The Endocannabinoid System in the Mediterranean Mussel Mytilus galloprovincialis: Possible Mediators of the Immune Activity?
Abstract
1. Introduction
2. Results
2.1. Characterization of the ECS in Mussel Hemocytes
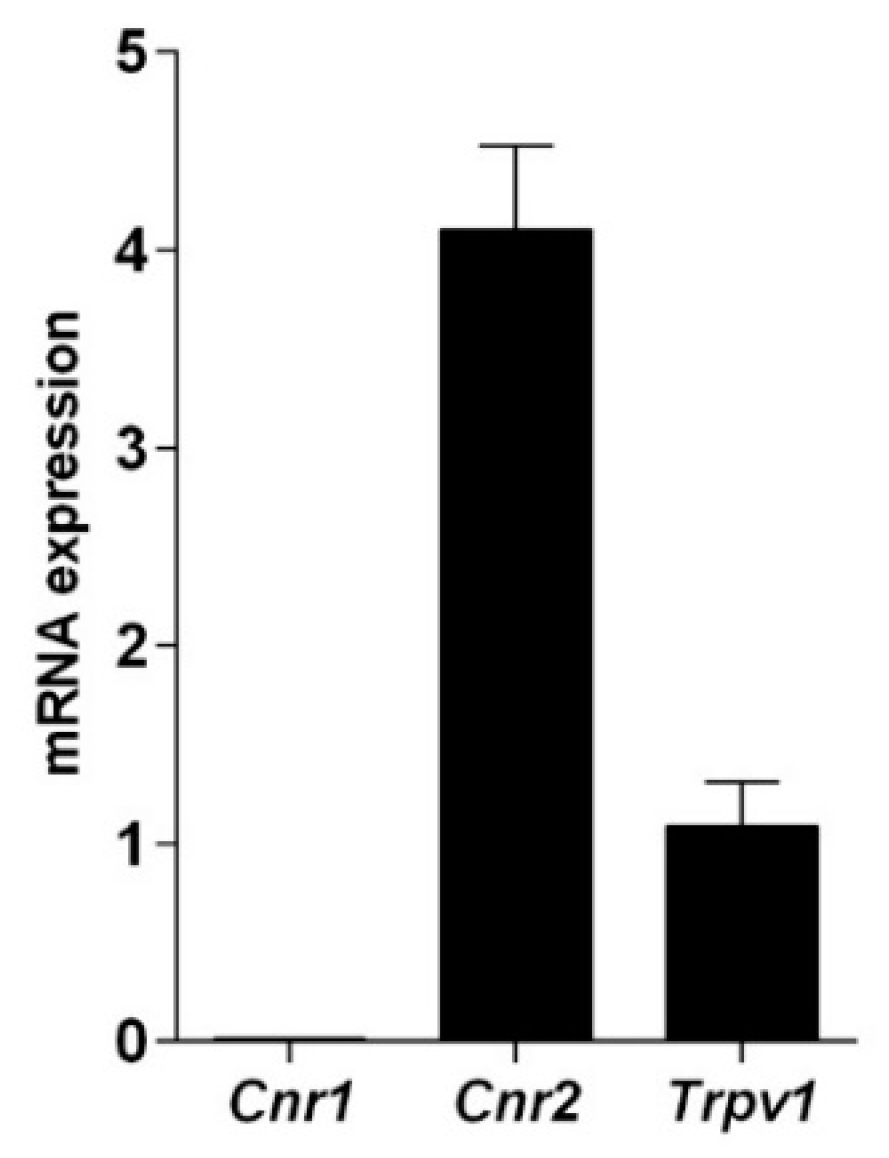
2.2. Cannabinoid and Vanilloid Receptors in Mussel Hemocytes
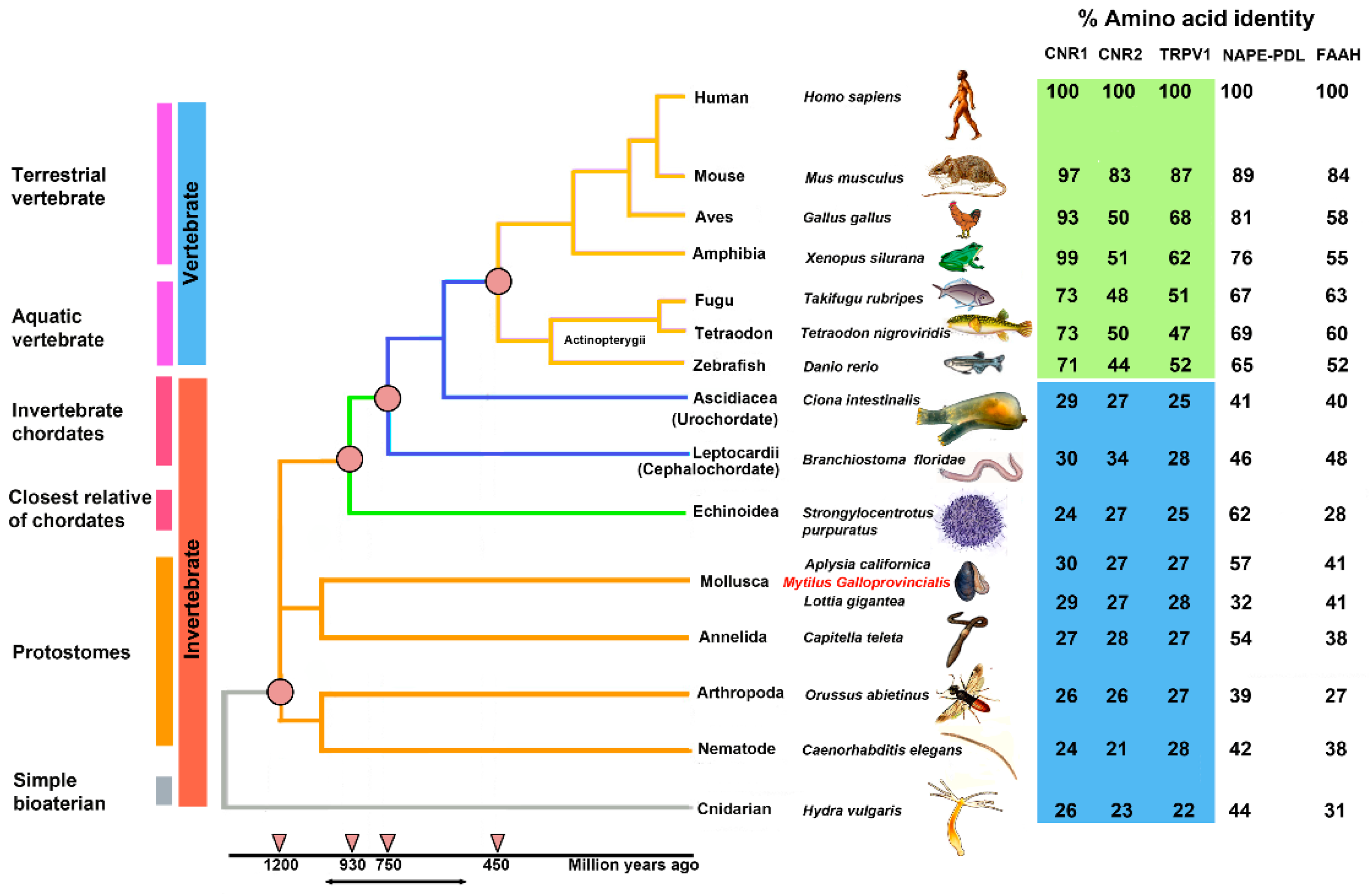
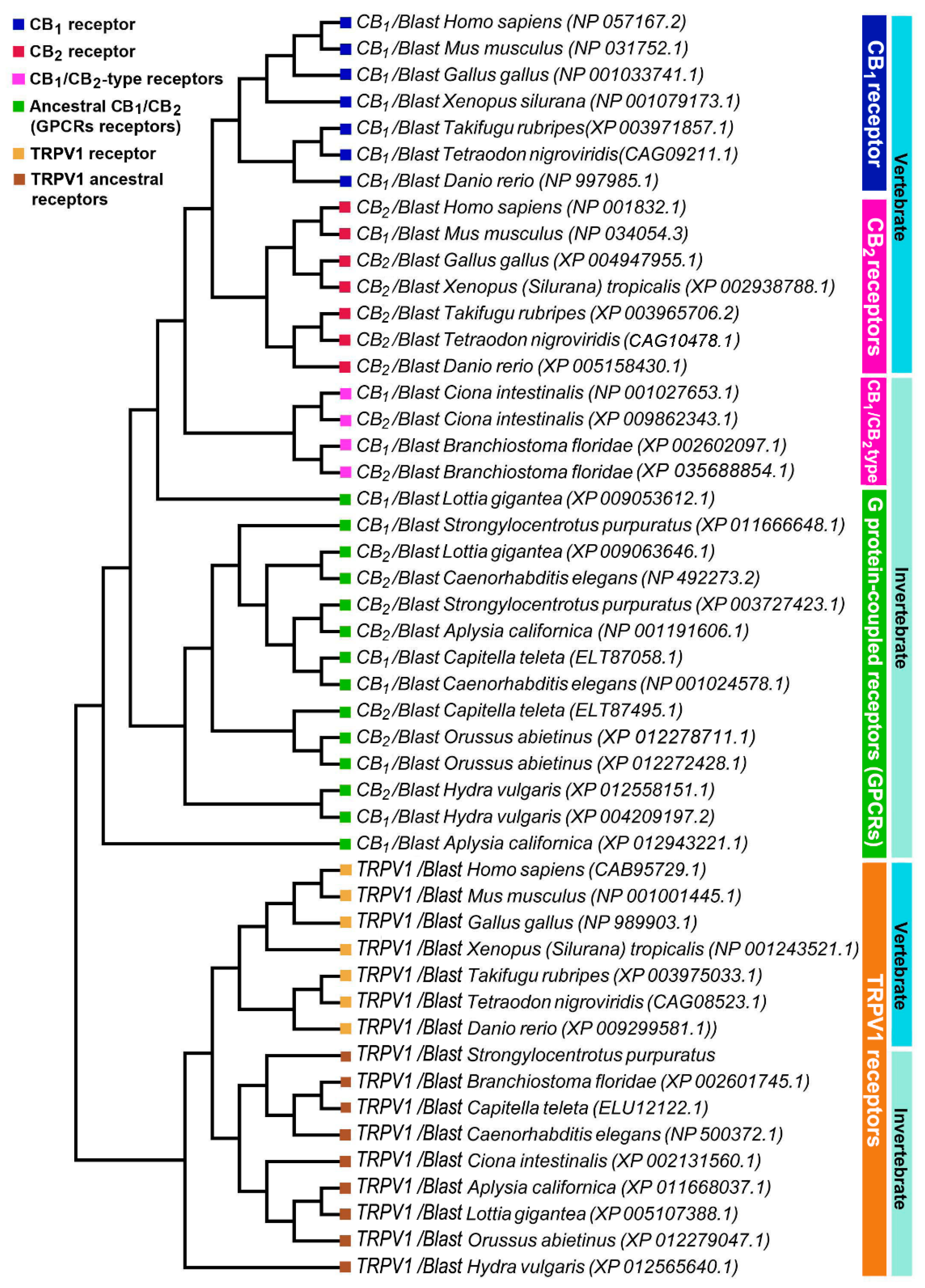
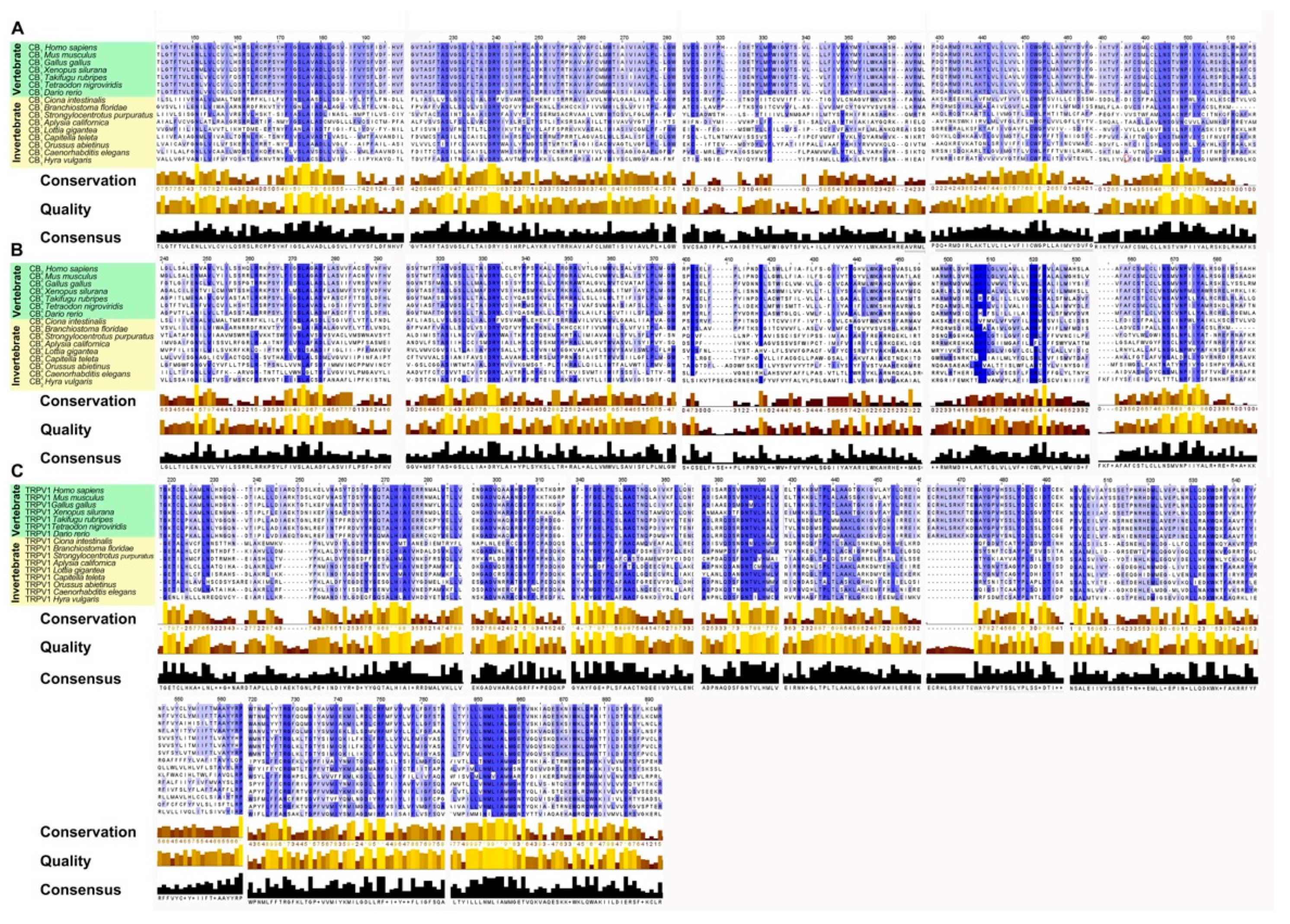
2.3. Phylogenetic Study of ECS
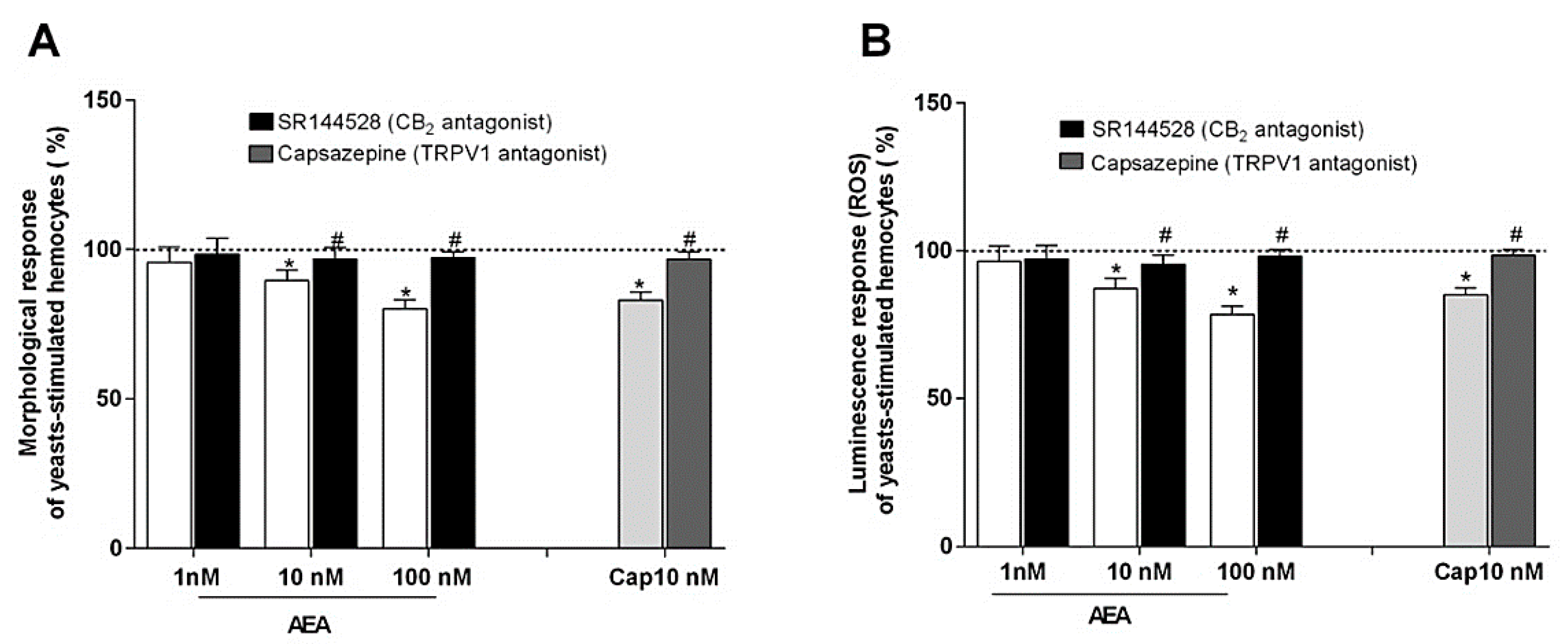
2.4. Effect of AEA on Phagocytosis Assay
3. Discussion
4. Materials and Methods
4.1. Samples Collection
4.2. Chemicals
4.3. Endogenous Levels of AEA, 2-AG and PEA
4.4. Endocannabinoid Metabolism
4.5. mRNA Expression of CB1, CB2 and TRPV1
| Gene | Forward (5′→3′) | Reverse (3′→5′) |
| Cnr1 | CCTTTTGCTGCCTAAATCCAC | CCACTGCTCAAACATCTGAC |
| Cnr2 | TCAACCCTGTCATCTATGCTC | AGTCAGTCCCAACACTCATC |
| Trpv1 | TCACCTACATCCTCCTGCTC | AAGTTCTTCCAGTGTCTGCC |
| β-actin | TGACCCAGATCATGTTTGAG | TTAATGTCACGCACGATTTCC |
4.6. Receptor Binding Assays
4.7. Phylogenetic Study
4.8. Phagocytosis Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Iannotti, F.A.; Di Marzo, V. The gut microbiome, endocannabinoids and metabolic disorders. J. Endocrinol. 2021, 248, R83–R97. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef]
- Solorzano, C.; Zhu, C.; Battista, N.; Astarita, G.; Lodola, A.; Rivara, S.; Mor, M.; Russo, R.; Maccarrone, M.; Antonietti, F.; et al. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc. Natl. Acad. Sci. USA 2009, 106, 20966–20971. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M. Missing Pieces to the Endocannabinoid Puzzle. Trends Mol. Med. 2020, 26, 263–272. