Effects of Overexpression of Neurosecretory Protein GL-Precursor Gene on Glucose Homeostasis and Insulin Sensitivity in Mice
Abstract
:1. Introduction
2. Results
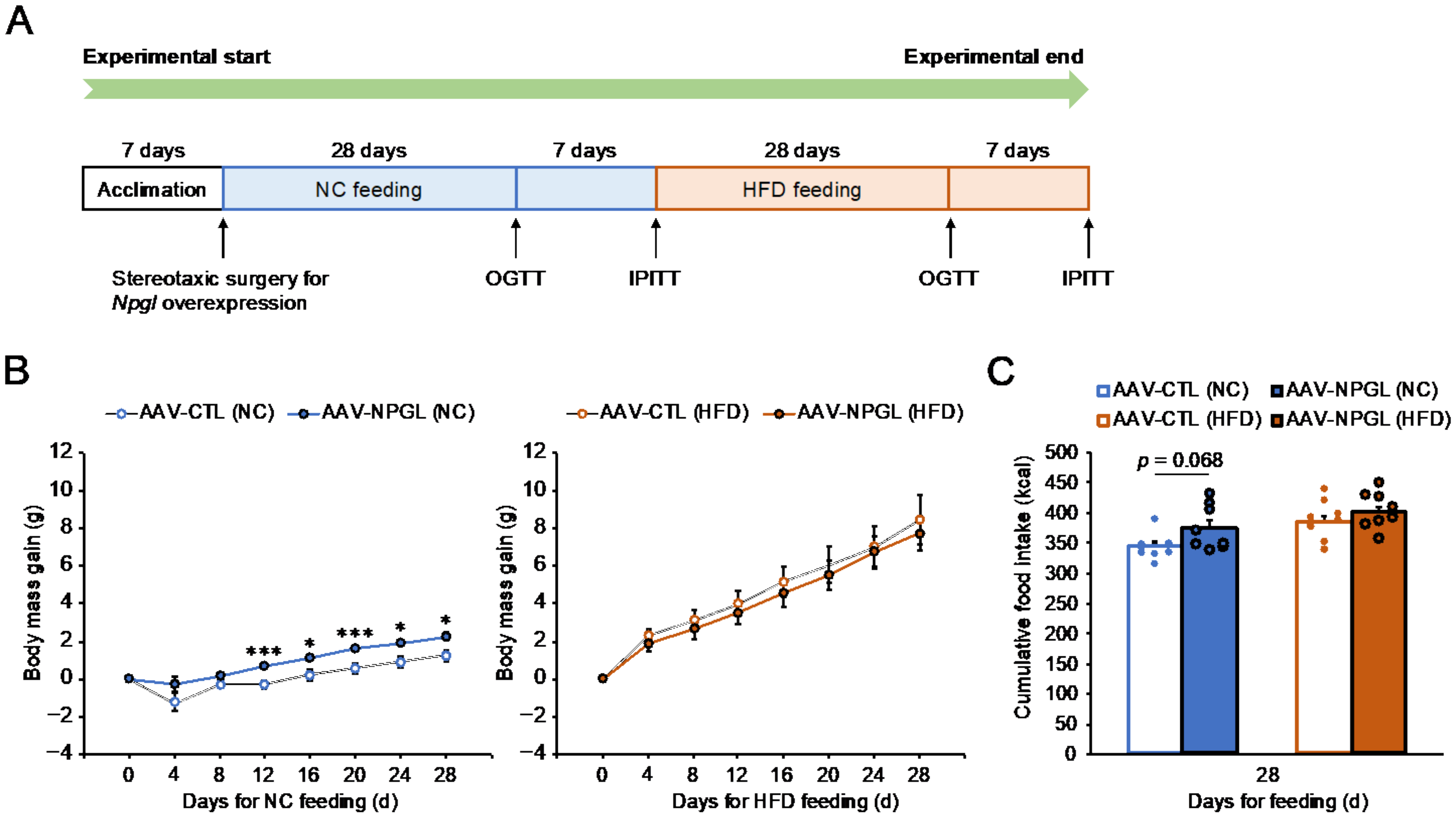
2.1. Effects of NPGL-Precursor Gene Overexpression on Food Intake and Body Mass Gain under NC and HFD Conditions
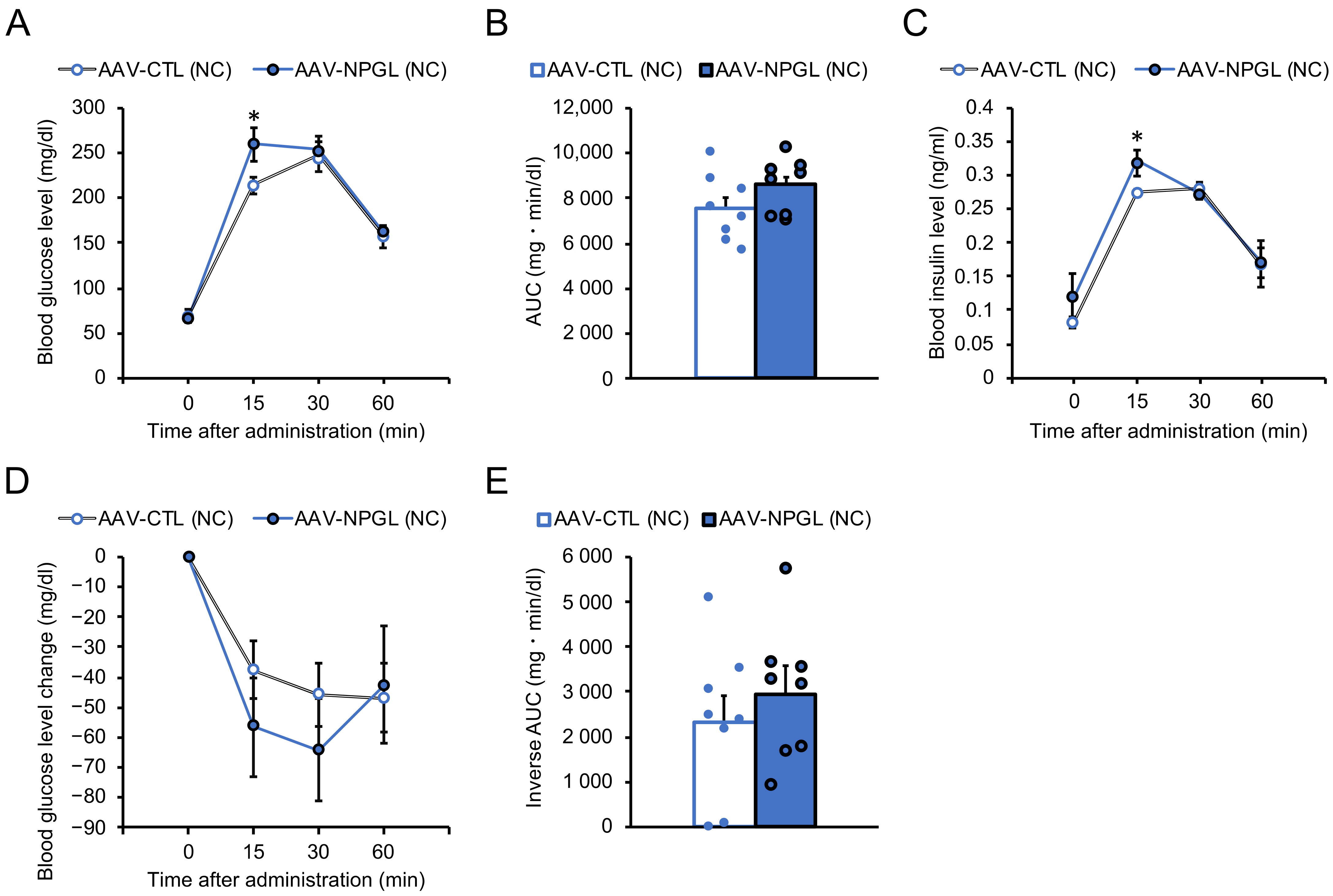
2.2. Effects of NPGL-Precursor Gene Overexpression on Glucose Homeostasis and Insulin Sensitivity under NC Conditions
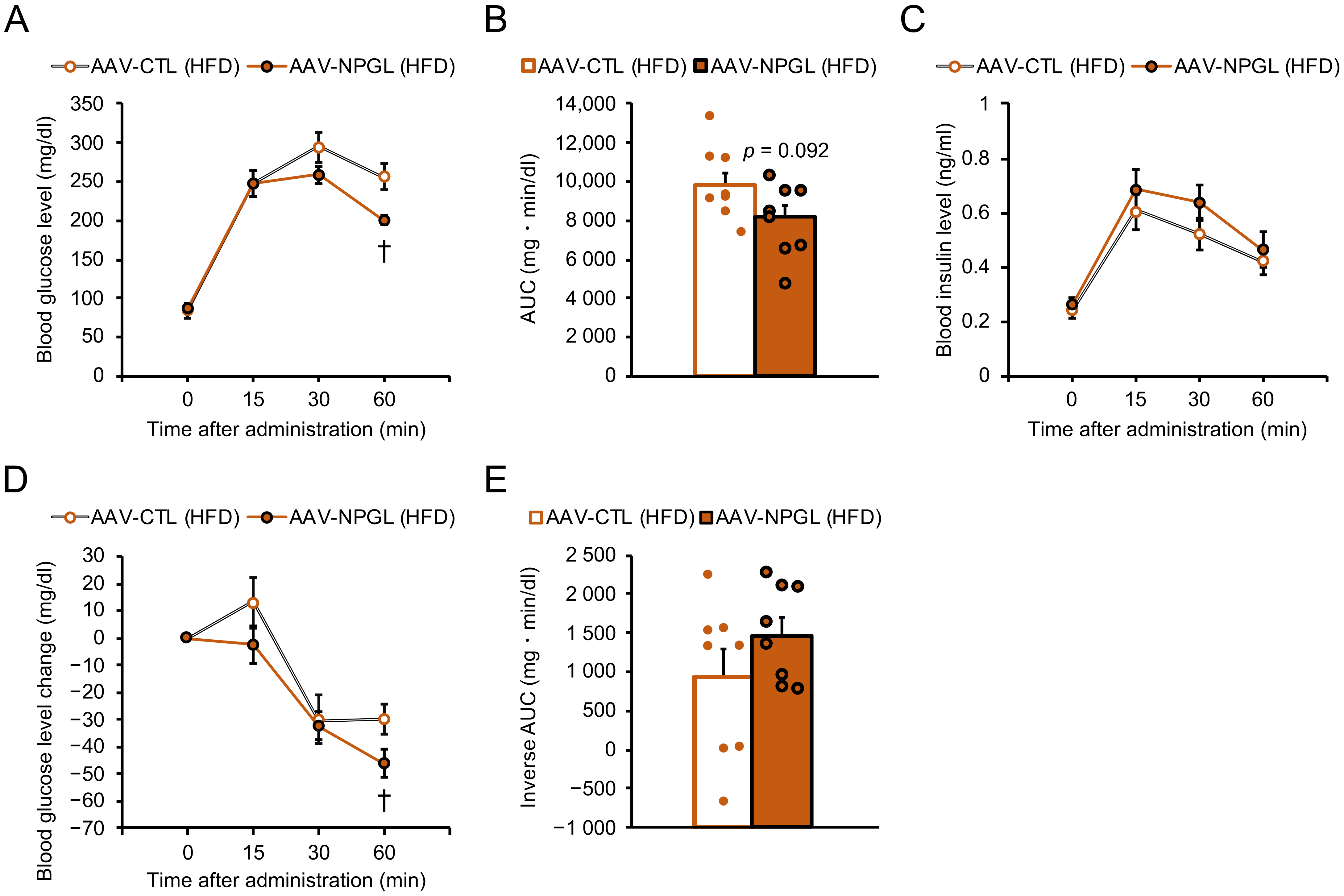
2.3. Effects of NPGL-Precursor Gene Overexpression on Glucose Homeostasis and Insulin Sensitivity under HFD Conditions
2.4. Effects of NPGL-Precursor Gene Overexpression on Body Composition and Blood Parameters
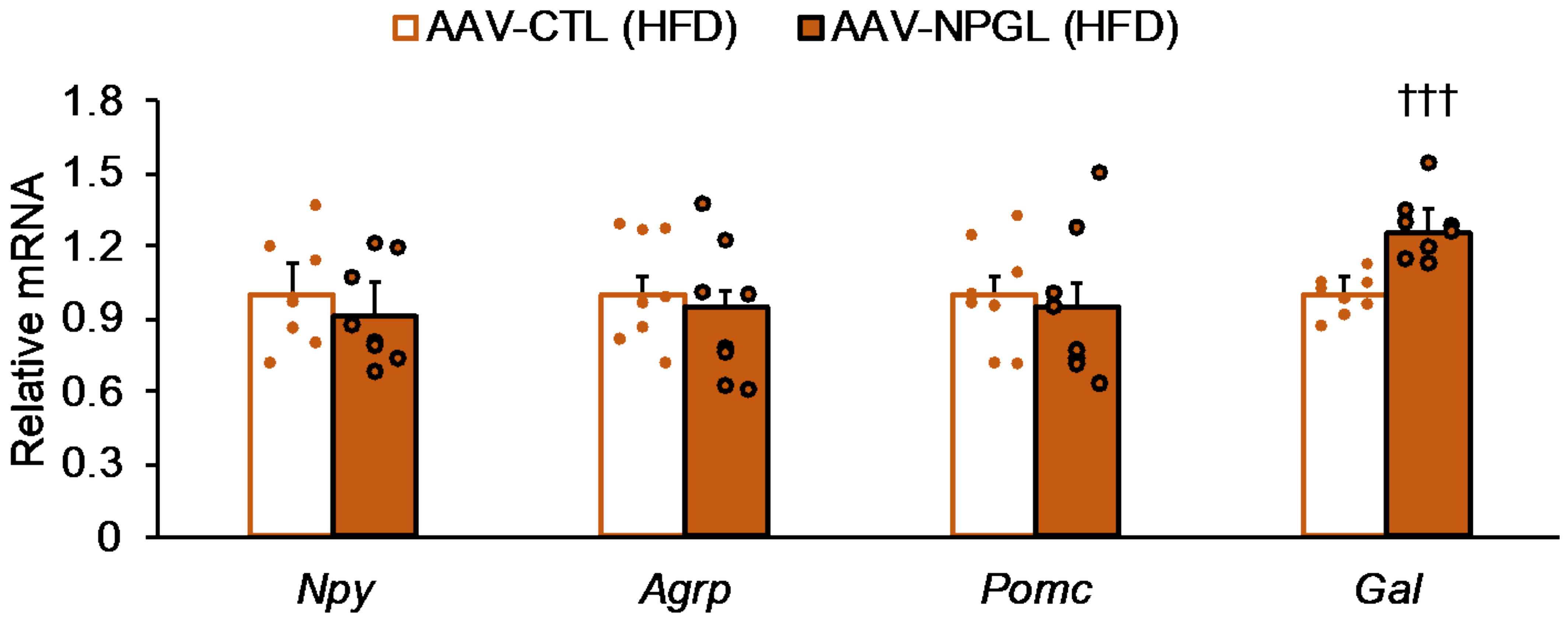
2.5. Effects of NPGL-Precursor Gene Overexpression on mRNA Expression of Neuropeptides and Genes Related to Gluconeogenesis and Glucose Uptake
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Production of AAV-Based Vectors
4.3. Npgl Overexpression
4.4. OGTT and IPITT
4.5. Quantitative RT-PCR
4.6. Blood Biochemical Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability Statement
Acknowledgments
Conflicts of Interest
References
- Haslam, D.W.; James, W.P.T. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The epidemiology of obesity: A big picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bouchard, C. Convergence between biological, behavioural and genetic determinants of obesity. Nat. Rev. Genet. 2017, 18, 731–748. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Porte, D. Diabetes, obesity, and the brain. Science 2005, 307, 375–379. