Concurrent Physiological and Pathological Angiogenesis in Retinopathy of Prematurity and Emerging Therapies
Abstract
1. Introduction
2. Physiological Angiogenesis in the Developing Retina
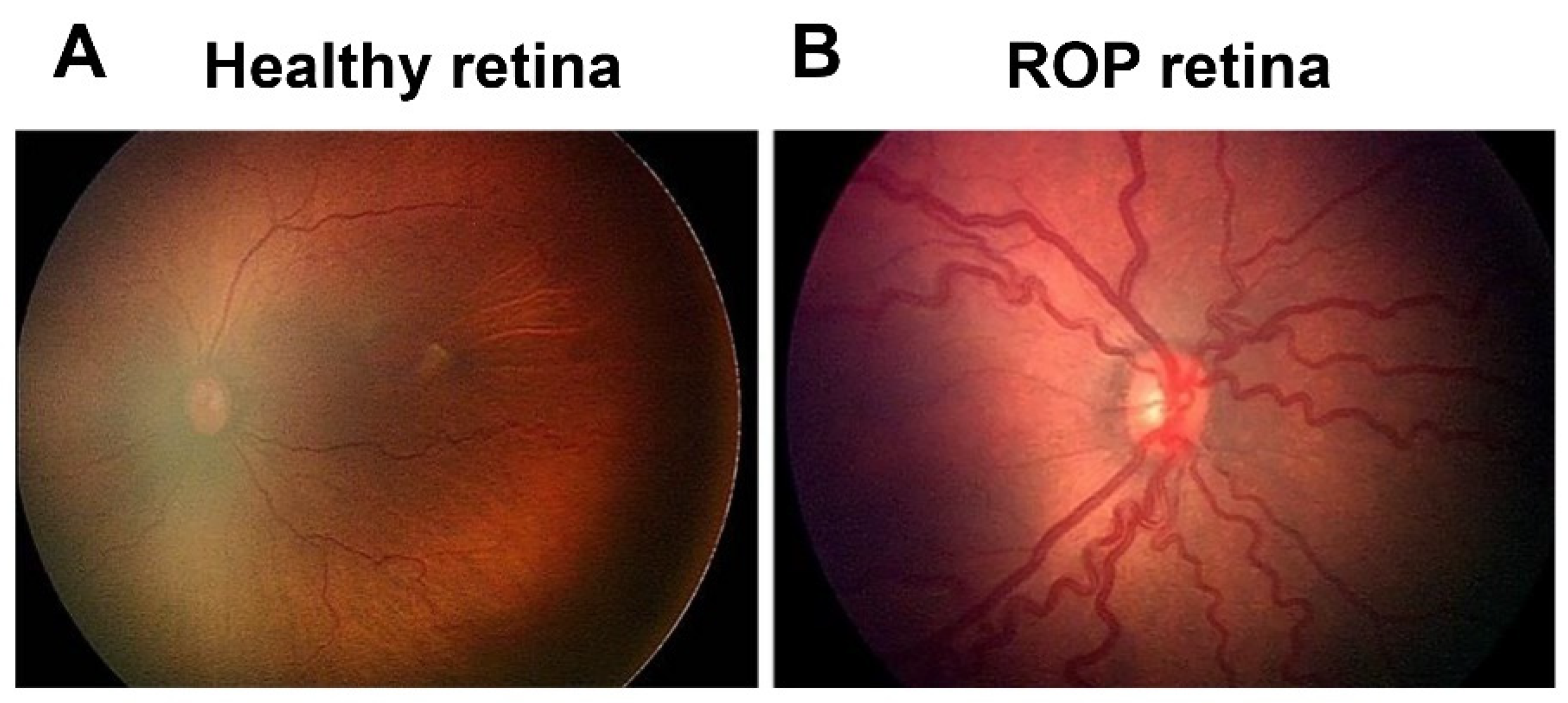
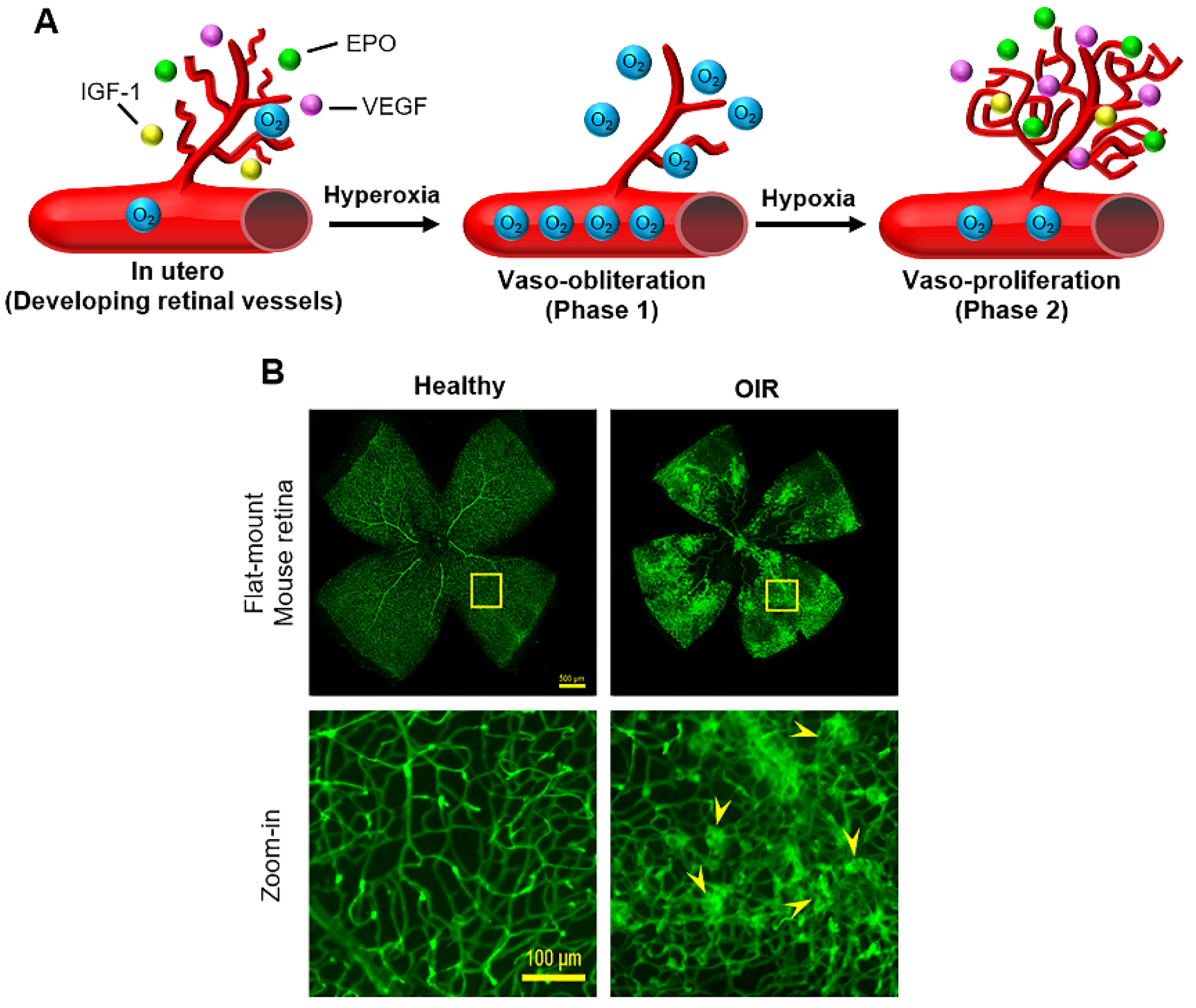
3. Two-Phase Theory of ROP with Pathological Angiogenesis
4. Molecular Mechanisms of ROP
4.1. Oxygen
4.2. VEGF
4.3. IGF-1
4.4. Other Molecular Regulators
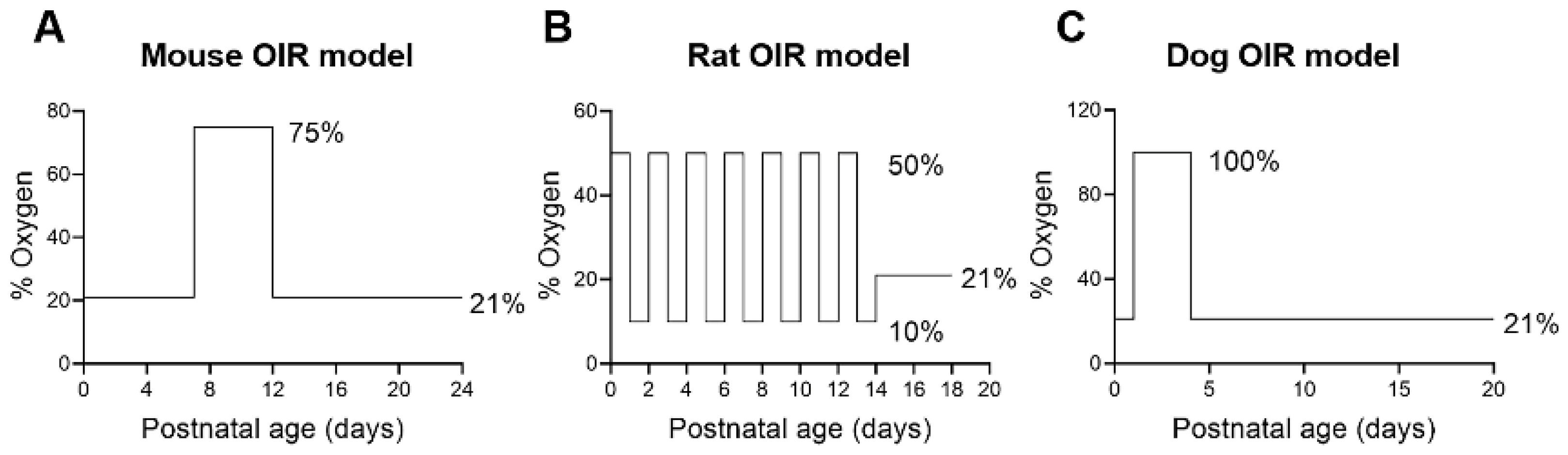
5. Animal Models of ROP
5.1. Mice
5.2. Rats
5.3. Felines
5.4. Dogs
6. Current and Emerging Therapies
6.1. Oxygen for ROP Prevention
6.2. Laser Therapy and Cryotherapy
6.3. Anti-VEGF Therapy
6.4. Other Therapies
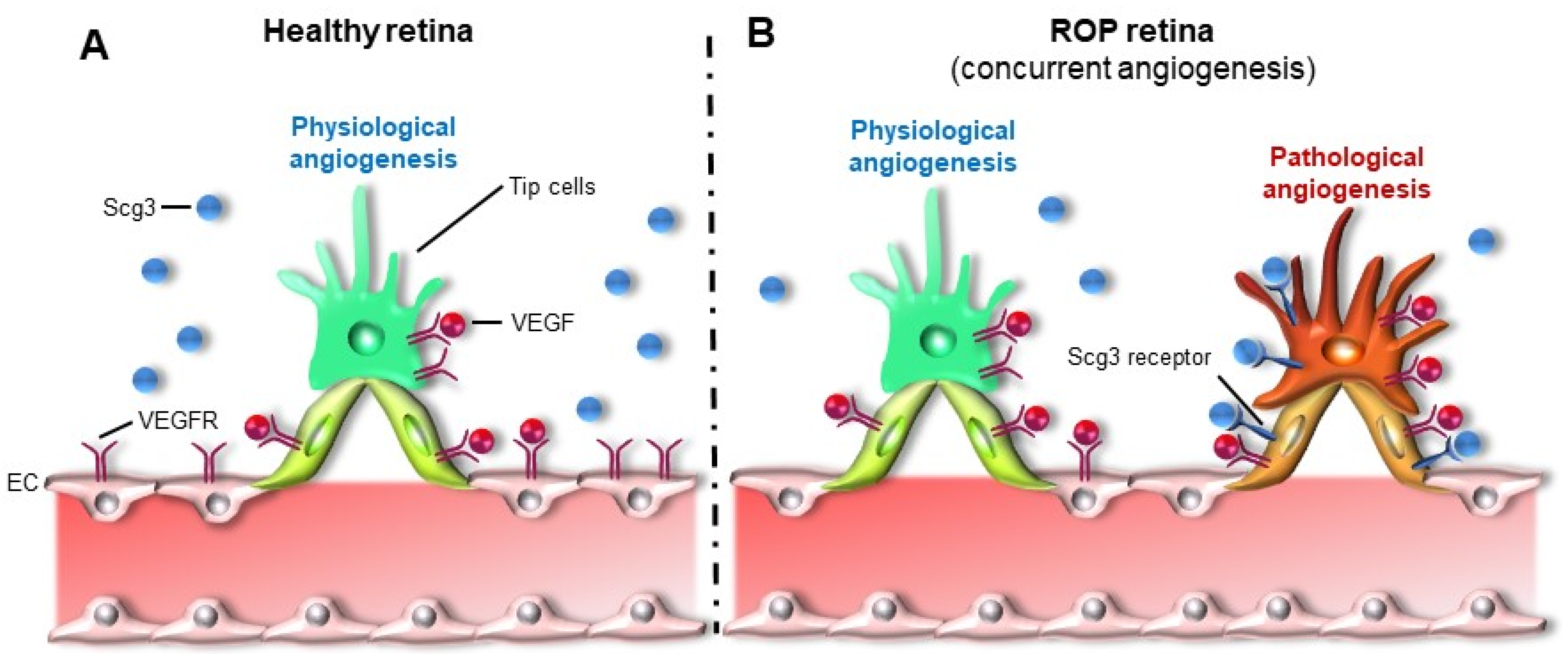
6.5. Scg3 Antagonist as An Emerging Therapy That Targets Pathological Angiogenesis
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| CNV | Choroidal neovascularization |
| DR | Diabetic retinopathy |
| EPO | Erythropoietin |
| GA | Gestational age |
| IGF-1 | Insulin-like growth factor-1 |
| IVNV | Intravitreal neovascularization |
| OIR | Oxygen-induced retinopathy |
| PUF | Polyunsaturated fatty acid |
| RNV | Retinal neovascularization |
| ROP | Retinopathy of prematurity |
| Scg3 | Secretogranin III |
| SpO2 | Oxygen saturation |
| VEGF | Vascular endothelial growth factor |
| VEGF | Vascular endothelial growth factor receptor |
References
- Hellström, A.; Smith, L.E.H.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef]
- Hartnett, M.E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 2015, 122, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E.; Lane, R.H. Effects of oxygen on the development and severity of retinopathy of prematurity. J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2013, 17, 229–234. [Google Scholar] [CrossRef]
- Blencowe, H.; Lawn, J.E.; Vazquez, T.; Fielder, A.; Gilbert, C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr. Res. 2013, 74 (Suppl. S1), 35–49. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Nwanyanwu, K. Retinopathy of Prematurity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, January 2021. [Google Scholar]
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2021, 49, 67–81. [Google Scholar] [CrossRef]
- Selvam, S.; Kumar, T.; Fruttiger, M. Retinal vasculature development in health and disease. Prog. Retin. Eye Res. 2015, 63, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.V.; Linsenmeier, R.A.; Chung, C.K.; Curcio, C.A. Photoreceptor inner segments in monkey and human retina: Mitochondrial density, optics, and regional variation. Vis. Neurosci. 2002, 19, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P. Eyeing central neurons in vascular growth and reparative angiogenesis. Blood 2012, 120, 2182–2194. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.E. Normal development of the hyaloid and retinal vessels in the rat. Br. J. Ophthalmol. 1959, 43, 385–393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, B.; Bezhadian, M.A.; Caldwell, R.B. Astrocytes modulate retinal vasculogenesis: Effects on endothelial cell differentiation. Glia 1995, 15, 1–10. [Google Scholar] [CrossRef]
- Bolton, D.P.; Cross, K.W. Further observations on cost of preventing retrolental fibroplasia. Lancet 1974, 1, 445–448. [Google Scholar] [CrossRef]
- Saugstad, O.D.; Aune, D. Optimal oxygenation of extremely low birth weight infants: A meta-analysis and systematic review of the oxygen saturation target studies. Neonatology 2014, 105, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, J.M.; Bloom, J.N.; Orge, F.; Schutt, A.; Schluchter, M.; Cheruvu, V.K.; Walsh, M.; Finer, N.; Martin, R.J. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J. Pediatr. 2010, 157, 69–73. [Google Scholar] [CrossRef]
- Cunningham, S.; Fleck, B.W.; Elton, R.A.; McIntosh, N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet 1995, 346, 1464–1465. [Google Scholar] [CrossRef]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef]
- Sato, T.; Kusaka, S.; Shimojo, H.; Fujikado, T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology 2009, 116, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Velez-Montoya, R.; Clapp, C.; Rivera, J.C.; Garcia-Aguirre, G.; Morales-Cantón, V.; Fromow-Guerra, J.; Guerrero-Naranjo, J.L.; Quiroz-Mercado, H. Intraocular and systemic levels of vascular endothelial growth factor in advanced cases of retinopathy of prematurity. Clin. Ophthalmol. 2010, 4, 947–953. [Google Scholar] [CrossRef]
- Pierce, E.A.; Avery, R.L.; Foley, E.D.; Aiello, L.P.; Smith, L.E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. USA 1995, 92, 905–909. [Google Scholar] [CrossRef]
- Pierce, E.A.; Foley, E.D.; Smith, L.E. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch. Ophthalmol. 1996, 114, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, S.; Kobayashi, M.; Goto, Y.; Hiraoka, M.; Harada, H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018, 109, 560–571. [Google Scholar] [CrossRef]
- Sears, J.E.; Hoppe, G.; Ebrahem, Q.; Anand-Apte, B. Prolyl hydroxylase inhibition during hyperoxia prevents oxygen-induced retinopathy. Proc. Natl. Acad. Sci. USA 2008, 105, 19898–19903. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed. Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Liegl, R.; Löfqvist, C.; Hellström, A.; Smith, L.E.H. IGF-1 in retinopathy of prematurity, a CNS neurovascular disease. Early Hum. Dev. 2016, 102, 13–19. [Google Scholar] [CrossRef]