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Matias, I.; Di Marzo, V.; Glass, M. Evolutionary origins of the endocannabinoid system. Gene 2006, 370, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Battista, N.; Meccariello, R.; Cobellis, G.; Fasano, S.; Di Tommaso, M.; Pirazzi, V.; Konje, J.C.; Pierantoni, R.; Maccarrone, M. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol. Cell. Endocrinol. 2012, 355, 1–14. [Google Scholar] [CrossRef]
- Elphick, M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3201–3215. [Google Scholar] [CrossRef]
- Clarke, T.; Johnson, R.; Simone, J.; Carlone, R. The Endocannabinoid System and Invertebrate Neurodevelopment and Regeneration. Int. J. Mol. Sci. 2021, 22, 2103. [Google Scholar] [CrossRef]
- Pacioni, G.; Rapino, C.; Zarivi, O.; Falconi, A.; Leonardi, M.; Battista, N.; Colafarina, S.; Sergi, M.; Bonfigli, A.; Miranda, M.; et al. Truffles contain endocannabinoid metabolic enzymes and anandamide. Phytochemistry 2015, 110, 104–110. [Google Scholar] [CrossRef]
- Salzet, M.; Breton, C.; Bisogno, T.; Di Marzo, V. Comparative biology of the endocannabinoid system possible role in the im-mune response. Eur. J. Biochem. 2000, 267, 4917–4927. [Google Scholar] [CrossRef]
- Salzet, M.; Stefano, G. The endocannabinoid system in invertebrates. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 353–361. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Melck, D.; Bisogno, T.; Milone, A.; Di Marzo, V. Finding of the endocannabinoid signalling system in Hydra, a very primitive organism: Possible role in the feeding response. Neuroscience 1999, 92, 377–387. [Google Scholar] [CrossRef]