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Morton, G.J.; Meek, T.H.; Schwartz, M.W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 2014, 15, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Kamegai, J.; Tamura, H.; Shimizu, T.; Ishii, S.; Sugihara, H.; Wakabayashi, I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 2001, 51, 2438–2443. [Google Scholar] [CrossRef] [Green Version]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Beutler, L.R.; Chen, Y.; Ahn, J.S.; Lin, Y.C.; Essner, R.A.; Knight, Z.A. Dynamics of gut-brain communication underlying hunger. Neuron 2017, 96, 461–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowley, M.A.; Smart, J.L.; Rubinstein, M.; Cerdán, M.G.; Diano, S.; Horvath, T.L.; Cone, R.D.; Low, M.J. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001, 411, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.; Ye, C.; Yang, Z.; Choi, B.; Chua, S.; Lowell, B.B. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011, 71, 142–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Koch, L.; Wunderlich, F.T.; Seibler, J.; Könner, A.C.; Hampel, B.; Irlenbusch, S.; Brabant, G.; Kahn, C.R.; Schwenk, F.; Brüning, J.C. Central insulin action regulates peripheral glucose and fat metabolism in mice. J. Clin. Investig. 2008, 118, 2132–2147. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [Green Version]
- Ukena, K.; Iwakoshi-Ukena, E.; Taniuchi, S.; Bessho, Y.; Maejima, S.; Masuda, K.; Shikano, K.; Kondo, K.; Furumitsu, M.; Tachibana, T. Identification of a cDNA encoding a novel small secretory protein, neurosecretory protein GL, in the chicken hypothalamic infundibulum. Biochem. Biophys. Res. Commun. 2014, 446, 298–303. [Google Scholar] [CrossRef]
- Ukena, K. Avian and murine neurosecretory protein GL participates in the regulation of feeding and energy metabolism. Gen. Comp. Endocrinol. 2018, 260, 164–170. [Google Scholar] [CrossRef]
