Methionine Diet Evoked Hyperhomocysteinemia Causes Hippocampal Alterations, Metabolomics Plasma Changes and Behavioral Pattern in Wild Type Rats
Abstract
:1. Introduction
2. Results
2.1. Level of Total Hcy in Plasma
2.2. NMR (Nuclear Magnetic Resonance) Analysis of Plasma Metabolites
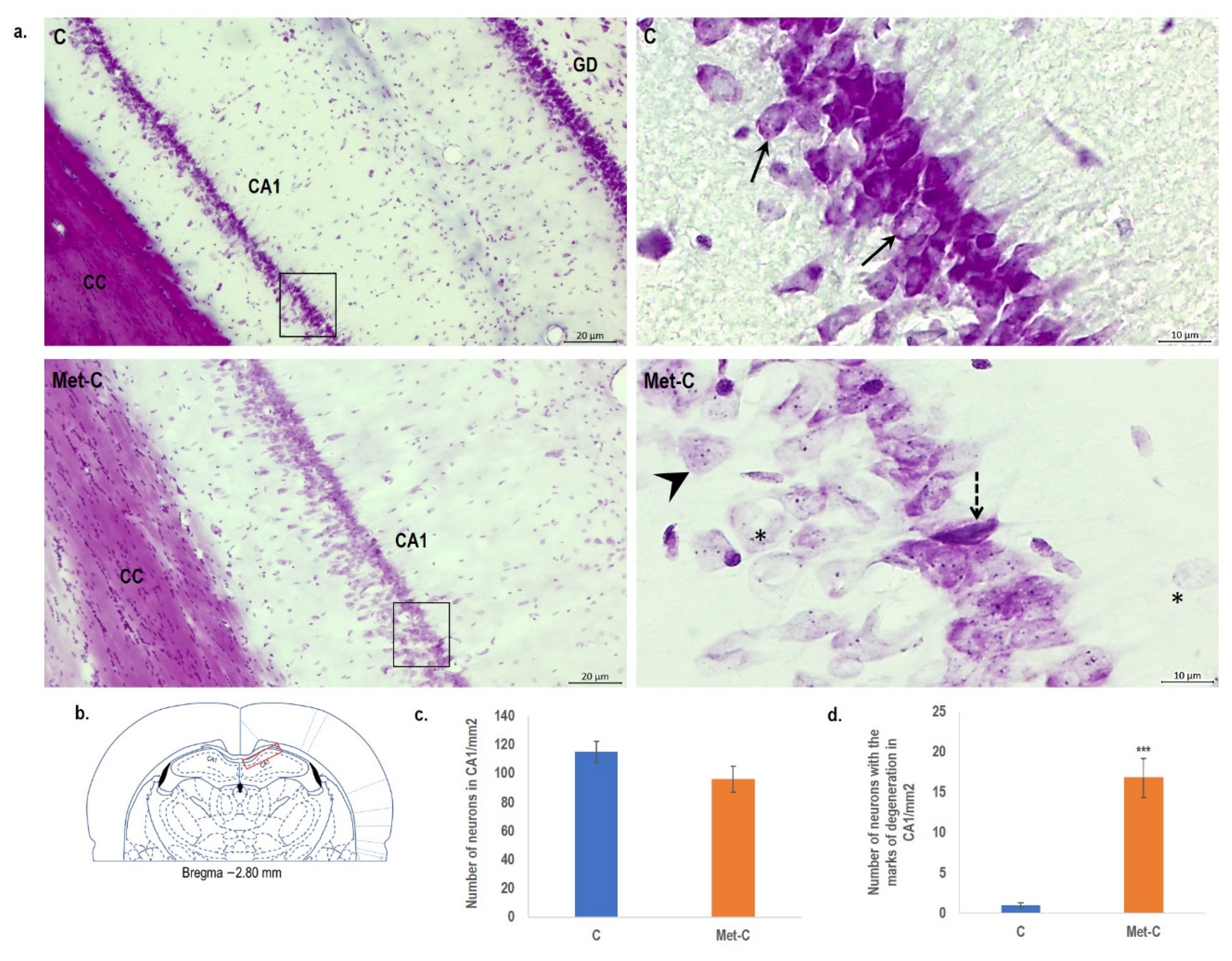
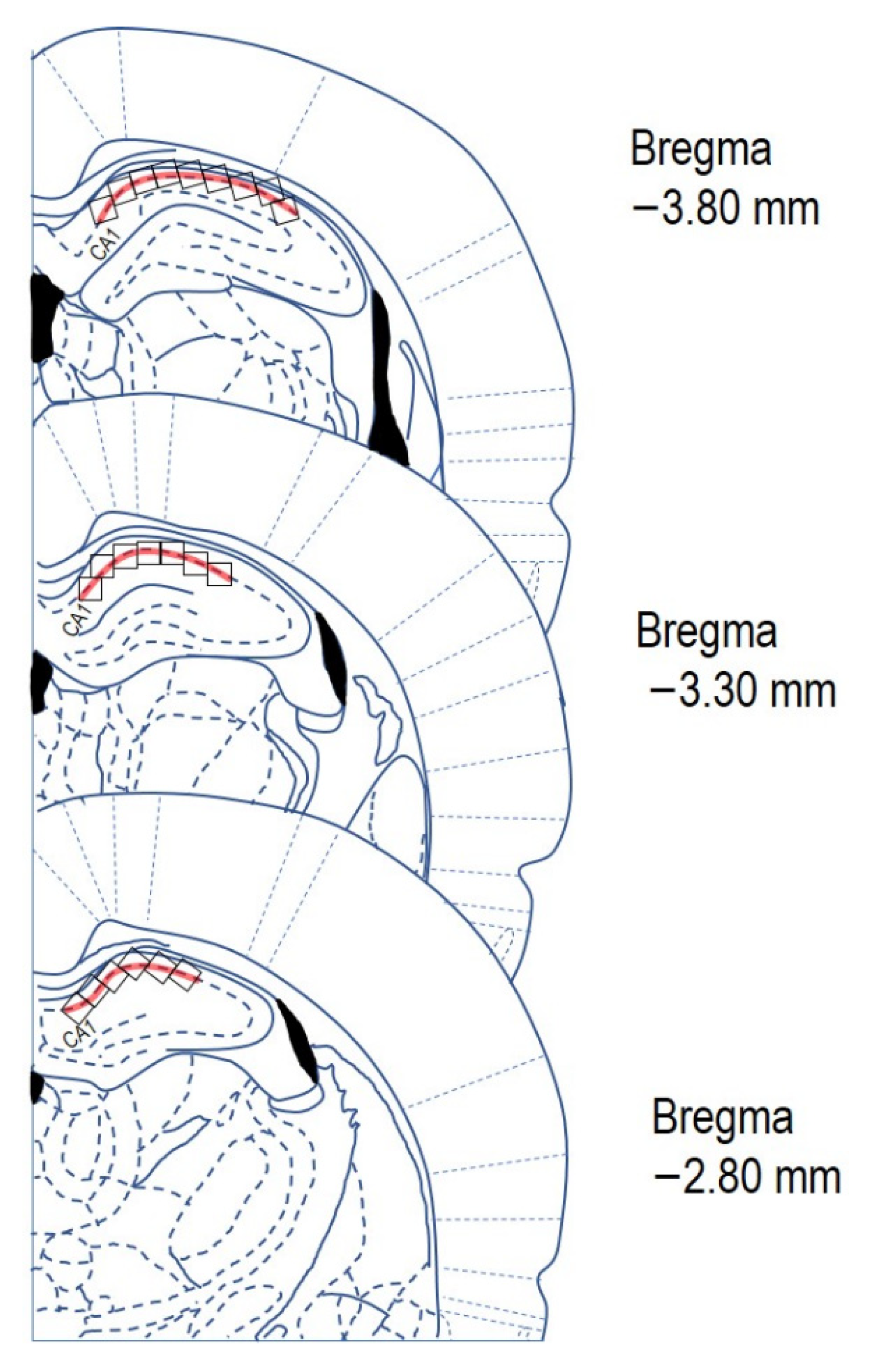
2.3. Cresyl Violet Staining
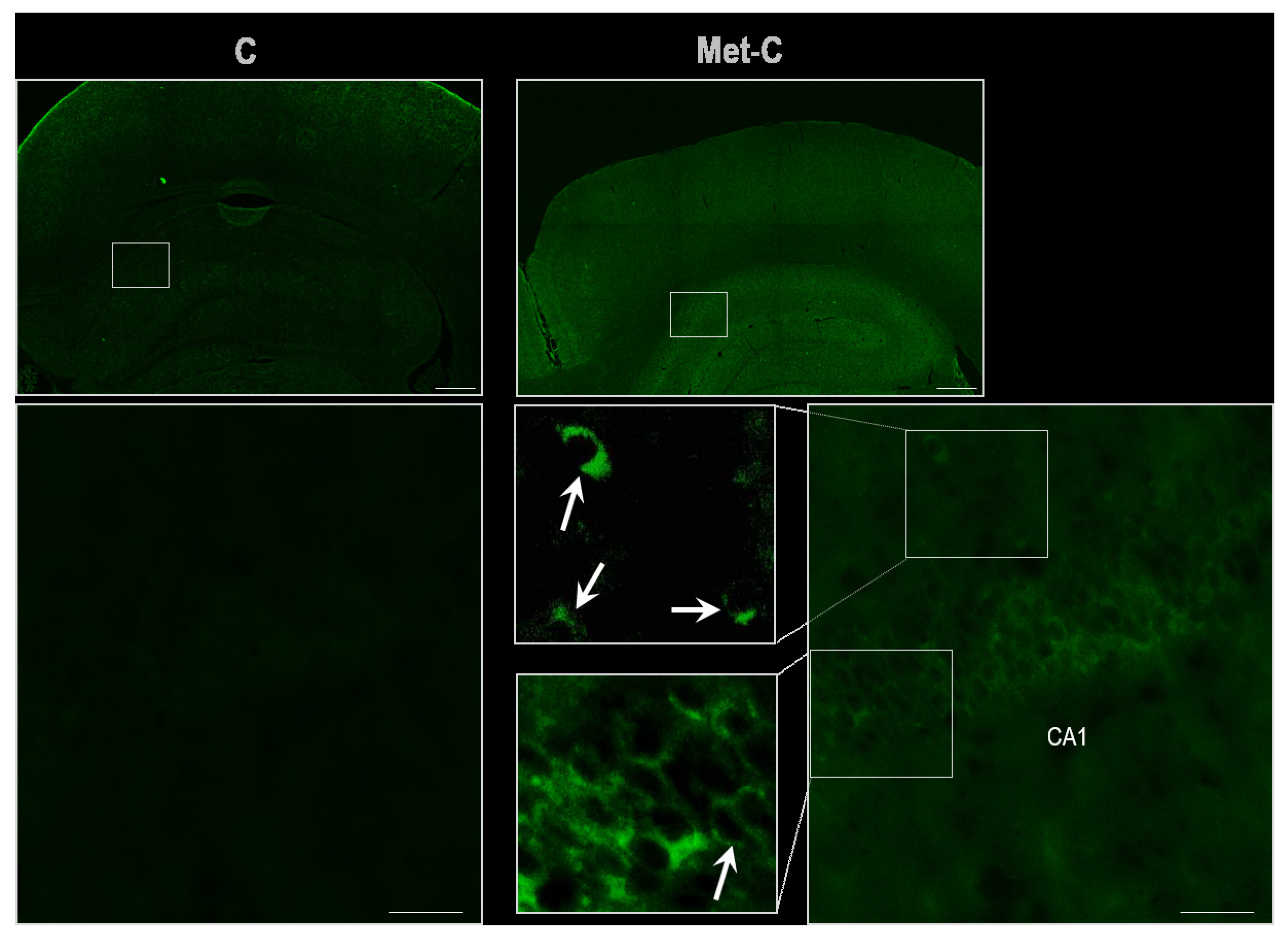
2.4. TUNEL (Terminal Transferase-Mediated dUTP Nick end Labeling) Assay
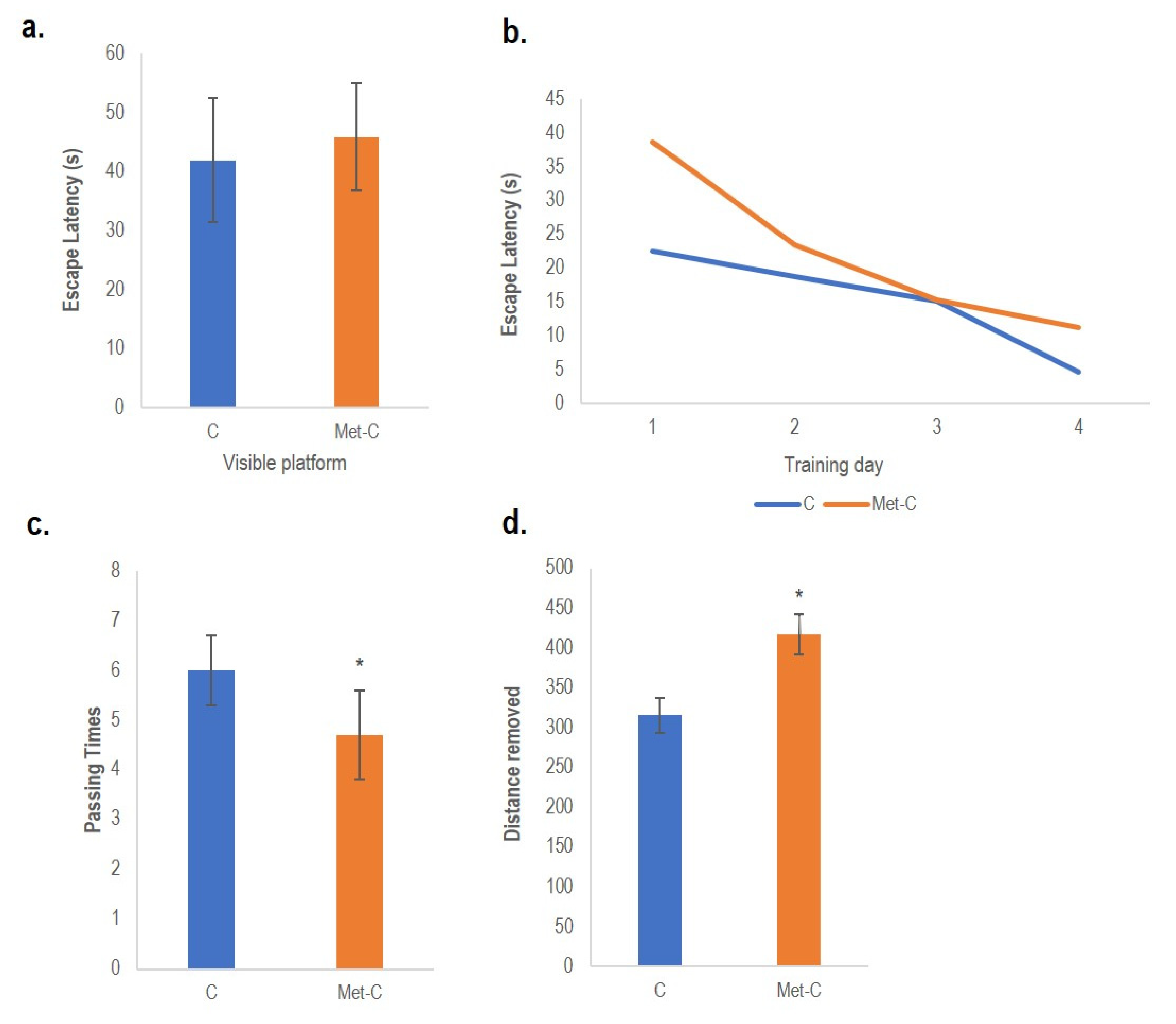
2.5. Behavioural Tests
3. Discussion
4. Materials and Methods
4.1. Induction of Mild hHcy by Met Enriched Diet
4.2. Experimental Groups of Animals
4.3. Behavioural Analysis
4.4. NMR Analysis of Plasma
4.5. Cresyl Violet Staining
4.6. In Situ Labelling of DNA Fragmenattion by TUNEL Assay
4.7. Quantitative Image Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA1 | Cornu ammonis 1 |
| Hcy | Homocysteine |
| hHcy | Hyperhomocysteinemia |
| Met | Methionine |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| NMR | Nuclear magnetic resonance |
References
- Lehotsky, J.; Kovalska, M.; Tomascova, A.; Kalenska, D.; Baranovicova, E.; Kaplan, P. Ischemic brain injury in hyperhomocysteinemic conditions and the development of Alzheimer’s disease. In Brain Ischemia: Alzheimer’s Disease Mechanisms, 1st ed.; Pluta, R., Ed.; Nova Science Pub Inc.: New York, NY, USA, 2019; pp. 115–156. [Google Scholar]
- Kovalska, M.; Hnilicova, P.; Kalenska, D.; Tothova, B.; Adamkov, M.; Lehotsky, J. Effect of Methionine Diet on Metabolic and Histopathological Changes of Rat Hippocampus. Int. J. Mol. Sci. 2019, 20, 6234. [Google Scholar] [CrossRef] [Green Version]
- Kovalska, M.; Tothova, B.; Kalenska, D.; Tomascova, A.; Kovalska, L.; Adamkov, M.; Lehotsky, J. Association of induced hyperhomocysteinemia with neurodegeneration in rat entorhinal cortex-hippocampal system after global brain ischemia: A progression of Alzheimer’s disease-like pathological features? Act. Nerv. Super. Rediviva 2019, 61, 31–38. [Google Scholar]
- Mattson, M.P. Gene–Diet Interactions in Brain Aging and Neurodegenerative Disorders. Determinants of Successful Aging: Developing an Integrated Research Agenda for the 21st Century 2. Ann. Intern. Med. 2003, 139, 441–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallaro, R.A.; Fuso, A.; d’Erme, M.; Miraglia, N.; Martire, S. Role of S-adenosylmethionine in the Modulation of Oxidative Stress-Related Neurodegeneration. Int. J. Clin. Nutr. Diet. 2016, 2, 109. [Google Scholar] [CrossRef] [Green Version]
