Genome-Wide Analyses of the Temperature-Responsive Genetic Loci of the Pectinolytic Plant Pathogenic Pectobacterium atrosepticum
Abstract
1. Introduction
2. Results
2.1. Transposon Mutagenesis and Visual Estimation of β-glucuronidase Activity
2.2. Quantitative Spectrophotometric and Fluorometric GUS Assay
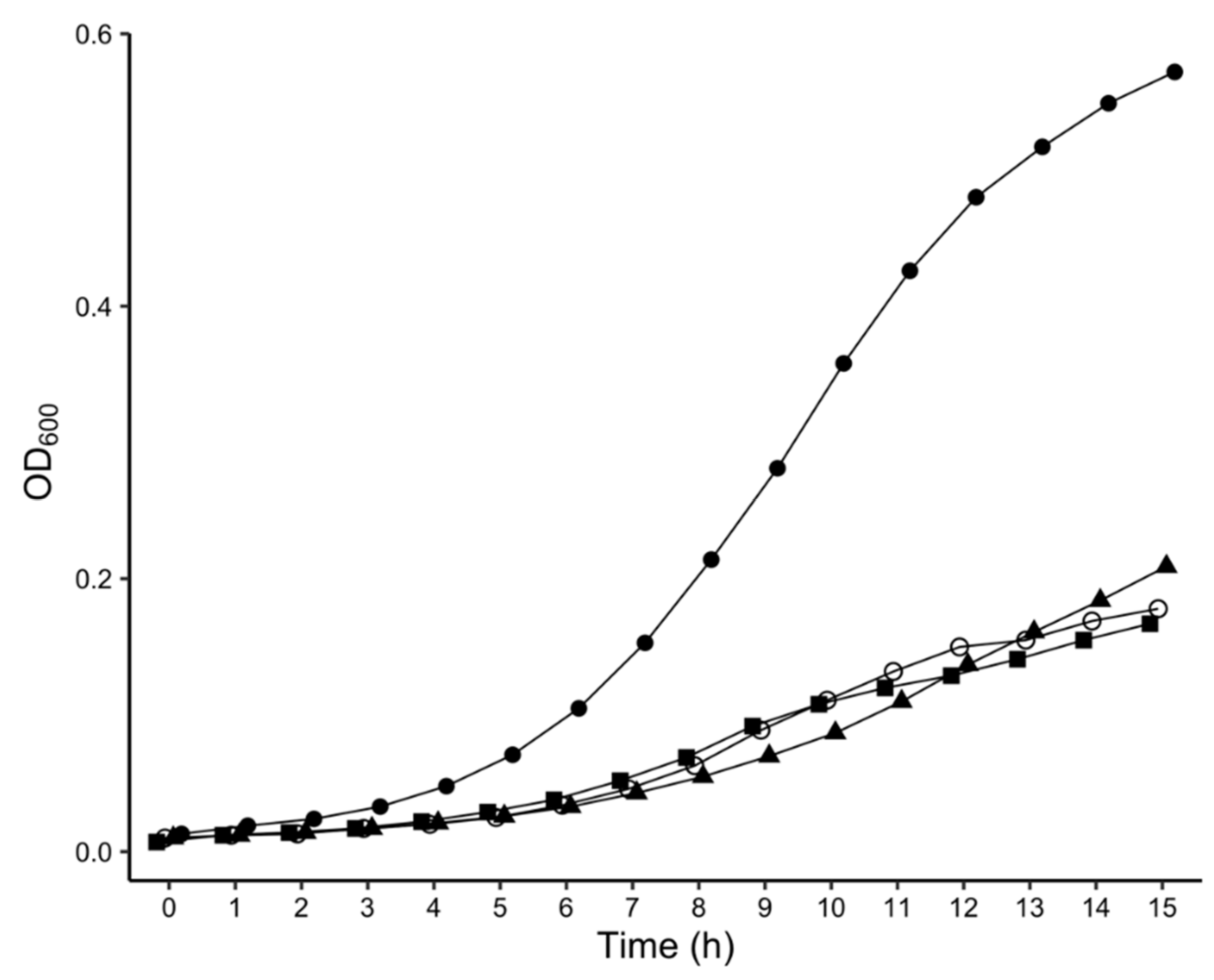
2.3. Phenotypic Characterization of P. atrosepticum Transposon Mutants
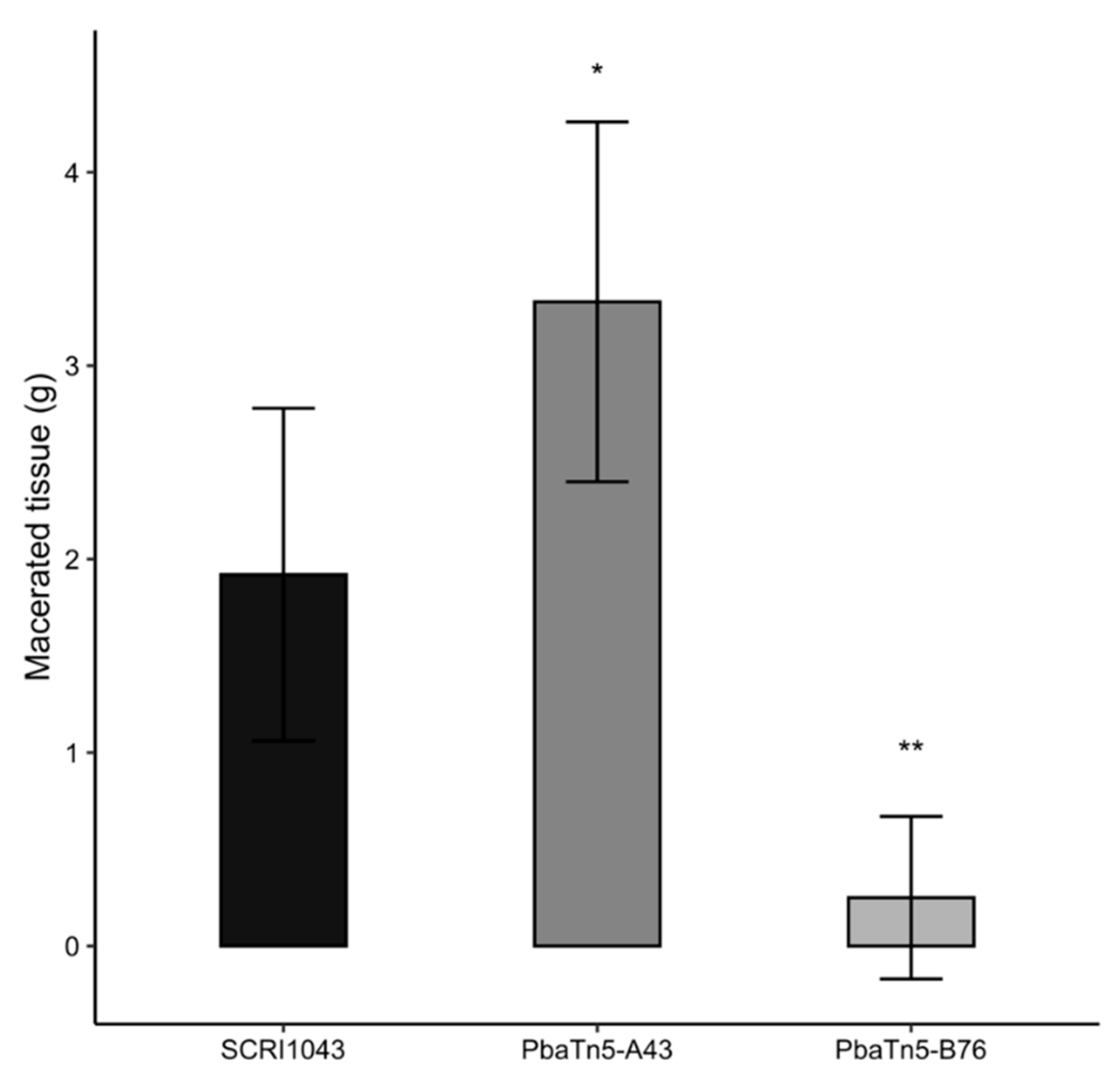
2.4. Virulence of P. atrosepticum Tn5 Mutants
2.5. Characterization of Transposon Insertion Sites
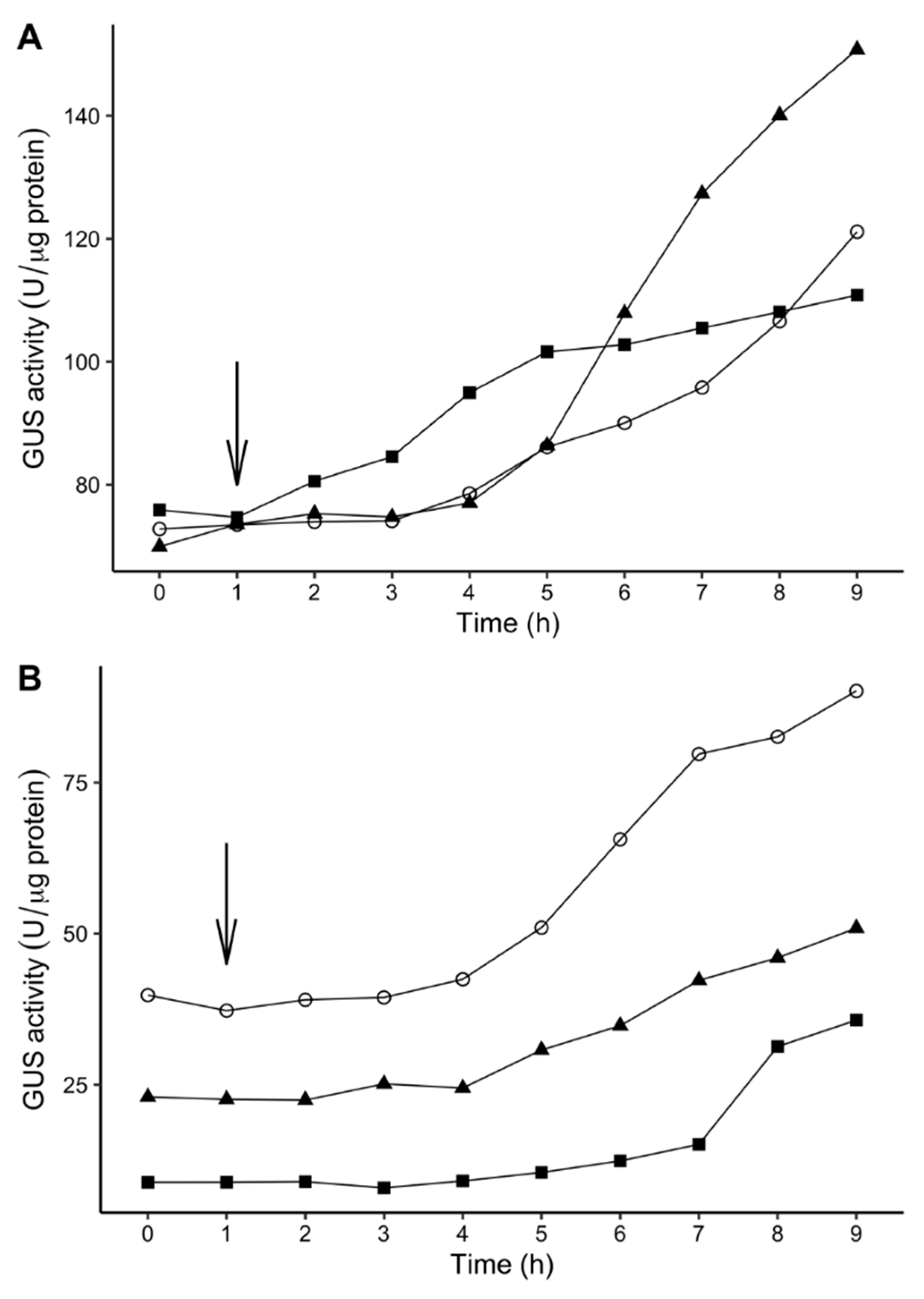
2.6. Time-Dependent Induction of Gene Expression Among P. atrosepticum Transposon Mutants
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Media Used
4.2. Transposon Mutagenesis of P. atrosepticum SCRI1043
4.3. Identification of P. atrosepticum Tn5 Mutants by PCR and Plating on Selective CVP Medium
4.4. Visual Estimation of β-glucuronidase Activity of P. atrosepticum Tn5 Mutants
4.5. Semi-Quantitative Assays to Assess the Rate of Gene Expression in P. atrosepticum Tn5 Mutants
4.6. Identification of Regions Flanking the Tn5 Transposon Insertion
4.7. Phenotypic Characterization of P. atrosepticum Transposon Mutants
4.8. Measurement of Bacterial Growth Rates
4.9. LPS Extraction and Analysis
4.10. Morphological Characterization of the P. atrosepticum SCRI1043 Tn5 Mutants by Electron Microscopy (TEM)
4.11. Time-Dependent Induction of Gene Expression in Tn5 Mutants
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 March 2021).