- Shikano, K.; Bessho, Y.; Kato, M.; Iwakoshi-Ukena, E.; Taniuchi, S.; Furumitsu, M.; Tachibana, T.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Localization and function of neurosecretory protein GM, a novel small secretory protein, in the chicken hypothalamus. Sci. Rep. 2018, 8, 704. [Google Scholar] [CrossRef] [Green Version]
- Shikano, K.; Kato, M.; Iwakoshi-Ukena, E.; Furumitsu, M.; Matsuura, D.; Masuda, K.; Tachibana, T.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Effects of chronic intracerebroventricular infusion of neurosecretory protein GL on body mass and food and water intake in chicks. Gen. Comp. Endocrinol. 2018, 256, 37–42. [Google Scholar] [CrossRef]
- Shikano, K.; Iwakoshi-Ukena, E.; Kato, M.; Furumitsu, M.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Neurosecretory protein GL induces fat accumulation in chicks. Front. Endocrinol. 2019, 10, 392. [Google Scholar] [CrossRef]
- Matsuura, D.; Shikano, K.; Saito, T.; Iwakoshi-Ukena, E.; Furumitsu, M.; Ochi, Y.; Sato, M.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Neurosecretory protein GL, a hypothalamic small secretory protein, participates in energy homeostasis in male mice. Endocrinology 2017, 158, 1120–1129. [Google Scholar] [CrossRef]
- Shikano, K.; Iwakoshi-Ukena, E.; Saito, T.; Narimatsu, Y.; Kadota, A.; Furumitsu, M.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Neurosecretory protein GL induces fat accumulation in mice. J. Endocrinol. 2020, 244, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iwakoshi-Ukena, E.; Shikano, K.; Kondo, K.; Taniuchi, S.; Furumitsu, M.; Ochi, Y.; Sasaki, T.; Okamoto, S.; Bentley, G.E.; Kriegsfeld, L.J.; et al. Neurosecretory protein GL stimulates food intake, de novo lipogenesis, and onset of obesity. Elife 2017, 6, e28527. [Google Scholar] [CrossRef] [Green Version]