- Tapia-Rojas, C.; Lindsay, C.B.; Montecinos-Oliva, C.; Arrazola, M.S.; Retamales, R.M.; Bunout, D.; Hirsch, S.; Inestrosa, N.C. Is L-methionine a trigger factor for Alzheimer’s-like neurodegeneration? Changes in Aβ oligomers, tau phosphorylation, synaptic proteins, Wnt signaling and behavioral impairment in wild-type mice. Mol. Neurodegener. 2015, 10, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabler, S.P.; Steegborn, C.; Wahl, M.C.; Oliveriusova, J.; Kraus, J.P.; Allen, R.H.; Wagner, C.; Mudd, S.H. Elevated plasma total homocysteine in severe methionine adenosyltransferase I/III deficiency. Metabolism 2002, 51, 981–988. [Google Scholar] [CrossRef]
- Mudd, S.H. Hypermethioninemias of genetic and non-genetic origin: A review. Am. J. Med. Genet. Part C Semin. Med. Genet. 2011, 157, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Ursin, G.; Veierod, M.B. Meat consumption and the risk of type 2 diabetes: A systematic review and meta-analysis of cohort studies. Diabetologia 2009, 52, 2277–2287. [Google Scholar] [CrossRef] [Green Version]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuru, M.; Muradashvili, N.; Kalani, A.; Lominadze, D.; Tyagi, N. High methionine, low folate and low vitamin B6/B12 (HM-LF-LV) diet causes neurodegeneration and subsequent short-term memory loss. Metab. Brain Dis. 2018, 33, 1923–1934. [Google Scholar] [CrossRef]
- Zhang, J.W.; Ma, Y.M.; Jing, L.; Wang, Y.L.; Zhang, J.Z. Synaptic remodeling and reduced expression of the transcription factors, HES1 and HES5, in the cortex neurons of cognitively impaired hyperhomocysteinemic mice. Pathol. Res. Pract. 2020, 216, 152953. [Google Scholar] [CrossRef] [PubMed]
- Schweinberger, B.M.; Wyse, A.T. Mechanistic basis of hypermethioninemia. Amino Acids 2016, 48, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J. Toxicity of methionine in humans. J. Nutr. 2006, 136 (Suppl. 6), 1722S–1725S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey-Mudd, S.N.; Braverman, M.; Pomper, M.; Tezcan, K.; Kronick, J.; Jayakar, P.; Garganta, C.; Ampola, M.G.; Levy, H.L.; McCandless, S.E.; et al. Infantile hypermethioninemia and hyperhomocysteinemia due to high methionine intake: A diagnostic trap. Mol. Genet. Metab. 2003, 79, 6–16. [Google Scholar] [CrossRef]
- Lawson-Yuen, A.; Levy, H.L. The use of betaine in the treatment of elevated homocysteine. Mol. Genet. Metab. 2006, 88, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wu, G.; Feng, C.; Yang, Y.; Li, B.; Ge, Y.; Jiang, Y.; Shi, Y.; Le, G. Dietary methionine restriction improves the impairment of cardiac function in middle-aged obese mice. Food Funct. 2020, 11, 1764–1778. [Google Scholar] [CrossRef]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef]