- Elphick, M.R.; Satou, Y.; Satoh, N. The invertebrate ancestry of endocannabinoid signalling: An orthologue of vertebrate cannabinoid receptors in the urochordate Ciona intestinalis. Gene 2003, 302, 95–101. [Google Scholar] [CrossRef]
- Schuel, H.; Goldstein, E.; Mechoulam, R.; Zimmerman, A.M.; Zimmerman, S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc. Natl. Acad. Sci. USA 1994, 91, 7678–7682. [Google Scholar] [CrossRef]
- Matias, I.; Bisogno, T.; Melck, D.; Vandenbulcke, F.; Verger-Bocquet, M.; De Petrocellis, L.; Sergheraert, C.; Breton, C.; Di Marzo, V.; Salzet, M. Evidence for an endocannabinoid system in the central nervous system of the leech Hirudo medicinalis. Mol. Brain Res. 2001, 87, 145–159. [Google Scholar] [CrossRef]
- Stefano, G.B.; Liu, Y.; Goligorsky, M.S. Cannabinoid Receptors Are Coupled to Nitric Oxide Release in Invertebrate Immunocytes, Microglia, and Human Monocytes. J. Biol. Chem. 1996, 271, 19238–19242. [Google Scholar] [CrossRef]
- Sepe, N.; De Petrocellis, L.; Montanaro, F.; Cimino, G.; Di Marzo, V. Bioactive long chain N-acylethanolamines in five species of edible bivalve molluscs: Possible implications for mollusk physiology and sea food industry. Biochim. Biophys. Acta 1998, 1389, 101–111. [Google Scholar] [CrossRef]
- Sunada, H.; Watanabe, T.; Hatakeyama, D.; Lee, S.; Forest, J.; Sakakibara, M.; Ito, E.; Lukowiak, K. Pharmacological effects of cannabinoids on learning and memory in Lymnaea. J. Exp. Biol. 2017, 220, 3026–3038. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Abood, M.E. CB1 and CB2 Receptor Pharmacology. Rapid Acting Antidepressants 2017, 80, 169–206. [Google Scholar] [CrossRef]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef] [PubMed]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef] [PubMed]
