The Contribution of Small Vessel Disease to Neurodegeneration: Focus on Alzheimer’s Disease, Parkinson’s Disease and Multiple Sclerosis
Abstract
1. Introduction
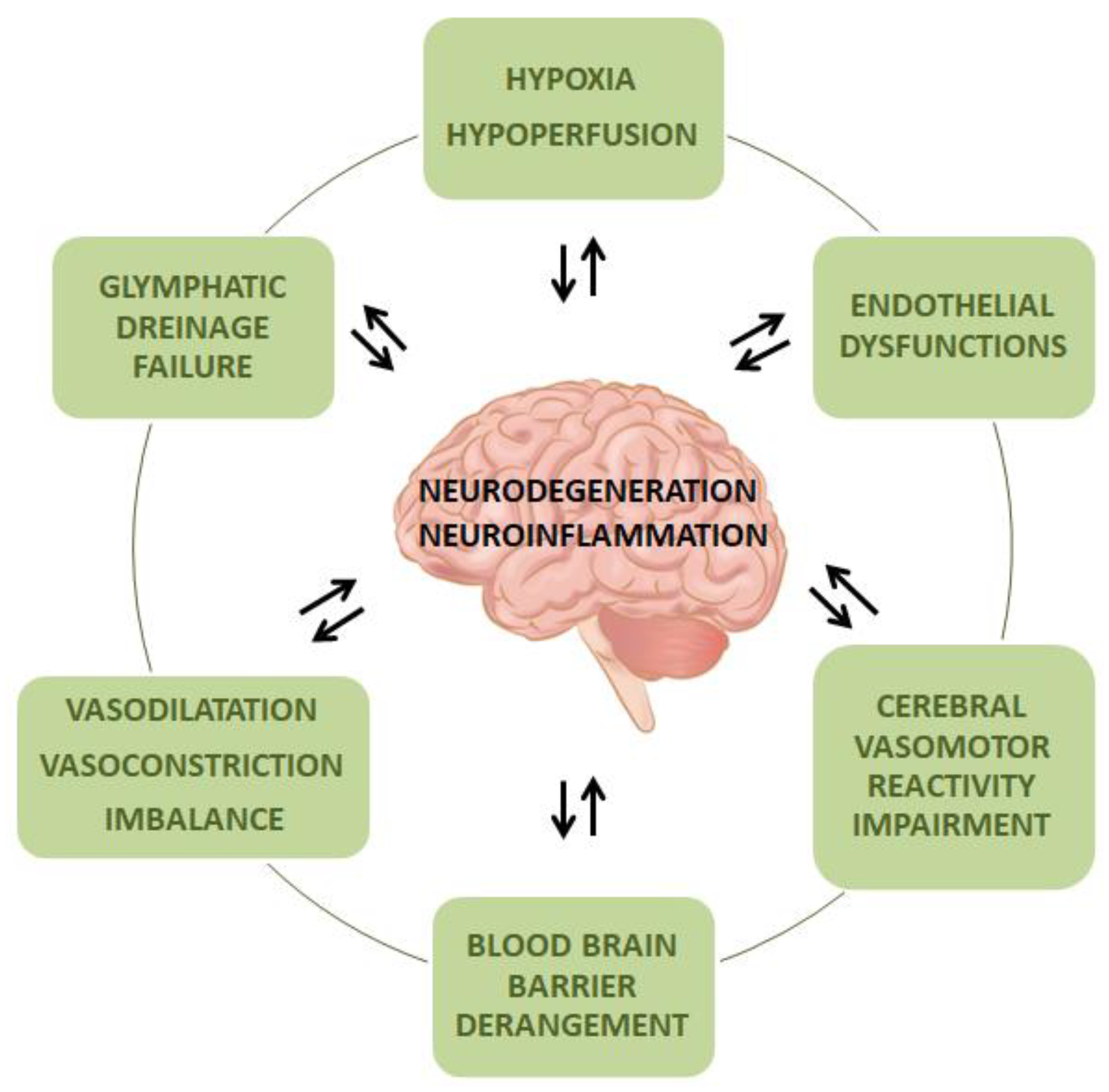
2. Common Molecular Mechanisms and Pathophysiological Aspects of Small Vessel Disease and Neurodegeneration
3. Role of Small Vessel Disease in Alzheimer’s Disease
4. Clinical Impact of Small Vessel Disease in Parkinson’s Disease
5. Small Vessel Disease as A Potential Contributor to Neurodegeneration in Multiple Sclerosis
6. Small Vessel Disease in the Spectrum of Frontotemporal Lobar Degeneration
7. Biomarkers for Small Vessel Disease: Beyond Neuroimaging
8. SVD as a Target for Possible Therapeutic Approaches
9. Concluding Remarks
Funding
Conflicts of Interest
References
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Charidimou, A.; Pantoni, L.; Love, S. The concept of sporadic cerebral small vessel disease: A road map on key definitions and current concepts. Int. J. Stroke 2016, 11, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, I.; Bianchi, S.; De Stefano, N.; Dichgans, M.; Dotti, M.T.; Duering, M.; Jouvent, E.; Korczyn, A.D.; Lesnik-Oberstein, S.A.J.; Malandrini, A.; et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: Update on clinical, diagnostic, and management aspects. BMC Med. 2017, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Skrobot, O.A.; Black, S.E.; Chen, C.; DeCarli, C.; Erkinjuntti, T.; Ford, G.A.; Kalaria, R.N.; O’Brien, J.; Pantoni, L.; Pasquier, F.; et al. Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimer’s Dement. 2018, 14, 280–292. [Google Scholar] [CrossRef]
- Debette, S.; Schilling, S.; Duperron, M.G.; Larsson, S.C.; Markus, H.S. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Przedborski, S. Neurodegeneration. In Neuroimmune Pharmacology; Springer: Cham, Switzerland, 2016; Volume 6, pp. 28–40. ISBN 9783319440224. [Google Scholar]
- Dichgans, M.; Wardlaw, J.; Smith, E.; Zietemann, V.; Seshadri, S.; Sachdev, P.; Biessels, G.J.; Fazekas, F.; Benavente, O.; Pantoni, L.; et al. METACOHORTS for the study of vascular disease and its contribution to cognitive decline and neurodegeneration: An initiative of the Joint Programme for Neurodegenerative Disease Research. Alzheimer’s Dement. 2016, 12, 1235–1249. [Google Scholar]
- Soto, C.; Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1332–1340. [Google Scholar] [CrossRef]
- Wang, X.; Xing, A.; Xu, C.; Cai, Q.; Liu, H.; Li, L. Cerebrovascular hypoperfusion induces spatial memory impairment, synaptic changes, and amyloid-β oligomerization in rats. J. Alzheimer’s Dis. 2010, 21, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Krajcer, W.; Kozniewska, E.; Lazarewicz, J.W.; Ksiezak-Reding, H. Differential changes in phosphorylation of tau at PHF-1 and 12E8 epitopes during brain ischemia and reperfusion in gerbils. Neurochem. Res. 2007, 32, 729–737. [Google Scholar] [CrossRef]
