Caffeine Inhibits Direct and Indirect Angiogenesis in Zebrafish Embryos
Abstract
1. Introduction
2. Results
2.1. Caffeine Inhibits Developmental Angiogenesis in Zebrafish Embryos
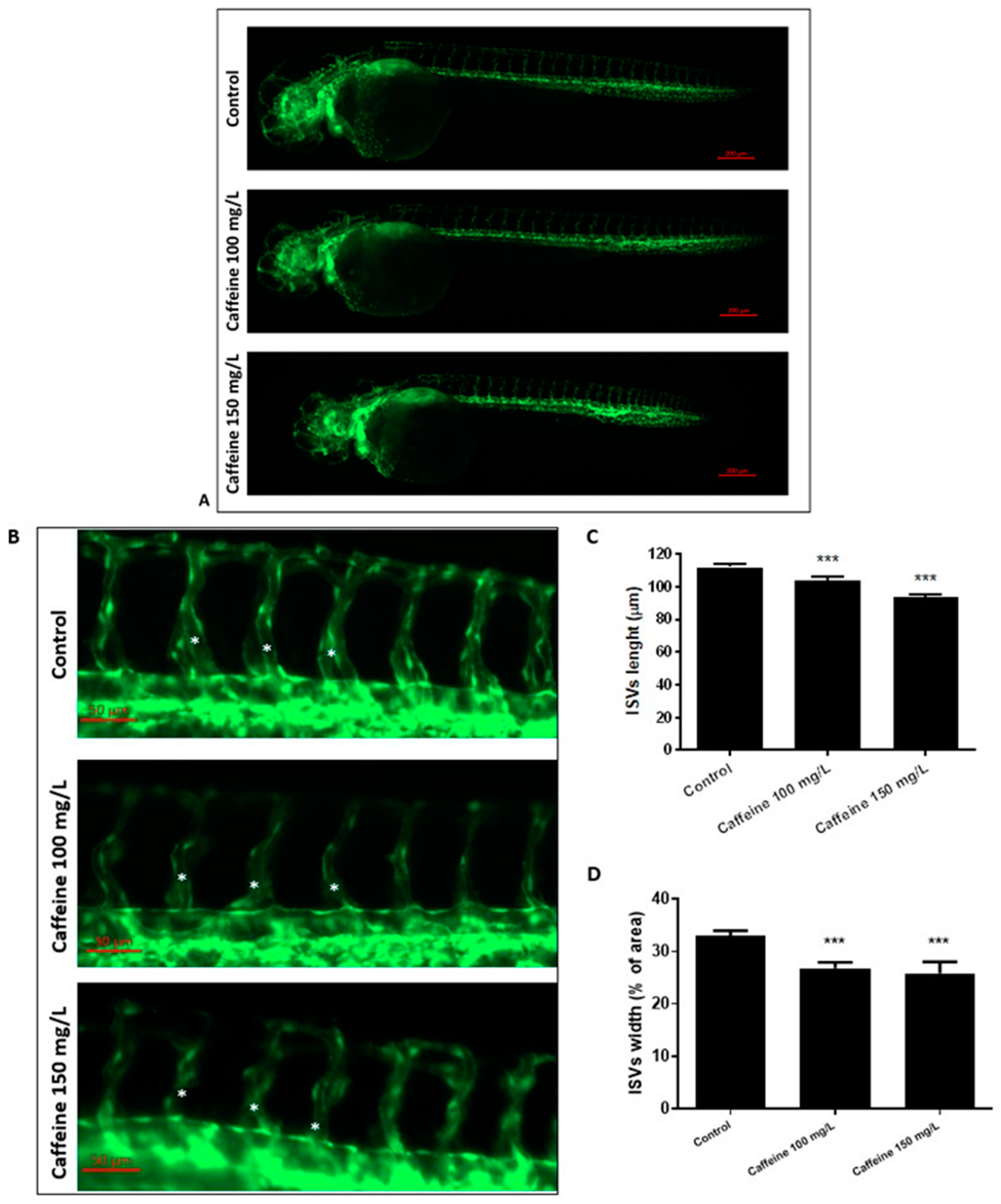
2.1.1. Effect of Caffeine on the Formation of ISVs
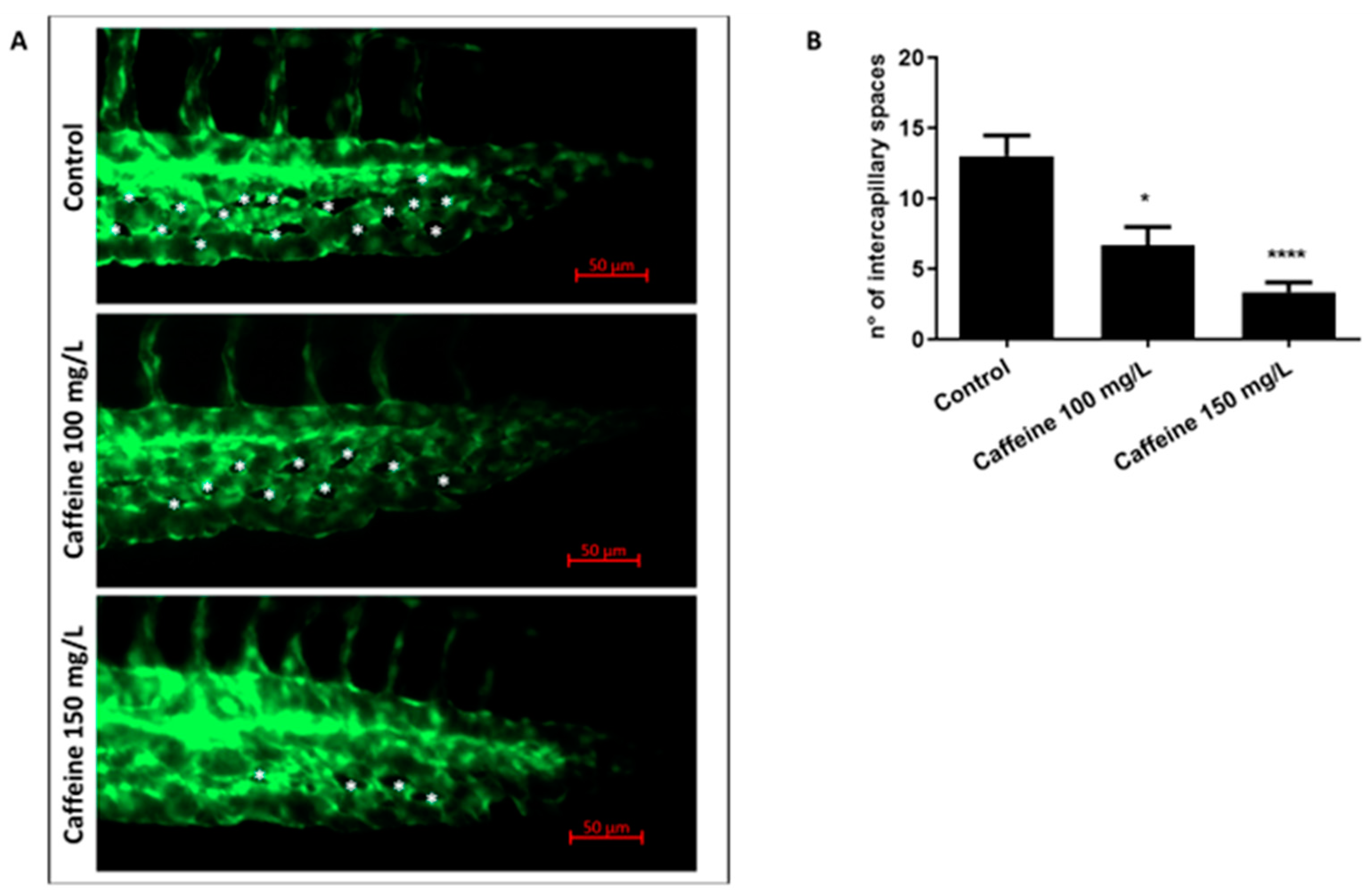
2.1.2. Caffeine Prevents the Remodeling of CVP
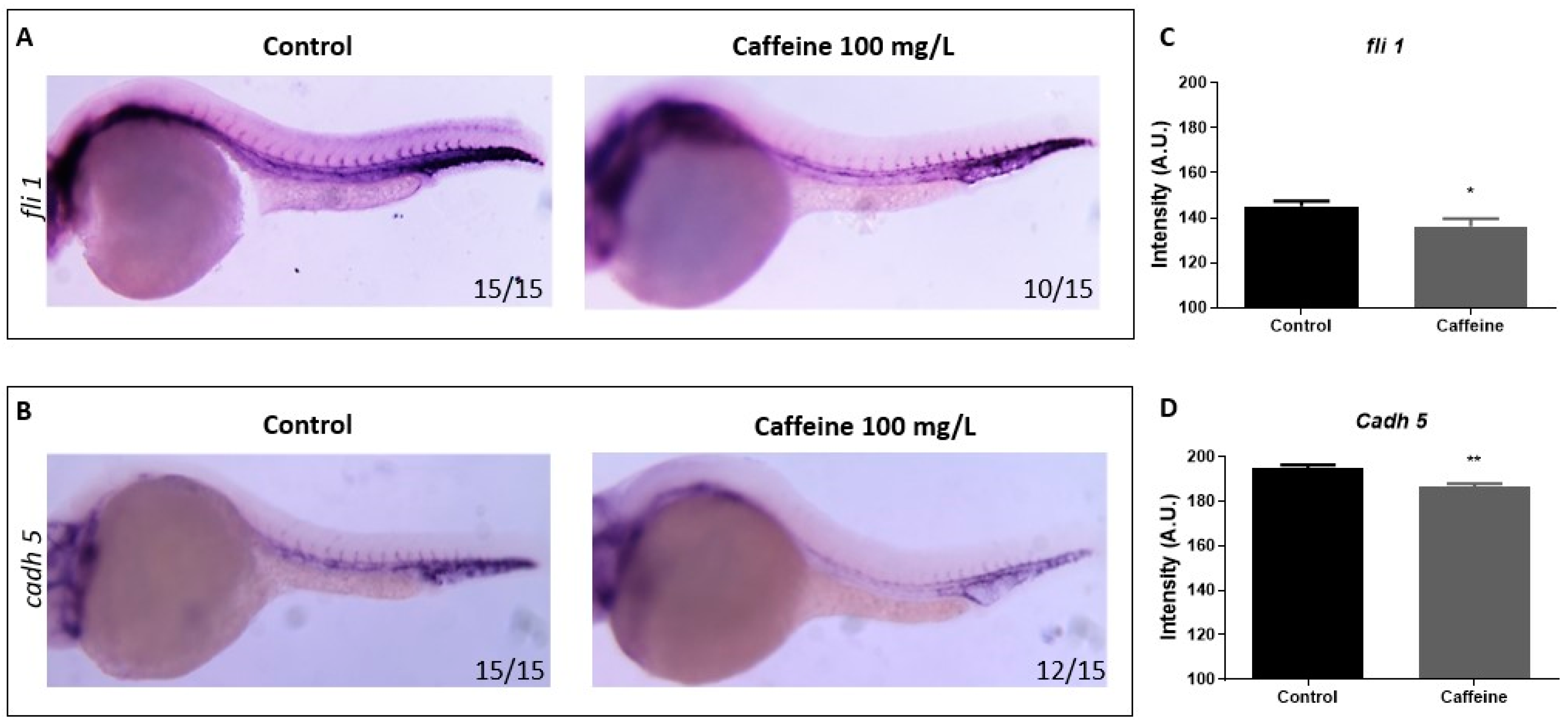
2.1.3. Caffeine Reduces the Expression of Vascular Markers fli1 and cadh5
2.1.4. Caffeine Inhibits the Formation of SIVP
2.2. Caffeine Inhibits FGF2 Mediated Angiogenesis
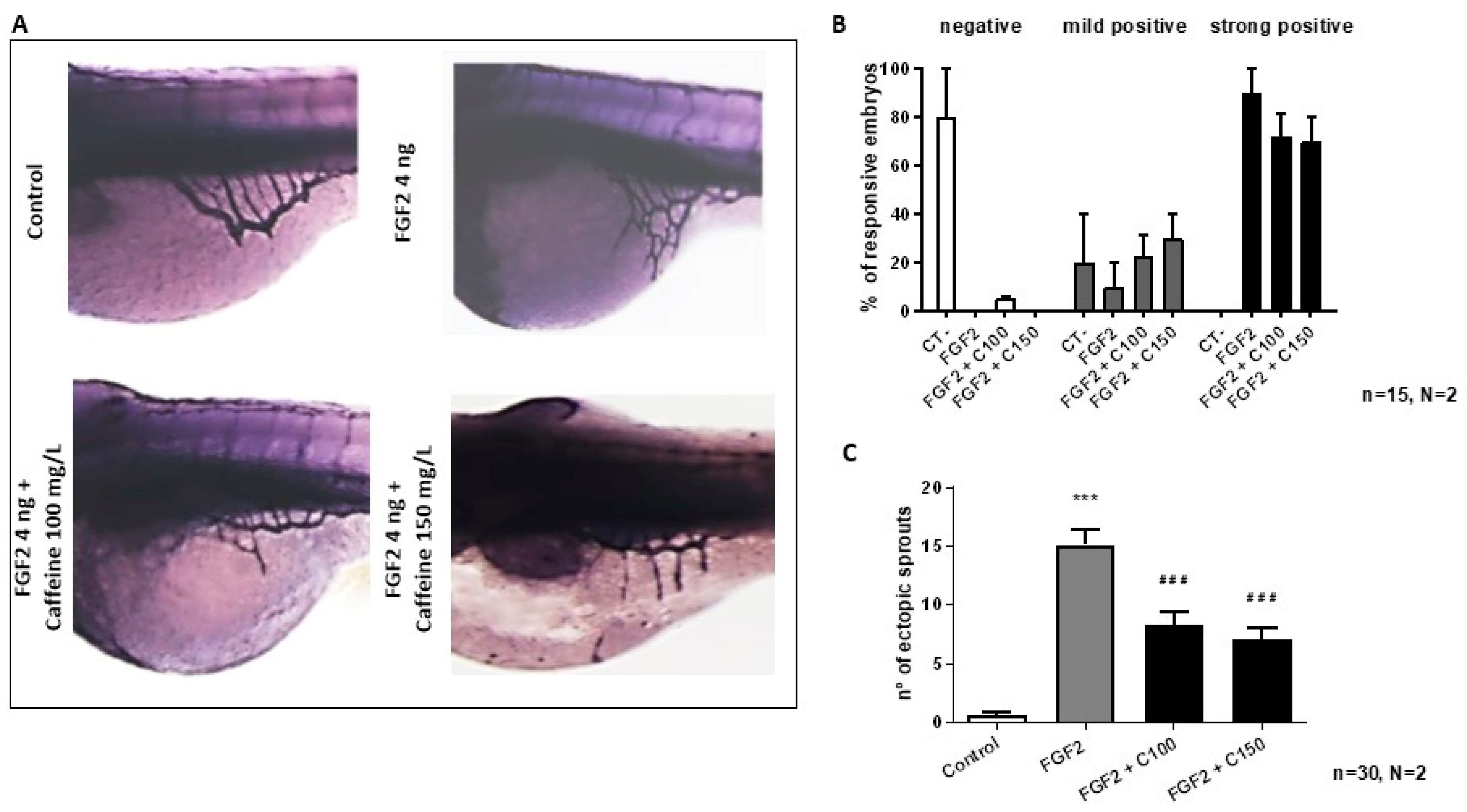
2.2.1. Caffeine Inhibits FGF2 Induced Neo-Angiogenesis
2.2.2. Caffeine Inhibits Neo-Angiogenesis Induced by Tumorigenic Cells
3. Discussion
4. Materials and Methods
4.1. Zebrafish Maintenance and Collection of Eggs
4.2. Caffeine Treatment
4.3. Cell Culture
4.4. Image Analysis of ISVs and CVP
4.5. WISH
4.6. AP Staining
4.7. ZFYM Assay
4.8. Phalloidin Staining
4.9. Zebrafish TX Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Szekanecz, Z.; Koch, A.E. Angiogenesis and its targeting in rheumatoid arthritis. Vascul. Pharmacol. 2009, 51, 1–7. [Google Scholar] [CrossRef]
- Jefferies, W.A.; Price, K.A.; Biron, K.E.; Fenninger, F.; Pfeifer, C.G.; Dickstein, D.L. Adjusting the compass: New insights into the role of angiogenesis in Alzheimer’s disease. Alzheimers Res. Ther. 2013, 5, 64. [Google Scholar] [CrossRef]