- Perombelon, M.C.M. Potato diseases caused by soft rot Erwinias: An overview of pathogenesis. Plant Pathol. 2002, 51, 1–12. [Google Scholar] [CrossRef]
- Czajkowski, R.; Grabe, G.J.; van der Wolf, J.M. Distribution of Dickeya spp. and Pectobacterium carotovorum subsp. carotovorum in naturally infected seed potatoes. Eur. J. Plant Pathol. 2009, 125, 263–275. [Google Scholar] [CrossRef]
- Toth, I.K.; van der Wolf, J.M.; Saddler, G.; Lojkowska, E.; Helias, V.; Pirhonen, M.; Tsror (Lahkim), L.; Elphinstone, J.G. Dickeya species: An emerging problem for potato production in Europe. Plant Pathol. 2011, 60, 385–399. [Google Scholar] [CrossRef]
- Gardan, L.; Gouy, C.; Christen, R.; Samson, R. Elevation of three subspecies of Pectobacterium carotovorum to species level: Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Khayi, S.; Cigna, J.; Chong, T.M.; Quetu-Laurent, A.; Chan, K.-G.; Helias, V.; Faure, D. Transfer of the potato plant isolates of Pectobacterium wasabiae to Pectobacterium parmentieri sp. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5379–5383. [Google Scholar] [CrossRef] [PubMed]
- Dees, M.W.; Lysøe, E.; Rossmann, S.; Perminow, J.; Brurberg, M.B. Pectobacterium polaris sp. nov., isolated from potato (Solanum tuberosum). Int. J. Syst. Evol. Microbiol. 2017, 67, 5222–5229. [Google Scholar] [CrossRef]
- Sarfraz, S.; Riaz, K.; Oulghazi, S.; Cigna, J.; Sahi, S.T.; Khan, S.H.; Faure, D. Pectobacterium punjabense sp. nov., isolated from blackleg symptoms of potato plants in Pakistan. Int. J. Syst. Evol. Microbiol. 2018, 68, 3551–3556. [Google Scholar] [CrossRef] [PubMed]
- Pedron, J.; Bertrand, C.; Taghouti, G.; Portier, P.; Barny, M.A. Pectobacterium aquaticum sp. nov., isolated from waterways. Int. J. Syst. Evol. Microbiol. 2019, 69, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Portier, P.; Pédron, J.; Taghouti, G.; Fischer-Le Saux, M.; Caullireau, E.; Bertrand, C.; Laurent, A.; Chawki, K.; Oulgazi, S.; Moumni, M.; et al. Elevation of Pectobacterium carotovorum subsp. odoriferum to species level as Pectobacterium odoriferum sp. nov., proposal of Pectobacterium brasiliense sp. nov. and Pectobacterium actinidiae sp. nov., emended description of Pectobacterium carotovorum and description of Pectobacterium versatile sp. nov., isolated from streams and symptoms on diverse plants. Int. J. Syst. Evol. Microbiol. 2019, 69, 3207–3216. [Google Scholar]
- Waleron, M.; Misztak, A.; Waleron, M.; Franczuk, M.; Wielgomas, B.; Waleron, K. Transfer of Pectobacterium carotovorum subsp. carotovorum strains isolated from potatoes grown at high altitudes to Pectobacterium peruviense sp. nov. Syst. Appl. Microbiol. 2018, 41, 85–93. [Google Scholar] [CrossRef]
- Waleron, M.; Misztak, A.; Waleron, M.; Jonca, J.; Furmaniak, M.; Waleron, K. Pectobacterium polonicum sp. nov. isolated from vegetable fields. Int. J. Syst. Evol. Microbiol. 2019, 69, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, M.; Waleron, M.; Schott, T.; Cleenwerck, I.; Misztak, A.; Waleron, K.; Pritchard, L.; Bakr, R.; Degefu, Y.; van der Wolf, J.; et al. Pectobacterium parvum sp. nov., having a Salmonella SPI-1-like type III secretion system and low virulence. Int. J. Syst. Evol. Microbiol. 2020, 70, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; Legendre, J.B.; Christen, R.; Fischer-Le Saux, M.; Achouak, W.; Gardan, L. Transfer of Pectobacterium chrysanthemi (Burkholder et al. 1953) Brenner et al. 1973 and Brenneria paradisiaca to the genus Dickeya gen. nov. as Dickeya chrysanthemi comb. nov. and Dickeya paradisiaca comb. nov. and delineation of four novel species, Dickeya dadantii sp. nov., Dickeya dianthicola sp. nov, Dickeya dieffenbachiae sp. nov. and Dickeya zeae sp. nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 1415–1427. [Google Scholar]
- Parkinson, N.; DeVos, P.; Pirhonen, M.; Elphinstone, J. Dickeya aquatica sp. nov., isolated from waterways. Int. J. Syst. Evol. Microbiol. 2014, 64, 2264–2266. [Google Scholar] [CrossRef] [PubMed]
- van der Wolf, J.M.; Nijhuis, E.H.; Kowalewska, M.J.; Saddler, G.S.; Parkinson, N.; Elphinstone, J.G.; Pritchard, L.; Toth, I.K.; Lojkowska, E.; Potrykus, M.; et al. Dickeya solani sp. nov., a pectinolytic plant-pathogenic bacterium isolated from potato (Solanum tuberosum). Int. J. Syst. Evol. Microbiol. 2014, 64, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.K.; Barny, M.; Czajkowski, R.; Elphinstone, J.G.; Li, X.; Pédron, J.; Pirhonen, M.; Van Gijsegem, F. Pectobacterium and Dickeya: Taxonomy and evolution. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 13–37. [Google Scholar]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Perombelon, M.C.M.; Kelman, A. Ecology of the soft rot Erwinias. Annu. Rev. Phytopathol. 1980, 18, 361–387. [Google Scholar] [CrossRef]
- Cother, E.J.; Gilbert, R.L. Presence of Erwinia chrysanthemi in two major river systems and their alpine sources in Australia. J. Appl. Bacteriol. 1990, 69, 729–738. [Google Scholar] [CrossRef]
- Laurila, J.; Hannukkala, A.; Nykyri, J.; Pasanen, M.; Hélias, V.; Garlant, L.; Pirhonen, M. Symptoms and yield reduction caused by Dickeya spp. strains isolated from potato and river water in Finland. Eur. J. Plant Pathol. 2010, 126, 249–262. [Google Scholar] [CrossRef]