- Guglielmotto, M.; Aragno, M.; Autelli, R.; Giliberto, L.; Novo, E.; Colombatto, S.; Danni, O.; Parola, M.; Smith, M.A.; Perry, G.; et al. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: Role of oxidative stress and HIF1α. J. Neurochem. 2009, 108, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, D.; Zhang, X.; Li, T.; Li, J.; Tang, Y.; Le, W. Hypoxia-induced Down-regulation of Neprilysin by histone modification in mouse primary cortical and Hippocampal neurons. PLoS ONE 2011, 6, e19229. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, L.F.; Meng, F.T.; Du, X.; Zhou, J.N. Acute hypoxia promote the phosphorylation of tau via ERK pathway. Neurosci. Lett. 2010, 474, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kahl, A.; Blanco, I.; Jackman, K.; Baskar, J.; Milaganur Mohan, H.; Rodney-Sandy, R.; Zhang, S.; Iadecola, C.; Hochrainer, K. Cerebral ischemia induces the aggregation of proteins linked to neurodegenerative diseases. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Desai, R.A.; Davies, A.L.; Tachrount, M.; Kasti, M.; Laulund, F.; Golay, X.; Smith, K.J. Cause and prevention of demyelination in a model multiple sclerosis lesion. Ann. Neurol. 2016, 79, 591–604. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Boycott, H.E.; Dallas, M.; Boyle, J.P.; Pearson, H.A.; Peers, C. Hypoxia suppresses astrocyte glutamate transport independently of amyloid formation. Biochem. Biophys. Res. Commun. 2007, 364, 100–104. [Google Scholar] [CrossRef]
- Lyros, E.; Bakogiannis, C.; Liu, Y.; Fassbender, K. Molecular Links Between Endothelial Dysfunction and Neurodegeneration in Alzheimer’s Disease. Curr. Alzheimer Res. 2014, 11, 18–26. [Google Scholar] [CrossRef]
- Koizumi, K.; Wang, G.; Park, L. Endothelial Dysfunction and Amyloid-β-Induced Neurovascular Alterations. Cell Mol. Neurobiol. 2016, 36, 155–165. [Google Scholar] [CrossRef]
- Guan, J.; Pavlovic, D.; Dalkie, N.; Waldvogel, H.J.; O’Carroll, S.J.; Green, C.R.; Nicholson, L.F.B. Vascular degeneration in parkinsons disease. Brain Pathol. 2013, 23, 154–164. [Google Scholar] [CrossRef]
- Sarkar, S.; Raymick, J.; Mann, D.; Bowyer, J.; Hanig, J.; Schmued, L.; Paule, M.; Chigurupati, S. Neurovascular Changes in Acute, sub-Acute and Chronic Mouse Models of Parkinson’s Disease. Curr. Neurovasc. Res. 2014, 11, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Smoliński, Ł.; Członkowska, A. Cerebral vasomotor reactivity in neurodegenerative diseases. Neurol. Neurochir. Pol. 2016, 50, 455–462. [Google Scholar] [CrossRef]
- Viticchi, G.; Falsetti, L.; Vernieri, F.; Altamura, C.; Bartolini, M.; Luzzi, S.; Provinciali, L.; Silvestrini, M. Vascular predictors of cognitive decline in patients with mild cognitive impairment. Neurobiol. Aging 2012, 33, 1127-e1. [Google Scholar] [CrossRef]
- Buratti, L.; Balestrini, S.; Altamura, C.; Viticchi, G.; Falsetti, L.; Luzzi, S.; Provinciali, L.; Vernieri, F.; Silvestrini, M. Markers for the Risk of Progression from Mild Cognitive Impairment to Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 883–890. [Google Scholar] [CrossRef]
- Camargo, C.H.F.; Martins, E.A.; Lange, M.C.; Hoffmann, H.A.; Luciano, J.J.; Young Blood, M.R.; Schafranski, M.D.; Ferro, M.M.; Miyoshi, E. Abnormal Cerebrovascular Reactivity in Patients with Parkinson’s Disease. Parkinsons Dis. 2015, 2015, 523041. [Google Scholar] [CrossRef] [PubMed]