- Cantara, S.; Donnini, S.; Morbidelli, L.; Giachetti, A.; Schulz, R.; Memo, M.; Ziche, M. Physiological levels of amyloid peptides stimulate the angiogenic response through FGF-2. FASEB J. 2004, 18, 1943–1945. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Dev. Biol. 2001, 230, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Samsa, L.A.; Qian, L.; Liu, J. Advances in the Study of Heart Development and Disease Using Zebrafish. J. Cardiovasc. Dev. Dis. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Chávez, M.N.; Aedo, G.; Fierro, F.A.; Allende, M.L.; Egaña, J.T. Zebrafish as an Emerging Model Organism to Study Angiogenesis in Development and Regeneration. Front. Physiol. 2016, 7, 56. [Google Scholar] [CrossRef]
- Goi, M.; Childs, S.J. Patterning mechanisms of the sub-intestinal venous plexus in zebrafish. Dev. Biol. 2016, 409, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, B.; Zhang, W.; Qian, Z.; Xiang, Y. Monitoring antiangiogenesis of bevacizumab in zebrafish. Drug Des. Devel. Ther. 2018, 12, 2423–2430. [Google Scholar] [CrossRef]
- Nicoli, S.; de Sena, G.; Presta, M. Fibroblast growth factor 2-induced angiogenesis in zebrafish: The zebrafish yolk membrane (ZFYM) angiogenesis assay. J. Cell Mol. Med. 2009, 13, 2061–2068. [Google Scholar] [CrossRef]
- Nicoli, S.; Ribatti, D.; Cotelli, F.; Presta, M. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 2007, 67, 2927–2931. [Google Scholar] [CrossRef]
- El-Kenawi, A.E.; El-Remessy, A.B. Angiogenesis inhibitors in cancer therapy: Mechanistic perspective on classification and treatment rationales. Br. J. Pharmacol. 2013, 170, 712–729. [Google Scholar] [CrossRef]