- Hellstrom, A.; Perruzzi, C.; Ju, M.; Engstrom, E.; Hard, A.L.; Liu, J.L.; Albertsson-Wikland, K.; Carlsson, B.; Niklasson, A.; Sjodell, L.; et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: Direct correlation with clinical retinopathy of prematurity. Proc. Natl. Acad. Sci. USA 2001, 98, 5804–5808. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebrouck, S.; Daniëls, H.; Moons, L.; Vanhole, C.; Carmeliet, P.; Zegher, F.D. Oxygen-induced retinopathy in mice: Amplification by neonatal IGF-I deficit and attenuation by IGF-I administration. Pediatr. Res. 2009, 65, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Kopchick, J.J.; Chen, W.; Knapp, J.; Kinose, F.; Daley, D.; Foley, E.; Smith, R.G.; Schaeffer, J.M. Essential role of growth hormone in ischemia-induced retinal neovascularization. Science 1997, 276, 1706–1709. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Shen, W.; Perruzzi, C.; Soker, S.; Kinose, F.; Xu, X.; Robinson, G.; Driver, S.; Bischoff, J.; Zhang, B.; et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med. 1999, 5, 1390–1395. [Google Scholar] [CrossRef]
- Kondo, T.; Vicent, D.; Suzuma, K.; Yanagisawa, M.; King, G.L.; Holzenberger, M.; Kahn, C.R. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularization. J. Clin. Investig. 2003, 111, 1835–1842. [Google Scholar] [CrossRef]
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef]
- Jarajapu, Y.P.R.; Cai, J.; Yan, Y.; Calzi, S.L.; Kielczewski, J.L.; Hu, P.; Shaw, L.C.; Firth, S.M.; Chan-Ling, T.; Boulton, M.E.; et al. Protection of blood retinal barrier and systemic vasculature by insulin-like growth factor binding protein-3. PLoS ONE 2012, 7, e39398. [Google Scholar] [CrossRef]
- Lofqvist, C.; Chen, J.; Connor, K.M.; Smith, A.C.; Aderman, C.M.; Liu, N.; Pintar, J.E.; Ludwig, T.; Hellstrom, A.; Smith, L.E. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc. Natl. Acad. Sci. USA 2007, 104, 10589–10594. [Google Scholar] [CrossRef]
- Chen, J.; Connor, K.M.; Aderman, C.M.; Smith, L.E.H. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Investig. 2008, 118, 526–533. [Google Scholar] [CrossRef]
- Chen, J.; Connor, K.M.; Aderman, C.M.; Willett, K.L.; Aspegren, O.P.; Smith, L.E. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1329–1335. [Google Scholar] [CrossRef]
- Romagnoli, C.; Tesfagabir, M.G.; Giannantonio, C.; Papacci, P. Erythropoietin and retinopathy of prematurity. Early Hum. Dev. 2011, 87 (Suppl. S1), S39–S42. [Google Scholar] [CrossRef]
- Querques, G.; Forte, R.; Souied, E.H. Retina and omega-3. J. Nutr. Metab. 2011, 2011, 748361. [Google Scholar] [CrossRef]
- SanGiovanni, J.P.; Chew, E.Y. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef] [PubMed]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef]
