Single-Walled Carbon Nanotubes Modify Leaf Micromorphology, Chloroplast Ultrastructure and Photosynthetic Activity of Pea Plants
Abstract
1. Introduction
2. Results
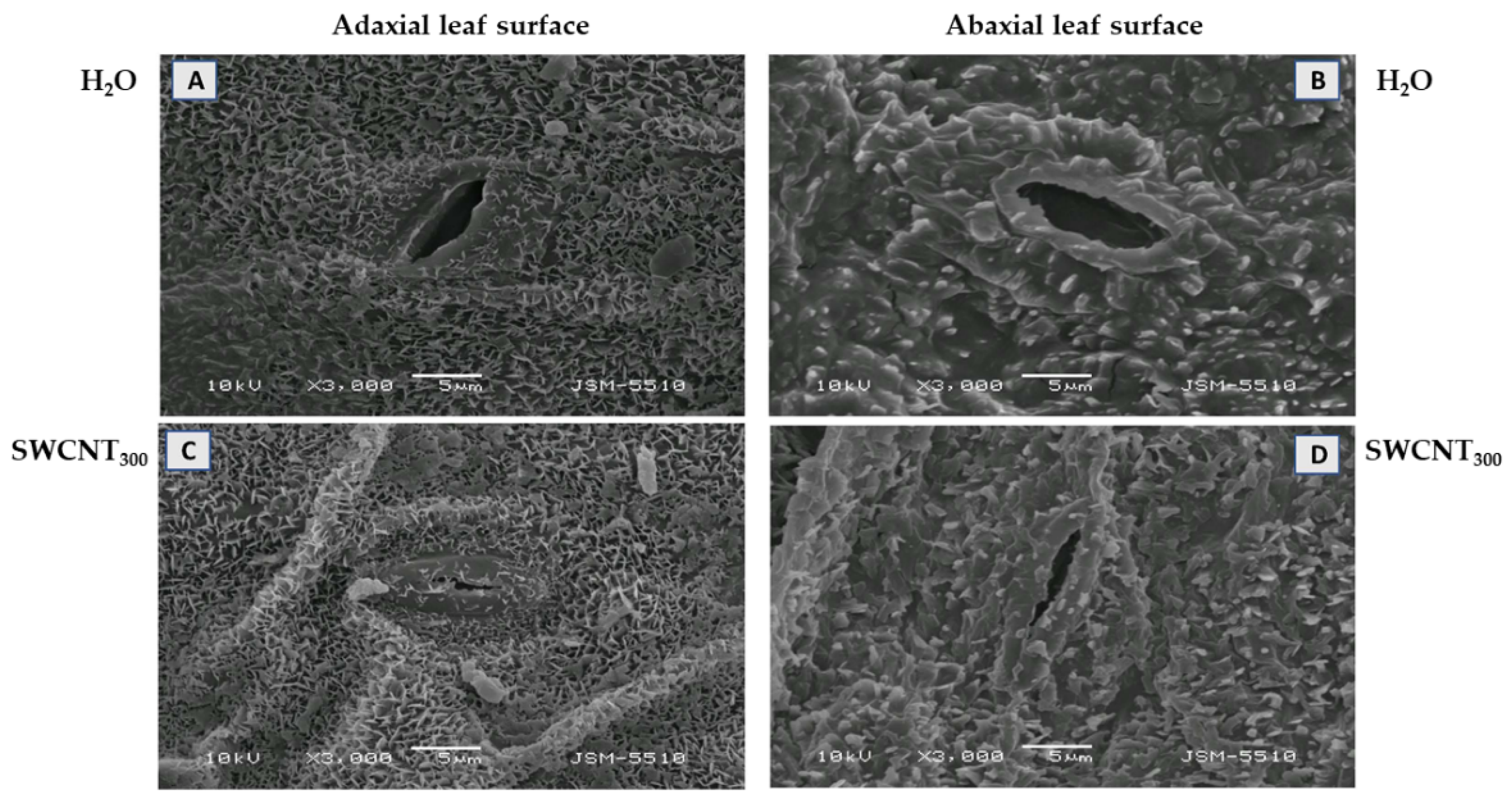
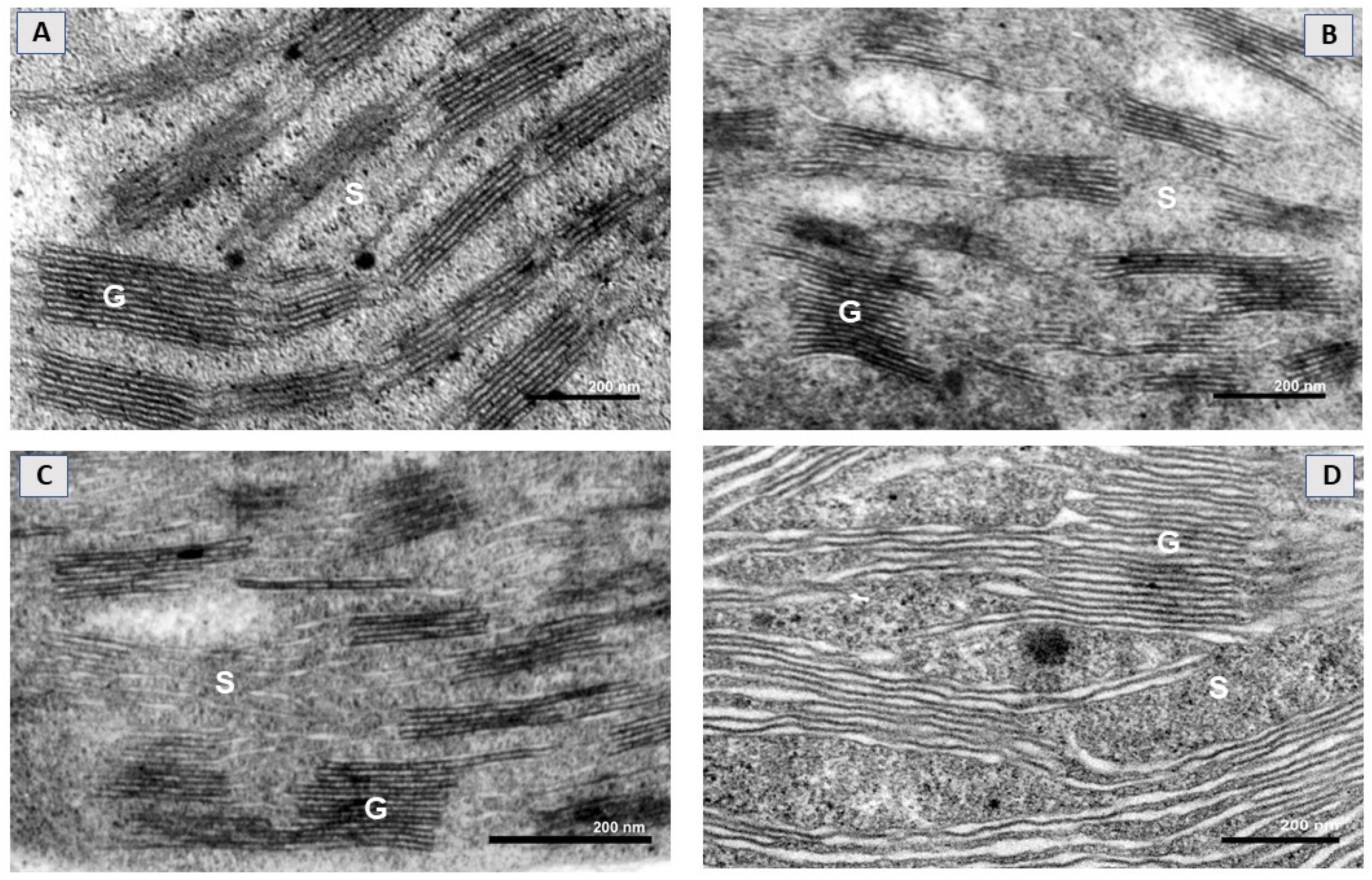
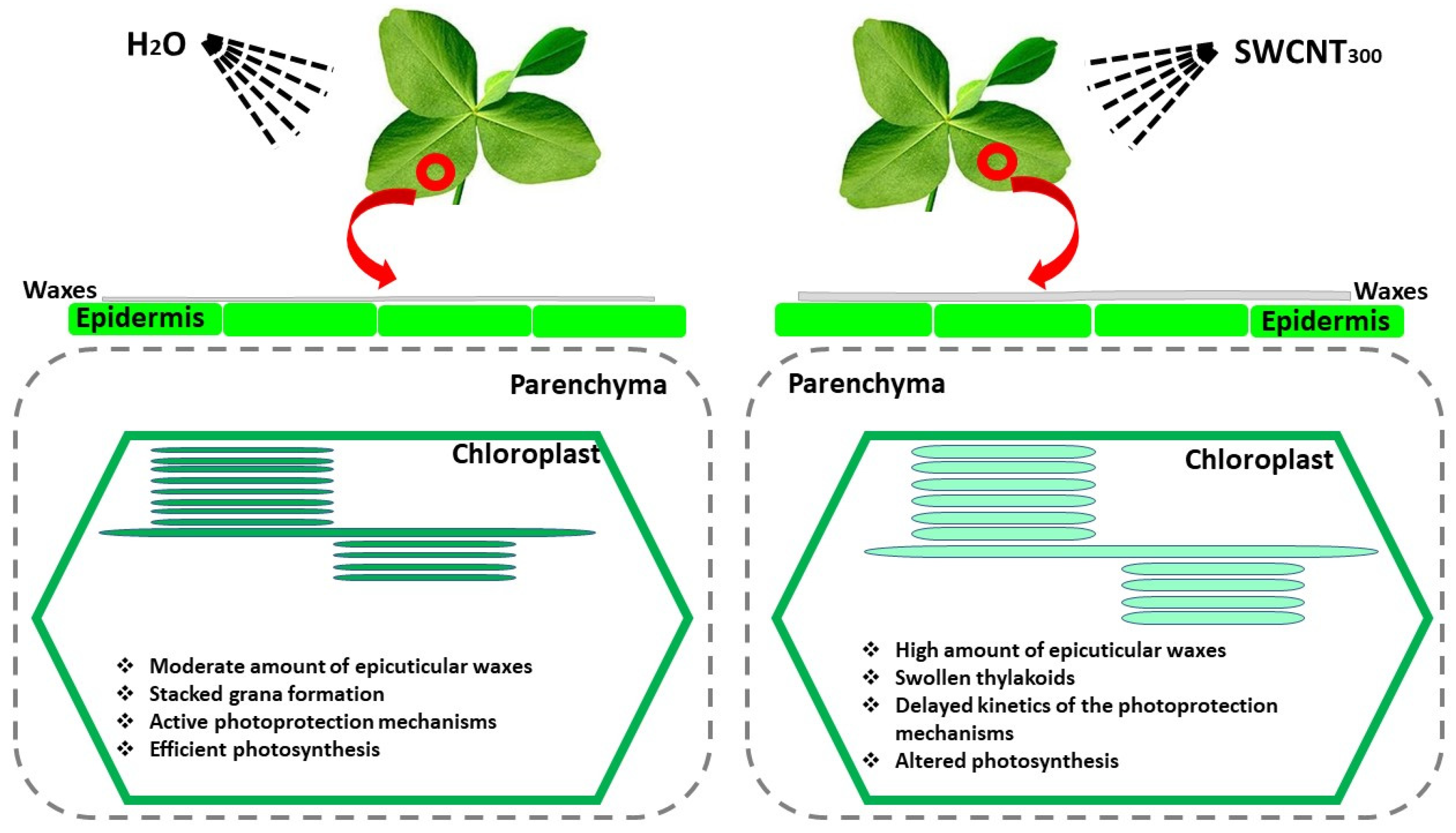
2.1. SWCNTs Affect Leaf Morphology and Chloroplast Ultrastructure
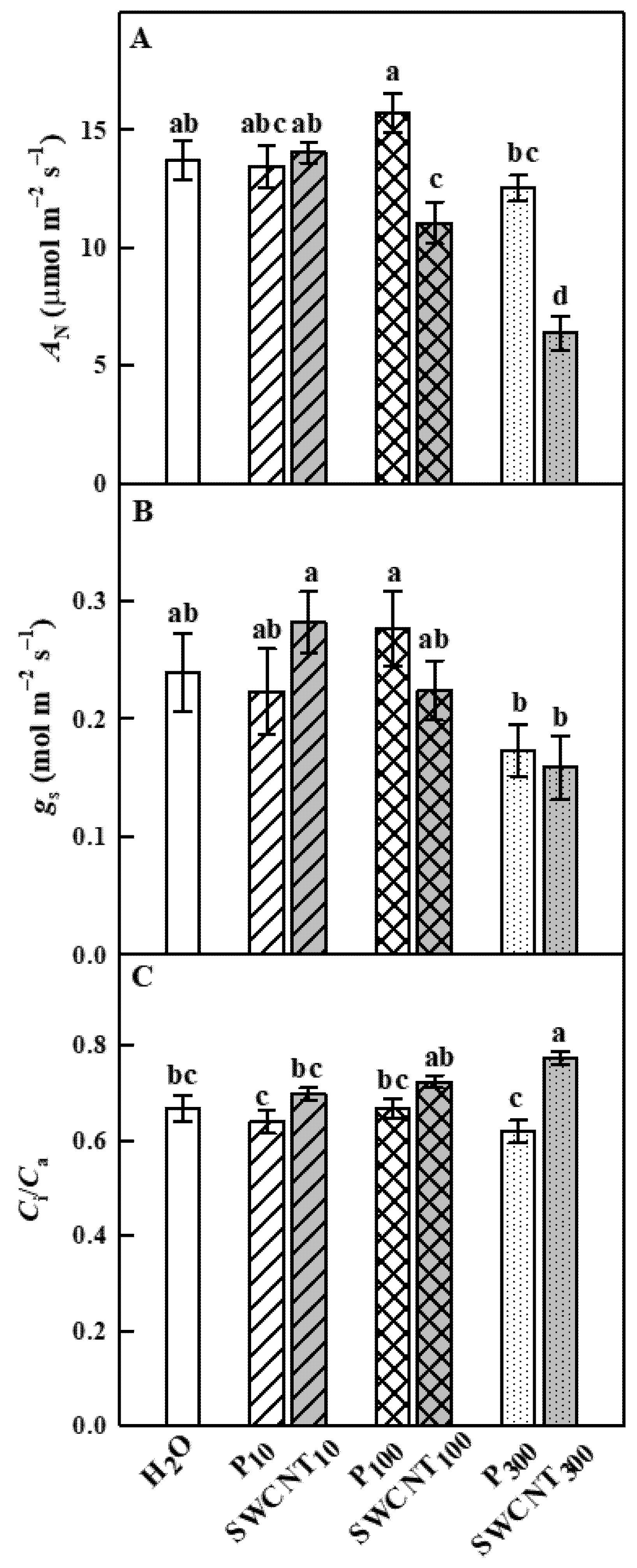
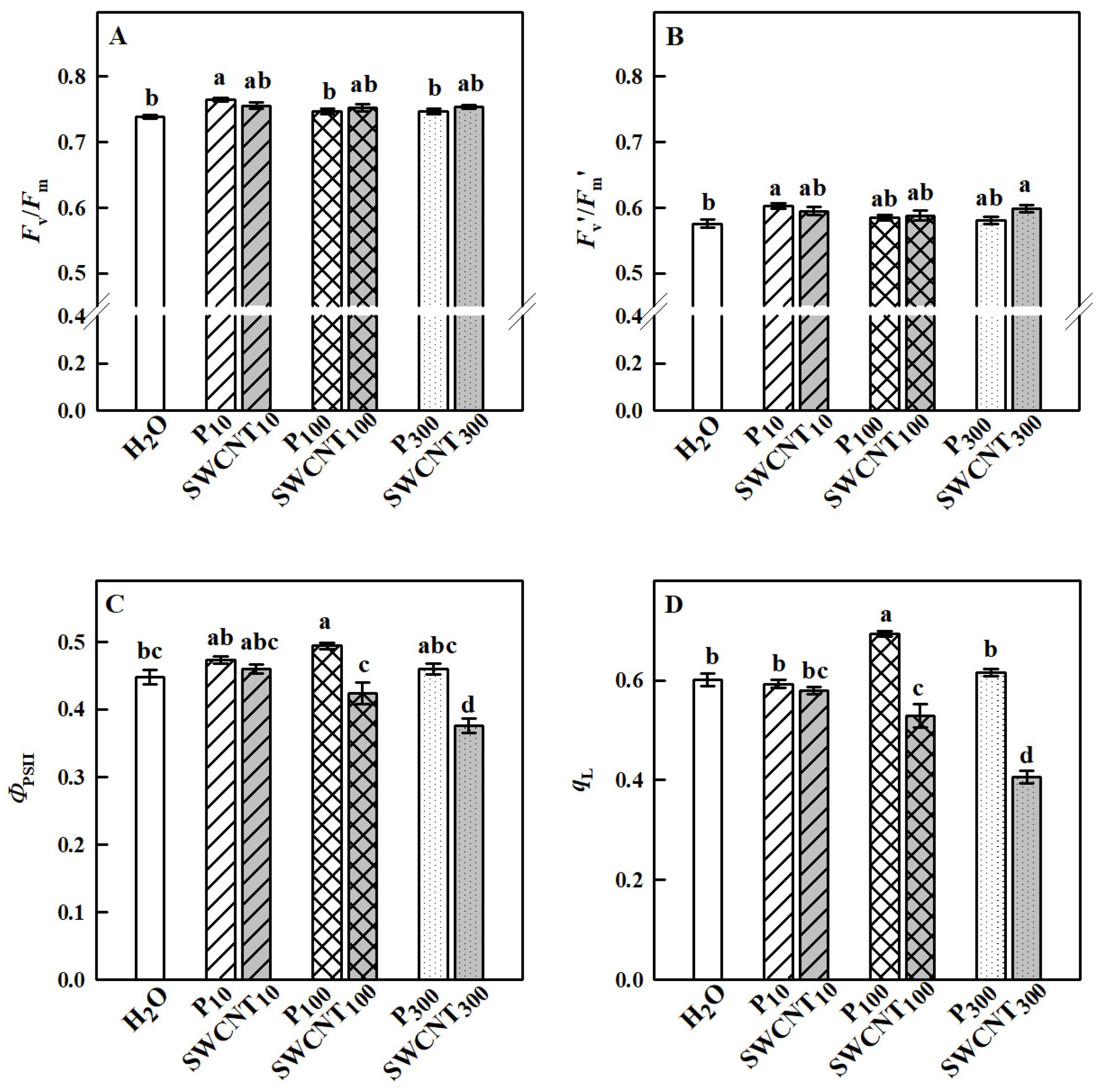
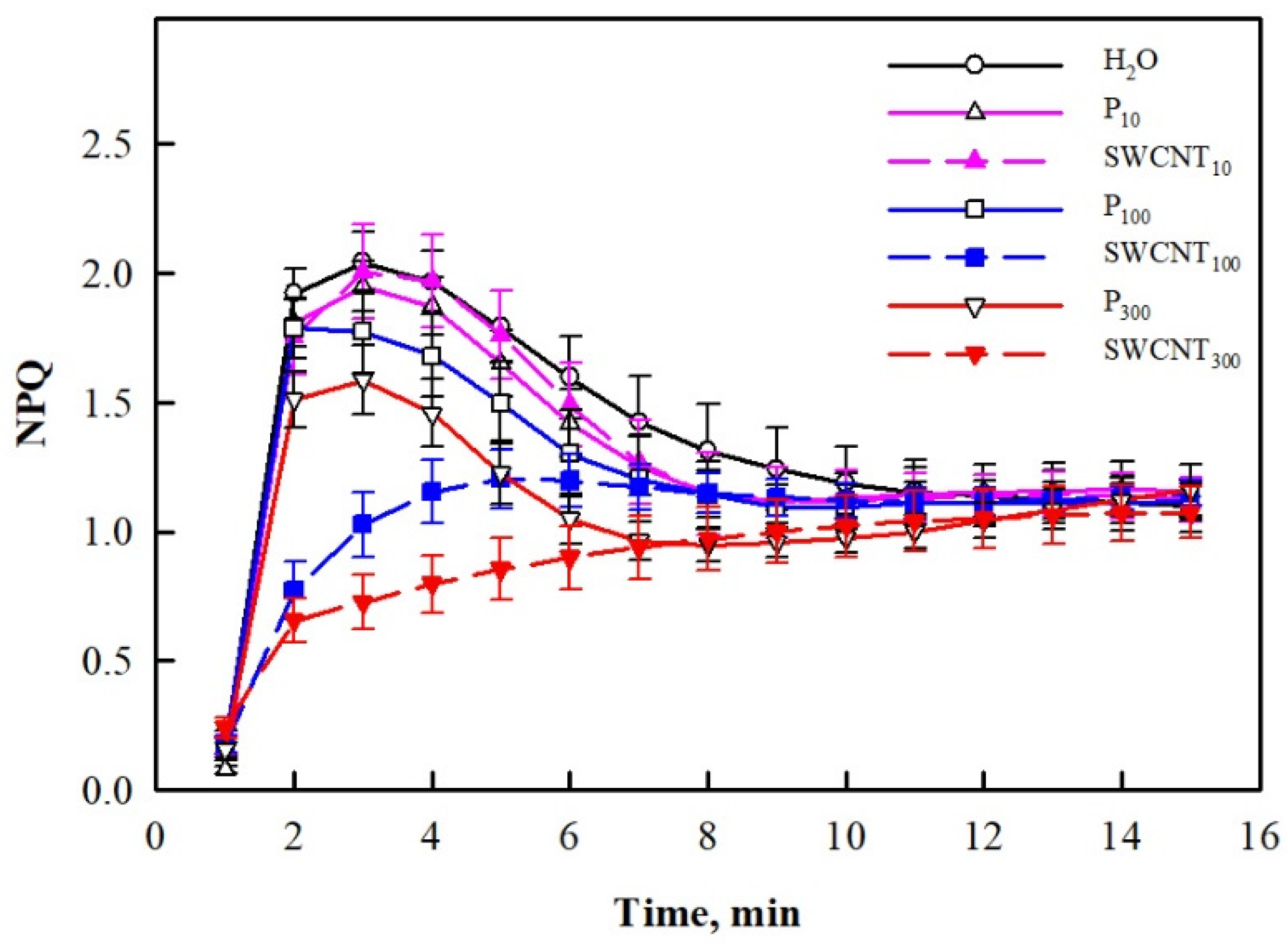
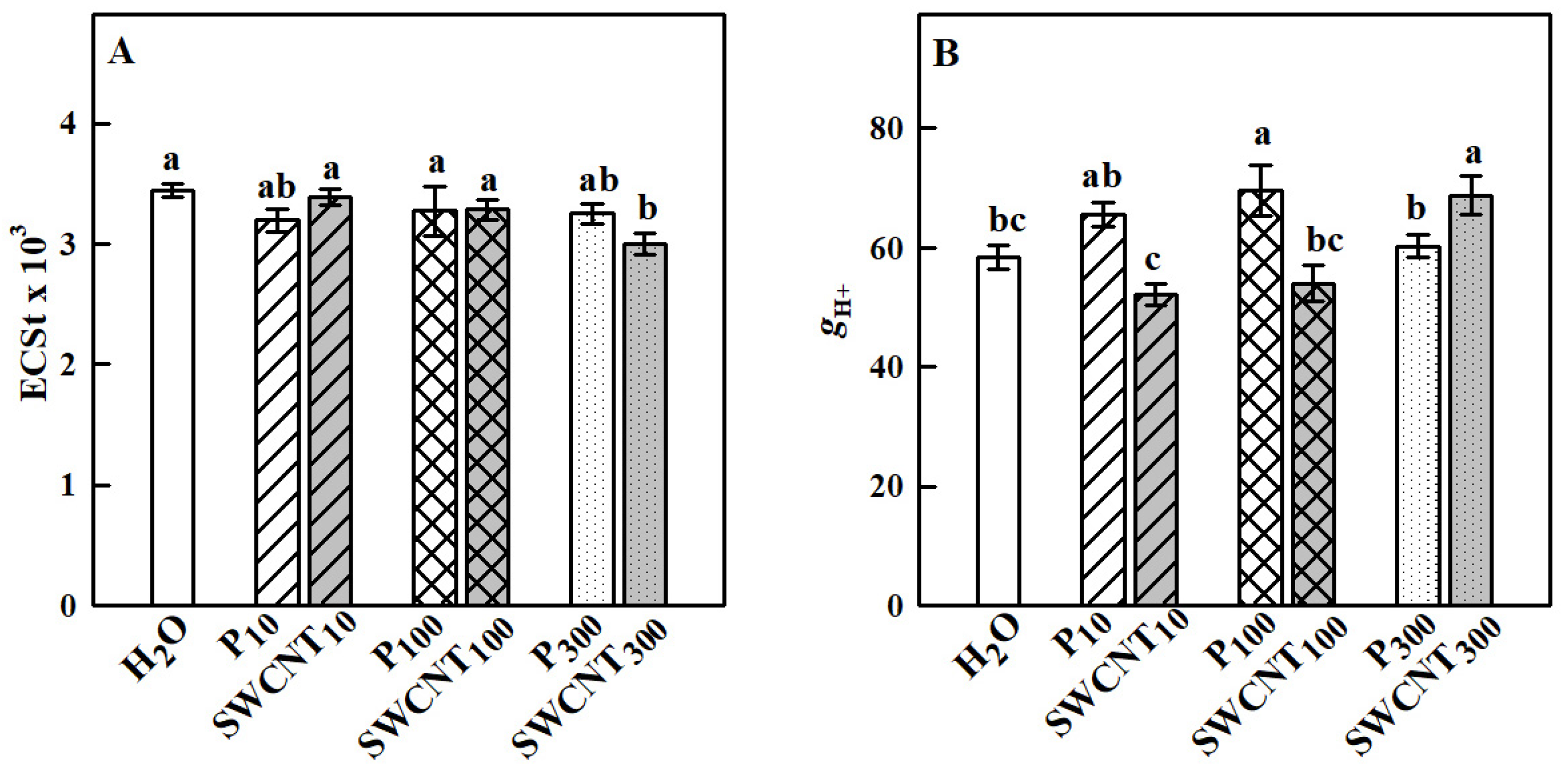
2.2. SWCNTs Affect CO2 Assimilation, Photosynthetic Light Reactions and Membrane Proton Permeability
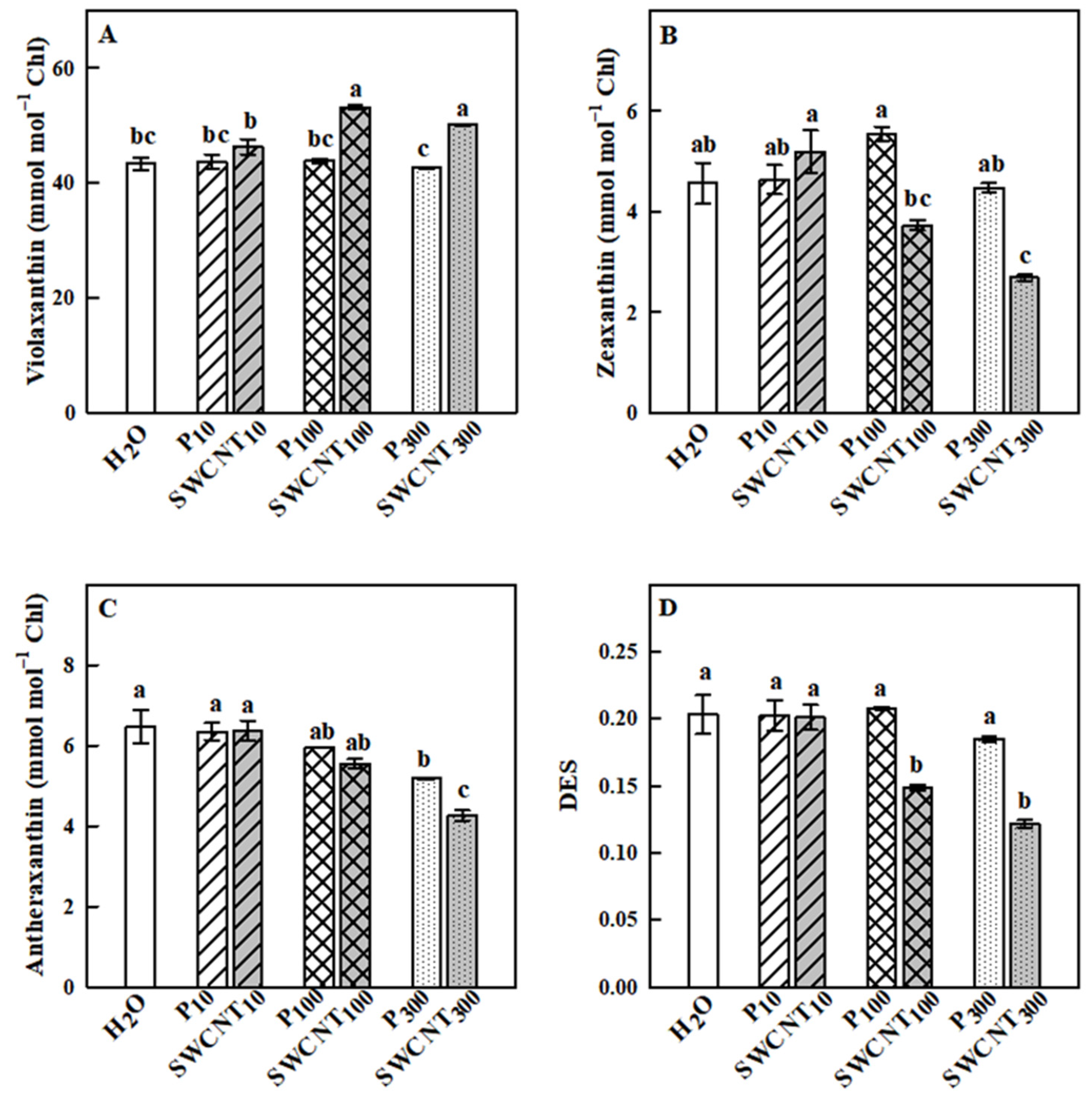
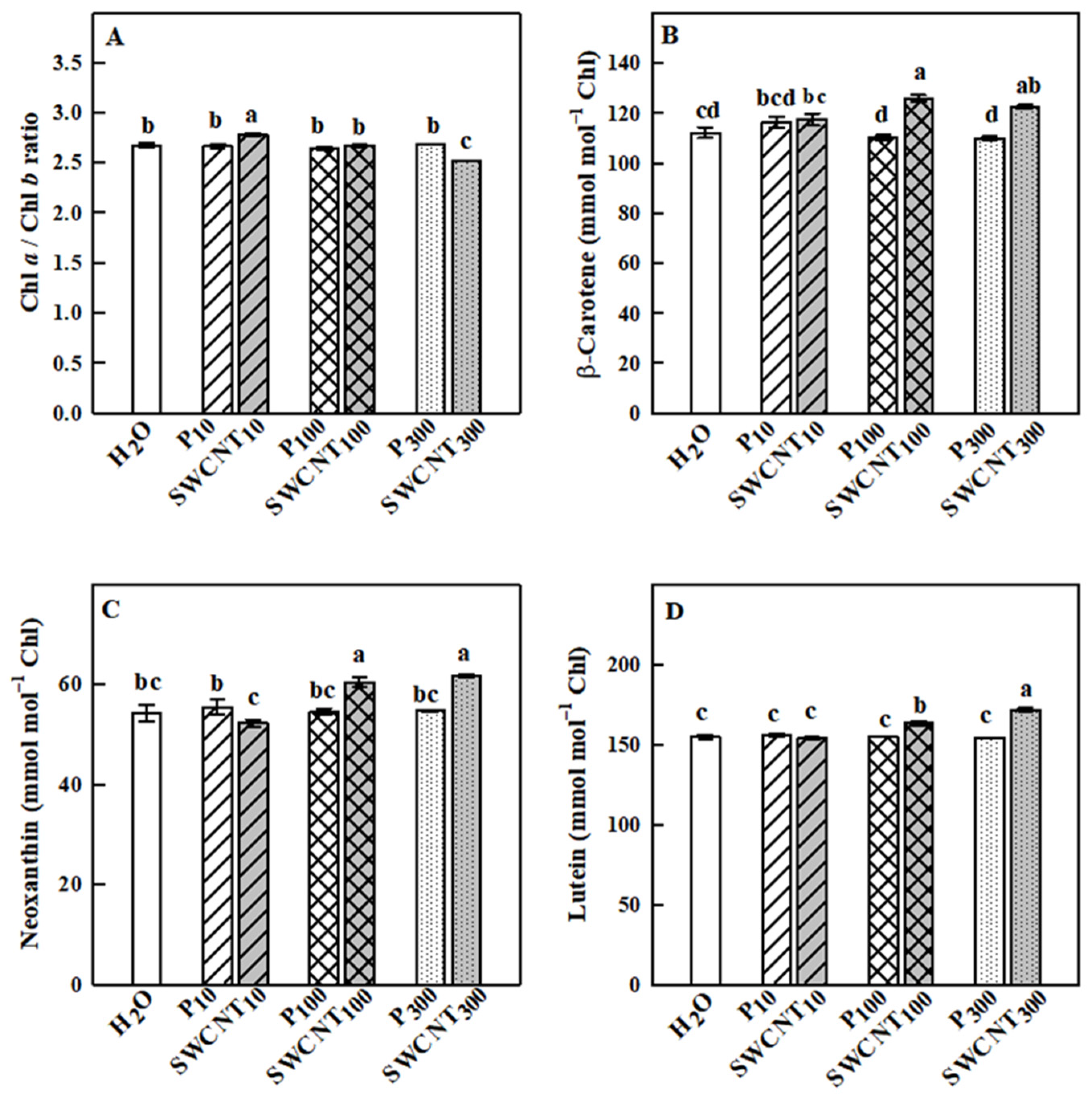
2.3. SWCNTs Affect Leaf Pigment Composition
3. Discussion
4. Materials and Methods
4.1. Single-Walled Carbon Nanotubes
4.2. Plant Material
4.3. Scanning and Transmission Electron Microscopy
4.4. Photosynthetic Gas Exchange Measurements
4.5. Chlorophyll Fluorescence Measurements and Analysis of Absorbance Changes
4.6. Pigment Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on synthesis, properties and plethora of applications in the field of biomedical science. Sen. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Silva, A.T.; Nguyen, A.; Ye, C.; Verchot, J.; Moon, J.H. Conjugated polymer nanoparticles for effective siRNA delivery to tobacco BY-2 protoplasts. BMC Plant Biol. 2010, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.-P.; Kuang, L.-Y.; Huang, C.-A.; Jane, W.-N.; Hung, Y.; Hsing, Y.-I.C.; Mou, C.-Y. A simple plant gene delivery system using mesoporous silica nanoparticles as carriers. J. Mater. Chem. B. 2013, 1, 5279–5287. [Google Scholar] [CrossRef]
- Lew, T.T.S.; Wong, M.H.; Kwak, S.-Y.; Sinclair, R.; Koman, V.B.; Strano, M.S. Rational design principles for the transport and subcellular distribution of nanomaterials into plant protoplasts. Small 2018, 14, 1802086. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Chen, J.; Han, H.; Yuan, Z. Evaluation and mechanism of antifungal effects of carbon nanomaterials in controlling plant fungal pathogen. Carbon 2014, 68, 798–806. