Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential
Abstract
1. Introduction
2. Chemistry, Occurrence and Isolation of Ursolic and Oleanolic Acids
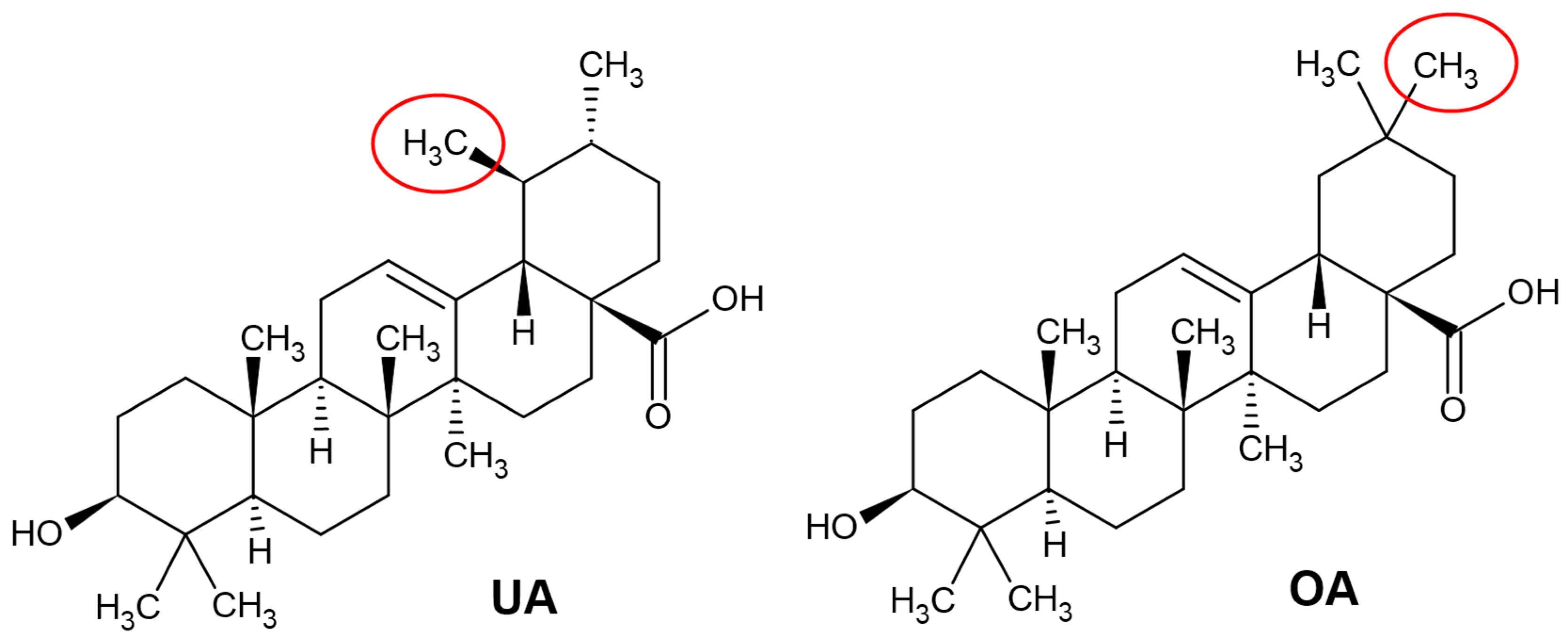
2.1. Chemical Structure of Ursolic and Oleanolic Acids
2.2. Natural Sources of Oleanolic and Ursolic Acids
2.3. Extraction of Ursolic and Oleanolic Acids from Plants
3. Bioavailability and Pharmacokinetic Properties of Ursolic and Oleanolic Acids
4. Neuroprotective Effects of Ursolic and Oleanolic Acids
4.1. Neuroprotective Effects of Ursolic and Oleanolic Acids in Neurodegeneration
| Ursolic Acid | ||||
| Brain Pathology | Experimental Model | Dosage | Beneficial Effects | Reference |
| Trauma/ischaemic damage | 1 h MCAO with 24 h reperfusion. Neonatal rat hippocampal slices after oxygen-glucose deprivation. | 10, 50 and 100 mg/kg. | Apoptosis ↓, Protection via AKT/mTOR/HIF-1α pathway, Bcl-2 ↑, Bad ↓ and Caspase 3 ↓ | [99] |
| 2 h MCAO and 48 h reperfusion. | Post-conditioning with 5, 10 or 20 mg/kg. | MMP-2 and MMP-9 ↓, TIMP1 ↑, PPARγ-positive cells ↑ | [91] | |
| MCAO rat model with 24 h reperfusion. | Post-conditioning with 130 mg/kg. | Nrf2 pathway activation, TLR4 ↓ and NF-kB ↓, MDA ↓ | [84] | |
| Mouse traumatic brain injury after 24 h. | Pre-conditioning with 100 mg/kg. | GP ↑, SOD ↑ and MDA ↓, apoptosis ↓ via Nrf2-ARE signalling pathway. | [83] | |
| Subarachnoid haemorrhage rat model by endovascular perforation. | Post-treatment 50 mg/kg. | SOD ↑, CAT ↑, GSH and GSSH ↑, MDA ↓, caspase-3 and-9 ↓ | [80] | |
| Excitotoxicity | Primary neuronal cultures from the hippocampus of 7-day-old rats were treated with 150 mM kainate for 2 h. | Pre-treatment for 10 min with 5, 10 or 15 μM. | Non-NMDA receptor modulation, intracellular ROS ↓, mitochondrial membrane potential stabilisation. | [79] |
| Inflammation | To mimic NF-κB pathway mice were subcutaneously injected with D-galactose. | 10 mg/kg/d for 8 weeks. | COX-2 ↓, iNOS ↓, IL-1β ↓, IL-6 ↓, TNF-α ↓, ROS ↓, advanced glycation end products ↓ | [90] |
| Multiple sclerosis | Multiple sclerosis mouse model. | Prolonged oral administration of 25 mg/kg. | MBP+ ↑, myelinated axons ↑, PPARγ pathway activation. | [93] |
| Multiple sclerosis mouse model. | Daily use of drinking water with 1 mg/mL for 6 weeks. | myelinated area in corpus callosum ↑, MBP ↑ | [92] | |