- Favrod-Coune, T.; Broers, B. The Health Effect of Psychostimulants: A Literature Review. Pharmaceuticals 2010, 3, 2333–2361. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bakre, M.; Yin, H.; Varner, J.A. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J. Clin. Investig. 2002, 110, 933–941. [Google Scholar] [CrossRef]
- Yeh, C.H.; Liao, Y.F.; Chang, C.Y.; Tsai, J.N.; Wang, Y.H.; Cheng, C.C.; Wen, C.C.; Chen, Y.H. Caffeine treatment disturbs the angiogenesis of zebrafish embryos. Drug Chem. Toxicol. 2012, 35, 361–365. [Google Scholar] [CrossRef]
- Li, H.; Jin, S.Y.; Son, H.J.; Seo, J.H.; Jeong, G.B. Caffeine-induced endothelial cell death and the inhibition of angiogenesis. Anat. Cell Biol. 2013, 46, 57–67. [Google Scholar] [CrossRef]
- Ma, Z.L.; Wang, G.; Lu, W.H.; Cheng, X.; Chuai, M.; Lee, K.K.; Yang, X. Investigating the effect of excess caffeine exposure on placental angiogenesis using chicken ’functional’ placental blood vessel network. J. Appl. Toxicol. 2016, 36, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Hsu, C.H.; Wen, Z.H.; Lin, C.S.; Agoramoorthy, G. Effect of caffeine, norfloxacin and nimesulide on heartbeat and VEGF expression of zebrafish larvae. J. Environ. Biol. 2011, 32, 179–183. [Google Scholar] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Saccone, S.; Federico, C.; Magro, G.; Cavallaro, S.; D’Agata, V. Caffeine Effect on HIFs/VEGF Pathway in Human Glioblastoma Cells Exposed to Hypoxia. Anticancer Agent. Med. Chem. 2018, 18, 1432–1439. [Google Scholar] [CrossRef]