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Chen, J.; Irin, F.; Puretzky, A.A.; Green, M.J.; Khodakovskaya, M.V. Interaction of carbon nanohorns with plants: Uptake and biological effects. Carbon 2015, 81, 607–619. [Google Scholar] [CrossRef]
- Lew, T.T.S.; Koman, V.B.; Silmore, K.S.; Seo, J.S.; Gordiichuk, P.; Kwak, S.-Y.; Park, M.; Ang, M.C.-Y.; Khong, D.T.; Lee, M.A.; et al. Real-time detection of wound-induced H2O2 signaling waves in plants with optical nanosensors. Nat. Plants 2020, 6, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Zhang, L.; Mattison, N.T.; O’Carroll, D.M.; Whelton, A.J.; Uddin, N.; Nguyen, T.; Huang, Q.; Henry, T.B.; Holbrook, R.D.; et al. Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9837–9856. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Tiwari, D.K.; Tripathi, D. Interaction of carbon nanotubes with plant system: A review. Carbon Lett. 2020. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon nanotubes as molecular transporters for walled plant cells. Nano. Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.P.; Landry, M.P.; Faltermeier, S.M.; McNicholas, T.P.; Iverson, N.M.; Boghossian, A.A.; Reuel, N.F.; Hilmer, A.J.; Sen, F.; Brew, J.A.; et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 2014, 13, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Paulus, G.L.; Maruyama, R.; Heller, D.A.; Kim, W.J.; Barone, P.W.; Lee, C.Y.; Choi, J.H.; Ham, M.H.; Song, C.; et al. Exciton antennas and concentrators from core-shell and corrugated carbon nanotube filaments of homogeneous composition. Nat. Mater. 2010, 9, 833–839. [Google Scholar] [CrossRef]
- Ghasemi-Kooch, M.; Dehestani, M. Interaction of photosynthetic pigments with single-walled carbon nanotube (15, 15): A molecular dynamics study. Adsorption 2018, 24, 43–51. [Google Scholar] [CrossRef]
