Low-Cost Biobased Coatings for AM60 Magnesium Alloys for Food Contact and Harsh Environment Applications
Abstract
1. Introduction
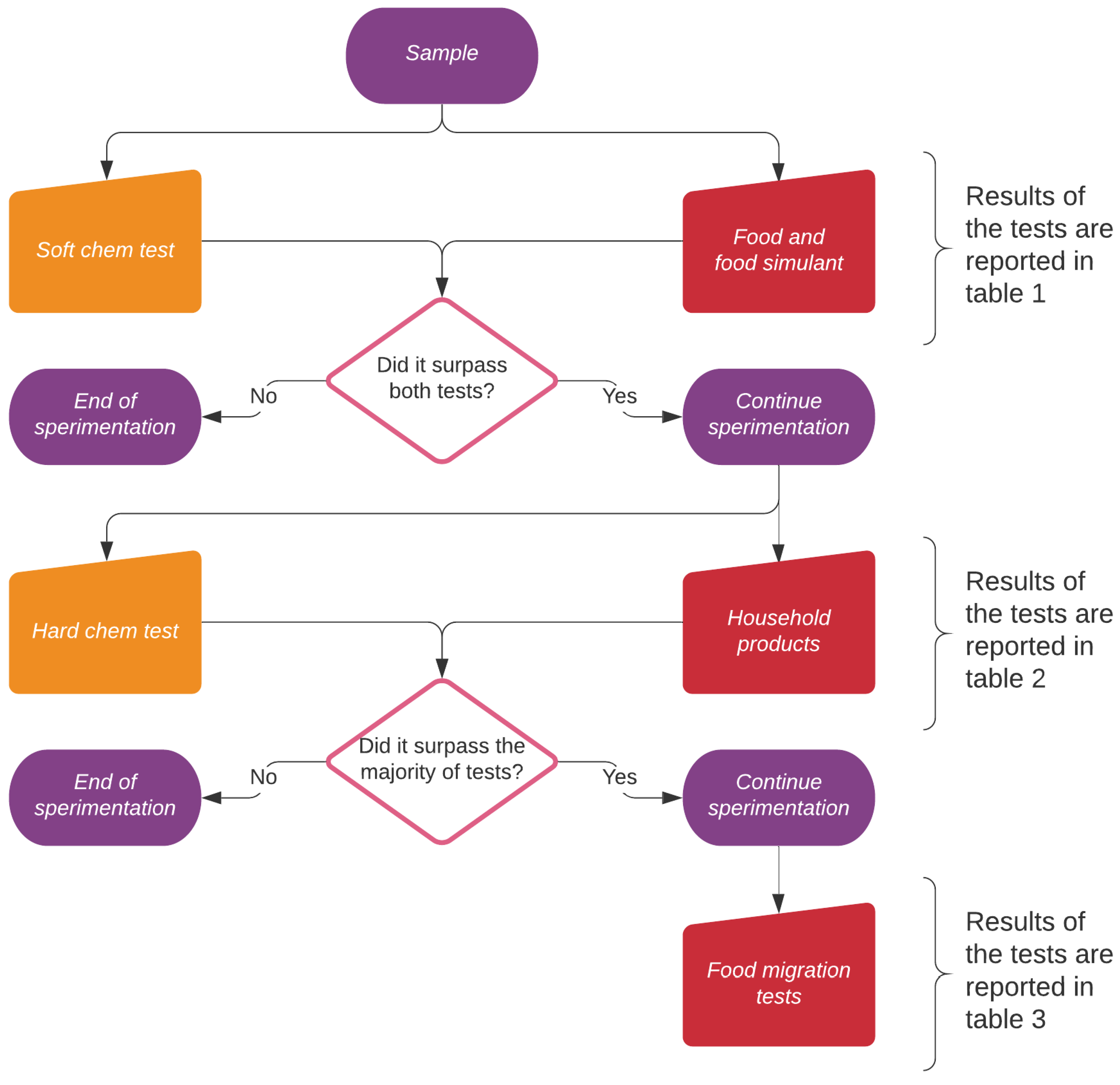
2. Results
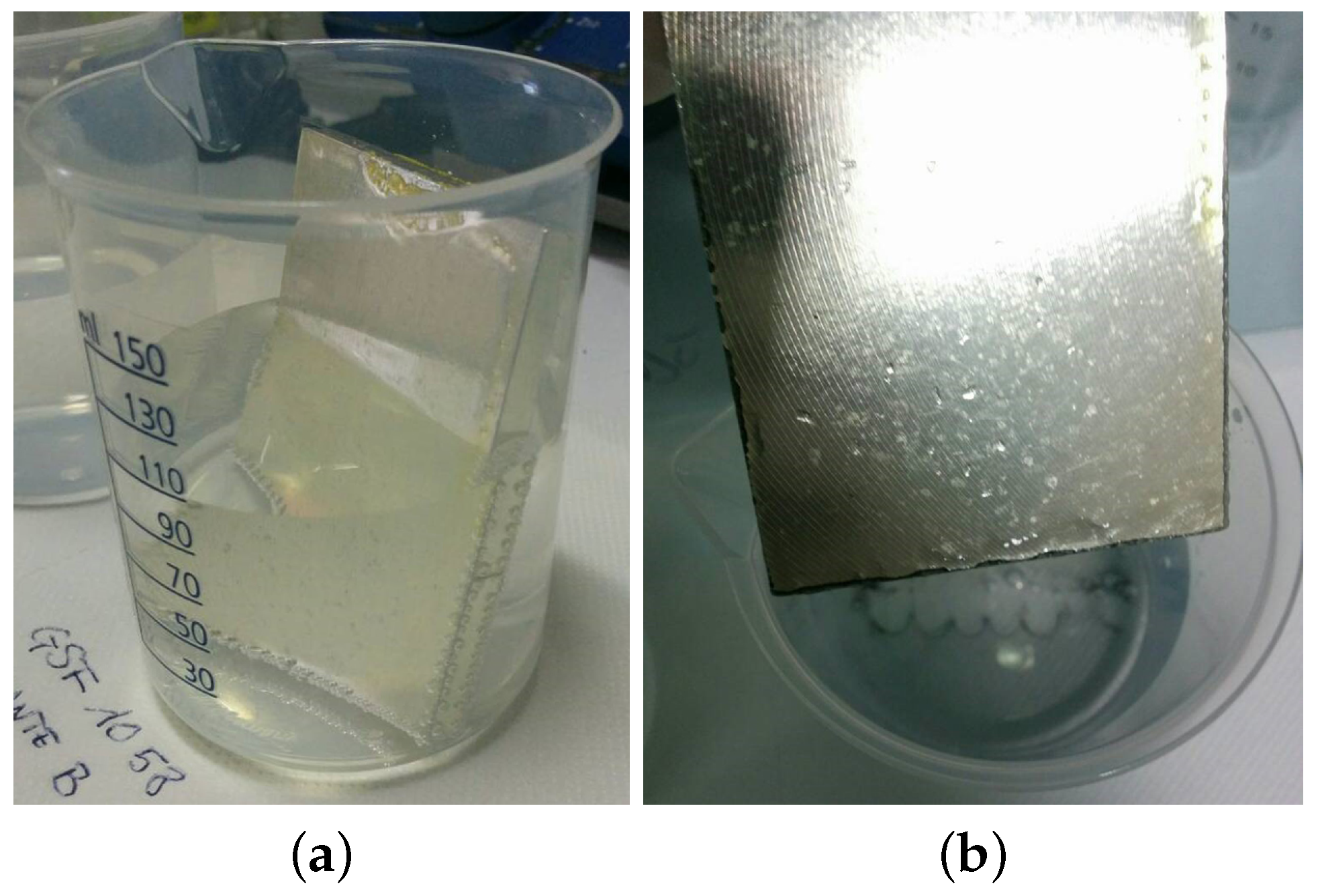
- the samples passing the first tests were subjected to hard chemical aggression and contact with real world household products (second level of Figure 1) and the results are given in Table 2, Section 2.1.1. Drop tests using aggressive solutions of strong acids, bases and salts were carried out at increasing concentrations and temperatures to assess possible usages in harsh environments and to simulate aging during long term usage. These tests allowed to understand the intrinsic shape-independent resistance of each coating;
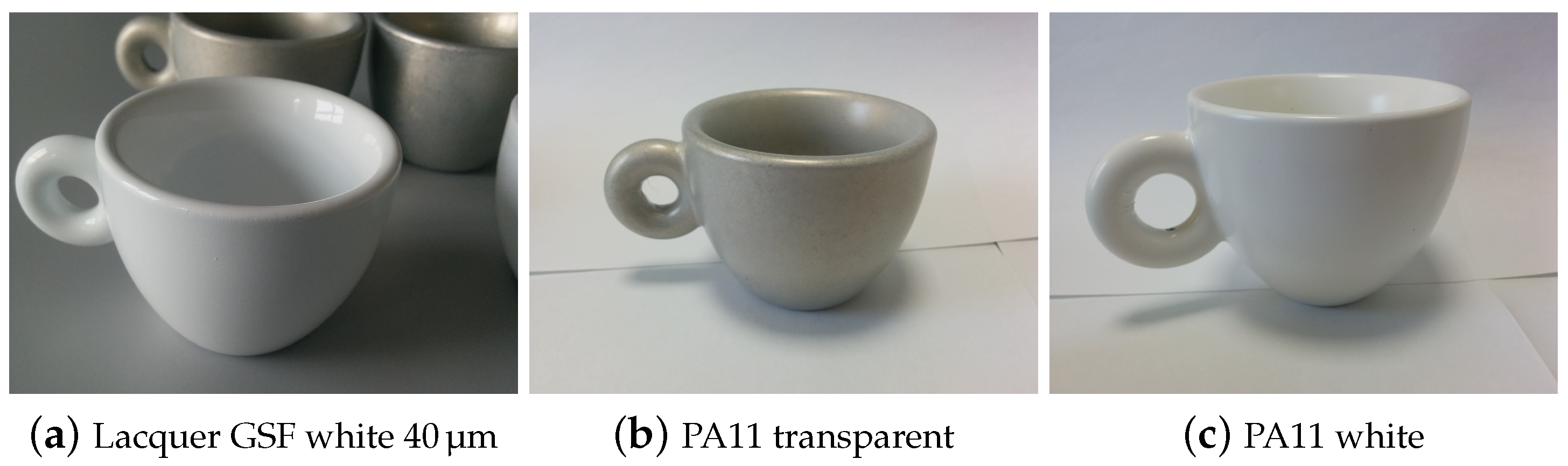
- the suitability for food contact applications of the best performing samples was then tested using the food simulant solutions described in the UNI EN 1186-3:2002 norm (third level of