Trichodermin Induces G0/G1 Cell Cycle Arrest by Inhibiting c-Myc in Ovarian Cancer Cells and Tumor Xenograft-Bearing Mice
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Evaluation of Cell Proliferation and Viability
2.3. Determination of Apoptosis
2.4. Determination of Cell Cycle Distribution
2.5. Western Blot Assay
2.6. Plasmid Transfection
2.7. Animal Experiments
2.8. Statistical Analysis
3. Results
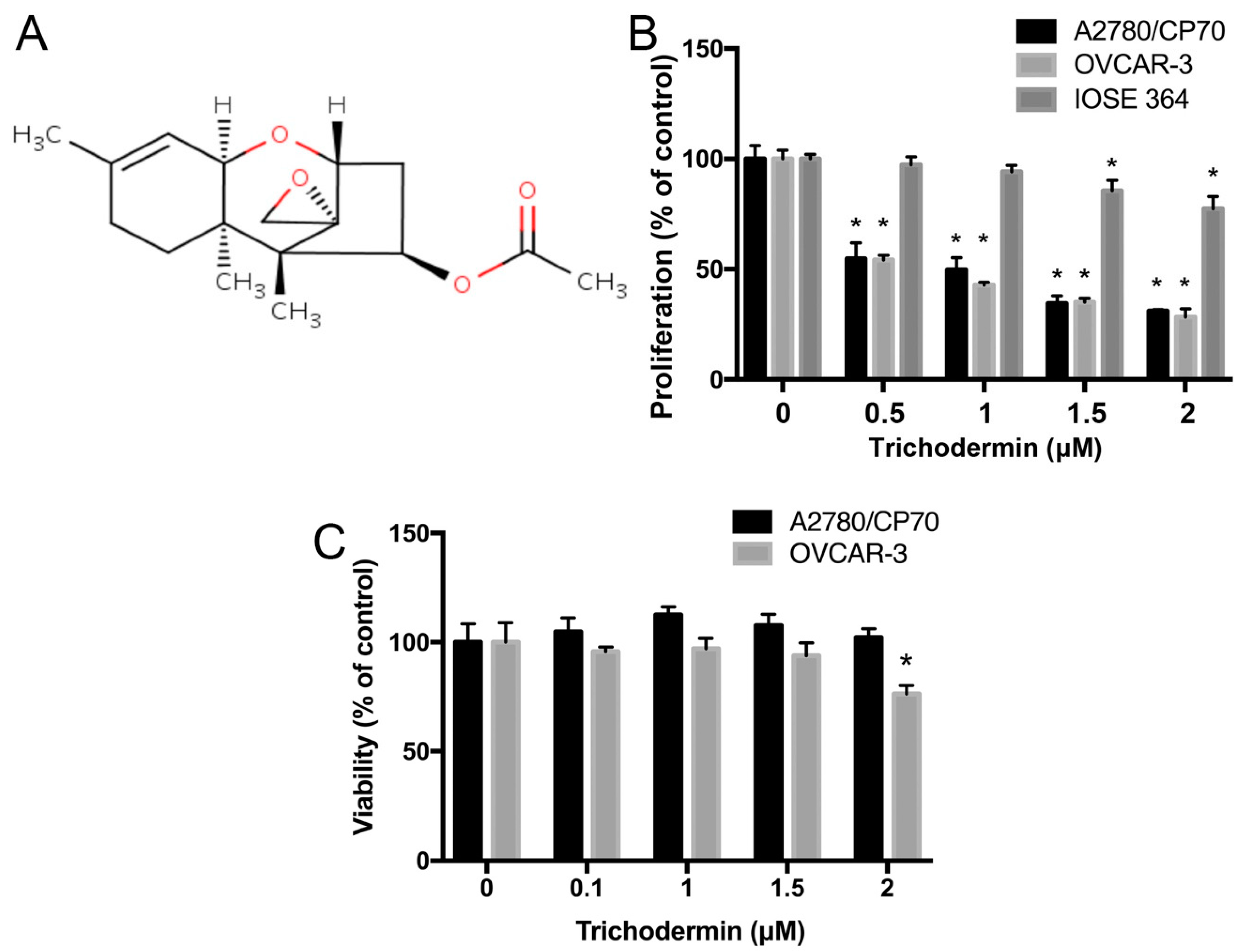
3.1. Trichodermin Specifically Lowers the Proliferation of Ovarian Cancer Cells
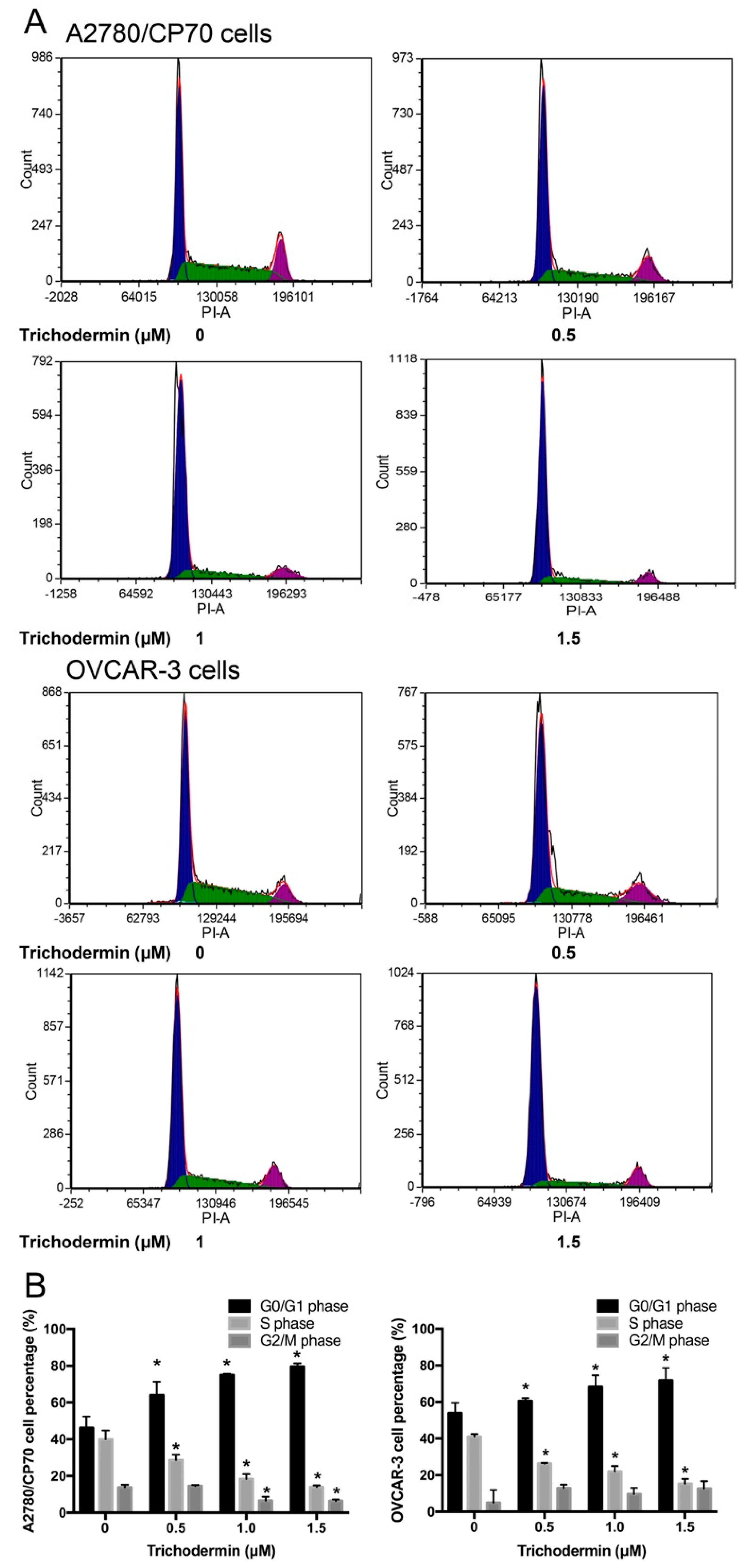
3.2. Trichodermin Suppresses Ovarian Cancer Cells Mainly via Inducing G0/G1 Cell Cycle Arrest
