2-Arachidonyl-lysophosphatidylethanolamine Induces Anti-Inflammatory Effects on Macrophages and in Carrageenan-Induced Paw Edema
Abstract
1. Introduction
2. Results
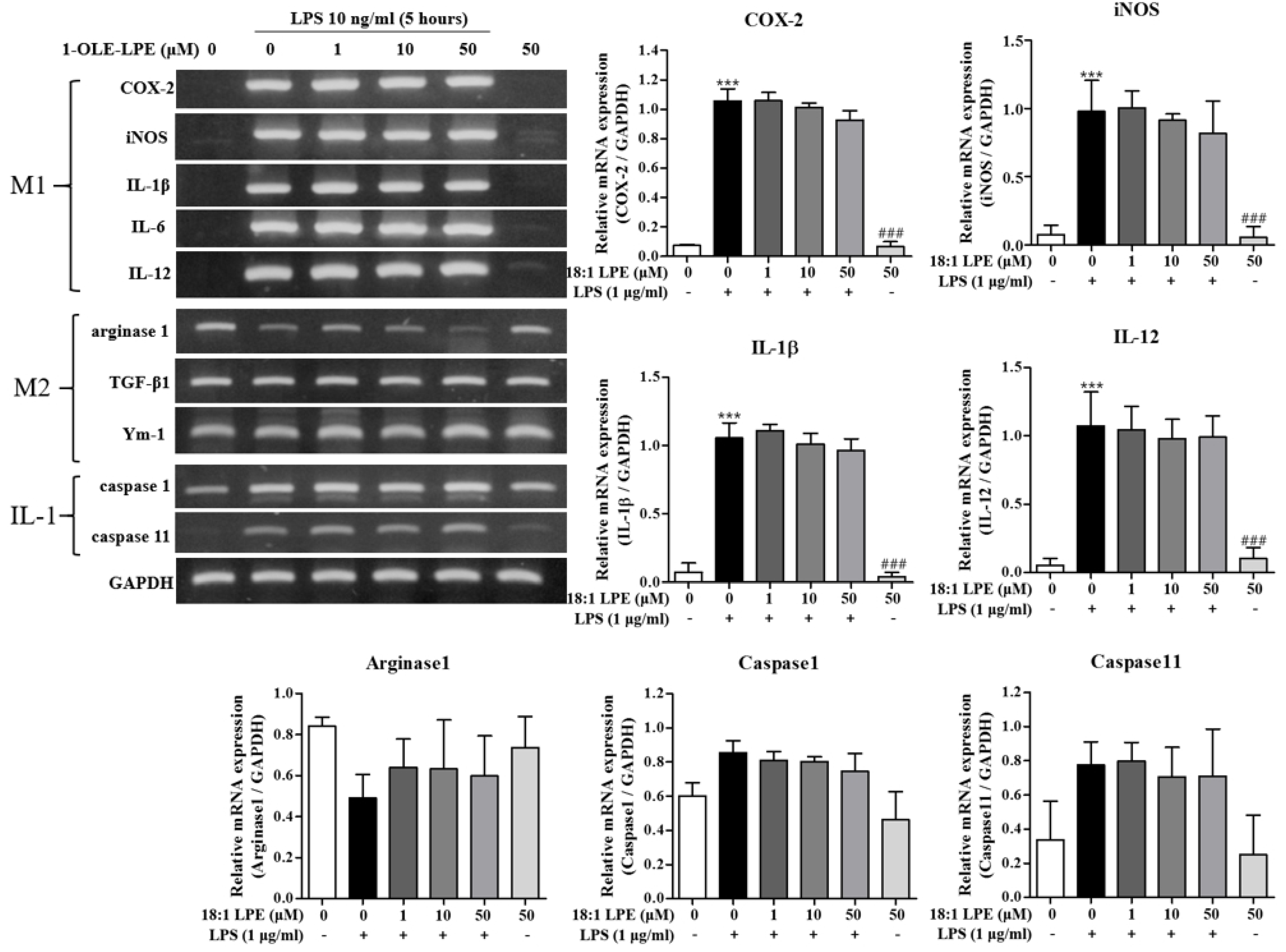
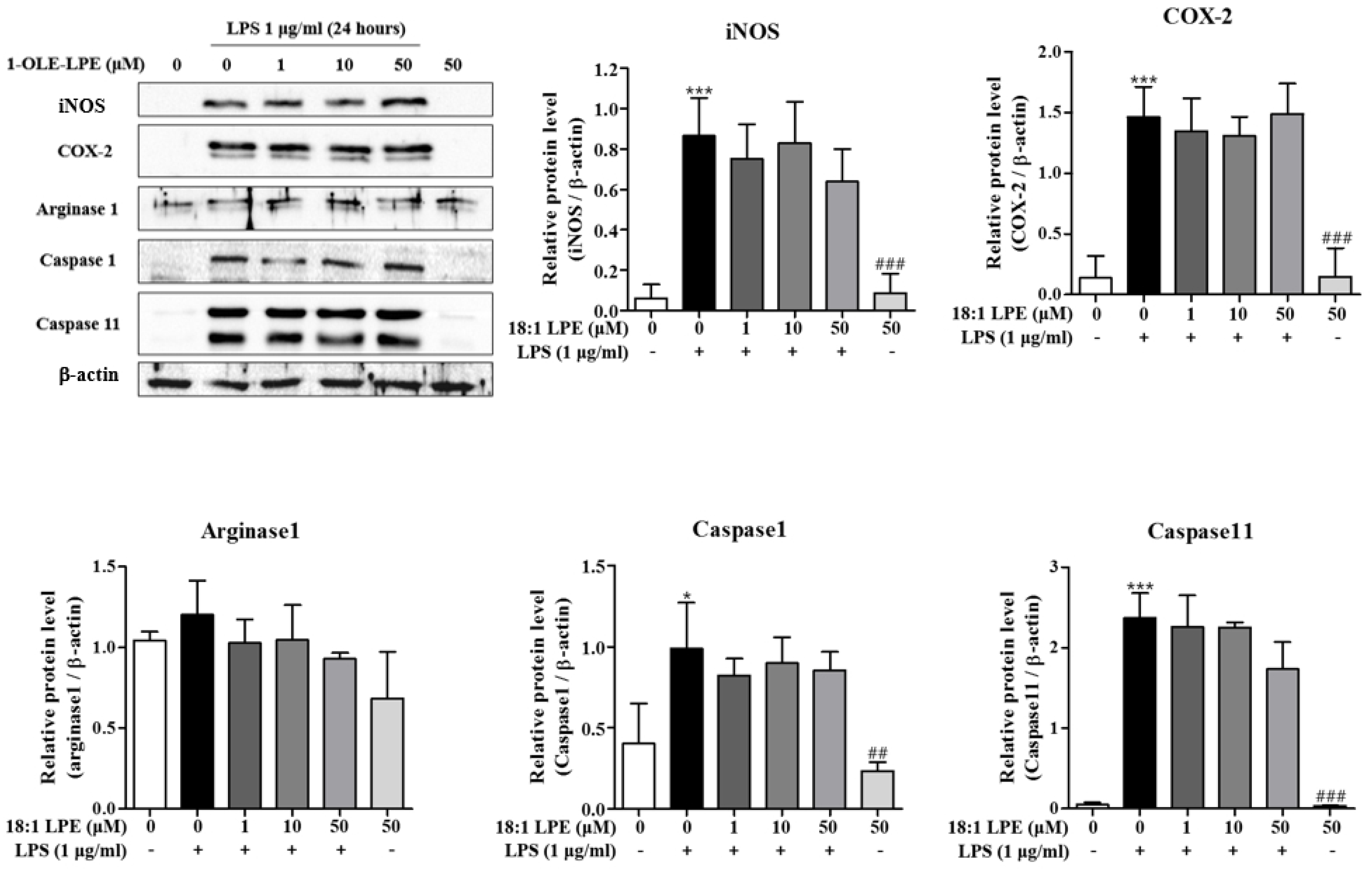
2.1. Effects of 2-ARA-LPE on Macrophage Phenotypes
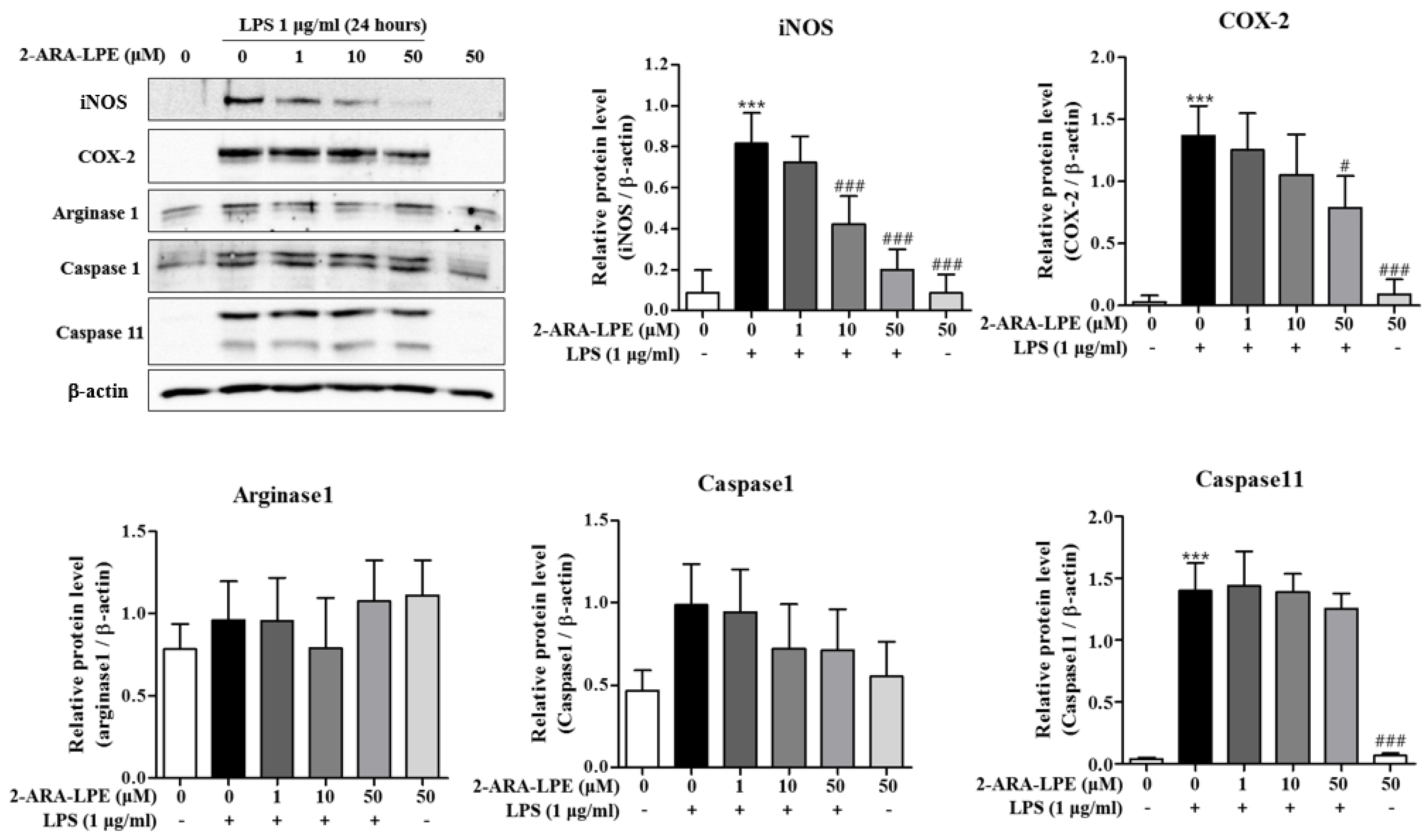
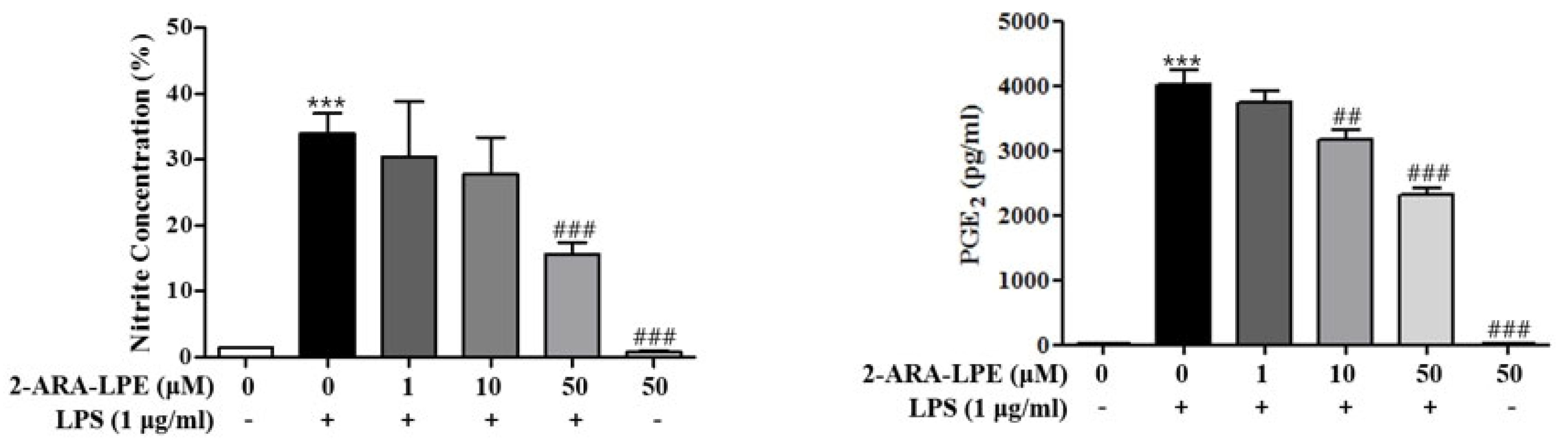
2.2. Effects of 2-ARA-LPE on COX-2 and iNOS Expression
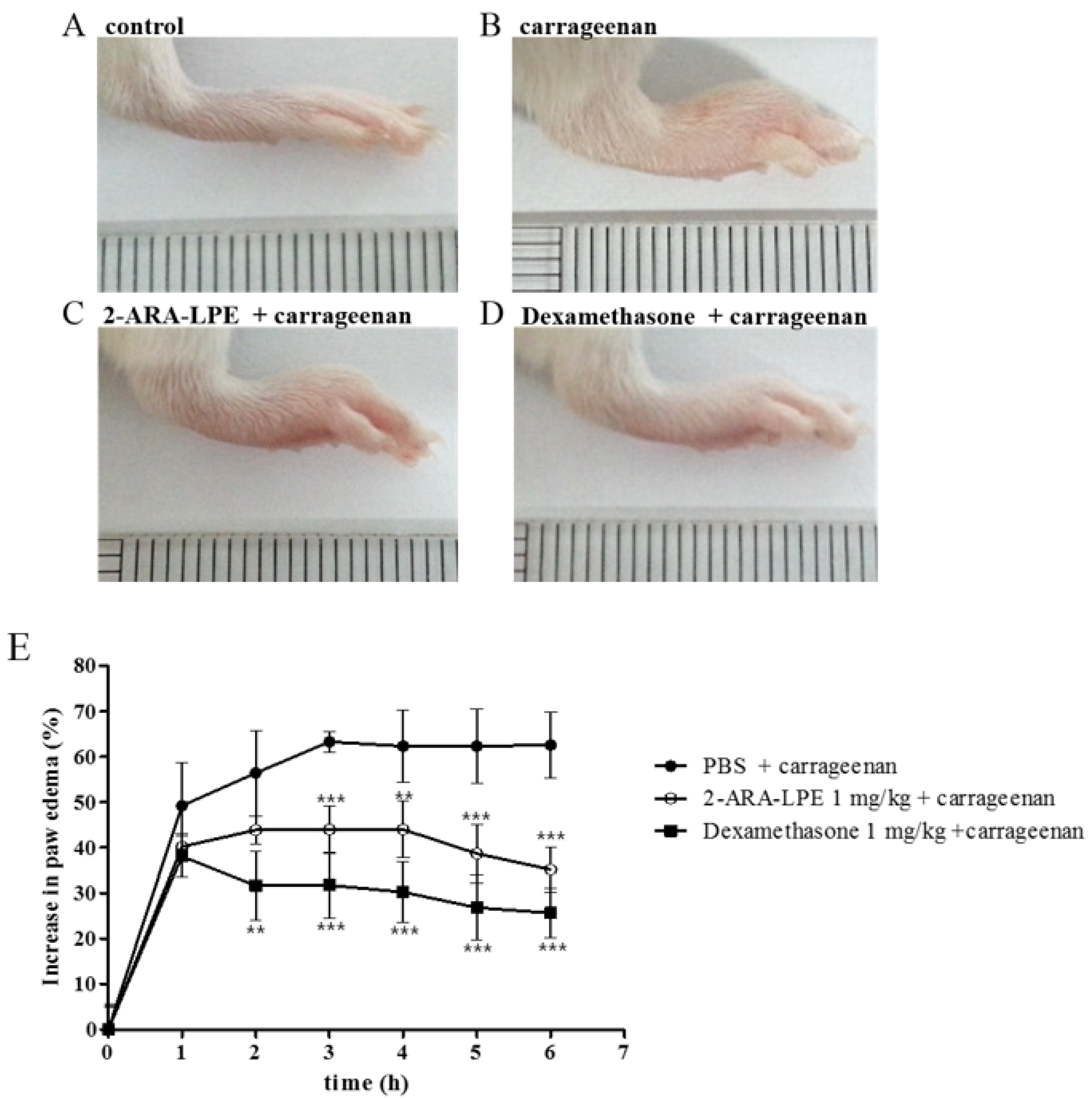
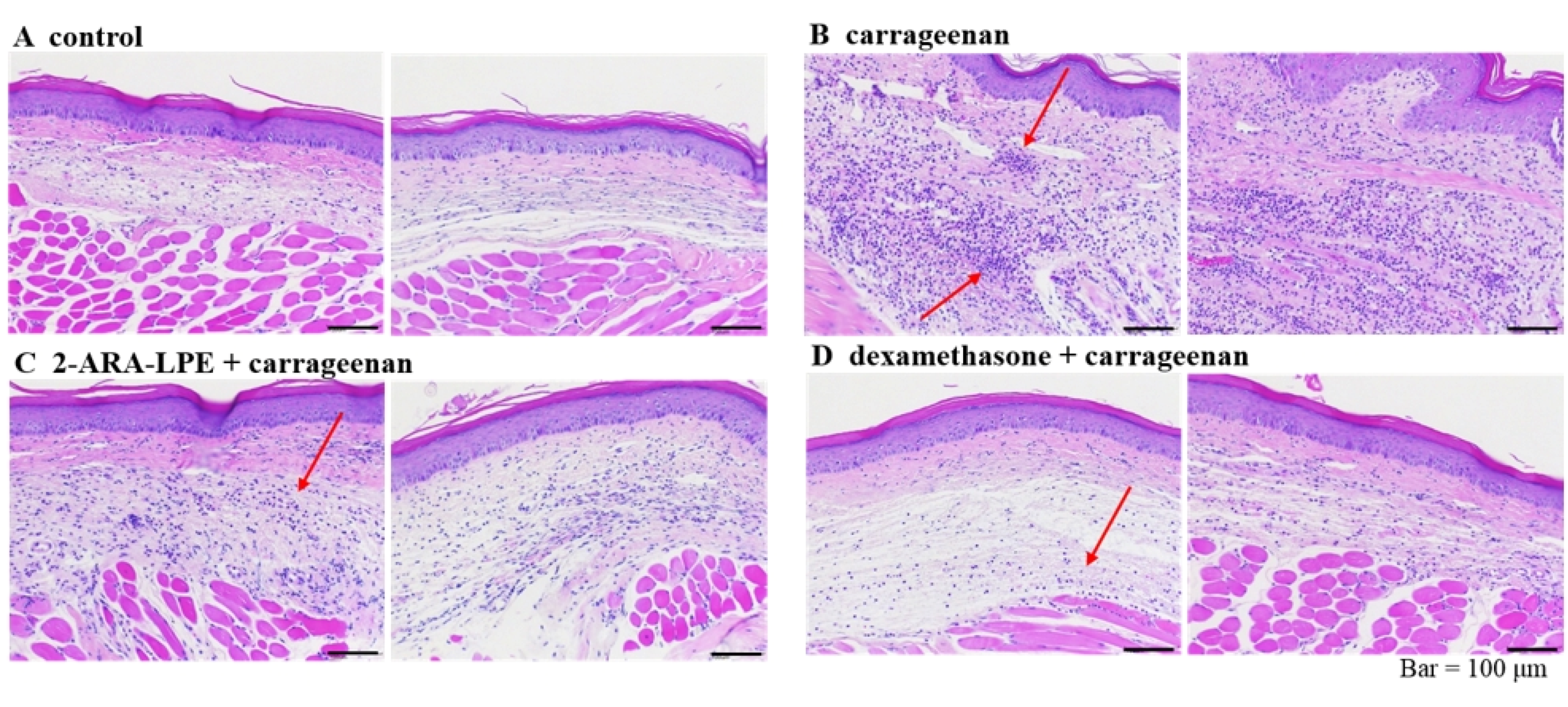
2.3. Effect of 2-ARA-LPE on Carrageenan-Induced Paw Edema
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Isolation and Culture of Mouse Peritoneal Macrophages
4.4. Reverse Transcriptase-PCR
4.5. Western Blot
4.6. Nitrites Measurement
4.7. PGE2 Production
4.8. Carrageenan-Induced Paw Edema Assay
4.9. Histology