- Tiscar, P.; Mosca, F. Defense mechanisms in farmed marine molluscs. Vet. Res. Commun. 2004, 28, 57–62. [Google Scholar] [CrossRef]
- Bouallegui, Y. Immunity in mussels: An overview of molecular components and mechanisms with a focus on the functional defenses. Fish Shellfish. Immunol. 2019, 89, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Bilfinger, T.V.; Rialas, C.M.; Deutsch, D.G. 2-arachidonyl-glycerol stimulates nitric oxide release from human immune and vascular tissues and invertebrate immunocytes by cannabinoid receptor 1. Pharmacol. Res. 2000, 42, 317–322. [Google Scholar] [CrossRef]
- McPartland, J.M. Phylogenomic and chemotaxonomix analysis of the endocannabinoid system. Brain Res. Brain Res. Rev. 2004, 45, 18–29. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833–855. [Google Scholar]
- Lourenço, C.R.; Nicastro, K.; Serrao, E.A.; Castilho, R.; Zardi, G.I. Behind the mask: Cryptic genetic diversity of Mytilus gallo-provincialis along southern European and northern African shores. J. Molluscan Stud. 2015, 81, 1–8. [Google Scholar] [CrossRef][Green Version]
- Oláh, A.; Szekanecz, Z.; Bíró, T. Targeting cannabinoid signaling in the immune system: “High”-ly exciting questions, possi-bilities, and challenges. Front. Immunol. 2017, 8, 1487. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Sacerdote, P.; Massi, P.; E Panerai, A.; Parolaro, D. In vivo and in vitro treatment with the synthetic cannabinoid CP55,940 decreases the in vitro migration of macrophages in the rat: Involvement of both CB1 and CB2 receptors. J. Neuroimmunol. 2000, 109, 155–163. [Google Scholar] [CrossRef]