Figure 1 and Table 3); finally, immersion tests were carried out (also using real world prototypes, e.g., coffee cups) to measure, by ICP-OES, the amount of metals released and to calculate the specific migration. Dipping tests allowed to introduce the shape effect, since edgy parts are very critical in favoring corrosion.
2.1. Drop Tests
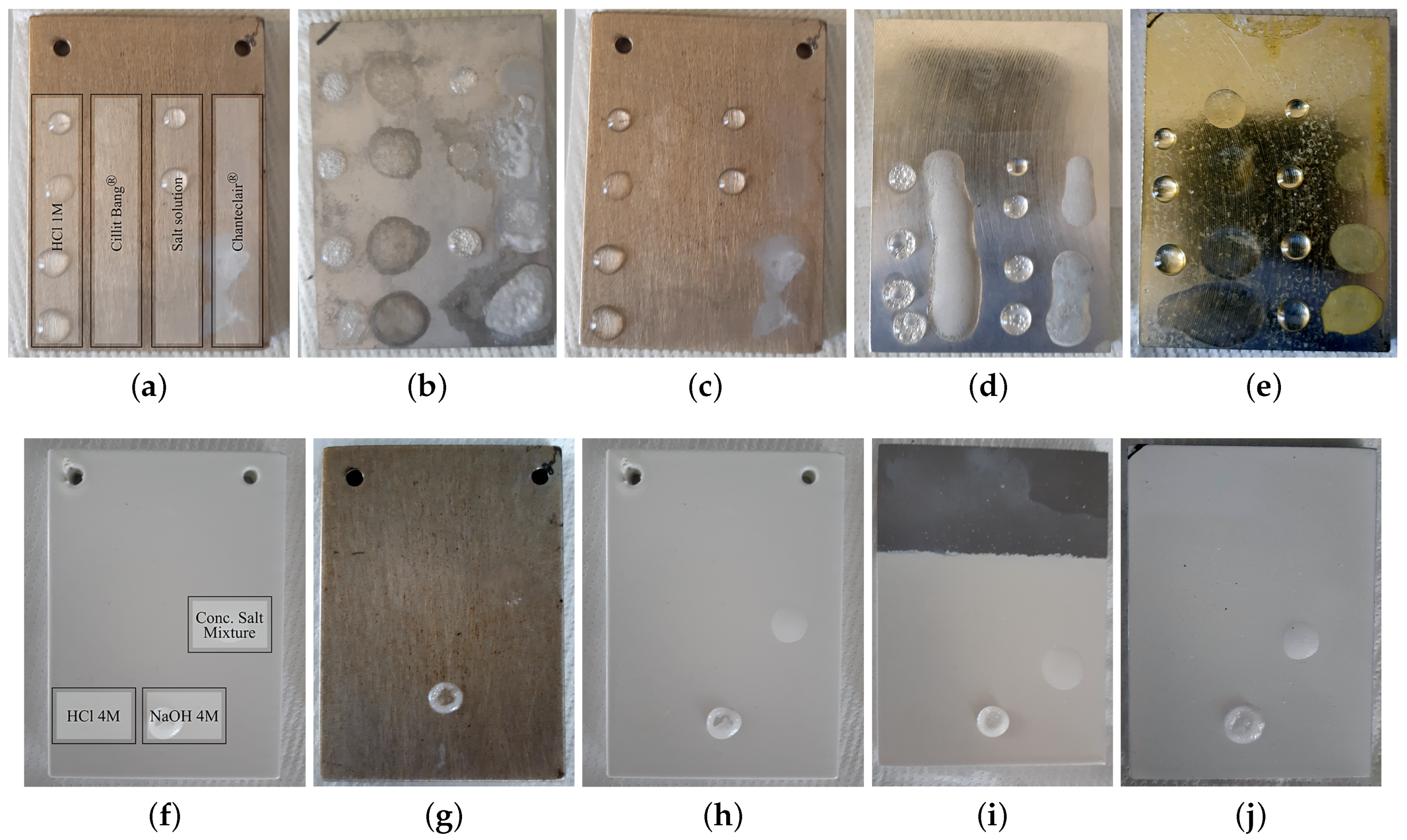
2.1.1. Chemical Aggression Tests
Soft Chemicals
Harsh Solutions and Household Products
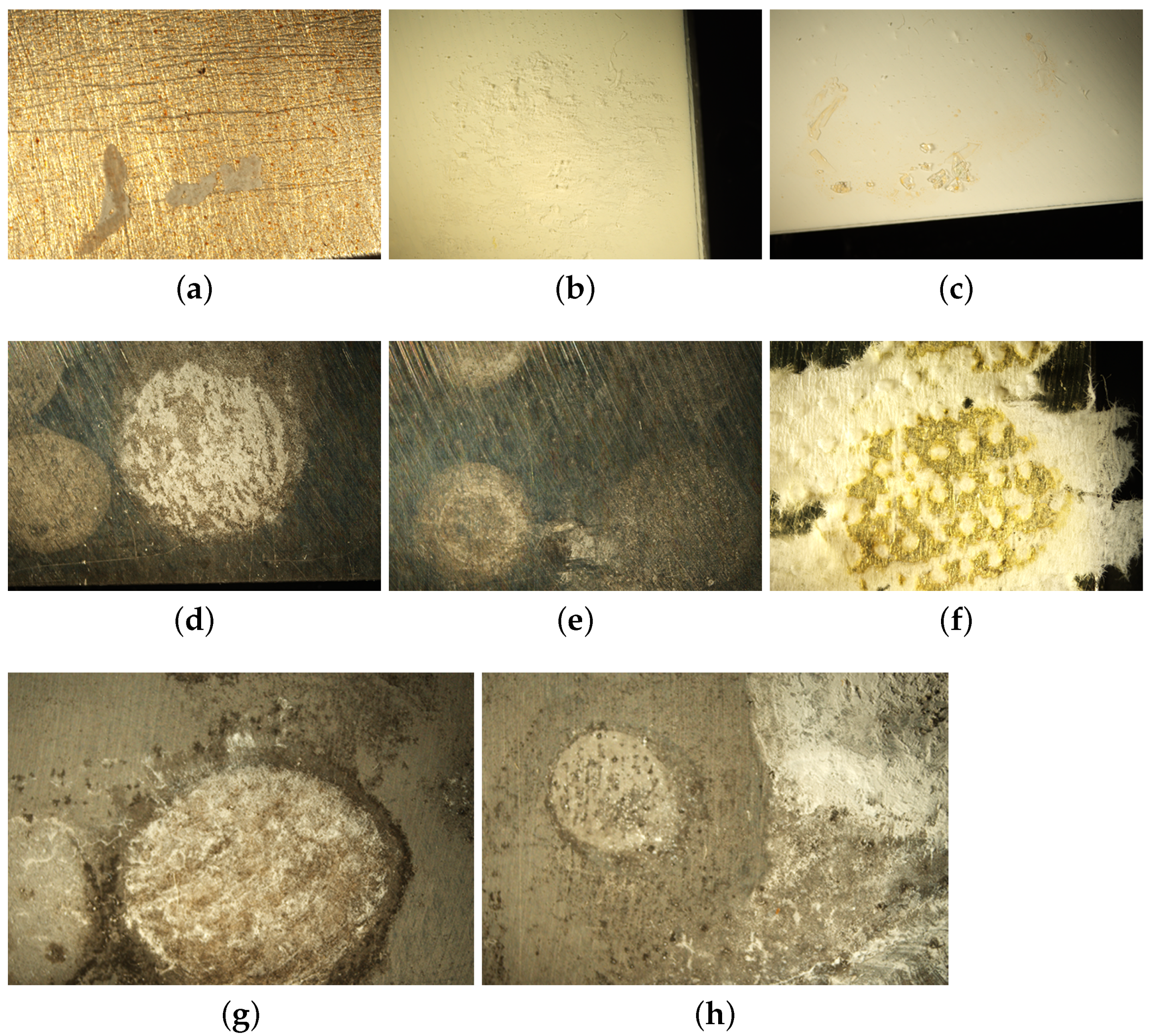
2.1.2. Optical Microscopy Analysis
2.1.3. Infrared Microscopy Analysis
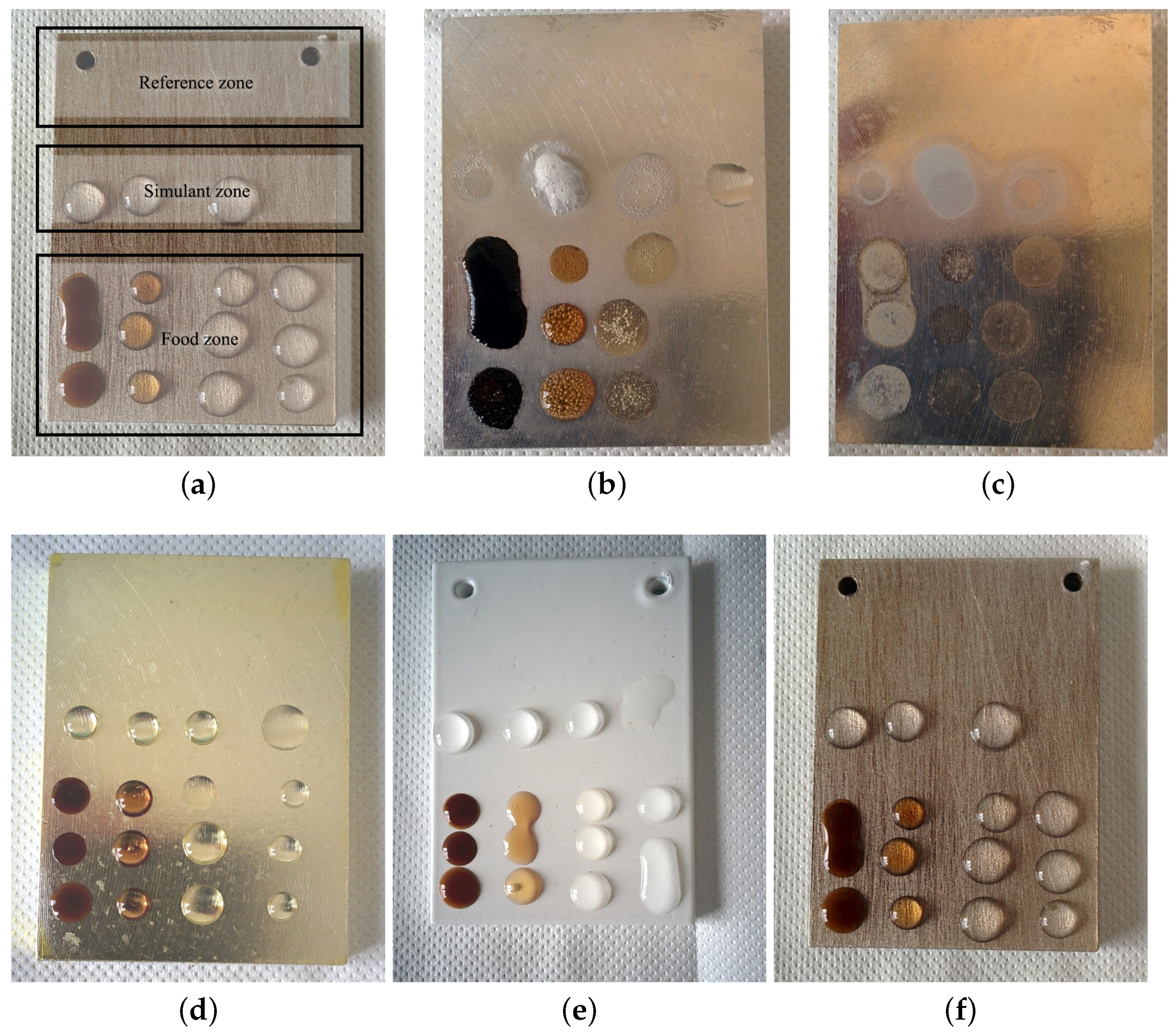
2.2. Food Contact Tests
2.2.1. Food Contact Drop Tests
2.2.2. Migration Tests by Dipping and Elemental ICP-OES Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Samples
5.2. Solutions
5.3. Tests and Analysis Methods
5.3.1. Drop Test
5.3.2. Specific Migration Test
5.3.3. ICP-OES Analysis for Specific Migration Test
5.3.4. ATR Analysis