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Im, D.-S. Intercellular Lipid Mediators and GPCR Drug Discovery. Biomol. Ther. 2013, 21, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Makide, K.; Kitamura, H.; Sato, Y.; Okutani, M.; Aoki, J. Emerging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat. 2009, 89, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Misra, U. Isolation of lysophosphatidylethanolamine from human serum. Biochim. et Biophys. Acta (BBA) Lipids Lipid Metab. 1965, 106, 371–378. [Google Scholar] [CrossRef]
- Park, K.S.; Lee, H.Y.; Lee, S.Y.; Kim, M.-K.; Kim, S.D.; Kim, J.M.; Yun, J.; Im, D.-S.; Bae, Y.-S. Lysophosphatidylethanolamine stimulates chemotactic migration and cellular invasion in SK-OV3 human ovarian cancer cells: Involvement of pertussis toxin-sensitive G-protein coupled receptor. FEBS Lett. 2007, 581, 4411–4416. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Lee, K.-P.; Kang, S.; Chung, H.-Y.; Bae, Y.-S.; Okajima, F.; Im, D.-S. Lysophosphatidylethanolamine utilizes LPA1 and CD97 in MDA-MB-231 breast cancer cells. Cell. Signal. 2013, 25, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Lee, K.-P.; Im, D.-S. Action and Signaling of Lysophosphatidylethanolamine in MDA-MB-231 Breast Cancer Cells. Biomol. Ther. 2014, 22, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-M.; Park, S.-J.; Im, D.-S. Lysophosphatidylethanolamine increases intracellular Ca2+ through LPA1 in PC-12 neuronal cells. Biochem. Biophys. Res. Commun. 2015, 461, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Suzuki, A.; Inoue, A.; Tokuhara, Y.; Kano, K.; Matsumoto, H.; Igarashi, K.; Ohkawa, R.; Nakamura, K.; Dohi, T.; et