- Uzuner, N.; Ozkan, S.; Cinar, N. Cerebrovascular reactivity in multiple sclerosis patients. Mult. Scler. 2007, 13, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Niwa, K.; Porter, V.A.; Kazama, K.E.N.; Cornfield, D.; Carlson, G.A.; Iadecola, C. Aβ-peptides enhance vasoconstriction in cerebral circulation. Am. J. Physiol. Hear. Circ. Physiol. 2001, 281, H2417–H2424. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. Cerebrovascular effects of amyloid-β peptides: Mechanisms and implications for Alzheimer’s dementia. Cell Mol. Neurobiol. 2003, 23, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, A.H.E.A.; Claassen, J.A.H.R. The cerebrovascular role of the cholinergic neural system in Alzheimer’s disease. Behav. Brain Res. 2011, 221, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.L.T.M.; Bohnen, N.I. Cholinergic dysfunction in parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2013, 13, 377. [Google Scholar] [CrossRef]
- Kooi, E.J.; Prins, M.; Bajic, N.; Beliën, J.A.M.; Gerritsen, W.H.; Van Horssen, J.; Aronica, E.; Van Dam, A.M.; Hoozemans, J.J.M.; Francis, P.T.; et al. Cholinergic imbalance in the multiple sclerosis hippocampus. Acta Neuropathol. 2011, 122, 313–322. [Google Scholar] [CrossRef]
- Palmer, J.C.; Barker, R.; Kehoe, P.G.; Love, S. Endothelin-1 is Elevated in Alzheimer’s Disease and Upregulated by Amyloid-β. J. Alzheimer’s Dis. 2012, 29, 853–861. [Google Scholar] [CrossRef] [PubMed]
- D’Haeseleer, M.; Beelen, R.; Fierens, Y.; Cambron, M.; Vanbinst, A.M.; Verborgh, C.; Demey, J.; De Keyser, J. Cerebral hypoperfusion in multiple sclerosis is reversible and mediated by endothelin-1. Proc. Natl. Acad. Sci. USA 2013, 110, 5654–5658. [Google Scholar] [CrossRef]
- Zhang, W.W.; Badonic, T.; Höög, A.; Jiang, M.H.; Ma, K.C.; Nie, X.J.; Olsson, Y. Astrocytes in Alzheimer’s disease express immunoreactivity to the vaso-constrictor endothelin-1. J. Neurol. Sci. 1994, 122, 90–96. [Google Scholar] [CrossRef]
- Haufschild, T.; Shaw, S.G.; Kesselring, J.; Flammer, J. Increased Endothelin-1 Plasma Levels in Patients With Multiple Sclerosis. J. Neuro Ophthalmol. 2001, 21, 37–38. [Google Scholar] [CrossRef][Green Version]
- Speciale, L.; Sarasella, M.; Ruzzante, S.; Caputo, D.; Mancuso, R.; Calvo, M.G.; Guerini, F.R.; Ferrante, P. Endothelin and nitric oxide levels in cerebrospinal fluid of patients with multiple sclerosis. Proc. J. Neuro Virol. 2000, 6, S62. [Google Scholar]
- Thal, D.R. The precapillary segment of the blood-brain barrier and its relation to perivascular drainage in Alzheimer’s disease and small vessel disease. Sci. World J. 2009, 9, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Wu, Z.; Zlokovic, B.V. RAGE (Yin) versus LRP (Yang) balance regulates Alzheimer amyloid β-peptide clearance through transport across the blood-brain barrier. Stroke 2004, 35, 2628–2631. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Sagare, A.; Hamm, K.; Parisi, M.; Lane, S.; Finn, M.B.; Holtzman, D.M.; Zlokovic, B.V. apoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Investig. 2008, 118, 4002–4013. [Google Scholar] [CrossRef]