- Dorogi, M.; Balint, Z.; Mikó, C.; Vileno, B.; Milas, M.; Hernadi, K.; Forró, L.; Varó, G.; Nagy, L. Stabilization effect of single-walled carbon nanotubes on the functioning of photosynthetic reaction centers. J. Phys. Chem. B 2006, 110, 21473–21479. [Google Scholar] [CrossRef]
- Mackowski, S. Hybrid nanostructures for efficient light harvesting. J. Phys. Cond. Mat. 2010, 22, 193102. [Google Scholar] [CrossRef]
- Hajdu, K.; Szabó, T.; Magyar, M.; Bencsik, G.; Németh, Z.; Nagy, K.; Magrez, A.; Forró, L.; Váró, G.; Hernádi, K.; et al. Photosynthetic reaction center protein in nanostructures. Phys. Stat. Sol. B 2011, 248, 2700–2703. [Google Scholar] [CrossRef]
- Nagy, L.; Magyar, M.; Szabó, T.; Hajdu, K.; Giotta, L.; Dorogi, M.; Milano, F. Photosynthetic machineries in nano-systems. Curr. Protein Pept. Sci. 2014, 15, 363–373. [Google Scholar] [CrossRef]
- Wiwatowski, K.; Dużyńska, A.; Świniarski, M.; Szalkowski, M.; Zdrojek, M.; Judek, J.; Mackowski, S.; Kaminska, I. Energy transfer from natural photosynthetic complexes to single-wall carbon nanotubes. J. Lumin. 2016, 170, 855–859. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Ann. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1898, 990, 87–92. [Google Scholar] [CrossRef]
- Olvera-González, E.; Alaniz-Lumbreras, D.; Ivanov-Tsonchev, R.; Villa-Hernández, J.; Rosa-Vargas, I.D.L.; López-Cruz, I.; Silos-Espino, H.; Lara-Herrera, A. Chlorophyll fluorescence emission of tomato plants as a response to pulsed light based LEDs. Plant Growth Regul. 2013, 69, 117–123. [Google Scholar] [CrossRef]
- Jenks, M.A.; Eigenbrode, S.D.; Lemieux, B. Cuticular waxes of Arabidopsis. Arab. Book 2002, 1, e0016. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Martínez, M.; Cambra, I.; Grbic, V.; Diaz, I. Understanding plant defence responses against herbivore attacks: An essential first step towards the development of sustainable resistance against pests. Transgen. Res. 2013, 22, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Riederer, M.; Schreiber, L. Waxes—The transport barriers of plant cuticles. In Waxes: Chemistry, Molecular Biology and Functions; Hamilton, R.J., Ed.; The Oily Press: Dundee, UK, 1995; pp. 131–156. [Google Scholar]