- Zhuo, J.M.; Portugal, G.S.; Kruger, W.D.; Wang, H.; Gould, T.J.; Pratico, D. Diet-induced hyperhomocysteinemia increases amyloid-beta formation and deposition in a mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2010, 7, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Chu, J.; Barrero, C.; Merali, S.; Praticò, D. Homocysteine Exacerbates β-Amyloid Pathology, Tau Pathology, and Cognitive Deficit in a Mouse Model of Alzheimer Disease with Plaques and Tangles. Ann. Neurol. 2014, 75, 851–863. [Google Scholar] [CrossRef]
- Ringman, J.M.; Coppola, G. New genes and new insights from old genes: Update on Alzheimer disease. Continuum 2013, 19, 358–371. [Google Scholar] [CrossRef] [Green Version]
- Sudduth, T.L.; Powell, D.K.; Smith, C.D.; Greenstein, A.; Wilcock, D.M. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J. Cereb. Blood Flow Metab. 2013, 33, 708–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchantchou, F.; Goodfellow, M.; Li, F.; Ramsue, L.; Miller, C.; Puche, A.; Fiskum, G. Hyperhomocysteinemia-Induced Oxidative Stress Exacerbates Cortical Traumatic Brain Injury Outcomes in Rats. Cell. Mol. Neurobiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kovalska, M.; Kovalska, L.; Tothova, B.; Mahmood, S.; Adamkov, M.; Lehotsky, J. Combination of hyperhomocysteinemia and ischemic tolerance in experimental model of global ischemia in rats. J. Physiol. Pharmacol. 2015, 66, 887–897. [Google Scholar]
- Kovalska, M.; Tothova, B.; Kovalska, L.; Tatarkova, Z.; Kalenska, D.; Tomascova, A.; Adamkov, M.; Lehotsky, J. Association of Induced Hyperhomocysteinemia with Alzheimer’s Disease-Like Neurodegeneration in Rat Cortical Neurons After Global Ischemia-Reperfusion Injury. Neurochem. Res. 2018, 43, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, B.; Kovalská, M.; Kalenská, D.; Tomascova, A.; Lehotsky, J. Histone Hyperacetylation as a Response to Global Brain Ischemia Associated with Hyperhomocysteinemia in Rats. Int. J. Mol. Sci. 2018, 19, 3147. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Gao, C.; Cueto, R.; Liu, L.; Fu, H.; Shao, Y.; Yang, W.Y.; Fang, P.; Choi, E.T.; Wu, Q.; et al. Homocysteine-methionine cycle is a metabolic sensor system controlling methylation-regulated pathological signalling. Redox Biol. 2020, 28, 101322. [Google Scholar] [CrossRef]
- Zhang, Z. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef]
- Liu, M.; Tang, H.; Nicholson, J.K.; Lindon, J.C. Use of 1H NMR-determined diffusion coefficients to characterize lipoprotein fractions in human blood plasma. Magn. Reson. Chem. 2002, 40, S83–S88. [Google Scholar] [CrossRef]