- Merighi, S.; Benini, A.; Mirandola, P.; Gessi, S.; Varani, K.; Simioni, C.; Leung, E.; Maclennan, S.; Baraldi, P.G.; Borea, P.A. Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol. Pharmacol. 2007, 72, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; He, P.C.; Li, A.Q.; Cao, K.X.; Yan, J.W.; Guo, S.; Jiang, L.; Yao, L.; Dai, X.Y.; Feng, D.; et al. Caffeine promotes angiogenesis through modulating endothelial mitochondrial dynamics. Acta Pharmacol. Sin. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Kim, K.J.; Ryu, S.J.; Lee, B.Y. Caffeine prevents LPS-induced inflammatory responses in RAW264.7 cells and zebrafish. Chem. Biol. Interact. 2016, 248, 1–7. [Google Scholar] [CrossRef]
- Ben Shoham, A.; Malkinson, G.; Krief, S.; Shwartz, Y.; Ely, Y.; Ferrara, N.; Yaniv, K.; Zelzer, E. S1P1 inhibits sprouting angiogenesis during vascular development. Development 2012, 139, 3859–3869. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H.; Liu, T.; Zacksenhaus, E.; Ben-David, Y. The ets transcription factor Fli-1 in development, cancer and disease. Oncogene 2015, 34, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Montero-Balaguer, M.; Swirsding, K.; Orsenigo, F.; Cotelli, F.; Mione, M.; Dejana, E. Stable vascular connections and remodeling require full expression of VE-cadherin in zebrafish embryos. PLoS ONE 2009, 4, e5772. [Google Scholar] [CrossRef] [PubMed]
- Niderla-Bielińska, J.; Bartkowiak, K.; Ciszek, B.; Czajkowski, E.; Jankowska-Steifer, E.; Krejner, A.; Ratajska, A. Pentoxifylline inhibits angiogenesis via decreasing Dll4 and Notch1 expression in mouse proepicardial explant cultures. Eur. J. Pharmacol. 2018, 827, 80–87. [Google Scholar] [CrossRef]