| Parkinson’s disease | Parkinson’s disease model established by rotenone infusions | 30-day administration of 5 and 10 mg/kg. | CAT ↑, SOD ↑, GSH ↑, MDA ↓, TNF- α ↓, improved mitochondria complex I enzymatic activity, MtCO1 gene expression ↑, tyrosine hydroxylase positive neurons ↑, glial fibrillary acidic protein ↓ | [81] |
| Parkinson’s disease rat model designed by intraperitoneal injections of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine | Orally 25 mg/kg for 21 d. | CAT ↑, GSH ↑, MDA ↓, tyrosine hydroxylase-positive dopaminergic neurons ↑, NF-κB ↓, TNF-α ↓, IFN-γ ↓, IL-12 ↓, IL-10 ↑, IL-4 ↑ | [82] | |
| Oleanolic Acid | ||||
| Brain Pathology | Experimental Model | Dosage | Beneficial Effects | Reference |
| Inflammation | Mouse microglial BV2 cell line activated by lipopolysaccharide. | Pre-treatment with 0.5–25 µM. | IL-1β ↓ , IL-6 ↓, TNF-α ↓, NO ↓, GSH ↑, iNOS ↑ | [16] |
| Parkinson’s disease | PC12 cell culture treated with 6- Hydroxydopamine. | Pre-treatment and post-treatment with 100 mg/kg. | Dopamine ↑, intracellular ROS ↓, neuronal cell survival ↑ | [85] |
| Ischaemic damage | Wistar rat focal cortical hypoxia induced by cobalt chloride injection. | Intraperitoneal injection with 6 mg/kg/d for 7 d. | Neuronal survival ↑, dendrite recovery ↑, astroglial and microglial reaction ↓. | [102] |
| Bilateral common carotid artery ligation in mice, and PC12 cells pre-treated with H2O2 | Pre-administration of 50 and 25 mg/kg, respectively. | Infarct zone size ↓, mitochondrial membrane potential ↑ and succinic dehydrogenase ↑, SOD ↑ and GP ↑, MDA ↓ | [86] | |
4.2. Glioblastoma and Ursolic/Oleanolic Acids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Ramos-Hryb, A.B.; Pazini, F.L.; Kaster, M.P.; Rodrigues, A.L.S. Therapeutic potential of ursolic acid to manage neurodegenerative and psychiatric diseases. CNS Drugs 2017, 31, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Ayeni, G.; Ibeji, C.U.; Islam, S. Modulatory effect of ursolic acid on neurodegenerative activities in oxidative brain injury: An ex vivo study. J. Food Biochem. 2021, 45, e13597. [Google Scholar] [CrossRef]
- Bacci, A.; Runfola, M.; Sestito, S.; Rapposelli, S. Beyond antioxidant effects: Nature-based templates unveil new strategies for neurodegenerative diseases. Antioxidants 2021, 10, 367. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef]