- Utter, S.; Tamboli, I.Y.; Walter, J.; Upadhaya, A.R.; Birkenmeier, G.; Pietrzik, C.U.; Ghebremedhin, E.; Thal, D.R. Cerebral small vessel disease-induced apolipoprotein e leakage is associated with alzheimer disease and the accumulation of amyloid β-protein in perivascular astrocytes. J. Neuropathol. Exp. Neurol. 2008, 67, 842–856. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Herz, J.; Bu, G. Apolipoprotein E and apolipoprotein E receptors: Normal biology and roles in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006312. [Google Scholar] [CrossRef] [PubMed]
- Yip, A.G.; McKee, A.C.; Green, R.C.; Wells, J.; Young, H.; Cupples, L.A.; Farrer, L.A. APOE, vascular pathology, and the AD brain. Neurology 2005, 65, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Attems, J.; Jellinger, K.A. The overlap between vascular disease and Alzheimer’s disease—Lessons from pathology. BMC Med. 2014, 12, 1–12. [Google Scholar] [CrossRef]
- Soldan, A.; Pettigrew, C.; Zhu, Y.; Wang, M.C.; Moghekar, A.; Gottesman, R.F.; Singh, B.; Martinez, O.; Fletcher, E.; Decarli, C.; et al. White matter hyperintensities and CSF Alzheimer disease biomarkers in preclinical Alzheimer disease. Neurology 2020, 94, e950–e960. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H.; Ecke, G.M.; Bettin, S.; Dietrich, J.; Gertz, H.J. Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. Int. J. Geriatr. Psychiatry 2000, 15, 803–812. [Google Scholar] [CrossRef]
- Debette, S.; Bombois, S.; Bruandet, A.; Delbeuck, X.; Lepoittevin, S.; Delmaire, C.; Leys, D.; Pasquier, F. Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke 2007, 38, 2924–2930. [Google Scholar] [CrossRef]
- Smith, E.E.; Egorova, S.; Blacker, D.; Killiany, R.J.; Muzikansky, A.; Dickerson, B.C.; Tanzi, R.E.; Albert, M.S.; Greenberg, S.M.; Guttmann, C.R.G. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch. Neurol. 2008, 65, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Staekenborg, S.S.; Koedam, E.L.G.E.; Henneman, W.J.P.; Stokman, P.; Barkhof, F.; Scheltens, P.; Van Der Flier, W.M. Progression of mild cognitive impairment to dementia contribution of cerebrovascular disease compared with medial temporal lobe atrophy. Stroke 2009, 40, 1269–1274. [Google Scholar] [CrossRef]
- Carmichael, O.; Schwarz, C.; Drucker, D.; Fletcher, E.; Harvey, D.; Beckett, L.; Jack, C.R.; Weiner, M.; DeCarli, C. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch. Neurol. 2010, 67, 1370–1378. [Google Scholar] [CrossRef]
- Esiri, M.M.; Joachim, C.; Sloan, C.; Christie, S.; Agacinski, G.; Bridges, L.R.; Wilcock, G.K.; Smith, A.D. Cerebral subcortical small vessel disease in subjects with pathologically confirmed alzheimer disease: A clinicopathologic study in the oxford project to investigate memory and ageing (OPTIMA). Alzheimer Dis. Assoc. Disord. 2014, 28, 30–35. [Google Scholar] [CrossRef]
- Ortner, M.; Kurz, A.; Alexopoulos, P.; Auer, F.; Diehl-Schmid, J.; Drzezga, A.; Förster, S.; Förstl, H.; Perneczky, R.; Sorg, C.; et al. Small vessel disease, but neither amyloid load nor metabolic deficit, is dependent on age at onset in Alzheimer’s disease. Proc. Biol. Psychiatry 2015, 77, 704–710. [Google Scholar] [CrossRef]