- Raborn, E.S.; Marciano-Cabral, F.; Buckley, N.E.; Martin, B.R.; Cabral, G.A. The Cannabinoid Delta-9-tetrahydrocannabinol Mediates Inhibition of Macrophage Chemotaxis to RANTES/CCL5: Linkage to the CB2 Receptor. J. Neuroimmune Pharmacol. 2008, 3, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Djeu, J.Y.; Wang, M.; Friedman, H. Adverse effect of delta 9-tetrahydrocannabinol on human neutrophil function. Adv. Exp. Med. Biol. 1991, 288, 57–62. [Google Scholar] [PubMed]
- Kraft, B.; Wintersberger, W.; Kress, H.G. Cannabinoid receptor-independent suppression of the superoxide generation of human neutrophils (PMN) by CP55 940, but not by anandamide. Life Sci. 2004, 75, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.A.; Burstein, S.H. Receptor mediation in cannabinoid stimulated arachidonic acid mobilization and anandamide synthesis. Life Sci. 1997, 60, 1563–1573. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Chuai, S.; Shen, X. Phospholipase C and phosphatidylinositol 3-kinase signaling are involved in the exogenous arachidonic acid-stimulated respiratory burst in human neutrophils. J. Leukoc. Biol. 2003, 74, 428–437. [Google Scholar] [CrossRef]
- Kraft, B.; Kress, H.G. Indirect CB2 receptor and mediator-dependent stimulation of human whole-blood neutrophils by ex-ogenous and endogenous cannabinoids. J. Pharmacol. Exp. Ther. 2005, 315, 641–647. [Google Scholar] [CrossRef]
- Sulcova, E.; Mechoulam, R.; Fride, E. Biphasic effects of anandamide. Pharmacol. Biochem. Behav. 1998, 59, 347–352. [Google Scholar] [CrossRef]
- Croxford, J.L.; Yamamura, T. Cannabinoids and the immune system: Potential for the treatment of inflammatory diseases? J. Neuroimmunol. 2005, 166, 3–18. [Google Scholar] [CrossRef]