- Proshkin, E.; Plyusnin, S.; Babak, T.; Lashmanova, E.; Maganova, F.; Koval, L.; Platonova, E.; Shaposhnikov, M.; Moskalev, A. Terpenoids as potential geroprotectors. Antioxidants 2020, 9, 529. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.H.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2013, 30, 1028–1065. [Google Scholar] [CrossRef]
- Guinda, A.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic triterpenoids from olive fruit and leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.Y.; Park, S.Y. Terpenoids as potential anti-Alzheimer’s disease therapeutics. Molecules 2012, 17, 3524–3538. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic acid—A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef]
- Castellano, J.M.; Garcia-Rodriguez, S.; Espinosa, J.M.; Millan-Linares, M.C.; Rada, M.; Perona, J.S. Oleanolic acid exerts a neuroprotective efect against microglial cell activation by modulating cytokine release and antioxidant defense systems. Biomolecules 2019, 9, 683. [Google Scholar] [CrossRef]
- Pollie, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. antimicrobial activity of oleanolic and ursolic acids: An Update. Extracts rich in both ursolic and oleanolic acids. J. Evid. Based Complementary Altern. Med. 2015, 2015, 620472. [Google Scholar]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef]
- Pironi, A.M.; de Araújo, P.R.; Fernandes, M.A.; Salgado, H.R.N.; Chorilli, M. Characteristics, biological properties and analytical methods of ursolic acid: A Rrview. Crit Rev. Anal. Chem. 2018, 48, 86–93. [Google Scholar] [CrossRef]
- Lim, S.W.; Hong, S.P.; Jeong, S.W.; Kim, B.; Bak, H.; Ryoo, H.C.; Lee, S.H.; Ahn, S.K. Simultaneous effect of ursolic acid and oleanolic acid on epidermal permeability barrier function and epidermal keratinocyte differentiation via peroxisome proliferator-activated receptor-α. J. Dermatol. 2007, 34, 625–634. [Google Scholar] [CrossRef]
- Lopez-Hortas, L.; Perez-Larran, P.; Gonzalez-Munoz, M.J.; Falque, E.; Dominguez, H. Recent developments on the extraction and application of ursolic acid. A review. Food Res. Int. 2018, 103, 130–149. [Google Scholar] [CrossRef]
- FooDB. FoodDB Website. Available online: https://foodb.ca/ (accessed on 20 April 2021).