- Stefaniak, J.D.; Su, L.; Mak, E.; Sheikh-Bahaei, N.; Wells, K.; Ritchie, K.; Waldman, A.; Ritchie, C.W.; O’Brien, J.T. Cerebral small vessel disease in middle age and genetic predisposition to late-onset Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 253–258. [Google Scholar] [CrossRef]
- Raz, N.; Yang, Y.; Dahle, C.L.; Land, S. Volume of white matter hyperintensities in healthy adults: Contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 361–369. [Google Scholar] [CrossRef]
- Groot, C.; Sudre, C.H.; Barkhof, F.; Teunissen, C.E.; van Berckel, B.N.M.; Seo, S.W.; Ourselin, S.; Scheltens, P.; Cardoso, M.J.; van der Flier, W.M.; et al. Clinical phenotype, atrophy, and small vessel disease in APOE ε2 carriers with Alzheimer disease. Neurology 2019, 91, e1851–e1859. [Google Scholar] [CrossRef]
- Ferreira, D.; Shams, S.; Cavallin, L.; Viitanen, M.; Martola, J.; Granberg, T.; Shams, M.; Aspelin, P.; Kristoffersen-Wiberg, M.; Nordberg, A.; et al. The contribution of small vessel disease to subtypes of Alzheimer’s disease: A study on cerebrospinal fluid and imaging biomarkers. Neurobiol. Aging 2018, 70, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Vizcarra, J.A.; Lang, A.E.; Sethi, K.D.; Espay, A.J. Vascular Parkinsonism: Deconstructing a Syndrome. Mov. Disord. 2015, 30, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Rektor, I.; Bohnen, N.I.; Korczyn, A.D.; Gryb, V.; Kumar, H.; Kramberger, M.G.; de Leeuw, F.E.; Pirtošek, Z.; Rektorová, I.; Schlesinger, I.; et al. An updated diagnostic approach to subtype definition of vascular parkinsonism—Recommendations from an expert working group. Park Relat. Disord. 2018, 49, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zijlmans, J.C.M.; Daniel, S.E.; Hughes, A.J.; Révész, T.; Lees, A.J. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov. Disord. 2004, 19, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Zijlmans, J.C.M.; Katzenschlager, R.; Daniel, S.E.; Lees, A.J.L. The L-dopa response in vascular parkinsonism. J. Neurol Neurosurg. Psychiatry 2004, 75, 545–547. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Fitzgerald, P.M.; Jankovic, J. Lower body parkinsonism: Evidence for vascular etiology. Mov. Disord. 1989, 4, 249–260. [Google Scholar] [CrossRef]
- Antonini, A.; Vitale, C.; Barone, P.; Cilia, R.; Righini, A.; Bonuccelli, U.; Abbruzzese, G.; Ramat, S.; Petrone, A.; Quatrale, R.; et al. The relationship between cerebral vascular disease and parkinsonism: The VADO study. Park Relat. Disord. 2012, 18, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.T.M.; Szewczyk-Królikowski, K.; Tomlinson, P.; Nithi, K.; Rolinski, M.; Murray, C.; Talbot, K.; Ebmeier, K.P.; Mackay, C.E.; Ben-Shlomo, Y. Predictors of cognitive impairment in an early stage Parkinson’s disease cohort. Mov. Disord. 2014, 29, 351–359. [Google Scholar] [CrossRef]
- Malek, N.; Lawton, M.A.; Swallow, D.M.A.; Grosset, K.A.; Marrinan, S.L.; Bajaj, N.; Barker, R.A.; Burn, D.J.; Hardy, J.; Morris, H.R.; et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease. Mov. Disord. 2016, 31, 1518–1526. [Google Scholar] [CrossRef]