al. Possible Involvement of Minor Lysophospholipids in the Increase in Plasma Lysophosphatidic Acid in Acute Coronary Syndrome. Arter. Thromb. Vasc. Biol. 2015, 35, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.D.; Kim, M.R.; Sok, D.-E. 2-Polyunsaturated Acyl Lysophosphatidylethanolamine Attenuates Inflammatory Response in Zymosan A-Induced Peritonitis in Mice. Lipids 2011, 46, 893–906. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, K.-P.; Kang, S.; Lee, J.; Sato, K.; Chung, H.Y.; Okajima, F.; Im, D.-S. Sphingosine 1-phosphate induced anti-atherogenic and atheroprotective M2 macrophage polarization through IL-4. Cell. Signal. 2014, 26, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.T.; Lee, Y.M.; Kang, G.J.; Yoo, E.S.; Hong, J.; Lee, S.M.; Lee, S.K.; Pyee, Y.; Chung, H.-J.; Moon, H.R.; et al. In vitro stability and in vivo anti-inflammatory efficacy of synthetic jasmonates. Bioorganic Med. Chem. 2012, 20, 4109–4116. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.-K.; Lee, J.-W.; Moon, E.-Y. Thymosin Beta-4, Actin-Sequestering Protein Regulates Vascular Endothelial Growth Factor Expression via Hypoxia-Inducible Nitric Oxide Production in HeLa Cervical Cancer Cells. Biomol. Ther. 2015, 23, 19–25. [Google Scholar] [CrossRef]
- Kang, J.; Lee, J.-H.; Im, D.-S. Topical Application of S1P2 Antagonist JTE-013 Attenuates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in Mice. Biomol. Ther. 2020, 28, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.Y.; Im, D.S. Pro-Inflammatory Role of S1P3 in Macrophages. Biomol. Ther. 2019, 27, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Park, S.