- Narimatsu, Y.; Fukumura, K.; Shikano, K.; Iwakoshi-Ukena, E.; Furumitsu, M.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Hypothalamic overexpression of neurosecretory protein GL leads to obesity in Mice. bioRxiv 2021. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol. 2008, 59, 55–92. [Google Scholar] [CrossRef] [PubMed]
- Andermann, M.L.; Lowell, B.B. Toward a wiring diagram understanding of appetite control. Neuron 2017, 95, 757–778. [Google Scholar] [CrossRef]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahrén, B.; Winzell, M.S.; Pacini, G. The augmenting effect on insulin secretion by oral versus intravenous glucose is exaggerated by high-fat diet in mice. J. Endocrinol. 2008, 197, 181–187. [Google Scholar] [CrossRef]
- Horakova, O.; Kroupova, P.; Bardova, K.; Buresova, J.; Janovska, P.; Kopecky, J.; Rossmeisl, M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep. 2019, 9, 6156. [Google Scholar] [CrossRef]
- Ruud, J.; Steculorum, S.M.; Bruning, J.C. Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat. Commun. 2017, 8, 15259. [Google Scholar] [CrossRef] [Green Version]
- Fang, P.; Yu, M.; Shi, M.; Bo, P.; Zhang, Z. Galanin peptide family regulation of glucose metabolism. Front. Neuroendocrinol. 2020, 56, 100801. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, K.; Shikano, K.; Narimatsu, Y.; Iwakoshi-Ukena, E.; Furumitsu, M.; Naito, M.; Ukena, K. Effects of neurosecretory protein GL on food intake and fat accumulation under different dietary nutrient compositions in rats. Biosci. Biotechnol. Biochem. 2021, zbab064. [Google Scholar] [CrossRef] [PubMed]