- Sporn, M.B.; Liby, K.T.; Yore, M.M.; Fu, L.; Lopchuk, J.M.; Gribble, G.W. New synthetic triterpenoids: Potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J. Nat. Prod. 2011, 74, 537–545. [Google Scholar] [CrossRef]
- Kowalski, R. Studies of selected plant raw materials as alternative sources of triterpenes of oleanolic and ursolic acid types. J. Agric. Food Chem. 2007, 55, 656–662. [Google Scholar] [CrossRef]
- Ludeña-Huaman, M.A.; Ramos-Inquiltupa, D.A. Determination of the content of ursolic and oleanolic acid in the cuticular wax of fruits of different species of Rosaceae. Rev. Colomb. Quim. 2019, 48, 15–20. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef]
- Santos, H.M., Jr.; Lopes, K.C.; Alves, D.S.; Carvalho, G.A.; Oliveira, D.F. Ursolic acid and cis-tiliroside produced by Merremia tomentosa affect oviposition of Leucoptera coffeella on coffee plants. Quim Nova 2018, 41, 302–309. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional uses, phytochemistry, and pharmacology of olea europaea (olive). Evid. Based Complementary Altern. Med. 2015, 541591. [Google Scholar] [CrossRef]
- Fischer, N.H.; Williamson, G.B.; Weidenhamer, J.D.; Richardson, D.R. In search of allelopathy in Florida scrub: The role of allelopathy. J. Chem. Ecol. 1994, 20, 1355–1380. [Google Scholar] [CrossRef]
- Wang, C.M.; Chen, H.T.; Li, T.C.; Weng, J.H.; Jhan, Y.L.; Lin, S.X.; Chou, C.H. The role of pentacyclic triterpenoids in the allelopathic effects of Alstonia scholaris. J. Chem. Ecol. 2014, 40, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Y.; Sheng, Y.; Zhao, D.; Lv, S.; Hu, Y.; Tao, J. Herbaceous peony (Paeonia lactiflora Pall.) as an alternative source of oleanolic and ursolic acids. Int. J. Mol. Sci. 2011, 12, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.C.; Maiti, P.; Chanotiya, C.S.; Shanker, K.; Ghosh, S. Methyl jasmonate-elicited transcriptional responses and pentacyclic triterpene biosynthesis in sweet basil. Plant. Physiol. 2021, 164, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, K.; Sun, C.; Chen, Q.; Zhang, W.; Li, X. Determination of oleanolic acid, ursolic acid and amygdalin in the flower of Eriobotrya japonica lindl. by HPLC. Biomed. Chromatogr. 2007, 21, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Zengin, G.; Maggi, F.; Malagoli, M.; Dall’Acqua, S. Triterpene acid and phenolics from ancient apples of Friuli Venezia Giulia as nutraceutical ingredients: LC-MS study and in vitro activities. Molecules 2019, 24, 1109. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Liaudanskas, M.; Kviklys, D.; Zymonė, K.; Raudonis, R.; Viškelis, J.; Uselis, N.; Janulis, V. Detection and analysis of triterpenic compounds in apple extracts. Int. J. Food Prop. 2018, 21, 1716–1727. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Liaudanskas, M.; Kviklys, D.; Gelvonauskienė, D.; Janulis, V. Qualitative and quantitative composition of triterpenic compounds in the fruit of apple old cultivars grown in Lithuania. Agriculture 2021, 108, 63–70. [Google Scholar]
- Viškelis, J.; Uselis, N.; Liaudanskas, M.; Lanauskas, J.; Bielicki, P.; Univer, T.; Lepsis, J.; Kviklys, D. Location effects across northeastern Europe on bioactive compounds in apple fruit. Agric. Food Sci. 2019, 28, 93–100. [Google Scholar] [CrossRef]
- Zhou, C.; Sheng, Y.; Zhao, D.; Wang, Z.; Tao, J. Variation of oleanolic and ursolic acid in the flesh of persimmon fruit among different cultivars. Molecules 2010, 15, 6580–6587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Daimaru, E.; Ohnishi, M.; Kinoshita, M.; Tokuji, Y. Oleanolic acid and ursolic acid in commercial dried fruits. Food Sci. Technol. Res. 2013, 19, 113–116. [Google Scholar] [CrossRef]
- Xia, E.Q.; Yu, Y.Y.; Xu, X.R.; Deng, G.F.; Guo, Y.J.; Li, H.B. Ultrasound-assisted extraction of oleanolic acid and ursolic acid from ligustrum lucidum ait. Ultrason. Sonochem. 2011, 19, 772–776. [Google Scholar] [CrossRef]