- Stahl, A.; Sapieha, P.; Connor, K.M.; Sangiovanni, J.P.; Chen, J.; Aderman, C.M.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Seaward, M.R.; et al. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circ. Res. 2010, 107, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Löfqvist, C.A.; Najm, S.; Hellgren, G.; Engström, E.; Sävman, K.; Nilsson, A.K.; Andersson, M.X.; Hård, A.L.; Smith, L.E.; Hellström, A. Association of Retinopathy of Prematurity With Low Levels of Arachidonic Acid: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Anti-VEGF therapy in the management of retinopathy of prematurity: What we learn from representative animal models of oxygen-induced retinopathy. Eye Brain 2016, 8, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef]
- De Schaepdrijver, L.; Simoens, P.; Lauwers, H. Development of the retinal circulation in the pig. Anat. Embryol. 1995, 192, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D’Amato, R.; Sullivan, R.; D’Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Ophthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Connor, K.M.; Krah, N.M.; Dennison, R.J.; Aderman, C.M.; Chen, J.; Guerin, K.I.; Sapieha, P.; Stahl, A.; Willett, K.L.; Smith, L.E. Quantification of oxygen-induced retinopathy in the mouse: A model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009, 4, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.E.; Wang, W.; Chen, X.; Caberoy, N.B.; Guo, F.; Shen, C.; Ji, Y.; Tian, H.; Wang, H.; Chen, R.; et al. Secretogranin III as a disease-associated ligand for antiangiogenic therapy of diabetic retinopathy. J. Exp. Med. 2017, 214, 1029–1047. [Google Scholar] [CrossRef]
- Ricci, B.; Calogero, G. Oxygen-induced retinopathy in newborn rats: Effects of prolonged normobaric and hyperbaric oxygen supplementation. Pediatrics 1988, 82, 193–198. [Google Scholar] [PubMed]
- Penn, J.S.; Henry, M.M.; Tolman, B.L. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr. Res. 1994, 36, 724–731. [Google Scholar] [CrossRef]
- Michaelson, I.C. Vascular morphogenesis in the retina of the cat. J. Anat. 1948, 82, 167–174. [Google Scholar]
- Ashton, N.; Ward, B.; Serpell, G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br. J. Ophthalmol. 1954, 38, 397–432. [Google Scholar] [CrossRef]
- Flower, R.W.; McLeod, D.S.; Lutty, G.A.; Goldberg, B.; Wajer, S.D. Postnatal retinal vascular development of the puppy. Investig. Ophthalmol. Vis. Sci. 1985, 26, 957–968. [Google Scholar]
- Lutty, G.A.; McLeod, D.S.; Bhutto, I.; Wiegand, S.J. Effect of VEGF trap on normal retinal vascular development and oxygen-induced retinopathy in the dog. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4039–4047. [Google Scholar] [CrossRef]