- Soethoudt, M.; Grether, U.; Fingerle, J.; Grim, T.W.; Fezza, F.; de Petrocellis, L.; Ullmer, C.; Rothenhäusler, B.; Perret, C.; van Gils, N.; et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat. Commun. 2017, 8, 13958. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Owsianik, G.; Voets, T.; Droogmans, G.; Nilius, B. Invertebrate TRP proteins as functional models for mammalian channels. Pflügers Arch.—Eur. J. Physiol. 2004, 449, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S.; Kawada, T.; Kim, B.-S.; Han, I.-S.; Choe, S.-Y.; Kurata, T.; Yu, R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003, 15, 299–306. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, H.-J.; Kim, G.E.; Cho, M.-H.; Yoon, S.-Y.; Davies, A.J.; Oh, S.B.; Lee, H.; Cho, Y.K.; Joo, C.H.; et al. Attenuation of natural killer cell functions by capsaicin through a direct and TRPV1-independent mechanism. Carcinogenesis 2014, 35, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.I.; A Kunde, D.; Crawford, A.; Geraghty, D.P. Expression of transient receptor potential vanilloid 1 (TRPV1) and 2 (TRPV2) in human peripheral blood. Mol. Immunol. 2007, 44, 1429–1435. [Google Scholar] [CrossRef]
- Oliveira, I.B.; Beiras, R.; Thomas, K.V.; Suter, M.J.-F.; Barroso, C.M. Acute toxicity of tralopyril, capsaicin and triphenylborane pyridine to marine invertebrates. Ecotoxicology 2014, 23, 1336–1344. [Google Scholar] [CrossRef]
- Fernandes, E.S.; A Fernandes, M.; E Keeble, J. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef]