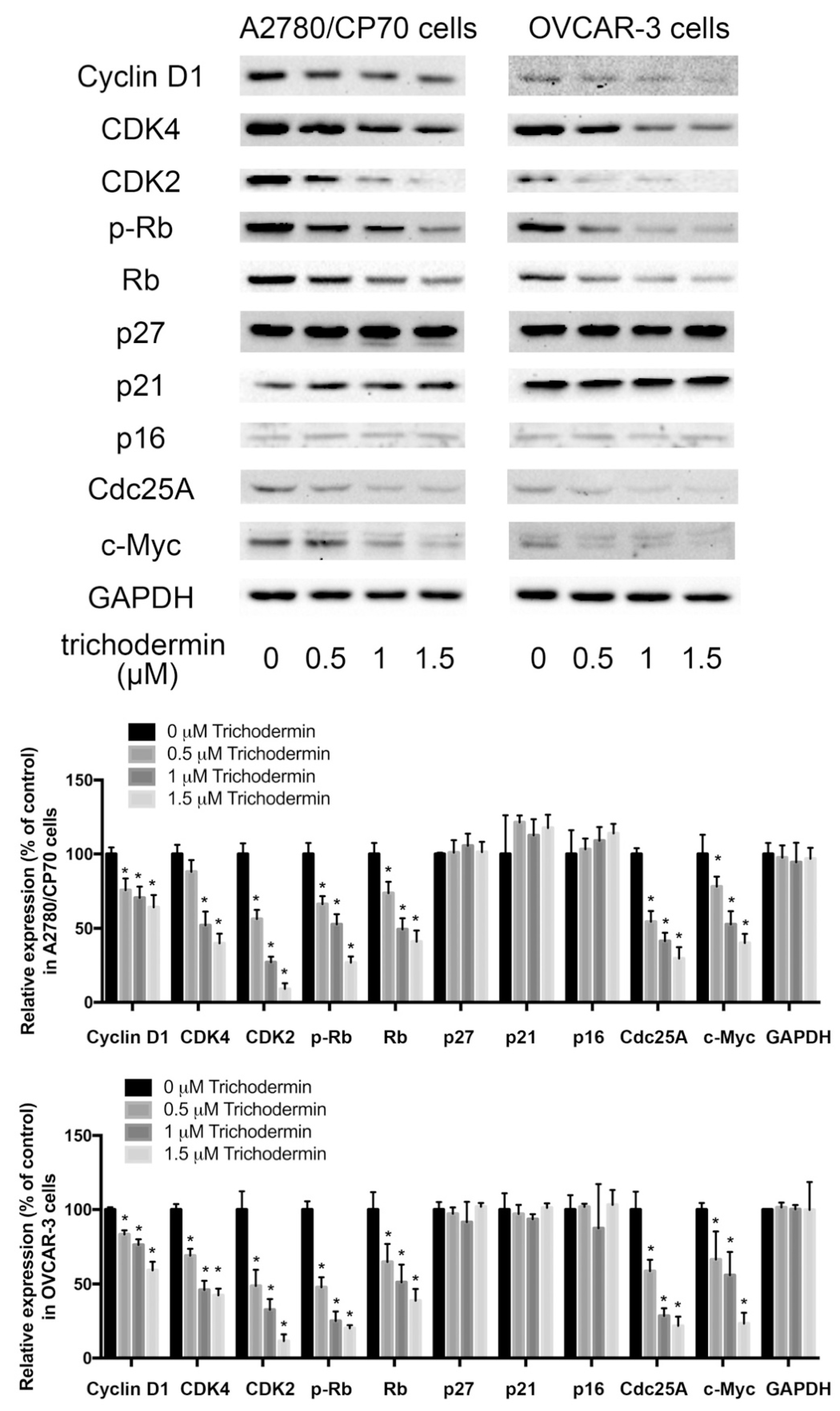
3.3. Trichodermin Inhibits Cyclin-Dependent Kinases (CDKs) and Cyclins
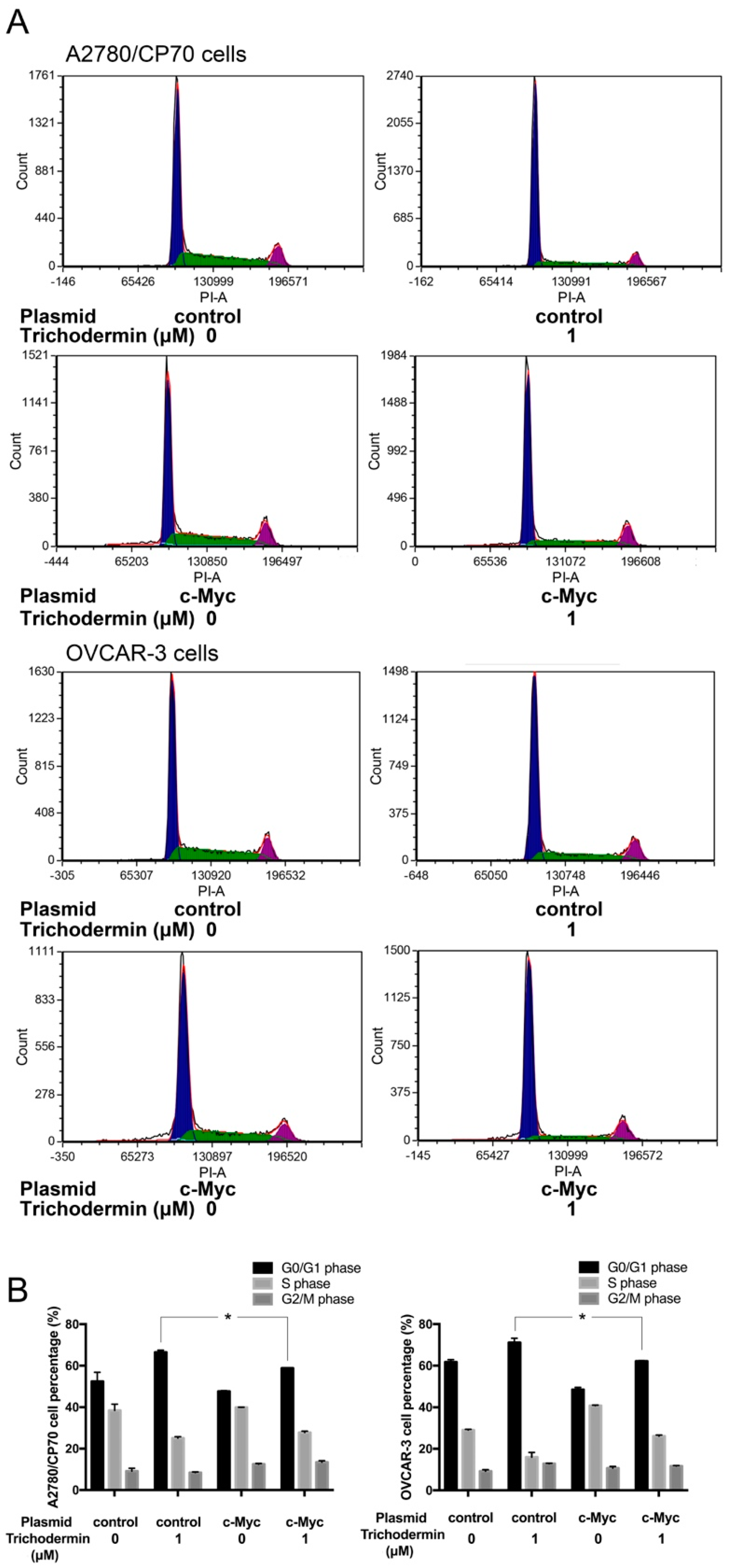
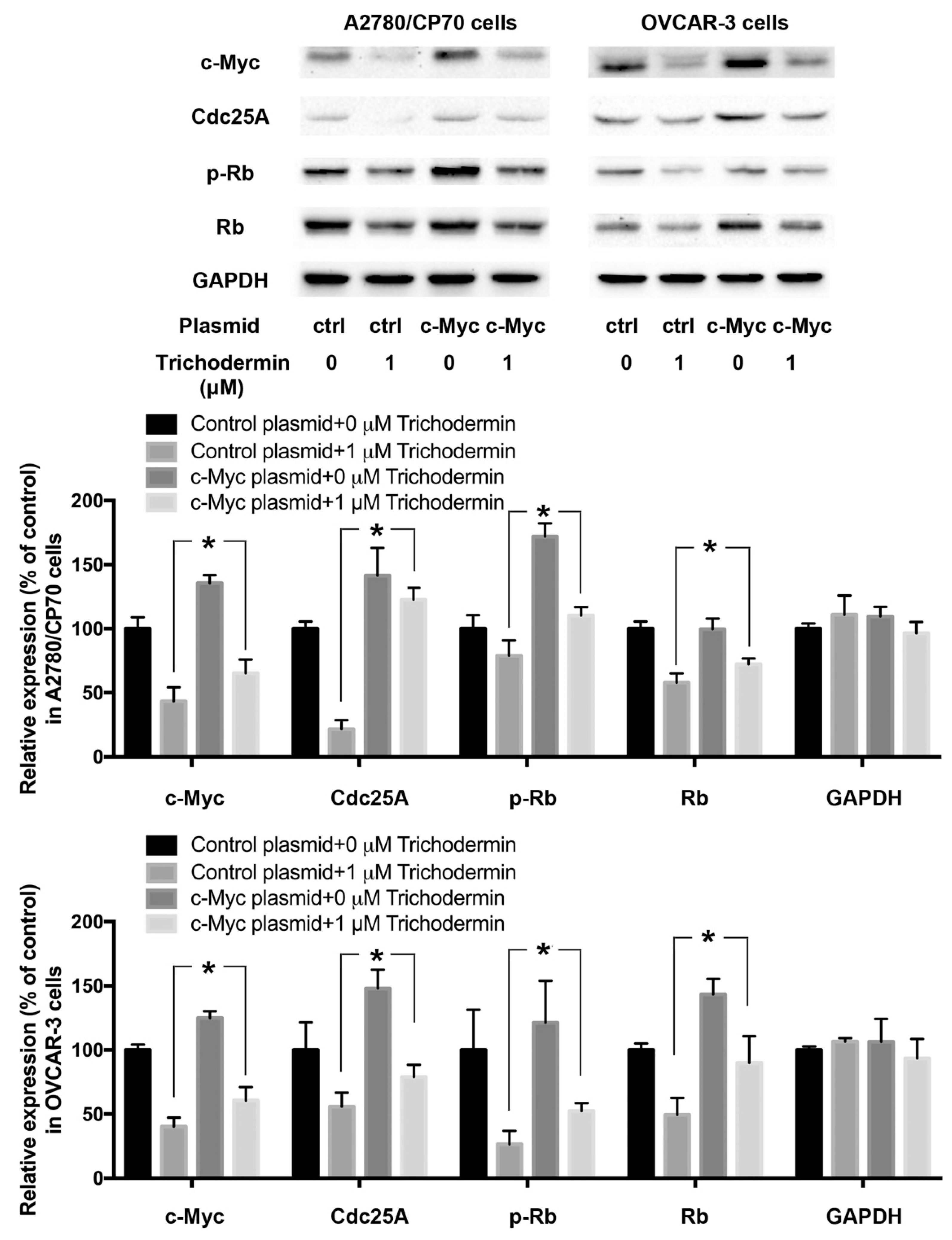
3.4. Trichodermin Induces G0/G1 Cell Cycle Arrest via Targeting c-Myc
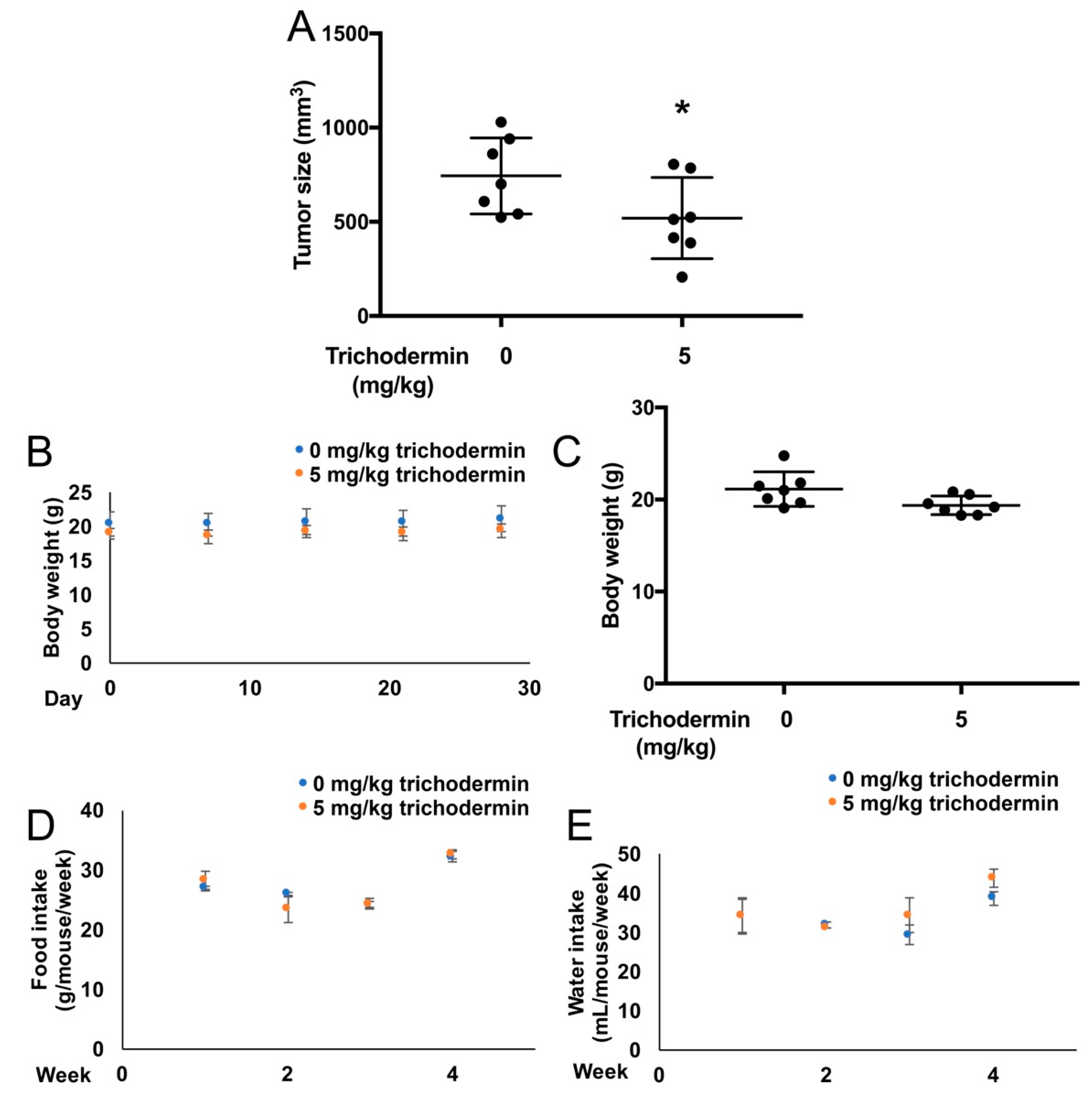
3.5. Trichodermin Reduces Ovarian Tumor Growth in Xenograft-Bearing Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.D.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Morgan, R.J., Jr.; Alvarez, R.D.; Armstrong, D.K.; Burger, R.A.; Chen, L.M.; Copeland, L.; Crispens, M.A.; Gershenson, D.M.; Gray, H.J.; Hakam, A.; et al. Ovarian cancer, version 2. 2013. J. Natl. Compr. Cancer Netw. 2013, 11, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Targeted Cancer Therapies. Available online: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet#what-are-the-side-effects-of-targeted-cancer-therapies (accessed on 27 July 2020).