- Tsror (Lahkim), L.; Lebiush, S.; Erlich, O.; Ben-Daniel, B.; van der Wolf, J. First report of latent infection of Cyperus rotundus cused by a biovar 3 Dickeya sp. (syn. Erwinia chrysanthemi) in Israel. New Dis. Rep. 2010, 22, 14. [Google Scholar] [CrossRef]
- Potrykus, M.; Golanowska, M.; Sledz, W.; Zoledowska, S.; Motyka, A.; Kolodziejska, A.; Butrymowicz, J.; Lojkowska, E. Biodiversity of Dickeya spp. isolated from potato plants and water sources in temperate climate. Plant Dis. 2016, 100, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Fikowicz-Krosko, J.; Wszalek-Rozek, K.; Smolarska, A.; Czajkowski, R. First report on isolation of soft rot Pectobacterium carotovorum subsp. carotovorum from symptomless bittersweet nightshade occurring in rural area in Poland. J. Plant Pathol. 2017, 99, 294. [Google Scholar]
- Pulatov, B.; Linderson, M.L.; Hall, K.; Jönsson, A.M. Modeling climate change impact on potato crop phenology, and risk of frost damage and heat stress in northern Europe. Agric. For. Meteorol. 2015, 214, 281–292. [Google Scholar] [CrossRef]
- du Raan, S.; Coutinho, T.A.; van der Waals, J.E. Cardinal temperature differences, determined in vitro, between closely related species and subspecies of pectinolytic bacteria responsible for blackleg and soft rot on potatoes. Eur. J. Plant Pathol. 2016, 144, 361–369. [Google Scholar] [CrossRef][Green Version]
- Golanowska, M.; Kielar, J.; Lojkowska, E. The effect of temperature on phenotypic features and the maceration ability of Dickeya solani strains isolated in Finland, Israel and Poland. Eur. J. Plant Pathol. 2017, 147, 803–817. [Google Scholar] [CrossRef][Green Version]
- Janse, J.D.; Ruissen, M.A. Characterization and classification of Erwinia chrysanthemi strains from several osts in the Netherlands. Phytopathology 1988, 78, 800–808. [Google Scholar] [CrossRef]
- Cazelles, O.; Schwärzel, R. Survey of bacterial diseases caused by Erwinia in seed potato fields in western Switzerland. Rev. Suisse d’Agric. 1992, 24, 215–218. [Google Scholar]
- Degefu, Y.; Potrykus, M.; Golanowska, M.; Virtanen, E.; Lojkowska, E. A new clade of Dickeya spp. plays a major role in potato blackleg outbreaks in north Finland. Ann. Appl. Biol. 2013, 162, 231–241. [Google Scholar] [CrossRef]
- Motyka, A.; Zoledowska, S.; Sledz, W.; Lojkowska, E. Molecular methods as tools to control plant diseases caused by Dickeya and Pectobacterium spp: A minireview. New Biotechnol. 2017, 39, 181–189. [Google Scholar] [CrossRef]
- Tsror (Lahkim), L.; Erlich, O.; Lebiush, S.; van der Wolf, J.; Czajkowski, R.; Mozes, G.; Sikharulidze, Z.; Ben-Daniel, B. First report of potato blackleg caused by a biovar 3 Dickeya sp. in Georgia. New Dis. Rep. 2011, 23, 1. [Google Scholar] [CrossRef]
- Ignatov, A.N.; Karlov, A.N.; Dzhalilov, F.S. Spreading of the blackleg of potatoes in Russia caused by bacteria of Dickeya genus. Zaschita Karantin Rastenij 2014, 11, 41–43. [Google Scholar]
- de Werra, P.; Bussereau, F.; Kellenberger, I.; Dupuis, B.; Schaerer, S.; Keiser, A. Potato: The Pectobacterium empire strikes back. Agrar. Schweiz 2015, 6, 256–263. [Google Scholar]
- van der Wolf, J.M.; de Haan, E.G.; Kastelein, P.; Krijger, M.; de Haas, B.H.; Velvis, H.; Mendes, O.; Kooman-Gersmann, M.; van der Zouwen, P.S. Virulence of Pectobacterium carotovorum subsp. brasiliense on potato compared with that of other Pectobacterium and Dickeya species under climatic conditions prevailing in the Netherlands. Plant Pathol. 2017, 66, 571–583. [Google Scholar] [CrossRef]
- Zoledowska, S.; Motyka, A.; Zukowska, D.; Sledz, W.; Lojkowska, E. Population structure and biodiversity of Pectobacterium parmentieri isolated from potato fields in temperate climate. Plant Dis. 2018, 102, 154–164. [Google Scholar] [CrossRef]
- Oulghazi, S.; Sarfraz, S.; Zaczek-Moczydłowska, M.A.; Khayi, S.; Ed-Dra, A.; Lekbach, Y.; Campbell, K.; Moleleki, L.N.; O’hanlon, R.; Faure, D. Pectobacterium brasiliense: Genomics, host range and disease management. Microorganisms 2021, 9, 106. [Google Scholar] [CrossRef]
- Śledź, W.; Jafra, S.; Waleron, M.; Łojkowska, E. Genetic diversity of Erwinia carotovora strains isolated from infected plants grown in Poland. Bull. OEPP/EPPO Bull. 2000, 30, 403–407. [Google Scholar] [CrossRef]
- Dees, M.W.; Lebecka, R.; Perminow, J.I.S.; Czajkowski, R.; Grupa, A.; Motyka, A.; Zoledowska, S.; Sliwka, J.; Lojkowska, E.; Brurberg, M.B. Characterization of Dickeya and Pectobacterium strains obtained from diseased potato plants in different climatic conditions of Norway and Poland. Eur. J. Plant Pathol. 2017, 148, 839–851. [Google Scholar] [CrossRef]
- Skelsey, P.; Humphris, S.N.; Campbell, E.J.; Toth, I.K. Threat of establishment of non-indigenous potato blackleg and tuber soft rot pathogens in Great Britain under climate change. PLoS ONE 2018, 13, e0205711. [Google Scholar] [CrossRef] [PubMed]
- van der Wolf, J.M.; Acuña, I.; De Boer, S.H.; Brurberg, M.B.; Cahill, G.; Charkowski, A.O.; Coutinho, T.; Davey, T.; Dees, M.W.; Degefu, Y.; et al. Diseases caused by Pectobacterium and Dickeya species around the world. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 215–261. [Google Scholar]
- Zaczek-Moczydłowska, M.A.; Fleming, C.C.; Young, G.K.; Campbell, K.; O’Hanlon, R. Pectobacterium and Dickeya species detected in vegetables in Northern Ireland. Eur. J. Plant Pathol. 2019, 154, 635–647. [Google Scholar] [CrossRef]