- Beyer, M.K.; Aarsland, D.; Greve, O.J.; Larsen, J.P. Visual rating of white matter hyperintensities in Parkinson’s disease. Mov. Disord. 2006, 21, 223–229. [Google Scholar] [CrossRef]
- Veselý, B.; Rektor, I. The contribution of white matter lesions (WML) to Parkinson’s disease cognitive impairment symptoms: A critical review of the literature. Park Relat. Disord. 2016, 22, S166–S170. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Sugiura, M.; Nishimura, Y.; Sakura, H. The effect of small vessel disease on motor and cognitive function in Parkinson’s disease. Clin. Neurol. Neurosurg. 2019, 182, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Kotagal, V.; Albin, R.L.; Müller, M.L.T.M.; Koeppe, R.A.; Frey, K.A.; Bohnen, N.I. Diabetes is associated with postural instability and gait difficulty in Parkinson disease. Park Relat. Disord. 2013, 19, 522–526. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Müller, M.L.T.M.; Zarzhevsky, N.; Koeppe, R.A.; Bogan, C.W.; Kilbourn, M.R.; Frey, K.A.; Albin, R.L. Leucoaraiosis, nigrostriatal denervation and motor symptoms in Parkinson’s disease. Brain 2011, 134, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Kotagal, V.; Albin, R.L.; Müller, M.L.T.M.; Koeppe, R.A.; Frey, K.A.; Bohnen, N.I. Modifiable cardiovascular risk factors and axial motor impairments in Parkinson disease. Neurology 2014, 82, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Liu, G.; Wang, X.; Wang, Z.; Ma, H.; Pan, Y.; Wang, D.; Wang, Y.; Feng, T. Effect of small vessel disease burden and lacunes on gait/posture impairment in Parkinson’s disease. Neurol. Sci. 2020, 41, 3617–3624. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis Prim. 2018. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Barkhof, F. The clinico-radiological paradox in multiple sclerosis revisited. Curr. Opin. Neurol. 2002, 15, 239–245. [Google Scholar] [CrossRef]
- Marrie, R.A.; Rudick, R.; Horwitz, R.; Cutter, G.; Tyry, T.; Campagnolo, D.; Vollmer, T. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology 2010, 74, 1041–1047. [Google Scholar] [CrossRef]
- Pichler, A.; Khalil, M.; Langkammer, C.; Pinter, D.; Ropele, S.; Fuchs, S.; Bachmaier, G.; Enzinger, C.; Fazekas, F. The impact of vascular risk factors on brain volume and lesion load in patients with early multiple sclerosis. Mult. Scler. J. 2019, 25, 48–54. [Google Scholar] [CrossRef]
- Geraldes, R.; Esiri, M.M.; Perera, R.; Yee, S.A.; Jenkins, D.; Palace, J.; Deluca, G.C. Vascular disease and multiple sclerosis: A post-mortem study exploring their relationships. Brain 2020, 143, 2998–3012. [Google Scholar] [CrossRef]
- D’Haeseleer, M.; Hostenbach, S.; Peeters, I.; El Sankari, S.; Nagels, G.; De Keyser, J.; D’Hooghe, M.B. Cerebral hypoperfusion: A new pathophysiologic concept in multiple sclerosis? J. Cereb. Blood Flow Metab. 2015, 35, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Sosa, S.M.; Smith, K.J. Understanding a role for hypoxia in lesion formation and location in the deep and periventricular white matter in small vessel disease and multiple sclerosis. Clin. Sci. 2017, 131, 2503–2524. [Google Scholar] [CrossRef] [PubMed]