- Baranovicova, E.; Kalenska, D.; Tomascova, A.; Holubcikova, S.; Lehotsky, J. Time-related metabolomics study in the rat plasma after global cerebral ischemia and reperfusion: Effect of ischemic preconditioning. IUBMB Life 2020, 72, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: London, UK, 2006. [Google Scholar]
- Jakubowski, H. Pathophysiological Consequences of Homocysteine Excess. J. Nutr. 2006, 136, 1741S–1749S. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Homocysteine Modification in Protein Structure/Function and Human Disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef]
- Selhub, J.; Troen, A.M. Sulfur amino acids and atherosclerosis: A role for excess dietary methionine. Ann. N. Y. Acad. Sci. 2016, 1363, 18–25. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Voutilainen, S.; Rissanen, T.H.; Happonen, P.; Mursu, J.; Laukkanen, J.A.; Poulsen, H.; Lakka, T.A.; Salonen, J.T. High dietary methionine intake increases the risk of acute coronary events in middle-aged men. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Dorszewska, J.; Florczak-Wyspiańska, J.; Oczkowska, A.; Kozubski, W. Homocysteine and asymmetric dimethylarginine concentrations in the plasma of Alzheimer’s disease patients with varying degrees of dementia. Adv. Alzheimer’s Dis. 2013, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Petráš, M.; Drgová, A.; Kovalská, M.; Tatarková, Z.; Tóthová, B.; Križanová, O.; Lehotský, J. Effect of Hyperhomocysteinemia on Redox Balance and Redox Defence Enzymes in Ischemia–Reperfusion Injury and/or After Ischemic Preconditioning in Rats. Cell. Mol. Neurobiol. 2017, 37, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Labinjoh, C.; Newby, D.E.; Wilkinson, I.B.; Megson, I.L.; MacCallum, H.; Melville, V.; Boon, N.A.; Webb, D.J. Effects of acute methionine loading and vitamin C on endogenous fibrinolysis, endothelium-dependent vasomotion and platelet aggregation. Clin. Sci. 2001, 100, 127–135. [Google Scholar]
- Chao, C.L.; Kuo, T.L.; Lee, Y.T. Effectsof methionine-induced hyperhomocysteinemia on endothelium-dependent vasodilation and oxidative status in healthy adults. Circulation 2000, 101, 485–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nestel, P.J.; Chronopoulos, A.; Cehun, M. Arterial stiffness is rapidly induced by raising the plasma homocy-steine concentration with methionine. Atherosclerosis 2003, 171, 83–86. [Google Scholar] [CrossRef]
- Romerio, S.C.; Linder, L.; Nyfeler, J. Acute hyperhomocysteinemia decreases NO bioavailability in healthy adults. Atherosclerosis 2004, 176, 337–344. [Google Scholar] [CrossRef]