- Perombelon, M.C.M. Potato blackleg: Epidemiology, host-pathogen interaction and control. Neth. J. Plant Pathol. 1992, 98, 135–146. [Google Scholar] [CrossRef]
- Stommel, J.R.; Goth, R.W.; Haynes, K.G.; Kim, S.H. Pepper (Capsicum annum) soft rot caused by Erwinia carotovora subsp. atroseptica. Plant Dis. 1996, 80, 1109–1112. [Google Scholar] [CrossRef]
- Baştaş, K.K.; Hekimhan, H.; Maden, S.; Tör, M. First report of bacterial stalk and head rot disease caused by Pectobacterium atrosepticum on sunflower in Turkey. Plant Dis. 2009, 93, 1352. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hibbing, M.E.; Kim, H.; Reedy, R.M.; Yedidia, I.; Breuer, J.; Breuer, J.; Glasner, J.D.; Perna, N.T.; Kelman, A.; et al. Host range and molecular phylogenies of the soft rot Enterobacterial genera Pectobacterium and Dickeya. Phytopathology 2007, 97, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Glasner, J.D.; Kim, H.; Jahn, C.E.; Ma, B.; Biehl, B.S.; Rissman, A.I.; Mole, B.; Yi, X.; Yang, C.; Dangl, J.L.; et al. Niche-specificity and the variable fraction of the Pectobacterium pan-genome. MPMI 2008, 21, 1549–1560. [Google Scholar] [CrossRef]
- De Boer, S.H.; Li, X.; Ward, L.J. Pectobacterium spp. associated with bacterial stem rot syndrome of potato in Canada. Phytopathology 2012, 102, 937–947. [Google Scholar] [CrossRef][Green Version]
- Molina, J.J.; Harrison, M.D. The role of Erwinia carotovora in the epidemiology of potato blackleg. II. The effect of soil temperature on disease severity. Am. Potato J. 1980, 57, 351–363. [Google Scholar] [CrossRef]
- Ali, H.F.; Ahmad, M.; Junaid, M.; Bibi, A.; Ali, A.; Sharif, M.; Ali, B.; Nawab, K.; Sadozai, A. Inoculum sources, disease incidence and severity of bacterial blackleg and soft rot of potato. Pak. J. Bot. 2012, 44, 825–830. [Google Scholar]
- Czajkowski, R.; De Boer, W.J.; Van der Zouwen, P.S.; Kastelein, P.; Jafra, S.; De Haan, E.G.; van den Bovenkamp, G.W.; van der Wolf, J.M. Virulence of “Dickeya solani” and Dickeya dianthicola biovar-1 and -7 strains on potato (Solanum tuberosum). Plant Pathol. 2013, 62, 597–610. [Google Scholar] [CrossRef]
- Hugouvieux-Cotte-Pattat, N.; Dominguez, H.; Robert-Baudouy, J. Environmental conditions affect transcription of the pectinase genes of Erwinia chrysanthemi 3937. J. Bacteriol. 1992, 174, 7807–7818. [Google Scholar] [CrossRef]
- Wei, Z.; Sneath, B.J.; Beer, S.V. Expression of Erwinia amylovora hrp genes in response to environmental stimuli. J. Bacteriol. 1992, 174, 1875–1882. [Google Scholar] [CrossRef]
- Ullrich, M.; Pen, A.; Bailey, A.; Bender, C.L. A modified two-component regulatory system is involved in temperature-dependent biosynthesis of the Pseudomonas syringae phytotoxin coronatine. J. Bacteriol. 1995, 177, 6160–6169. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, K.; Fouts, D.E.; Rehm, A.H.; Hill, A.R.; Collmer, A.; Alfano, J.R. The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (Type III) protein secretion system in a temperature- and pH-sensitive manner. J. Bacteriol. 1999, 181, 4790–4797. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, A.; Li, H.; Weingart, H.; Aufhammer, S.; Burse, A.; Finis, K.; Schenk, A.; Ullrich, M.S. Thermoregulated expression of virulence factors in plant-associated bacteria. Arch. Microbiol. 2001, 176, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Smadja, B.; Latour, X.; Trigui, S.; Burini, J.F.; Chevalier, S.; Orange, N. Thermodependence of growth and enzymatic activities implicated in pathogenicity of two Erwinia carotovora subspecies (Pectobacterium spp.). Can. J. Microbiol. 2004, 50, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lanham, P.G.; Mcllravey, K.I.; Perombelon, M.C.M. Production of cell wall dissolving enzymes by Erwinia carotovora subsp. atroseptica in vitro at 27 °C and 30.5 °C. J. Appl. Bacteriol. 1991, 70, 20–24. [Google Scholar] [CrossRef]
- Ullrich, M.S.; Schergaut, M.; Boch, J.; Ullrich, B. Temperature-responsive genetic loci in the plant pathogen Pseudomonas syringae pv. glycinea. Microbiology 2000, 146, 2457–2468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goyer, C.; Ullrich, M.S. Identification of low-temperature-regulated genes in the fire blight pathogen Erwinia amylovora. Can. J. Microbiol. 2006, 52, 468–475. [Google Scholar] [CrossRef]
- Czajkowski, R.; Kaczyńska, N.; Jafra, S.; Narajczyk, M.; Lojkowska, E. Temperature-responsive genetic loci in pectinolytic plant pathogenic Dickeya solani. Plant Pathol. 2017, 66, 584–594. [Google Scholar] [CrossRef]
- Hinton, J.C.D.; Sidebotham, J.M.; Hyman, L.J.; Prombdon, M.C.M.; Salmond, G.P.C. Isolation and characterisation of transposon-induced mutants of Erwinia carotovora subsp. atroseptica exhibiting reduced virulence. Mol. Gen. Genet. 1989, 217, 141–148. [Google Scholar] [CrossRef]
- Bell, K.S.; Sebaihia, M.; Pritchard, L.; Holden, M.T.G.; Hyman, L.J.; Holeva, M.C.; Thomson, N.R.; Bentley, S.D.; Churcher, L.J.C.; Mungall, K.; et al. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc. Natl. Acad. Sci. USA 2004, 101, 11105–11110. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.J.; Sessitsch, A.; Corbo, J.C.; Giller, K.E.; Akkermans, D.L.; Jefferson, R. Beta-glucuronidase (Gus) transposons for ecological and genetic-studies of Rhizobia and other gram-negative bacteria. Microbiology 1995, 141, 1691–1705. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. EggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef] [PubMed]