- Schreiber, L.; Kirsch, T.; Riederer, M. Diffusion through cuticles: Principles and models. In Plant Cuticles an Integrated Functional Approach; Kerstiens, G., Ed.; BIOS, Scientific Publishers Ltd.: Oxford, UK, 1996; pp. 109–119. [Google Scholar]
- Sánchez, F.J.; Manzanares, M.; de Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Residual transpiration rate, epicuticular wax load and leaf colour of pea plants in drought conditions. Influence on harvest index and canopy temperature. Eur. J. Agron. 2001, 15, 57–70. [Google Scholar] [CrossRef]
- Raffaele, S.; Leger, A.; Roby, D. Very long chain fatty acid and lipid signaling in the response of plants to pathogens. Plant Signal. Behav. 2009, 4, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, T.; Griffiths, D.W. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef]
- Chichiriccò, G.; Poma, A. Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 2015, 5, 851–873. [Google Scholar] [CrossRef]
- Shen, C.-X.; Zhang, Q.F.; Li, J.; Bi, F.-C.; Yao, N. Induction of programmed cell death in Arabidopsis and rice by single wall carbon nanotubes. Am. J. Bot. 2010, 97, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, H. Molecular crowding and order in photosynthetic membranes. Trends Plant Sci. 2008, 13, 201–207. [Google Scholar] [CrossRef]
- Fristedt, R.; Willig, A.; Granath, P.; Crévecoeur, M.; Rochaix, J.-D.; Vener, A.V. Phosphorylation of Photosystem II controls functional macroscopic folding of photosynthetic membranes in Arabidopsis. Plant Cell 2009, 21, 3950–3964. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. Drought and oxidative load in the leaves of C-3 plants: A predominant role for photorespiration? Ann. Bot. 2002, 89, 841–850. [Google Scholar] [CrossRef]
- Di Marco, G.; Iannelli, M.A.; Loreto, F. Relationship between photosynthesis and photorespiration in field-grown wheat leaves. Photosynthetica 1994, 30, 45–51. [Google Scholar]