- Troen, A.M.; Shea-Budgell, M.; Shukitt-Hale, B.; Smith, D.E.; Selhub, J.; Rosenberg, I.H. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 12474–12479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Guo, Y.; Zhu, Z. Effects of hyperhomocysteinemia on ischemic cerebral small vessel disease and analysis of inflammatory mechanisms. Int. J. Neurosci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Jiang, Y.; Zhang, S.; Tie, T.; Cheng, Y.; Su, X.; Man, Z.; Hou, J.; Sun, L.; Tian, M.; et al. The association between homocysteine and ischemic stroke subtypes in Chinese: A meta-analysis. Medicine 2020, 99, e19467. [Google Scholar] [CrossRef]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Andjus, P.R. Cellular markers of neuroinflammation and neurogenesis after ischemic brain injury in the long-term survival rat model. Brain Struct. Funct. 2012, 217, 411–420. [Google Scholar]
- Pluta, R.; Ułamek-Kozioł, M.; Kocki, J.; Bogucki, J.; Januszewski, S.; Bogucka-Kocka, A.; Czuczwar, S.J. Expression of the Tau Protein and Amyloid Protein Precursor Processing Genes in the CA3 Area of the Hippocampus in the Ischemic Model of Alzheimer’s Disease in the Rat. Mol. Neurobiol. 2020, 57, 1281–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radenovic, L.; Nenadic, M.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J.; Andjus, P.R.; Pluta, R. Heterogeneity in brain distribution of activated microglia and astrocytes in a rat ischemic model of Alzheimer’s disease after 2 years of survival. Aging 2020, 12, 12251–12267. [Google Scholar] [CrossRef]
- Ugolini, F.; Lana, D.; Nardiello, P.; Nosi, D.; Pantano, D.; Casamenti, F.; Giovannini, M.G. Different Patterns of Neurodegeneration and Glia Activation in CA1 and CA3 Hippocampal Regions of TgCRND8 Mice. Front. Aging Neurosci. 2018, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, Y.; Zhao, Z.; Li, P.; Xie, Z. Hydrogen Sulfide Overproduction Is Involved in Acute Ischemic Cerebral Injury under Hyperhomocysteinemia. Front. Neurosci. 2020, 14, 582851. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, B.; Kovalská, M.; Kalenská, D.; Tomascova, A.; Lehotsky, J. Effect of methionine-induced hyperhomocysteinemia on neurodegeneration in experimental conditions. Act. Nerv. Super. Rediviva 2017, 59, 101–107. [Google Scholar]
- Kovalska, M.; Hnilicova, P.; Kalenska, D.; Tomascova, A.; Adamkov, M.; Lehotsky, J. Effect of Methionine Diet on Time-Related Metabolic and Histopathological Changes of Rat Hippocampus in the Model of Global Brain Ischemia. Biomolecules 2020, 10, 1128. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Wei, H.J.; Dong, J.F.; Zhang, J.N. Methionine diet-induced hyperhomocysteinemia accelerates cerebral aneurysm formation in rats. Neurosci. Lett. 2011, 494, 139–144. [Google Scholar] [CrossRef]