- Battista, N.; Di Sabatino, A.; Di Tommaso, M.; Biancheri, P.; Rapino, C.; Vidali, F.; Papadia, C.; Montana, C.; Pasini, A.; Lanzini, A.; et al. Abnormal anandamide metabolism in celiac disease. J. Nutr. Biochem. 2012, 23, 1245–1248. [Google Scholar] [CrossRef]
- Maccarrone, M.; Lorenzon, T.; Bari, M.; Melino, G.; Finazzi-Agrò, A. Anandamide Induces Apoptosis in Human Cells via Vanilloid Receptors. J. Biol. Chem. 2000, 275, 31938–31945. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.V.; Fezza, F.; Baldassarri, S.; Gasperi, V.; Bertoni, A.; Pasquariello, N.; Finazzi-Agrò, A.; Sinigaglia, F.; Avigliano, L.; Maccarrone, M. Expression of the endocannabinoid system in the bi-potential HEL cell line: Commitment to the megakaryoblastic lineage by 2-arachidonoylglycerol. J. Mol. Med. 2009, 87, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Sigrist, C.J.A.; De Castro, E.; Cerutti, L.; Cuche, B.A.; Hulo, N.; Bridge, A.; Bougueleret, L.; Xenarios, I. New and continuing developments at PROSITE. Nucleic Acids Res. 2012, 41, D344–D347. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—a multiple sequence alignment editor and analysis workbenchi. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Narcisi, V.; Calzetta, A.; Gioia, L.; Finoia, M.G.; Latini, M.; Tiscar, P.G. Effects of high temperature and exposure to air on mussel (Mytilus galloprovincialis, Lmk 1819) hemocyte phagocytosis: Modulation of spreading and oxidative response. Tissue Cell 2013, 45, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Salzet, M. Invertebrate molecular neuroimmune processes. Brain Res. Rev. 2000, 34, 69–79. [Google Scholar] [CrossRef]
- Coccurello, R.; Maccarrone, M. Hedonic Eating and the “Delicious Circle”: From Lipid-Derived Mediators to Brain Dopamine and Back. Front. Neurosci. 2018, 12, 271. [Google Scholar] [CrossRef]