- Seelaar, H.; Rohrer, J.D.; Pijnenburg, Y.A.L.; Fox, N.C.; Van Swieten, J.C. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: A review. J. Neurol. Neurosurg. Psychiatry 2011, 82, 476–486. [Google Scholar] [CrossRef]
- Thal, D.R.; von Arnim, C.A.F.; Griffin, W.S.T.; Mrak, R.E.; Walker, L.; Attems, J.; Arzberger, T. Frontotemporal lobar degeneration FTLD-tau: Preclinical lesions, vascular, and Alzheimer-related co-pathologies. J. Neural Transm. 2015, 122, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- De Guio, F.; Jouvent, E.; Biessels, G.J.; Black, S.E.; Brayne, C.; Chen, C.; Cordonnier, C.; De Leeuw, F.E.; Dichgans, M.; Doubal, F.; et al. Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. J. Cereb. Blood Flow Metab. 2016, 36, 1319–1337. [Google Scholar] [CrossRef]
- Fredman, P.; Wallin, A.; Blennow, K.; Davidsson, P.; Gottfries, C.; Svennerholm, L. Sulfatide as a biochemical marker in cerebrospinal fluid of patients with vascular dementia. Acta Neurol. Scand. 1992, 85, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Tullberg, M.; Månsson, J.E.; Fredman, P.; Lekman, A.; Blennow, K.; Ekman, R.; Rosengren, L.E.; Tisell, M.; Wikkelso, C. CSF sulfatide distinguishes between normal pressure hydrocephalus and subcortical arteriosclerotic encephalopathy. J. Neurol Neurosurg. Psychiatry 2000, 69, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, M.; Zetterberg, H.; Edman, Å.; Blennow, K.; Wallin, A.; Andreasson, U. Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 27, 665–676. [Google Scholar] [CrossRef]
- Jonsson, M.; Zetterberg, H.; Van Straaten, E.; Lind, K.; Syversen, S.; Edman, Å.; Blennow, K.; Rosengren, L.; Pantoni, L.; Inzitari, D.; et al. Cerebrospinal fluid biomarkers of white matter lesions—Cross-sectional results from the LADIS study. Eur. J. Neurol. 2010, 17, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, M.; Andreasson, U.; Rolstad, S.; Nordlund, A.; Lind, K.; Zetterberg, H.; Edman, Å.; Blennow, K.; Wallin, A. Subcortical vascular dementia biomarker pattern in mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009, 28, 348–356. [Google Scholar] [CrossRef]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol Neurosurg. Psychiatry 2019, 90, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Öhrfelt, A.; Andreasson, U.; Simon, A.; Zetterberg, H.; Edman, Å.; Potter, W.; Holder, D.; Devanarayan, V.; Seeburger, J.; Smith, A.D.; et al. Screening for New Biomarkers for Subcortical Vascular Dementia and Alzheimer’s Disease. Dement. Geriatr Cogn Dis Extra 2011, 1, 31–42. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Chu, K.; Ko, S.Y.; Lee, S.T.; Sinn, D.I.; Park, D.K.; Kim, J.M.; Song, E.C.; Kim, M.; Roh, J.K. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke 2006, 37, 2744–2750. [Google Scholar] [CrossRef]