- Haverkort, A.J.; Verhagen, A. Climate change and its repercussions for the potato supply chain. Potato Res. 2008, 51, 223–237. [Google Scholar] [CrossRef]
- Schaap, B.F.; Blom-Zandstra, M.; Hermans, C.M.L.; Meerburg, B.G.; Verhagen, J. Impact changes of climatic extremes on arable farming in the north of the Netherlands. Reg. Environ. Chang. 2011, 11, 731–741. [Google Scholar] [CrossRef]
- White-Ziegler, C.A.; Um, S.; Perez, N.M.; Berns, A.L.; Malhowski, A.J.; Young, S. Low temperature (23 °C) increases expression of biofilm-, cold-shock- and RpoS-dependent genes in Escherichia coli K-12. Microbiology 2008, 154, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Dersch, P.; Kneip, S.; Bremer, E. The nucleoid-associated DNA-binding protein H-NS is required for the efficient adaptation of Escherichia coli K-12 to a cold environment. Mol. Gen. Genet. 1994, 245, 255–259. [Google Scholar] [CrossRef]
- White-Ziegler, C.A.; Davis, T.R. Genome-wide identification of H-NS-controlled, temperature-regulated genes in Escherichia coli K-12. J. Bacteriol. 2009, 191, 1106–1110. [Google Scholar] [CrossRef]
- Nasser, W.; Faelen, M.; Hugouvieux-Cotte-Pattat, N.; Reverchon, S. Role of the nucleoid-associated protein H-NS in the synthesis of virulence factors in the phytopathogenic bacterium Erwinia chrysanthemi. MPMI 2001, 14, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, E.C.; Glover, J.R.; Singer, M.A.; Lindquist, S. HSP100/Clp proteins: A common mechanism explains diverse functions. Trends Biochem. Sci. 1996, 21, 289–296. [Google Scholar] [CrossRef]
- Chan, K.-G.; Priya, K.; Chang, C.; Yamin, A.; Rahman, A.; Tee, K.K.; Yin, W.-F. Transcriptome analysis of Pseudomonas aeruginosa PAO1 grown at both body and elevated temperatures. PeerJ 2016, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Thoden, J.B.; Holden, H.M. The molecular architecture of glucose-1-phosphate uridylyltransferase. Protein Sci. 2007, 16, 432–440. [Google Scholar] [CrossRef]
- Weissborn, A.C.; Liu, Q.; Rumley, M.K.; Kennedy, E.P. UTP: α-D-glucose-1-phosphate uridylyltransferase of Escherichia coli: Isolation and DNA sequence of the galU Gene and purification of the enzyme. J. Bacteriol. 1994, 176, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.D.; Waldor, M.K. Enterohemorrhagic Escherichia coli O157:H7 gal mutants are sensitive to bacteriophage P1 and defective in intestinal colonization. Infect. Immun. 2007, 75, 1661–1666. [Google Scholar] [CrossRef][Green Version]
- Priebe, G.P.; Dean, C.R.; Zaidi, T.; Meluleni, G.J.; Coleman, F.T.; Coutinho, Y.S.; Noto, M.J.; Urban, T.A.; Pier, G.B.; Goldberg, J.B. The galU gene of Pseudomonas aeruginosa is required for corneal infection and efficient systemic spread following pneumonia but not for infection confined to the lung. Infect. Immun. 2004, 72, 4224–4232. [Google Scholar] [CrossRef][Green Version]
- Deng, W.-L.; Lin, Y.-C.; Lin, R.-H.; Wei, C.-F.; Huang, Y.-C.; Peng, H.-L.; Huang, H.-C. Effects of galU mutation on Pseudomonas syringae—plant interactions. MPMI 2010, 23, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Nesper, J.; Lauriano, C.M.; Klose, K.E.; Kapfhammer, D.; Kraiß, A.; Reidl, J. Characterization of Vibrio cholerae O1 El Tor galU and galE mutants: Influence on lipopolysaccharide structure, colonization, and biofilm formation. Infect. Immun. 2001, 69, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sagaram, U.S.; Kim, J.; Wang, N. Requirement of the galU gene for polysaccharide production by and pathogenicity and growth in planta of Xanthomonas citri subsp. citri. Appl. Environ. Microbiol. 2010, 76, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Wandersman, C.; Letoffe, S. Involvement of lipopolysaccharide in the secretion of Escherichia coli α-haemolysin and Erwinia chrysanthemi proteases. Mol. Microbiol. 1993, 7, 141–150. [Google Scholar] [CrossRef]
- Wang, L.E.I.; Reeves, P.R. Involvement of the galactosyl-1-phosphate transferase encoded by the Salmonella enterica rfbP gene in O-antigen subunit processing. J. Bacteriol. 1994, 176, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Yang, J.; Liu, Q.; Alamuri, P.; Roland, K.L.; Curtiss, R. Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar typhimurium. Infect. Immun. 2011, 79, 4227–4239. [Google Scholar] [CrossRef]
- Shibata, S.; Yip, E.S.; Quirke, K.P.; Ondrey, J.M.; Visick, K.L. Roles of the structural symbiosis polysaccharide (syp) genes in host colonization, biofilm formation, and polysaccharide biosynthesis in Vibrio fischeri. J. Bacteriol. 2012, 194, 6736–6747. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, L.; Mainprize, I.L.; Naismith, J.H.; Whitfield, C. Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram-negative bacteria. Microbiol. Mol. Biol. Rev. 2009, 73, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Bogino, P.C.; Oliva, M.; de las, M.; Sorroche, F.G.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef]