| ECS Element | Control | Inhibitor |
|---|---|---|
| Endogenous levels of AEA a | 1.90 ± 0.16 | − |
| Endogenous levels of 2-AG a | 43.5 ± 7.2 | − |
| Endogenous levels of PEA a | 0.40 ± 0.07 | − |
| NAPE-PLD b | 120.8 ± 0.6 | N.A. |
| FAAH b | 66.3 ± 6.6 | 20.3 ± 3 c |
| DAGL b | 200 ± 3 | 123 ± 7 d |
| MAGL b | 350 ± 10 | 252 ± 22 e |
| NAAA b | 96.9 ± 19.6 | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca, F.; Zarivi, O.; Battista, N.; Maccarrone, M.; Tiscar, P.G. The Endocannabinoid System in the Mediterranean Mussel Mytilus galloprovincialis: Possible Mediators of the Immune Activity? Int. J. Mol. Sci. 2021, 22, 4954. https://doi.org/10.3390/ijms22094954
Mosca F, Zarivi O, Battista N, Maccarrone M, Tiscar PG. The Endocannabinoid System in the Mediterranean Mussel Mytilus galloprovincialis: Possible Mediators of the Immune Activity? International Journal of Molecular Sciences. 2021; 22(9):4954. https://doi.org/10.3390/ijms22094954
Chicago/Turabian StyleMosca, Francesco, Osvaldo Zarivi, Natalia Battista, Mauro Maccarrone, and Pietro Giorgio Tiscar. 2021. "The Endocannabinoid System in the Mediterranean Mussel Mytilus galloprovincialis: Possible Mediators of the Immune Activity?" International Journal of Molecular Sciences 22, no. 9: 4954. https://doi.org/10.3390/ijms22094954
APA StyleMosca, F., Zarivi, O., Battista, N., Maccarrone, M., & Tiscar, P. G. (2021). The Endocannabinoid System in the Mediterranean Mussel Mytilus galloprovincialis: Possible Mediators of the Immune Activity? International Journal of Molecular Sciences, 22(9), 4954. https://doi.org/10.3390/ijms22094954