5.3.5. Electron and Optical Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Aghion, E.; Bronfin, B.; Eliezer, D. The role of magnesium industry in protecting the environment. J. Mater. Process. Technol. 2001, 117, 381–385. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Moosbrugger, C. Introduction to Magnesium Alloys. In Engineering Properties of Magnesium Alloys; ASM International: Novelty, OH, USA, 2017; pp. 1–10. [Google Scholar]
- Song, G. Recent progress in corrosion protection of magnesium alloys. Adv. Eng. Mater. 2005, 7, 563. [Google Scholar] [CrossRef]
- Majumdar, J.D.; Galun, R.; Mordike, B.L.; Manna, I. Effect of laser surface melting on corrosion and wear resistance of a commercial magnesium alloy. Mater. Sci. Eng. 2003, A361, 119–129. [Google Scholar] [CrossRef]
- Hu, R.G.; Zhang, S.; Bu, J.F.; Lin, C.J.; Song, G.L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2011, 73, 129. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, H.; Liu, P.; Zhang, C.; Li, W.; Chen, X.; Ma, F. Electrophoretic deposition of graphene oxide on NiTi alloy for corrosion prevention. Vacuum 2019, 161, 276–282. [Google Scholar] [CrossRef]
- Parco, M. Investigation of HVOF spraying on magnesium alloys. Surf. Coat. Technol. 2006, 201, 3269–3274. [Google Scholar] [CrossRef]
- Raj, R.V. The characteristics study of HVOF coated AZ91D magnesium alloy with silicon carbide and stainless steel. AIP Conf. Proc. 2019, 2015, 020024. [Google Scholar]
- Rakoch, A.G.; Monakhova, E.P.; Khabibullina, Z.V.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Gladkova, A.A. Plasma electrolytic oxidation of AZ31 and AZ91 magnesium alloys: Comparison of coatings formation mechanism. J. Magnes. Alloy. 2020, 8, 587–600. [Google Scholar] [CrossRef]
- Sinebryukhov, S.L.; Sidorova, M.V.; Egorkin, V.S.; Nedozorov, P.M.; Ustinov, A.Y.; Volkova, E.F.; Gnedenkov, S.V. Protective oxide coatings on Mg-Mn-Ce, Mg-Zn-Zr, Mg-Al-Zn-Mn, Mg-Zn-Zr-Y, and Mg-Zr-Nd magnesium-based alloys. Prot. Met. Phys. Chem. Surf. Vol. 2012, 48, 678–687. [Google Scholar] [CrossRef]