- Townsend, A.J.; Saccon, F.; Giovagnetti, V.; Wilson, S.; Ungerer, P.; Ruban, A.V. The causes of altered chlorophyll fluorescence quenching induction in the Arabidopsis mutant lacking all minor antenna complexes. Biochim. Biophys. Acta 2018, 1859, 666–675. [Google Scholar] [CrossRef]
- Wang, C.; Yamamoto, H.; Shikanai, T. Role of cyclic electron transport around photosystem I in regulating proton motive force. Biochim. Biophys. Acta 2015, 1847, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Blatt, M.R.; Chen, Z.-H. Ion transport at the plant plasma membrane. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2018. [Google Scholar] [CrossRef]
- Krause, G.H.; Jahns, P. Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: Characterization and function. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 463–495. [Google Scholar] [CrossRef]
- Allen, J.F.; Bennett, J.; Steinback, K.E.; Arntzen, C.J. Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 1981, 291, 25–29. [Google Scholar] [CrossRef]
- Krause, G.H. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant. 1988, 74, 566–574. [Google Scholar] [CrossRef]
- Holzwarth, A.R.; Miloslavina, Y.; Nilkens, M.; Jahns, P. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting. Chem. Phys. Lett. 2009, 483, 262–267. [Google Scholar] [CrossRef]
- Nilkens, M.; Kress, E.; Lambrev, P.; Miloslavina, Y.; Müller, M.; Holzwarth, A.R.; Jahns, P. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim. Biophys. Acta 2010, 1797, 466–475. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Shukla, M.K.; Watanabe, A.; Wilson, S.; Giovagnetti, V.; Moustafa, E.I.; Minagawa, J.; Ruban, A.V. A novel method produces native LHCII aggregates from the photosynthetic membrane revealing their role in non-photochemical quenching. J. Biol. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Avenson, T.J.; Cruz, J.A.; Kanazawa, A.; Kramer, D.M. Regulating the proton budget of higher plant photosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9709–9713. [Google Scholar] [CrossRef]