- McDonald, M.E.; Salinas, E.A.; Devor, E.J.; Newtson, A.M.; Thiel, K.W.; Goodheart, M.J.; Bender, D.P.; Smith, B.J.; Leslie, K.K.; Gonzalez-Bosquet, J. Molecular characterization of non-responders to chemotherapy in serous ovarian cancer. Int. J. Mol. Sci. 2019, 20, 1175. [Google Scholar] [CrossRef]
- Chase, D.M.; Mathur, N.; Tewari, K.S. Drug discovery in ovarian cancer. Recent Pat. Anticancer Drug Discov. 2010, 5, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ma, H.; Huang, F.; Zhu, D.; Bi, J.; Ke, Y.; Zhang, T. Correlation of bevacizumab-induced hypertension and outcomes of metastatic colorectal cancer patients treated with bevacizumab: A systematic review and meta-analysis. World J. Surg. Oncol. 2013, 11, 306. [Google Scholar] [CrossRef]
- Cheng, S.; Swanson, K.; Eliaz, I.; McClintick, J.N.; Sandusky, G.E.; Sliva, D. Pachymic acid inhibits growth and induces apoptosis of pancreatic cancer in vitro and in vivo by targeting er stress. PLoS ONE 2015, 10, e0122270. [Google Scholar] [CrossRef]
- Deng, Q.; Yu, X.; Xiao, L.; Hu, Z.; Luo, X.; Tao, Y.; Yang, L.; Liu, X.; Chen, H.; Ding, Z.; et al. Neoalbaconol induces energy depletion and multiple cell death in cancer cells by targeting pdk1-pi3-k/akt signaling pathway. Cell Death Dis. 2013, 4, e804. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, Y.; Rankin, G.O.; Rojanasakul, Y.; Cutler, S.J.; Tu, Y.; Chen, Y.C. Chaetoglobosin k induces apoptosis and g2 cell cycle arrest through p53-dependent pathway in cisplatin-resistant ovarian cancer cells. Cancer Lett. 2015, 356, 418–433. [Google Scholar] [CrossRef]
- Chen, X.; Lei, B.X.; Wen, T.C.; Zeng, Q. The anticancer activity of endophytic fungi trichoderma sp. from nothapodytes pittosporoides. Lishizhen Med. Mater. Med. Res. 2017, 28, 522–525. [Google Scholar]
- Malmierca, M.G.; Cardoza, R.E.; Alexander, N.J.; McCormick, S.P.; Hermosa, R.; Monte, E.; Gutierrez, S. Involvement of trichoderma trichothecenes in the biocontrol activity and induction of plant defense-related genes. Appl. Environ. Microbiol. 2012, 78, 4856–4868. [Google Scholar] [CrossRef]
- Stafford, M.E.; McLaughlin, C.S. Trichodermin, a possible inhibitor of the termination process of protein synthesis. J. Cell Physiol. 1973, 82, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Wang, S.W.; Lee, T.H.; Tzeng, W.P.; Hsiao, C.J.; Liu, S.C.; Tang, C.H. Trichodermin induces cell apoptosis through mitochondrial dysfunction and endoplasmic reticulum stress in human chondrosarcoma cells. Toxicol. Appl. Pharmacol. 2013, 272, 335–344. [Google Scholar] [CrossRef]
- Chien, M.H.; Lee, T.H.; Lee, W.J.; Yeh, Y.H.; Li, T.K.; Wang, P.C.; Chen, J.J.; Chow, J.M.; Lin, Y.W.; Hsiao, M.; et al. Trichodermin induces c-jun n-terminal kinase-dependent apoptosis caused by mitotic arrest and DNA damage in human p53-mutated pancreatic cancer cells and xenografts. Cancer Lett. 2017, 388, 249–261. [Google Scholar] [CrossRef]
- Culter, H.G.; LeFiles, J.H. Trichodermin: Effects on plants. Plant Cell Physiol. 1978, 19, 177–182. [Google Scholar]
- Gol’dberg, L.E.; Filippos’iants, S.T.; Shepelevtseva, N.G.; Vertogradova, T.P. Toxicity, pharmacokinetics and pharmacodynamics of soviet-made doxorubicin. Antibiotiki 1983, 28, 298–303. [Google Scholar] [PubMed]
