M. Serum and hair zinc levels in breast cancer: A meta-analysis. Sci. Rep. 2015, 5, 12249. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S.; Uzun, N.; Tarakcioglu, M.; Adli, M.; Aksoy, A.; Okumus, S.; Akyüz, M.; Demir, E.; Orkmez, M. Zinc administration modulates radiation-induced oxidative injury in lens of rat. Pharmacogn. Mag. 2012, 8, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Iodice, P.; Del Río, A.; Mellone, M.C.; Catalano, G.; Federico, P. Effects of selenium and zinc supplementation on nutritional status in patients with cancer of digestive tract. Eur. J. Clin. Nutr. 2001, 55, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Dierichs, L.; Kloubert, V.; Rink, L. Cellular zinc homeostasis modulates polarization of THP-1-derived macrophages. Eur. J. Nutr. 2017, 57, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc in Human Health: Effect of Zinc on Immune Cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shastri, A.A.; Lombardo, J.; Okere, S.C.; Higgins, S.; Smith, B.C.; DeAngelis, T.; Palagani, A.; Hines, K.; Monti, D.A.; Volpe, S.; et al. Personalized Nutrition as a Key Contributor to Improving Radiation Response in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 175. https://doi.org/10.3390/ijms23010175
Shastri AA, Lombardo J, Okere SC, Higgins S, Smith BC, DeAngelis T, Palagani A, Hines K, Monti DA, Volpe S, et al. Personalized Nutrition as a Key Contributor to Improving Radiation Response in Breast Cancer. International Journal of Molecular Sciences. 2022; 23(1):175. https://doi.org/10.3390/ijms23010175
Chicago/Turabian StyleShastri, Anuradha A., Joseph Lombardo, Samantha C. Okere, Stephanie Higgins, Brittany C. Smith, Tiziana DeAngelis, Ajay Palagani, Kamryn Hines, Daniel A. Monti, Stella Volpe, and et al. 2022. "Personalized Nutrition as a Key Contributor to Improving Radiation Response in Breast Cancer" International Journal of Molecular Sciences 23, no. 1: 175. https://doi.org/10.3390/ijms23010175
APA StyleShastri, A. A., Lombardo, J., Okere, S. C., Higgins, S., Smith, B. C., DeAngelis, T., Palagani, A., Hines, K., Monti, D. A., Volpe, S., Mitchell, E. P., & Simone, N. L. (2022). Personalized Nutrition as a Key Contributor to Improving Radiation Response in Breast Cancer. International Journal of Molecular Sciences, 23(1), 175. https://doi.org/10.3390/ijms23010175