- Wang, L.; Ren, B.; Hui, Y.; Chu, C.; Zhao, Z.; Zhang, Z.; Zhao, B.; Shi, R.; Ren, J.; Dai, X. Methionine Restriction Regulates Cognitive Function in High-Fat Diet-Fed Mice: Roles of Diurnal Rhythms of SCFAs Producing- and Inflammation-Related Microbes. Mol. Nutr. Food Res. 2020, 64. [Google Scholar] [CrossRef]
- Singhal, N.K.; Freeman, E.; Arning, E.; Wasek, B.; Clements, R.; Sheppard, C.; Blake, P.; Bottiglieri, T.; McDonough, J. Dysregulation of methionine metabolism in multiple sclerosis. Neurochem. Int. 2018, 112, 1–4. [Google Scholar] [CrossRef]
- Sudduth, T.L.; Weekman, E.M.; Price, B.R.; Gooch, J.L.; Woolums, A.; Norris, C.N.; Wilcock, D.M. Time-course of glial changes in the hyperhomocysteinemia model of vascular cognitive impairment and dementia (VCID). Neuroscience 2017, 341, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Weekman, E.M.; Sudduth, T.L.; Price, B.R.; Woolums, A.E.; Hawthorne, D.; Seaks, C.S.; Wilcock, D.M. Time course of neuropathological events in hyperhomocysteinemic amyloid depositing mice reveals early neuroinflammatory changes that precede amyloid changes and cerebrovascular events. J. Neuroinflamm. 2019, 16, 284. [Google Scholar] [CrossRef]
- Seminotti, B.; Zanatta, Â.; Ribeiro, R.T.; da Rosa, M.S.; Wyse, A.T.S.; Leipnitz, G.; Wajner, M. Disruption of Brain Redox Homeostasis, Microglia Activation and Neuronal Damage Induced by Intracerebroventricular Administration of S-Adenosylmethionine to Developing Rats. Mol. Neurobiol. 2019, 56, 2760–2773. [Google Scholar] [CrossRef] [PubMed]
- de S Moreira, D.; Figueiró, P.W.; Siebert, C.; Prezzi, C.A.; Rohden, F.; Guma, F.C.R.; Manfredini, V.; Wyse, A.T.S. Chronic Mild Hyperhomocysteinemia Alters Inflammatory and Oxidative/Nitrative Status and Causes Protein/DNA Damage, as well as Ultrastructural Changes in Cerebral Cortex: Is Acetylsalicylic Acid Neuroprotective? Neurotox. Res. 2018, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Figueiró, P.W.; Moreira, D.S.; Dos Santos, T.M.; Prezzi, C.A.; Rohden, F.; Faccioni-Heuser, M.C.; Manfredini, V.; Netto, C.A.; Wyse, A.T.S. The neuroprotective role of melatonin in a gestational hypermethioninemia model. Int. J. Dev. Neurosci. 