- McLeod, D.S.; D’Anna, S.A.; Lutty, G.A. Clinical and histopathologic features of canine oxygen-induced proliferative retinopathy. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1918–1932. [Google Scholar]
- Askie, L.M.; Darlow, B.A.; Finer, N.; Schmidt, B.; Stenson, B.; Tarnow-Mordi, W.; Davis, P.G.; Carlo, W.A.; Brocklehurst, P.; Davies, L.C.; et al. Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. J. Am. Med. Assoc. 2018, 319, 2190–2201. [Google Scholar] [CrossRef] [PubMed]
- Hellström, A.; Hård, A.-L. Screening and novel therapies for retinopathy of prematurity—A review. Early Hum. Dev. 2019, 138, 104846. [Google Scholar] [CrossRef]
- Anderson, C.G.; Benitz, W.E.; Madan, A. Retinopathy of prematurity and pulse oximetry: A national survey of recent practices. J. Perinatol. 2004, 24, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Newnam, K.M. Oxygen saturation limits and evidence supporting the targets. Adv. Neonatal Care 2014, 14, 403–409. [Google Scholar] [CrossRef]
- The STOP-ROP Multicenter Study Group. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP), a randomized, controlled trial. I: Primary outcomes. Pediatrics 2000, 105, 295–310. [Google Scholar] [CrossRef]
- Askie, L.M.; Henderson-Smart, D.J.; Irwig, L.; Simpson, J.M. Oxygen-saturation targets and outcomes in extremely preterm infants. N. Engl. J. Med. 2003, 349, 959–967. [Google Scholar] [CrossRef]
- Cummings, J.J.; Polin, R.A. Oxygen Targeting in Extremely Low Birth Weight Infants. Pediatrics 2018, 138, e20161576. [Google Scholar] [CrossRef]
- Houston, S.K.; Wykoff, C.C.; Berrocal, A.M.; Hess, D.J.; Murray, T.G. Laser treatment for retinopathy of prematurity. Lasers Med. Sci. 2013, 28, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Suelves, A.M.; Shulman, J.P. Current screening and treatments in retinopathy of prematurity in the US. Eye Brain 2016, 8, 37–43. [Google Scholar] [CrossRef][Green Version]
- Fong, G.H.; Rossant, J.; Gertsenstein, M.; Breitman, M.L. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995, 376, 66–70. [Google Scholar] [CrossRef]
- Shalaby, F.; Rossant, J.; Yamaguchi, T.P.; Gertsenstein, M.; Wu, X.F.; Breitman, M.L.; Schuh, A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 1995, 376, 62–66. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [Google Scholar] [CrossRef]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Bressler, N.M.; Bressler, S.B.; Brucker, A.J.; Ferris, F.L.; Hampton, G.R.; Jhaveri, C.; et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef]
- Chung, E.J.; Kim, J.H.; Ahn, H.S.; Koh, H.J. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z.; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef]