- Calkins, J.O.; Umasankar, Y.; O’Neill, H.; Ramasamy, R.P. High photo-electrochemical activity of thylakoid-carbon nanotube composites for photosynthetic energy conversion. Energy Environ. Sci. 2013, 6, 1891–1900. [Google Scholar] [CrossRef]
- Müller-Moulé, P.; Conklin, P.L.; Niyogi, K.K. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2002, 128, 970–977. [Google Scholar] [CrossRef]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef]
- Marin, E.; Nussaume, L.; Quesada, A.; Gonneau, M.; Sotta, B.; Hugueney, P.; Frey, A.; Marion-Poll, A. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J. 1996, 5, 2331–2342. [Google Scholar] [CrossRef]
- Demmig, B.; Winter, K.; Krüger, A.; Czygan, F.-C. Photoinhibition and zeaxanthin formation in intact leaves. A possible role of the xanthophyll cycle in the dissipation of excess light. Plant Physiol. 1987, 84, 218–224. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Rock, C.D.; Zeevart, J.A.D. The ABA mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc. Natl Acad. Sci. USA 1991, 88, 7496–7499. [Google Scholar] [CrossRef]
- Schwarz, N.; Armbruster, U.; Iven, T.; Brückle, L.; Melzer, M.; Feussner, I.; Jahns, P. Tissue-specific accumulation and regulation of zeaxanthin epoxidase in Arabidopsis reflect the multiple functions of the enzyme in plastids. Plant Cell Physiol. 2015, 56, 346–357. [Google Scholar] [CrossRef]
- Kirchhoff, H. Diffusion of molecules and macromolecules in thylakoid membranes. Biochim. Biophys. Acta 2014, 1837, 495–502. [Google Scholar] [CrossRef]
- Färber, A.; Jahns, P. The xanthophyll cycle of higher plants: Influence of antenna size and membrane organization. Biochim. Biophys. Acta 1998, 1363, 47–58. [Google Scholar] [CrossRef]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef]
- Telfer, A. What is β-carotene doing in the photosystem II reaction centre? Philos. Trans. R. Soc. B 2002, 357, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Ramel, F.; Birtic, S.; Ginies, C.; Soubigou-Taconnat, L.; Triantaphylidès, C.; Havaux, M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5535–5540. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef]