- Martin, J. The influence of metallurgical state of substrate on the efficiency of plasma electrolytic oxidation (PEO) process on magnesium alloy. Mater. Des. 2019, 178, 107859. [Google Scholar] [CrossRef]
- Borg, P.; Lê, G.; Lebrun, S.; Pées, B. Example of industrial valorisation of derivative products of Castor oil. Lipides Trop. 2009, 16, 211–214. [Google Scholar] [CrossRef][Green Version]
- Pey, J.L. Corrosion protection of pipes, fittings and component pieces of water treatment and pumping stations. Anti-Corros. Methods Mater. 1997, 44, 94–99. [Google Scholar] [CrossRef]
- Fernández-Álvarez, M.; Velasco, F.; Bautista, A.; Lobo, F.C.M.; Fernes, E.M.; Reis, R.L. Manufacturing and Characterization of Coatings from Polyamide Powders Functionalized with Nanosilica. Polymers 2020, 12, 2298. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.L.; Longo, A.; Androsch, R. Polyamide 11/Poly(butylene succinate) Bio-Based Polymer Blends. Materials 2019, 12, 2833. [Google Scholar] [CrossRef] [PubMed]
- Moshynets, O.; Bardeau, J.F.; Tarasyuk, O.; Makhno, S.; Cherniavska, T.; Dzhuzha, O.; Rogalsky, S. Antibiofilm Activity of Polyamide 11 Modified with Thermally Stable Polymeric Biocide Polyhexamethylene Guanidine 2-Naphtalenesulfonate. Int. J. Mol. Sci. 2019, 20, 348. [Google Scholar] [CrossRef]
- Baldi, G.; Cioni, A.; Dami, V.; Barzanti, A.; Lorenzi, G.; Marchese, E.M.; Bitossi, M. Ceramers, Their Application and Use. International Application Number PCT/IB2012/050997, 7 September 2012. [Google Scholar]
- Mirone, G.; Pesce, R. Photo-Crosslinkable Multi-Coating System Having Improved Gas Barrier Properties. U.S. Patent US7341791B2, 11 March 2008. [Google Scholar]
- UNI EN 1186-3:2003. Materials and Articles in Contact with Foodstuffs-Plastics-Guide to the Selection of Conditions and Test Methods for Overall Migration. 2003. Available online: http://store.uni.com/catalogo/uni-en-1186-3-2003 (accessed on 26 April 2021).
- Polyamide 11 Coatings Polyamide 11 Coatingsabrasion Resistance Study. Available online: https://www.rilsanfinepowders.com/export/sites/rilsanfinepowders/.content/medias/rfp-downloads/rfp-literature/rfp-cs-polyamide-11-coatings-abrasion-resistance-study.pdf (accessed on 26 April 2021).
- Dongguan Dechuang Hardware Co. Available online: https://dgdechuang.en.alibaba.com/ (accessed on 26 April 2021).
- Baldi, G.; Cioni, A.; Valentina, D.A.M.I. Polymeric Glass Based Compositions for Vitreous Coating. U.S. Patent 9,637,642, 2 May 2017. [Google Scholar]
- King, W.D. The physics of a stove-top espresso machine. Am. J. Phys. 2008, 76, 558. [Google Scholar] [CrossRef]
- UNI EN 1186-1:2003. Materials and Articles in Contact with Foodstuffs-Plastics-Test Methods for Overall Migration into Aqueous Food Simulants by Total Immersion. 2003. Available online: http://store.uni.com/catalogo/uni-en-1186-1-2003 (accessed on 26 April 2021).