- Lepore, D.; Quinn, G.E.; Molle, F.; Baldascino, A.; Orazi, L.; Sammartino, M.; Purcaro, V.; Giannantonio, C.; Papacci, P.; Romagnoli, C. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: Report on fluorescein angiographic findings. Ophthalmology 2014, 121, 2212–2219. [Google Scholar] [CrossRef]
- Vogel, R.N.; Strampe, M.; Fagbemi, O.E.; Visotcky, A.; Tarima, S.; Carroll, J.; Costakos, D.M. Foveal Development in Infants Treated with Bevacizumab or Laser Photocoagulation for Retinopathy of Prematurity. Ophthalmology 2018, 125, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Wright, T.; Isaac, M.; Westall, C.; Mireskandari, K.; Tehrani, N.N. Macular morphology following unilateral bevacizumab injection for retinopathy of prematurity: An OCT study. J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2017, 21, 499–501.e1. [Google Scholar] [CrossRef] [PubMed]
- Atchaneeyasakul, L.-O.; Trinavarat, A. Choroidal ruptures after adjuvant intravitreal injection of bevacizumab for aggressive posterior retinopathy of prematurity. J. Perinatol. 2010, 30, 497–499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, W.-C.; Yeh, P.-T.; Chen, S.-N.; Yang, C.-M.; Lai, C.-C.; Kuo, H.-K. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: A multicenter study in taiwan. Ophthalmology 2011, 118, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Choi, K.S.; Lee, S.J. Delayed-onset retinal detachment after an intravitreal injection of ranibizumab for zone 1 plus retinopathy of prematurity. J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2010, 14, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.K.; Berrocal, A.M.; Murray, T.G.; Rich, R.; Major, J.C.; Hess, D.; Johnson, R.A. Retinal detachment despite aggressive management of aggressive posterior retinopathy of prematurity. J. Pediatr. Ophthalmol. Strabismus 2010, 47, e1–e4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mintz-Hittner, H.A.; Geloneck, M.M.; Chuang, A.Z. Clinical Management of Recurrent Retinopathy of Prematurity after Intravitreal Bevacizumab Monotherapy. Ophthalmology 2016, 123, 1845–1855. [Google Scholar] [CrossRef]
- Stahl, A.; Lepore, D.; Fielder, A.; Fleck, B.; Reynolds, J.D.; Chiang, M.F.; Li, J.; Liew, M.; Maier, R.; Zhu, Q.; et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): An open-label randomised controlled trial. Lancet 2019, 394, 1551–1559. [Google Scholar] [CrossRef]
- Wu, W.-C.; Shih, C.-P.; Lien, R.; Wang, N.-K.; Chen, Y.-P.; Chao, A.-N.; Chen, K.-J.; Chen, T.-L.; Hwang, Y.-S.; Lai, C.-C. Serum vascular endothelial growth factor after bevacizumab or ranibizumab treatment for retinopathy of prematurity. Retina 2017, 37, 694–701. [Google Scholar] [CrossRef]