2019, 78, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.S.P.; Viau, C.M.; Saffi, J.; Saffi, J.; Zanusso Costa, M.; da Silva, T.M.; Oliveira, P.S.; Hofstatter Azambuja, J.; Barschak, A.G.; Braganhol, E.; et al. Acute administration of methionine and/or methionine sulfoxide impairs redox status and induces apoptosis in rat cerebral cortex. Metab. Brain Dis. 2017, 32, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Belloli, S.; Zanotti, L.; Murtaj, V.; Mazzon, C.; Di Grigoli, G.; Monterisi, C.; Masiello, V.; Iaccarino, L.; Cappelli, A.; Poliani, P.L.; et al. F-VC701-PET and MRI in the in vivo neuroinflammation assessment of a mouse model of multiple sclerosis. J. Neuroinflamm. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, X.; Liu, J.; Xie, X.; Cui, X.; Zhu, Y. Homocysteine accelerates senescence of endothelial cells via DNA hypomethylation of human telomerase reverse transcriptase. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Ungvari, Z.; Tarantini, S.; Angelia, C.; Csiszar, A.; Prodan, C. Advances in Cardiovascular Geroscience Cerebral microhemorrhages: Mechanisms, consequences, and prevention. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1128–H1143. [Google Scholar] [CrossRef] [PubMed]
- Cassel, R.; Bordiga, P.; Carcaud, J.; Simon, F.; Beraneck, M.; Le Gall, A.; Benoit, A.; Bouet, V.; Philoxene, B.; Besnard, S.; et al. Morphological and functional correlates of vestibular synaptic deafferentation and repair in a mouse model of acute-onset vertigo. Dis. Model Mech. 2019, 12, dmm039115. [Google Scholar] [CrossRef] [Green Version]
- Ruan, H.B.; Crawford, P.A. Ketone bodies as epigenetic modifiers. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 260–266. [Google Scholar] [CrossRef]
- Su, X.; Wellen, K.E.; Rabinowitz, J.D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 2016, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Shan, W.; Zhu, F.; Wu, J.; Wang, Q. Ketone Bodies in Neurological Diseases: Focus on Neuroprotection and Underlying Mechanisms. Front. Neurol. 2019, 10, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Alachkar, A.; Sanathara, N.; Belluzzi, J.D.; Wang, Z.; Civelli, O. A methionine-induced animal model of schizophrenia: Face and predic-tive validity. Int. J. Neuropsychopharmacol. 2015, 18, pyv054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streck, E.L.; Bavaresco, C.S.; Netto, C.A.; de Sousa Neto, A. Chronic hyperhomocysteinemia provokes a memory deficit in rats in the Morris water maze task. Behav. Brain Res. 2004, 153, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Algaidi, S.A.; Christie, L.A.; Jenkinson, A.M.; Whalley, L.; Riedel, G.; Plattt, B. Long-term homocysteine exposure induces alterations in spatial learning, hippocampal signalling and synaptic plasticity. Exp. Neurol. 2006, 197, 8–21. [Google Scholar] [CrossRef]