- Bakkiyanathan, A.; Nathan, J.R.; Ravikumar, S.; Gopalakrishnan, T.S.; Aruldas, F.M.M.; Malathi, R. Anti-angiogenic effects of theophylline on developing zebrafish (Danio rerio) embryos. Biomed. Prev. Nutr. 2012, 2, 174–178. [Google Scholar] [CrossRef]
- Beis, D.; Bartman, T.; Jin, S.-W.; Scott, I.C.; D’Amico, L.A.; Ober, E.A.; Verkade, H.; Frantsve, J.; Field, H.A.; Wehman, A.; et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 2005, 132, 4193–4204. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.M.; Guarienti, M.; Memo, M. Zebrafish Embryo as an In Vivo Model for Behavioral and Pharmacological Characterization of Methylxanthine Drugs. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Hans, C.; McCollum, C.W.; Bondesson, M.B.; Gustafsson, J.A.; Shah, S.K.; Merchant, F.A. Automated analysis of zebrafish images for screening toxicants. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; Volume 2013, pp. 3004–3007. [Google Scholar] [CrossRef] [PubMed]
- Khatri, D.; Zizioli, D.; Tiso, N.; Facchinello, N.; Vezzoli, S.; Gianoncelli, A.; Memo, M.; Monti, E.; Borsani, G.; Finazzi, D. Down-regulation of coasy, the gene associated with NBIA-VI, reduces Bmp signaling, perturbs dorso-ventral patterning and alters neuronal development in zebrafish. Sci. Rep. 2016, 6, 37660. [Google Scholar] [CrossRef]
- Serbedzija, G.N.; Flynn, E.; Willett, C.E. Zebrafish angiogenesis: A new model for drug screening. Angiogenesis 1999, 3, 353–359. [Google Scholar] [CrossRef]