- Delgado, T.C.; Pinheiro, D.; Caldeira, M.; Castro, M.M.; Geraldes, C.F.; López-Larrubia, P.; Cerdán, S.; Jones, J.G. Sources of hepatic triglyceride accumulation during high-fat feeding in the healthy rat. NMR Biomed. 2009, 22, 310–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, J.A.; Carvalho, F.; Pearson, M.; Horton, J.D.; Browning, J.D.; Jones, J.G.; Burgess, S.C. A high-fat diet suppresses de novo lipogenesis and desaturation but not elongation and triglyceride synthesis in mice. J. Lipid Res. 2014, 55, 2541–2553. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Wahed, A.; Guilmeau, S.; Postic, C. Sweet sixteenth for ChREBP: Established roles and future goals. Cell Metab. 2017, 26, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Dentin, R.; Benhamed, F.; Pégorier, J.P.; Foufelle, F.; Viollet, B.; Vaulont, S.; Girard, J.; Postic, C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J. Clin. Investig. 2005, 2843–2854. [Google Scholar] [CrossRef] [Green Version]
- Caron, A.; Richard, D. Neuronal systems and circuits involved in the control of food intake and adaptive thermogenesis. Ann. N. Y. Acad. Sci. 2017, 1391, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Lieu, L.; Chau, D.; Afrin, S.; Dong, Y.; Alhadeff, A.L.; Betley, J.N.; Williams, K.W. Effects of metabolic state on the regulation of melanocortin circuits. Physiol. Behav. 2020, 224, 113039. [Google Scholar] [CrossRef]
- Takahashi, K.A.; Cone, R.D. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 2005, 146, 1043–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baver, S.B.; Hope, K.; Guyot, S.; Bjørbaek, C.; Kaczorowski, C.; O’Connell, K.M.S. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 2014, 34, 5486–5496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, S.; Sato, T.; Tateyama, M.; Kageyama, H.; Maejima, Y.; Nakata, M.; Hirako, S.; Matsuo, T.; Kyaw, S.; Shiuchi, T.; et al. Activation of AMPK-regulated CRH neurons in the PVH is sufficient and necessary to induce