- Dong, C.; Beis, K.; Nesper, J.; Brunkan-LaMontagne, A.L.; Clarke, B.R.; Whitfield, C.; Naismith, J.H. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature 2006, 444, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Yuan, B.; Liu, J.; Zhu, D.; Wu, Y.; Wang, M.; Jia, R.; Sun, K.; Yang, Q.; Chen, S.; et al. Identification of a wza-like gene involved in capsule biosynthesis, pathogenicity and biofilm formation in Riemerella anatipestifer. Microb. Pathog. 2017, 107, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-C.; Lin, T.-L.; Hsieh, P.-F.; Yang, H.-C.; Wang, J.-T. Isolation of genes involved in biofilm formation of a Klebsiella pneumoniae strain causing pyogenic liver abscess. PLoS ONE 2011, 6, e23500. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Harayama, S.; Iino, T.; Hazelbauer, G.L. Molecular cloning and characterization of genes required for ribose transport and utilization in Escherichia coli K-12. J. Bacteriol. 1984, 158, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Hope, J.N.; Bell, W.; Hermodson, M.A.; Groarkeg, J.M. Ribokinase from Escherichia coli K12. J. Biol. Chem. 1986, 261, 7663–7668. [Google Scholar] [CrossRef]
- Romeo, T.; Wang, X.; Desplas, R.L. Novel Genes Involved in the Escherichia coli Biofilm Formation and Uses Thereof. US Patent Appl. Publ. No. US20050032093A1, 2005. [Google Scholar]
- Beenken, K.E.; Dunman, P.M.; Mcaleese, F.; Macapagal, D.; Murphy, E.; Projan, S.J.; Blevins, J.S.; Smeltzer, M.S. Global gene expression in Staphylococcus aureus biofilms. J. Bacteriol. 2004, 186, 4665–4684. [Google Scholar] [CrossRef]
- Lee, C.M.; Monson, R.E.; Adams, R.M.; Salmond, G.P.C. The LacI-family transcription factor, RbsR, is a pleiotropic regulator of motility, virulence, siderophore and antibiotic production, gas vesicle morphogenesis and flotation in Serratia. Front. Microbiol. 2017, 8, 1678. [Google Scholar] [CrossRef]
- Czajkowski, R.; Krzyzanowska, D.; Karczewska, J.; Atkinson, S.; Przysowa, J.; Lojkowska, E.; Williams, P.; Jafra, S. Inactivation of AHLs by Ochrobactrum sp. A44 depends on the activity of a novel class of AHL acylase. Environ. Microbiol. Rep. 2011, 3, 59–68. [Google Scholar] [CrossRef]
- Frechon, D.; Exbrayat, P.; Helias, V.; Hyman, L.J.; Jouan, B.; Llop, P.; Lopez, M.M.; Payet, N.; Perombelon, M.C.M.; Toth, I.K.; et al. Evaluation of a PCR kit for the detection of Erwinia carotovora subsp. atroseptica on potato tubers. Potato Res. 1998, 41, 163–173. [Google Scholar] [CrossRef]
- Hélias, V.; Hamon, P.; Huchet, E.; Wolf, J.V.D.; Andrivon, D. Two new effective semiselective crystal violet pectate media for isolation of Pectobacterium and Dickeya. Plant Pathol. 2012, 61, 339–345. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Bittinger, M.A.; Handelsman, J. Identification of genes in the rosR regulon of Rhizobium etli. J. Bacteriol. 2000, 182, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.-N.; Yang, C.-H.; Charkowski, A.O. The response regulator HrpY of Dickeya dadantii 3937 regulates virulence genes not linked to the hrp cluster. Mol. Plant. Microbe. Interact. 2008, 21, 304–314. [Google Scholar] [CrossRef]
- Jahn, C.E.; Willis, D.K.; Charkowski, A.O. The flagellar sigma factor FliA is required for Dickeya dadantii virulence. MPMI 2008, 21, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, S.; Van Gijsegem, F.; Rouve, M.; Kotoujansky, A.; Robert-Baudouy, J. Organization of a pectate lyase gene family in Erwinia chrysanthemi. Gene 1986, 49, 215–224. [Google Scholar] [CrossRef]
- Py, B.; Bortoli-German, I.; Haiech, J.; Chippaux, M.; Barras, F. Cellulase EGZ of Erwinia chrysanthemi: Structural organization and importance of His98 and Glul33 residues for catalysis. Protein Eng. 1991, 4, 325–333. [Google Scholar] [CrossRef]
- Wandersman, C.; Andro, T.; Bertheau, Y. Extracellular protease in Erwinia chrysanthemi. J. Gen. Microbiol. 1986, 132, 899–906. [Google Scholar] [CrossRef][Green Version]
- Nykyri, J.; Mattinen, L.; Niemi, O.; Adhikari, S.; Kõiv, V.; Somervuo, P.; Fang, X.; Auvinen, P.; Mäe, A.; Palva, E.T.; et al. Role and regulation of the Flp/Tad pilus in the virulence of Pectobacterium atrosepticum SCRI1043 and Pectobacterium wasabiae SCC3193. PLoS ONE 2013, 8, e73718. [Google Scholar] [CrossRef]
- Czajkowski, R.; de Boer, W.J.; Velvis, H.; van der Wolf, J.M. Systemic colonization of potato plants by a soilborne, green fluorescent protein-tagged strain of Dickeya sp. biovar 3. Phytopathology 2010, 100, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Sprouffske, K.; Wagner, A. Growthcurver: An R package for obtaining interpretable metrics from microbial growth curves. BMC Bioinform. 2016, 17, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Apicella, M.A.; Griffiss, J.M.; Schneider, H. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzymol. 1994, 235, 242–252. [Google Scholar] [PubMed]
- Tsai, C.M.; Frasch, C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar] [CrossRef]
- Czajkowski, R.; van der Wolf, J.M.; Krolicka, A.; Ozymko, Z.; Narajczyk, M.; Kaczynska, N.; Lojkowska, E. Salicylic acid can reduce infection symptoms caused by Dickeya solani in tissue culture grown potato (Solanum tuberosum L.) Plants. Eur. J. Plant Pathol. 2014, 141, 545–558. [Google Scholar] [CrossRef][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Found. Stat. Comput.: Vienna, Austria, 2016; Available online: http://www.R-project.org/ (accessed on 30 March 2021).
- Levene, H. Robust tests for equality of variances. Contrib. Probab. Stat. Essays Honor Harold Hotell. 1960, 1, 278–292. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one or two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]