- Gao, Y.; Rankin, G.O.; Tu, Y.; Chen, Y.C. Theaflavin-3, 3′-digallate decreases human ovarian carcinoma ovcar-3 cell-induced angiogenesis via akt and notch-1 pathways, not via mapk pathways. Int. J. Oncol. 2016, 48, 281–292. [Google Scholar] [CrossRef]
- Sexl, V.; Diehl, J.A.; Sherr, C.J.; Ashmun, R.; Beach, D.; Roussel, M.F. A rate limiting function of cdc25a for s phase entry inversely correlates with tyrosine dephosphorylation of cdk2. Oncogene 1999, 18, 573–582. [Google Scholar] [CrossRef]
- Blomberg, I.; Hoffmann, I. Ectopic expression of cdc25a accelerates the g(1)/s transition and leads to premature activation of cyclin e- and cyclin a-dependent kinases. Mol. Cell Biol. 1999, 19, 6183–6194. [Google Scholar] [CrossRef] [PubMed]
- Salvi, N. Intrinsically Disordered Proteins Dynamics, Binding, and Function, 1st ed.; Elsevier: Waltham, MA, USA, 2019. [Google Scholar]
- Tashiro, H.; Miyazaki, K.; Okamura, H.; Iwai, A.; Fukumoto, M. C-myc over-expression in human primary ovarian tumours: Its relevance to tumour progression. Int. J. Cancer 1992, 50, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, J. The Molecular Basis of Cancer, 3rd ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2008; p. 757. [Google Scholar]
- Mitra, A.K.; Davis, D.A.; Tomar, S.; Roy, L.; Gurler, H.; Xie, J.; Lantvit, D.D.; Cardenas, H.; Fang, F.; Liu, Y.Y.; et al. In vivo tumor growth of high-grade serous ovarian cancer cell lines. Gynecol. Oncol. 2015, 138, 372–377. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Godtfredsen, W.O.; Vangedal, S. Trichodermin, a new sesquiterpene antibiotic. Acta Chem. Scand. 1965, 19, 1088–1102. [Google Scholar] [CrossRef]
- Wang, G.P.; Zheng, B.Q.; Zhou, Z.Z.; Zhang, C.L. Optimization of fermentation conditions for trichodermin by the mutant strain ul60-11 of trichoderma taxi. Chin. J. Biol. Control 2010, 26, 486–491. [Google Scholar]
- Wei, C.M.; Campbell, I.M.; McLaughlin, C.S.; Vaughan, M.H. Letter: Binding of trichodermin to mammalian ribosomes and its inhibition by other 12,13-epoxytrichothecenes. Mol. Cell Biochem. 1974, 3, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Barbacid, M.; Vazquez, D. Binding of (acetyl-14c)trichodermin to the peptidyl transferase centre of eukaryotic ribosomes. Eur. J. Biochem. 1974, 44, 437–444. [Google Scholar] [CrossRef]
- Carrasco, L.; Barbacid, M.; Vazquez, D. The trichodermin group of antibiotics, inhibitors of peptide bond formation by eukaryotic ribosomes. Biochim. Biophys. Acta 1973, 312, 368–376. [Google Scholar] [CrossRef]
- Choi, S.U.; Choi, E.J.; Kim, K.H.; Kim, N.Y.; Kwon, B.M.; Kim, S.U.; Bok, S.H.; Lee, S.Y.; Lee, C.O. Cytotoxicity of trichothecenes to human solid tumor cells in vitro. Arch. Pharm. Res. 1996, 19, 6–11. [Google Scholar] [CrossRef]
- Quaranta, V.; Tyson, D.; Frick, P. Cell cycle, cancer cell cycle and oncogene addiction. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; pp. 341–343. [Google Scholar]
- Shehata, M.; Waterhouse, P.D.; Casey, A.E.; Fang, H.; Hazelwood, L.; Khokha, R. Proliferative heterogeneity of murine epithelial cells in the adult mammary gland. Commun. Biol. 2018, 1, 111. [Google Scholar] [CrossRef]