- Calvo, P.M.; Pastor, A.M.; de la Cruz, R.R. Vascular endothelial growth factor: An essential neurotrophic factor for motoneurons? Neural Regen. Res. 2018, 13, 1181–1182. [Google Scholar] [CrossRef]
- Arima, M.; Akiyama, M.; Fujiwara, K.; Mori, Y.; Inoue, H.; Seki, E.; Nakama, T.; Tsukamoto, S.; Ochiai, M.; Ohga, S.; et al. Neurodevelopmental outcomes following intravitreal bevacizumab injection in Japanese preterm infants with type 1 retinopathy of prematurity. PLoS ONE 2020, 15, e0230678. [Google Scholar] [CrossRef]
- Morin, J.; Luu, T.M.; Superstein, R.; Ospina, L.H.; Lefebvre, F.; Simard, M.-N.; Shah, V.; Shah, P.S.; Kelly, E.N. Neurodevelopmental Outcomes Following Bevacizumab Injections for Retinopathy of Prematurity. Pediatrics 2016, 137, e20153218. [Google Scholar] [CrossRef]
- Tang, F.; LeBlanc, M.E.; Wang, W.; Liang, D.; Chen, P.; Chou, T.-H.; Tian, H.; Li, W. Anti-secretogranin III therapy of oxygen-induced retinopathy with optimal safety. Angiogenesis 2019, 22, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, C.C.; Mitton, K.P.; Dailey, W.; Massoll, C.; Roumayah, K.; Guzman, E.; Tarabishy, N.; Cheng, M.; Drenser, K.A. Effects of anti-VEGF treatment on the recovery of the developing retina following oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Beharry, K.D.; Valencia, G.B.; Lazzaro, D.R.; Aranda, J.V. Pharmacologic interventions for the prevention and treatment of retinopathy of prematurity. Semin. Perinatol. 2016, 40, 189–202. [Google Scholar] [CrossRef]
- Ley, D.; Hallberg, B.; Hansen-Pupp, I.; Dani, C.; Ramenghi, L.A.; Marlow, N.; Beardsall, K.; Bhatti, F.; Dunger, D.; Higginson, J.D.; et al. rhIGF-1/rhIGFBP-3 in Preterm Infants: A Phase 2 Randomized Controlled Trial. J. Pediatr. 2019, 206, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Brion, L.P.; Bell, E.F.; Raghuveer, T.S. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2003, CD003665. [Google Scholar] [CrossRef]
- Law, M.R.; Wijewardene, K.; Wald, N.J. Is routine vitamin E administration justified in very low-birthweight infants? Dev. Med. Child Neurol. 1990, 32, 442–450. [Google Scholar] [CrossRef]
- Du, Y.; He, Y.; Wang, Y.-L.; Zhou, J.-G.; Chen, C. The efficacy and safety of inositol supplementation in preterm infants to prevent retinopathy of prematurity: A systematic review and meta-analysis. BMC Ophthalmol. 2019, 19, 135. [Google Scholar] [CrossRef]
- Kapoor, V.; Malviya, M.N.; Soll, R. Lipid emulsions for parenterally fed preterm infants. Cochrane Database Syst. Rev. 2019, 6, CD013163. [Google Scholar] [CrossRef]
- Stritzke, A.; Kabra, N.; Kaur, S.; Robertson, H.L.; Lodha, A. Oral propranolol in prevention of severe retinopathy of prematurity: A systematic review and meta-analysis. J. Perinatol. 2019, 39, 1584–1594. [Google Scholar] [CrossRef]