- Formaggio, E.; Cinque, G.; Bassi, R. Functional architecture of the major Light-harvesting complex from higher plants. J. Mol. Biol. 2001, 314, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.D.; Georgiev, G.L. Fabrication of super-macroporous nanocomposites by deposition of carbon nanotubes onto polymer cryogels. Eur. Polym. J. 2012, 48, 1366–1373. [Google Scholar] [CrossRef]
- Petrov, P.; Georgiev, G.; Momekova, D.; Momekov, G.; Tsvetanov, C.B. UV-assisted grafting of polymers: A method towards biocompatible carbon nanotubes. Polymer 2010, 51, 2465–2471. [Google Scholar] [CrossRef]
- Velikova, V.; Tsonev, T.; Barta, C.; Centritto, M.; Koleva, D.; Stefanova, M.; Busheva, M.; Loreto, F. BVOC emissions, photosynthetic characteristics and changes in chloroplast ultra-structure of Platanus orientalis L. exposed to elevated CO2 and high temperature. Environ. Pollut. 2009, 157, 2629–2637. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photoche, mical and non-photochemical components calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Temperature dependence of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta 1991, 184, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Kuhlgert, S.; Austic, G.; Zegarac, R.; Osei-Bonsu, I.; Hoh, D.; Chilvers, M.I.; Roth, M.G.; Bi, K.; TerAvest, D.; Weebadde, P.; et al. MultispeQ Beta: A tool for large-scale plant phenotyping connected to the open PhotosynQ network. R. Soc. Open Sci. 2016, 3, 160592. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, A.; Kramer, D.M. In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc. Natl. Acad. Sci. USA 2002, 99, 12789–12794. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ostendorf, E.; Kohzuma, K.; Hoh, D.; Strand, D.D.; Sato-Cruz, M.; Savage, L.; Cruz, J.A.; Fisher, N.; Froehlich, J.E.; et al. Chloroplast ATP synthase modulation of the thylakoid proton motive force: Implications for photosystem I and photosystem II photoprotection. Front. Plant Sci. 2017, 8, 719. [Google Scholar] [CrossRef]