dietary preference for carbohydrate over fat. Cell Rep. 2018, 22, 706–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Holstein-Rathlou, S.; BonDurant, L.D.; Peltekian, L.; Naber, M.C.; Yin, T.C.; Claflin, K.E.; Urizar, A.I.; Madsen, A.N.; Ratner, C.; Holst, B.; et al. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 2016, 23, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukdar, S.; Owen, B.M.; Song, P.; Hernandez, G.; Zhang, Y.; Zhou, Y.; Scott, W.T.; Paratala, B.; Turner, T.; Smith, A.; et al. FGF21 regulates sweet and alcohol preference. Cell Metab. 2016, 23, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Nagy, C.; Einwallner, E. Study of in vivo glucose metabolism in high-fat diet-fed mice using oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). J. Vis. Exp. 2018, 131, 56672. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 1997. [Google Scholar]

| Gene | Sense Primer (5′ to 3′) | Antisense Primer (5′ to 3′) |
|---|---|---|
| Npgl | GGAACCATGGCTTAGGAAGG | TCTAAGGAGCTGAGAATATGCA |
| Npy | TATCTCTGCTCGTGTGTTTG | GATTGATGTAGTGTCGCAGA |
| Agrp | TGTTCCCAGAGTTCCCAGGTC | GCATTGAAGAAGCGGCAGTAGCAC |
| Pomc | AGCTGCCTTTCCGCGACA | ATCTATGGAGGTCTGAAGCA |
| Gal | GAGCCTTGATCCTGCACTGA | AGTGGCTGACAGGGTCACAA |
| Acc | TCCGCACTGACTGTAACCACAT | TGCTCCGCACAGATTCTTCA |
| Fas | AGGGGTCGACCTGGTCCTCA | GCCATGCCCAGAGGGTGGTT |
| Chrebpα | CGACACTCACCCACCTCTTC | TTGTTCAGCCGGATCTTGTC |
| Cpt1a | CCTGGGCATGATTGCAAAG | GGACGCCACTCACGATGTT |
| Atgl | AACACCAGCATCCAGTTCAA | GGTTCAGTAGGCCATTCCTC |
| Hsl | GCTGGGCTGTCAAGCACTGT | GTAACTGGGTAGGCTGCCAT |
| Gapdh | AAGGTCATCCCAGAGCTGAA | CTGCTTCACCACCTTCTTGA |
| Slc2a4 | GTAACTTCATTGTCGGCATGG | AGCTGAGATCTGGTCAAACG |
| Cd36 | TCCTCTGACATTTGCAGGTCTATC | AAAGGCATTGGCTGGAAGAA |
| Pgc1α | GCAACATGCTCAAGCCAAAC | TGCAGTTCCAGAGAGTTCCA |