- Filippi, L.; Cavallaro, G.; Bagnoli, P.; Monte, M.D.; Fiorini, P.; Donzelli, G.; Tinelli, F.; Araimo, G.; Cristofori, G.; Marca, G.L.; et al. Oral propranolol for retinopathy of prematurity: Risks, safety concerns, and perspectives. J. Pediatr. 2013, 163, 1570–1577.e6. [Google Scholar] [CrossRef]
- Filippi, L.; Cavallaro, G.; Berti, E.; Padrini, L.; Araimo, G.; Regiroli, G.; Raffaeli, G.; Bozzetti, V.; Tagliabue, P.; Tomasini, B.; et al. Propranolol 0.2% Eye Micro-Drops for Retinopathy of Prematurity: A Prospective Phase IIB Study. Front. Pediatr. 2019, 7, 180. [Google Scholar] [CrossRef]
- Rong, X.; Tian, H.; Yang, L.; Li, W. Function-first ligandomics for ocular vascular research and drug target discovery. Exp. Eye Res. 2019, 182, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Chikaraishi, Y.; Izuta, H.; Ogata, N.; Shimazawa, M.; Matsumura, M.; Hara, H. Role of soluble vascular endothelial growth factor receptor-1 in the vitreous in proliferative diabetic retinopathy. Ophthalmology 2008, 115, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.E.; Wang, W.; Ji, Y.; Tian, H.; Liu, D.; Zhang, X.; Li, W. Secretogranin III as a novel target for the therapy of choroidal neovascularization. Exp. Eye Res. 2019, 181, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, D.M.; Rinchik, E.M.; Russell, L.B.; Ottiger, H.P.; Sutcliffe, J.G.; Copeland, N.G.; Jenkins, N.A. Genetic ablation of a mouse gene expressed specifically in brain. EMBO J. 1990, 9, 395–399. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, C.; Webster, K.A.; Bhatt, A.; Tian, H.; Su, G.; Li, W. Concurrent Physiological and Pathological Angiogenesis in Retinopathy of Prematurity and Emerging Therapies. Int. J. Mol. Sci. 2021, 22, 4809. https://doi.org/10.3390/ijms22094809
Dai C, Webster KA, Bhatt A, Tian H, Su G, Li W. Concurrent Physiological and Pathological Angiogenesis in Retinopathy of Prematurity and Emerging Therapies. International Journal of Molecular Sciences. 2021; 22(9):4809. https://doi.org/10.3390/ijms22094809
Chicago/Turabian StyleDai, Chang, Keith A. Webster, Amit Bhatt, Hong Tian, Guanfang Su, and Wei Li. 2021. "Concurrent Physiological and Pathological Angiogenesis in Retinopathy of Prematurity and Emerging Therapies" International Journal of Molecular Sciences 22, no. 9: 4809. https://doi.org/10.3390/ijms22094809
APA StyleDai, C., Webster, K. A., Bhatt, A., Tian, H., Su, G., & Li, W. (2021). Concurrent Physiological and Pathological Angiogenesis in Retinopathy of Prematurity and Emerging Therapies. International Journal of Molecular Sciences, 22(9), 4809. https://doi.org/10.3390/ijms22094809