- Nghiem, P.; Park, P.K.; Kim, Y.S.; Vaziri, C.; Schreiber, S.L. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc. Natl. Acad. Sci. USA 2001, 98, 9092–9097. [Google Scholar] [CrossRef]
- Zhang, Z.Y. Mechanistic studies on protein tyrosine phosphatases. Prog. Nucleic Acid Res. Mol. Biol. 2003, 73, 171–220. [Google Scholar]
- Reyes-Gonzalez, J.M.; Armaiz-Pena, G.N.; Mangala, L.S.; Valiyeva, F.; Ivan, C.; Pradeep, S.; Echevarria-Vargas, I.M.; Rivera-Reyes, A.; Sood, A.K.; Vivas-Mejia, P.E. Targeting c-myc in platinum-resistant ovarian cancer. Mol. Cancer Ther. 2015, 14, 2260–2269. [Google Scholar] [CrossRef]
- Dang, C.V.; O’Donnell, K.A.; Zeller, K.I.; Nguyen, T.; Osthus, R.C.; Li, F. The c-myc target gene network. Semin. Cancer Biol. 2006, 16, 253–264. [Google Scholar] [CrossRef]
- Novetsky, A.P.; Thompson, D.M.; Zighelboim, I.; Thaker, P.H.; Powell, M.A.; Mutch, D.G.; Goodfellow, P.J. Lithium chloride and inhibition of glycogen synthase kinase 3beta as a potential therapy for serous ovarian cancer. Int. J. Gynecol. Cancer 2013, 23, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, D.; Liu, D.; Yin, L.; Kharbanda, S.; Kufe, D. Muc1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005, 65, 10413–10422. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zou, L.; Yao, Q.; Zhang, Y.; Gan, L.; Tang, L. Silencing dek downregulates cervical cancer tumorigenesis and metastasis via the dek/p-ser9-gsk-3beta/p-tyr216-gsk-3beta/beta-catenin axis. Oncol. Rep. 2017, 38, 1035–1042. [Google Scholar] [CrossRef]
- Averett, C.; Arora, S.; Zubair, H.; Singh, S.; Bhardwaj, A.; Singh, A.P. Chapter nine—Molecular targets of honokiol: A promising phytochemical for effective cancer management. Enzymes 2014, 36, 175–193. [Google Scholar] [PubMed]
- Zhang, J.Y.; Tao, L.Y.; Liang, Y.J.; Yan, Y.Y.; Dai, C.L.; Xia, X.K.; She, Z.G.; Lin, Y.C.; Fu, L.W. Secalonic acid d induced leukemia cell apoptosis and cell cycle arrest of g(1) with involvement of gsk-3beta/beta-catenin/c-myc pathway. Cell Cycle 2009, 8, 2444–2450. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Miles, S.L.; Dasgupta, P.; Rankin, G.O.; Cutler, S.; Chen, Y.C. Trichodermin Induces G0/G1 Cell Cycle Arrest by Inhibiting c-Myc in Ovarian Cancer Cells and Tumor Xenograft-Bearing Mice. Int. J. Mol. Sci. 2021, 22, 5022. https://doi.org/10.3390/ijms22095022
Gao Y, Miles SL, Dasgupta P, Rankin GO, Cutler S, Chen YC. Trichodermin Induces G0/G1 Cell Cycle Arrest by Inhibiting c-Myc in Ovarian Cancer Cells and Tumor Xenograft-Bearing Mice. International Journal of Molecular Sciences. 2021; 22(9):5022. https://doi.org/10.3390/ijms22095022
Chicago/Turabian StyleGao, Ying, Sarah L. Miles, Piyali Dasgupta, Gary O. Rankin, Stephen Cutler, and Yi Charlie Chen. 2021. "Trichodermin Induces G0/G1 Cell Cycle Arrest by Inhibiting c-Myc in Ovarian Cancer Cells and Tumor Xenograft-Bearing Mice" International Journal of Molecular Sciences 22, no. 9: 5022. https://doi.org/10.3390/ijms22095022
APA StyleGao, Y., Miles, S. L., Dasgupta, P., Rankin, G. O., Cutler, S., & Chen, Y. C. (2021). Trichodermin Induces G0/G1 Cell Cycle Arrest by Inhibiting c-Myc in Ovarian Cancer Cells and Tumor Xenograft-Bearing Mice. International Journal of Molecular Sciences, 22(9), 5022. https://doi.org/10.3390/ijms22095022