- Onozato, M.; Uta, A.; Magarida, A.; Fukuoka, N.; Ichiba, H.; Tsujino, N.; Funatogawa, T.; Tagata, H.; Nemoto, T.; Mizuno, M.; et al. Alterations in methionine to homocysteine ratio in individuals with first-episode psychosis and those with at-risk mental state. Clin. Biochem. 2020, 77, 48–53. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek-Kozioł, M. The role of degenerative pathways in the development of irreversible consequences after brain ischemia. Neural Regen. Res. 2019, 14, 982–983. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, P.; Tatarkova, Z.; Kmetova-Sivonova, M.; Racay, P.; Lehotsky, J. Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems. Int. J. Mol. Sci. 2020, 21, 7698. [Google Scholar] [CrossRef] [PubMed]
| Metabolite | p | % Change |
|---|---|---|
| Glucose | p < 0.05 | 21 |
| Acetate | p < 0.05 | 20 |
| Lipoproteins | p < 0.05 | −59 |
| 3-hydroxybutyrate | p < 0.05 | 53 |
| Phenylalanine | p < 0.05 | −17 |
| Tryptophan | p < 0.05 | −39 |
| Tyrosine | p < 0.05 | −23 |
| Histidine | p < 0.05 | −11 |
| Leucine | p < 0.08 | −14 |
| Isoleucine | p < 0.08 | −11 |
| Valine | p < 0.08 | −14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalska, M.; Baranovicova, E.; Kalenska, D.; Tomascova, A.; Adamkov, M.; Kovalska, L.; Lehotsky, J. Methionine Diet Evoked Hyperhomocysteinemia Causes Hippocampal Alterations, Metabolomics Plasma Changes and Behavioral Pattern in Wild Type Rats. Int. J. Mol. Sci. 2021, 22, 4961. https://doi.org/10.3390/ijms22094961
Kovalska M, Baranovicova E, Kalenska D, Tomascova A, Adamkov M, Kovalska L, Lehotsky J. Methionine Diet Evoked Hyperhomocysteinemia Causes Hippocampal Alterations, Metabolomics Plasma Changes and Behavioral Pattern in Wild Type Rats. International Journal of Molecular Sciences. 2021; 22(9):4961. https://doi.org/10.3390/ijms22094961
Chicago/Turabian StyleKovalska, Maria, Eva Baranovicova, Dagmar Kalenska, Anna Tomascova, Marian Adamkov, Libusa Kovalska, and Jan Lehotsky. 2021. "Methionine Diet Evoked Hyperhomocysteinemia Causes Hippocampal Alterations, Metabolomics Plasma Changes and Behavioral Pattern in Wild Type Rats" International Journal of Molecular Sciences 22, no. 9: 4961. https://doi.org/10.3390/ijms22094961
APA StyleKovalska, M., Baranovicova, E., Kalenska, D., Tomascova, A., Adamkov, M., Kovalska, L., & Lehotsky, J. (2021). Methionine Diet Evoked Hyperhomocysteinemia Causes Hippocampal Alterations, Metabolomics Plasma Changes and Behavioral Pattern in Wild Type Rats. International Journal of Molecular Sciences, 22(9), 4961. https://doi.org/10.3390/ijms22094961