| Ucp1 | CAAAAACAGAAGGATTGCCGAAA | TCTTGGACTGAGTCGTAGAGG |
| Dio2 | CCACCTTCTTGACTTTGCCA | GGTGAGCCTCATCAATGTATAC |
| G6pase | ACTGTGGGCATCAATCTCCTC | CGGGACAGACAGACGTTCAGC |
| Pepck | GTGCTGGAGTGGATGTTCGG | CTGGCTGATTCTCTGTTTCAGG |
| Slc2a2 | GGCTAATTTCAGGACTGGTT | TTTCTTTGCCCTGACTTCCT |
| Fgf21 | CCTCTAGGTTTCTTTGCCAACAG | AAGCTGCAGGCCTCAGGAT |
| Actb | GGCACCACACCTTCTACAAT | AGGTCTCAAACATGATCTGG |
| Rps18 | CCTGAGAAGTTCCAGCACAT | TTCTCCAGCCCTCTTGGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukumura, K.; Narimatsu, Y.; Moriwaki, S.; Iwakoshi-Ukena, E.; Furumitsu, M.; Ukena, K. Effects of Overexpression of Neurosecretory Protein GL-Precursor Gene on Glucose Homeostasis and Insulin Sensitivity in Mice. Int. J. Mol. Sci. 2021, 22, 4681. https://doi.org/10.3390/ijms22094681
Fukumura K, Narimatsu Y, Moriwaki S, Iwakoshi-Ukena E, Furumitsu M, Ukena K. Effects of Overexpression of Neurosecretory Protein GL-Precursor Gene on Glucose Homeostasis and Insulin Sensitivity in Mice. International Journal of Molecular Sciences. 2021; 22(9):4681. https://doi.org/10.3390/ijms22094681
Chicago/Turabian StyleFukumura, Keisuke, Yuki Narimatsu, Shogo Moriwaki, Eiko Iwakoshi-Ukena, Megumi Furumitsu, and Kazuyoshi Ukena. 2021. "Effects of Overexpression of Neurosecretory Protein GL-Precursor Gene on Glucose Homeostasis and Insulin Sensitivity in Mice" International Journal of Molecular Sciences 22, no. 9: 4681. https://doi.org/10.3390/ijms22094681
APA StyleFukumura, K., Narimatsu, Y., Moriwaki, S., Iwakoshi-Ukena, E., Furumitsu, M., & Ukena, K. (2021). Effects of Overexpression of Neurosecretory Protein GL-Precursor Gene on Glucose Homeostasis and Insulin Sensitivity in Mice. International Journal of Molecular Sciences, 22(9), 4681. https://doi.org/10.3390/ijms22094681