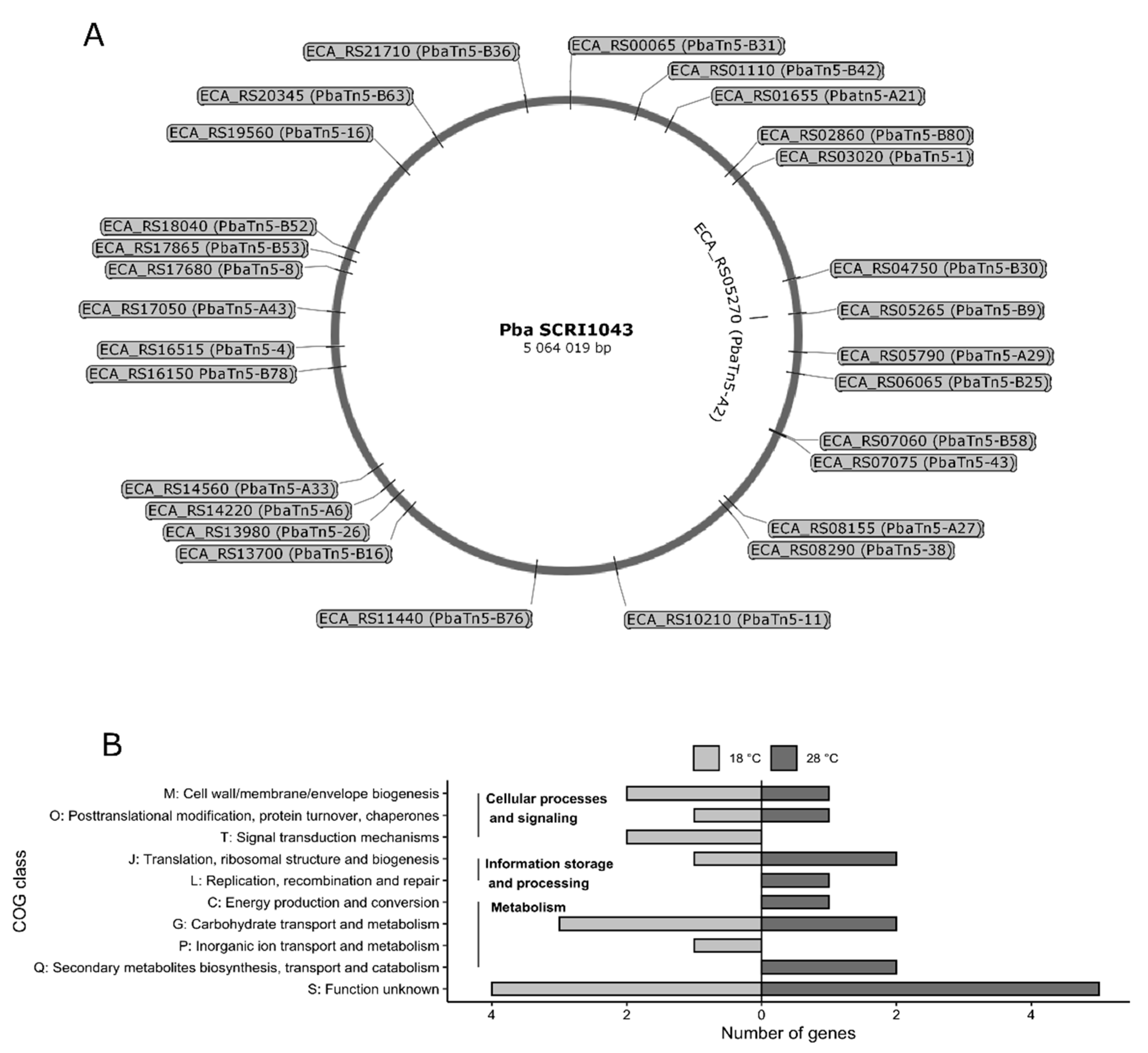
| Number | Mutant | % Identity a; Protein, Accession Number; Gene b; TU c | Predicted Function | Fold Induction d of GUS Activity | Function Group (COGs) e | Differential Phenotype |
|---|---|---|---|---|---|---|
| Mutants with increased GUS activity at 18 °C | ||||||
| 1 | PbaTn5-38 | 99; CAG74583.1; ECA_RS08290; PTU | Transcriptional regulator | 1.6 | COG2336T | WT f |
| 2 | PbaTn5-43 | 100; WbaP; CAG74330.1; ECA_RS07075 (rfbP); MTU | UDP-phosphate galactose phosphotransferase | 2.0 | COG2148M | enhanced biofilm formation, reduced growth |
| 3 | PbaTn5-A6 | 95; H-NS; CAG75793.1; ECA_RS14220; MTU | DNA-binding protein H-NS | 1.5 | COG2916S ENOG501RF2B | WT |
| 4 | PbaTn5-A21 | 98; CAG73240.1; ECA_RS01655; PTU | Putative phosphoheptose isomerase | 1.5 | COG0279G | WT |
| 5 | PbaTn5-A27 | 100; CAG74551.1; ECA_RS08155; MPU | Hypothetical protein ECA1647 | 1.9 | ENOG502FKDA | WT |
| 6 | PbaTn5-A29 | 100; CAG74078.1; ECA_RS05790; PTU | Putative 50S ribosomal protein L31 | 1.6 | COG0254J | WT |
| 7 | PbaTn5-B9 | 90; CAG73972.1; ECA_RS05265; PTU | Putative integrase | 2.1 | COG4688S | WT |
| 8 | PbaTn5-B16 | 100; CAG75691.1; ECA_RS13700; MTU | Putative glutatione S-transferase | 1.5 | COG0625O | WT |
| 9 | PbaTn5-B31 | 100; CAG72938.1; ECA_RS00065; PTU | Ribokinase | 2.6 | COG0524G | reduced biofilm formation |
| 10 | PbaTn5-B36 | 100; CAG77284.1; ECA_RS21710; PTU | IIABC component of phosphoenolpyruvate-dependent sugar phosphotransferase (PTS) system | 1.5 | COG1263G | WT |
| 11 | PbaTn5-B52 | 100; AIK15419.1; ECA_RS18040; MTU | Transcriptional regulator, XRE family | 2.2 | ENOG501MXWB | WT |
| 12 | PbaTn5-B63 | 100; CAG77013.1; ECA_RS20345; MTU | Putative IucA/IucC family siderophore biosynthesis protein | 2.0 | COG4264P | WT |
| 13 | PbaTn5-B76 | 96; GalU; CAG75232.1; ECA_RS11440 (galU); MTU | UTP-glucose-1-phosphate uridylyltransferase | 1.6 | COG1210M | reduced swimming motility, reduced exoenzyme production, reduced growth, enhance biofilm formation, reduced ability to macerate potato tissue, altered LPS synthesis, altered lactose fermentation |
| 14 | PbaTn5-B78 | 100; MucB, RseB; CAG76180.1; ECA_RS16150; MTU | Sigma-E factor regulatory protein | 1.8 | COG3026T | WT |
| Mutants with increased GUS activity at 28 °C | ||||||
| 15 | PbaTn5-1 | 98; CAG73522.1; ECA_RS03020; PTU | Cfa-β-ketoacyl synthase | 2.2 | COG0304IQ | WT |
| 16 | PbaTn5-4 | 100; ClpB; CAG76243.1; ECA_RS16515; MTU | Chaperone protein ClpB | 2.8 | COG0542O | WT |
| 17 | PbaTn5-8 | 100; CAG76476.1; ECA_RS17680; MTU | d-galactarate dehydratase | 2.9 | COG2721G | WT |
| 18 | PbaTn5-11 | 85; CAG74973.1; ECA_RS10210; PTU | Putative cytochrome P450 | 3.8 | COG2124Q | WT |
| 19 | PbaTn5-16 | 100; CAG76859.1; ECA_RS19560; MTU | Putative exported protein | 2.0 | ENOG502C5YQ | WT |
| 20 | PbaTn5-26 | 91; CAG75749.1; ECA_RS13980; MTU | Metallo-β-lactamase | 2.8 | COG0491GM | WT |
| 21 | PbaTn5-A2 | 93; CAG73973.1; ECA_RS05270; PTU | Conserved hypothetical protein | 5.0 | ENOG502E3II | WT |
| 22 | PbaTn5-A33 | 89; CAG75864.1; ECA_RS14560; MTU | Amidohydrolase; putative peptidase | 4.7 | COG1473S | WT |
| 23 | PbaTn5-A43 | 100; CAG76360.1; ECA_RS17050; PTU | Putative exported protein | 2.3 | ENOG502ASC5 | enhanced ability to macerate potato tissue |
| 24 | PbaTn5-B25 | 77; HybO; CAG74135.1; ECA_RS06065; PTU | Hydrogenase-2 small subunit | 2.7 | COG1740C | WT |
| 25 | PbaTn5-B30 | 100; CAG73872.1; ECA_RS04750; MTU | AAA family ATPase | 2.2 | COG0419L | WT |
| 26 | PbaTn5-B42 | 99; WP_011091854.1; ECA_RS01110; MTU | Elongation factor Tu | 2.3 | COG0050J | WT |
| 27 | PbaTn5-B53 | 99; CAG76514.1; ECA_RS17865; PTU | RNA ligase RtcB family protein | 1.6 | COG1690J | WT |
| 28 | PbaTn5-B58 | 98; Wza; CAG74327.1; ECA_RS07060; PTU | Putative polysaccharide export protein | 1.7 | COG1596M | enhanced biofilm formation, reduced growth |
| 29 | PbaTn5-B80 | 100; CAG73490.1; ECA_RS02860; PTU | Putative membrane protein | 1.8 | ENOG5028UFW | WT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczynska, N.; Lojkowska, E.; Narajczyk, M.; Czajkowski, R. Genome-Wide Analyses of the Temperature-Responsive Genetic Loci of the Pectinolytic Plant Pathogenic Pectobacterium atrosepticum. Int. J. Mol. Sci. 2021, 22, 4839. https://doi.org/10.3390/ijms22094839
Kaczynska N, Lojkowska E, Narajczyk M, Czajkowski R. Genome-Wide Analyses of the Temperature-Responsive Genetic Loci of the Pectinolytic Plant Pathogenic Pectobacterium atrosepticum. International Journal of Molecular Sciences. 2021; 22(9):4839. https://doi.org/10.3390/ijms22094839
Chicago/Turabian StyleKaczynska, Natalia, Ewa Lojkowska, Magdalena Narajczyk, and Robert Czajkowski. 2021. "Genome-Wide Analyses of the Temperature-Responsive Genetic Loci of the Pectinolytic Plant Pathogenic Pectobacterium atrosepticum" International Journal of Molecular Sciences 22, no. 9: 4839. https://doi.org/10.3390/ijms22094839
APA StyleKaczynska, N., Lojkowska, E., Narajczyk, M., & Czajkowski, R. (2021). Genome-Wide Analyses of the Temperature-Responsive Genetic Loci of the Pectinolytic Plant Pathogenic Pectobacterium atrosepticum. International Journal of Molecular Sciences, 22(9), 4839. https://doi.org/10.3390/ijms22094839