- Gierczik, K.; Vukušić, T.; Kovács, L.; Székely, A.; Szalai, G.; Milošević, S.; Kocsy, G.; Kutasi, K.; Galiba, G. Plasma-activated water to improve the stress tolerance of barley. Plasma Process. Polym. 2020, 17, 1900123. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Mantoura, R.F.C.; Bjørnland, T. Data for the identification of 47 key phytoplankton pigments. In Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Jeffrey, S.W., Mantoura, R.F.C., Wright, S.W., Eds.; UNESCO Publishing: Paris, France, 1997; pp. 447–560. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velikova, V.; Petrova, N.; Kovács, L.; Petrova, A.; Koleva, D.; Tsonev, T.; Taneva, S.; Petrov, P.; Krumova, S. Single-Walled Carbon Nanotubes Modify Leaf Micromorphology, Chloroplast Ultrastructure and Photosynthetic Activity of Pea Plants. Int. J. Mol. Sci. 2021, 22, 4878. https://doi.org/10.3390/ijms22094878
Velikova V, Petrova N, Kovács L, Petrova A, Koleva D, Tsonev T, Taneva S, Petrov P, Krumova S. Single-Walled Carbon Nanotubes Modify Leaf Micromorphology, Chloroplast Ultrastructure and Photosynthetic Activity of Pea Plants. International Journal of Molecular Sciences. 2021; 22(9):4878. https://doi.org/10.3390/ijms22094878
Chicago/Turabian StyleVelikova, Violeta, Nia Petrova, László Kovács, Asya Petrova, Dimitrina Koleva, Tsonko Tsonev, Stefka Taneva, Petar Petrov, and Sashka Krumova. 2021. "Single-Walled Carbon Nanotubes Modify Leaf Micromorphology, Chloroplast Ultrastructure and Photosynthetic Activity of Pea Plants" International Journal of Molecular Sciences 22, no. 9: 4878. https://doi.org/10.3390/ijms22094878
APA StyleVelikova, V., Petrova, N., Kovács, L., Petrova, A., Koleva, D., Tsonev, T., Taneva, S., Petrov, P., & Krumova, S. (2021). Single-Walled Carbon Nanotubes Modify Leaf Micromorphology, Chloroplast Ultrastructure and Photosynthetic Activity of Pea Plants. International Journal of Molecular Sciences, 22(9), 4878. https://doi.org/10.3390/ijms22094878