- Simoneau, C. Guidelines on Testing Conditions for Articles. In Contact with Foodstuffs (with a Focus on Kitchenware), 1st ed.; A CRL-NRL-FCM Publication: Luxembourg, 2009. [Google Scholar]


| Sample Name 1 | Soft Chemical Aggression | Food Simulant Drop Test | Food Drop Test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCl 1M | Salt Mixture | MilliQ H2O | Acetic Ac. 3% | EtOH 10% | Oil | Acetic Ac. 10% | Coffee | Cola® | White Wine | |
| • Nanoc. Easysol | Fail | Fail | Pass | Fail | Pass | Pass | Fail | Fail | Fail | Fail |
| • Ceramic techsol | Fail | Pass | Pass | Fail | Pass | Pass | Fail | Pass | Fail | Fail |
| • Siliconic white | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| • Siliconic brown | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| • Lacquer GSF 12 | Fail | Fail | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| • Lacquer GSF 30 | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| • Lacquer GSF white 13 | - | - | Pass | Pass | Pass | Pass | Fail | Pass | Pass | Pass |
| • Lacquer GSF white 40 | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| • PA11 transparent | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| • PA11 white | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass | Pass |
| Sample Name | Hard Chemical Aggression | Household Drop Test | |||
|---|---|---|---|---|---|
| HCl 4M | NaOH 4M | Conc. Salt Mixture | Chanteclair® | Chillit Bang® | |
| • Siliconic white | Pass | Fail | Pass | Pass | Pass |
| • Siliconic brown | Pass | Fail | Pass | Pass | Pass |
| • Lacquer GSF 30 | Fail | Fail | Fail | Fail | Fail |
| • Lacquer GSF white 40 | Pass | Pass | Pass | Pass | Pass |
| • PA11 transparent | Pass | Pass | Pass | Pass | Pass |
| • PA11 white | Pass | Pass | Pass | Pass | Pass |
| Sample Name | Migration Test and Coffee Test | Migration Test on Prototypes | |
|---|---|---|---|
| Acetic Ac. 3% on Flat Samples | Simulants A B C in Cups | Coffee | |
| • Siliconic white | Fail | Fail | Fail |
| • Siliconic brown | Fail | - | - |
| • Lacquer GSF white 40 | Pass | Pass | Pass |
| • PA11 transparent | Pass | Pass | Pass |
| • PA11 white | Pass | Pass | Pass |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangolini, B.; Lopresti, M.; Conterosito, E.; Rombolà, G.; Palin, L.; Gianotti, V.; Milanesio, M. Low-Cost Biobased Coatings for AM60 Magnesium Alloys for Food Contact and Harsh Environment Applications. Int. J. Mol. Sci. 2021, 22, 4915. https://doi.org/10.3390/ijms22094915
Mangolini B, Lopresti M, Conterosito E, Rombolà G, Palin L, Gianotti V, Milanesio M. Low-Cost Biobased Coatings for AM60 Magnesium Alloys for Food Contact and Harsh Environment Applications. International Journal of Molecular Sciences. 2021; 22(9):4915. https://doi.org/10.3390/ijms22094915
Chicago/Turabian StyleMangolini, Beatrice, Mattia Lopresti, Eleonora Conterosito, Giuseppe Rombolà, Luca Palin, Valentina Gianotti, and Marco Milanesio. 2021. "Low-Cost Biobased Coatings for AM60 Magnesium Alloys for Food Contact and Harsh Environment Applications" International Journal of Molecular Sciences 22, no. 9: 4915. https://doi.org/10.3390/ijms22094915
APA StyleMangolini, B., Lopresti, M., Conterosito, E., Rombolà, G., Palin, L., Gianotti, V., & Milanesio, M. (2021). Low-Cost Biobased Coatings for AM60 Magnesium Alloys for Food Contact and Harsh Environment Applications. International Journal of Molecular Sciences, 22(9), 4915. https://doi.org/10.3390/ijms22094915