-J.; Lee, A.-Y.; Huang, J.; Chung, H.-Y.; Im, D.-S. Ginsenoside Rg3 promotes inflammation resolution through M2 macrophage polarization. J. Ginseng Res. 2018, 42, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Im, D.S. Deficiency of Sphingosine-1-Phosphate Receptor 2 (S1P2) Attenuates Bleomycin-Induced Pulmonary Fibrosis. Biomol. Ther. 2019, 27, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Cao, J.; Huang, J.; Liu, S.; Im, D.S.; Yoo, J.-W.; Jung, J.H. The In Vitro and In Vivo Anti-Inflammatory Effects of a Phthalimide PPAR-γ Agonist. Mar. Drugs 2017, 15, 7. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-J.; Im, D.-S. 2-Arachidonyl-lysophosphatidylethanolamine Induces Anti-Inflammatory Effects on Macrophages and in Carrageenan-Induced Paw Edema. Int. J. Mol. Sci. 2021, 22, 4865. https://doi.org/10.3390/ijms22094865
Park S-J, Im D-S. 2-Arachidonyl-lysophosphatidylethanolamine Induces Anti-Inflammatory Effects on Macrophages and in Carrageenan-Induced Paw Edema. International Journal of Molecular Sciences. 2021; 22(9):4865. https://doi.org/10.3390/ijms22094865
Chicago/Turabian StylePark, Soo-Jin, and Dong-Soon Im. 2021. "2-Arachidonyl-lysophosphatidylethanolamine Induces Anti-Inflammatory Effects on Macrophages and in Carrageenan-Induced Paw Edema" International Journal of Molecular Sciences 22, no. 9: 4865. https://doi.org/10.3390/ijms22094865
APA StylePark, S.-J., & Im, D.-S. (2021). 2-Arachidonyl-lysophosphatidylethanolamine Induces Anti-Inflammatory Effects on Macrophages and in Carrageenan-Induced Paw Edema. International Journal of Molecular Sciences, 22(9), 4865. https://doi.org/10.3390/ijms22094865