- Nicoli, S.; Presta, M. The zebrafish/tumor xenograft angiogenesis assay. Nat. Protoc. 2007, 2, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, S.; Cui, C.; Ten Dijke, P. Invasive Behavior of Human Breast Cancer Cells in Embryonic Zebrafish. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basnet, R.M.; Zizioli, D.; Muscò, A.; Finazzi, D.; Sigala, S.; Rossini, E.; Tobia, C.; Guerra, J.; Presta, M.; Memo, M. Caffeine Inhibits Direct and Indirect Angiogenesis in Zebrafish Embryos. Int. J. Mol. Sci. 2021, 22, 4856. https://doi.org/10.3390/ijms22094856
Basnet RM, Zizioli D, Muscò A, Finazzi D, Sigala S, Rossini E, Tobia C, Guerra J, Presta M, Memo M. Caffeine Inhibits Direct and Indirect Angiogenesis in Zebrafish Embryos. International Journal of Molecular Sciences. 2021; 22(9):4856. https://doi.org/10.3390/ijms22094856
Chicago/Turabian StyleBasnet, Ram Manohar, Daniela Zizioli, Alessia Muscò, Dario Finazzi, Sandra Sigala, Elisa Rossini, Chiara Tobia, Jessica Guerra, Marco Presta, and Maurizio Memo. 2021. "Caffeine Inhibits Direct and Indirect Angiogenesis in Zebrafish Embryos" International Journal of Molecular Sciences 22, no. 9: 4856. https://doi.org/10.3390/ijms22094856
APA StyleBasnet, R. M., Zizioli, D., Muscò, A., Finazzi, D., Sigala, S., Rossini, E., Tobia, C., Guerra, J., Presta, M., & Memo, M. (2021). Caffeine Inhibits Direct and Indirect Angiogenesis in Zebrafish Embryos. International Journal of Molecular Sciences, 22(9), 4856. https://doi.org/10.3390/ijms22094856