- Terpolilli, N.A.; Kim, S.W.; Thal, S.C.; Kataoka, H.; Zeisig, V.; Nitzsche, B.; Klaesner, B.; Zhu, C.; Schwarzmaier, S.; Meissner, L.; et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ. Res. 2012, 110, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Garry, P.S.; Ezra, M.; Rowland, M.J.; Westbrook, J.; Pattinson, K.T.S. The role of the nitric oxide pathway in brain injury and its treatment—From bench to bedside. Exp. Neurol. 2015, 263, 235–243. [Google Scholar] [CrossRef]
- Liu, B.; Gao, H.M.; Wang, J.Y.; Jeohn, G.H.; Cooper, C.L.; Hong, J.S. Role of nitric oxide in inflammation-mediated neurodegeneration. Proc. Ann. N. Y. Acad. Sci. 2002, 962, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Spuch, C.; Antequera, D.; Portero, A.; Orive, G.; Hernández, R.M.; Molina, J.A.; Bermejo-Pareja, F.; Pedraz, J.L.; Carro, E. The effect of encapsulated VEGF-secreting cells on brain amyloid load and behavioral impairment in a mouse model of Alzheimer’s disease. Biomaterials 2010, 31, 5608–5618. [Google Scholar] [CrossRef]
- Storkebaum, E.; Lambrechts, D.; Dewerchin, M.; Moreno-Murciano, M.P.; Appelmans, S.; Oh, H.; Van Damme, P.; Rutten, B.; Man, W.Y.; De Mol, M.; et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat. Neurosci. 2005, 8, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, M.; Ralph, G.S.; Storkebaum, E.; Walmsley, L.E.; Mitrophanous, K.A.; Kingsman, S.M.; Carmellet, P.; Mazarakis, N.D. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature 2004, 429, 413–417. [Google Scholar] [CrossRef]
| Pathophysiological Mechanisms | CSF Biomarkers | Patients | Main Findings |
|---|---|---|---|
| Demyelination and re-myelination processes | sulfatide | VaD vs. AD and HC VaD vs. NPH | ↑ in VaD ↑ in VaD |
| MBP | VaD vs. HC | ↑ in VaD | |
| Axonal damage | NfL | SVD | ↑ in SVD ↑ in MCI, predicting VaD |
| Vascular remodeling | AAT | SVD and MD vs. AD | ↑ in SVD and MD |
| PAI-1 | SVD and MD vs. AD | ↑ in SVD and MD | |
| TIMP-1 | SVD and MD vs. AD | ↑ in SVD and MD | |
| ApoH | SVD and MD vs. AD | ↑ in SVD and MD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolini Paoletti, F.; Simoni, S.; Parnetti, L.; Gaetani, L. The Contribution of Small Vessel Disease to Neurodegeneration: Focus on Alzheimer’s Disease, Parkinson’s Disease and Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 4958. https://doi.org/10.3390/ijms22094958
Paolini Paoletti F, Simoni S, Parnetti L, Gaetani L. The Contribution of Small Vessel Disease to Neurodegeneration: Focus on Alzheimer’s Disease, Parkinson’s Disease and Multiple Sclerosis. International Journal of Molecular Sciences. 2021; 22(9):4958. https://doi.org/10.3390/ijms22094958
Chicago/Turabian StylePaolini Paoletti, Federico, Simone Simoni, Lucilla Parnetti, and Lorenzo Gaetani. 2021. "The Contribution of Small Vessel Disease to Neurodegeneration: Focus on Alzheimer’s Disease, Parkinson’s Disease and Multiple Sclerosis" International Journal of Molecular Sciences 22, no. 9: 4958. https://doi.org/10.3390/ijms22094958
APA StylePaolini Paoletti, F., Simoni, S., Parnetti, L., & Gaetani, L. (2021). The Contribution of Small Vessel Disease to Neurodegeneration: Focus on Alzheimer’s Disease, Parkinson’s Disease and Multiple Sclerosis. International Journal of Molecular Sciences, 22(9), 4958. https://doi.org/10.3390/ijms22094958