- Xia, E.Q.; Wang, B.W.; Xu, X.R.; Zhu, L.; Song, Y.; Li, H.B. Microwave-assisted extraction of oleanolic acid and ursolic acid from ligustrum lucidum. ait. Int. J. Mol. Sci. 2011, 12, 5319–5329. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, L.; Cheng, N.; Jis, M.; Zhang, Y. Extraction optimization of oleanolic and ursolic acids from pomegranate (Punica granatum L.) flowers. Food Bioprod. Process. 2014, 92, 321–327. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Cognigni, A.; De Faria, E.L.P.; Silvestr, A.J.D.; Zirbs, R.; Freire, M.G.; Bica, K. Valorization of olive tree leaves: Extraction of oleanolic acid using aqueous solutions of surface-active ionic liquids. Sep. Purif. Technol. 2018, 204, 30–37. [Google Scholar] [CrossRef]
- Kosior, M.J.; Sowa, I.; Kocjan, R.; Nowak, R. Effect of different extraction techniques on quantification of oleanolic and ursolic acid in Lamii albi flos. Ind. Crops Prod. 2013, 44, 373–377. [Google Scholar] [CrossRef]
- Fan, J.P.; Liao, D.D.; Zhang, X.H. Ultrasonic assisted extraction of ursolic acid from apple pomace: A novel and facile technique. Sep. Sci. Technol. 2016, 51, 1344–1350. [Google Scholar] [CrossRef]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.H.Y.; Du, Z.; Wang, G.N.; Chan, H.M.; Chang, Q.; Lai, L.C.M.; Chow, A.H.L.; Zheng, Y. Spray freeze drying with polyvinylpyrrolidone and sodium caprate for improved dissolution and oral bioavailability of oleanolic acid, a BCS Class IV compound. Int. J. Pharm. 2011, 404, 148–158. [Google Scholar] [CrossRef]
- Gao, S.; Basu, S.; Yang, Z.; Deb, A.; Hu, M. bioavailability challenges associated with development of saponins as therapeutic and chemopreventive agents. Curr. Drug Targets 2012, 13, 1885–1899. [Google Scholar] [CrossRef]
- Kalani, K.; Yadav, D.K.; Khan, F.; Srivastava, S.K.; Suri, N. Pharmacophore, QSAR, and ADME based semisynthesis and in vitro evaluation of ursolic acid analogs for anticancer activity. J. Mol. Model. 2012, 18, 3389–3413. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Song, Y.; Chan, H.; Bi, C.; Yang, X.; Yan, R.; Wang, Y.; Zheng, Y. Development and characterisation of ursolic acid nanocrystals without stabiliser having improved dissolution rate and in vitro anticancer activity. AAPS PharmSciTech 2014, 15, 11–19. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, X.; Du, P.; Zhang, H.; Zhang, T. Dual strategies to improve oral bioavailability of oleanolic acid: Enhancing water-solubility, permeability and inhibiting cytochrome P450 isozymes. Eur. J. Pharm. Biopharm. 2016, 99, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, Z.; Zu, Y.; Zhao, C.; Sun, X.; Zhang, Z.; Zhang, L. Physicochemical properties and oral bioavailability of ursolic acid nanoparticles using supercritical anti-solvent (SAS) process. Food Chem. 2012, 132, 319–325. [Google Scholar] [CrossRef]
- Eloy, J.O.; Marchetti, J.M. Solid dispersions containing ursolic acid in Poloxamer 407 and PEG 6000: A comparative study of fusion and solvent methods. Powder Technol. 2014, 253, 98–106. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, Y.; Yang, Z.; Niu, R.; Gao, K.; Yang, B.; Liao, X.; Zhang, J. Solid inclusion complexes of oleanolic acid with amino-appended β-cyclodextrins (ACDs): Preparation, characterization, water solubility and anticancer activity. Mater. Sci. Eng. C 2016, 69, 68–76. [Google Scholar] [CrossRef]
- Qiang, Z.; Ye, Z.; Hauck, C.; Murphy, P.A.; Mc Coy, J.A.; Widrlechne, M.P.; Reddy, M.B.; Hendrich, S. Permeability of rosmarinic acid in Prunella vulgaris and ursolic acid in Salvia officinalis extracts across Caco-2 cell monolayers. J. Ethnopharmacol. 2011, 137, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Jinhua, W.; Ying, Z.; Yuhua, L. PXR–ABC drug transporters/CYP-mediated ursolic acid transport and metabolism in vitro and vivo. Arch. Pharm. 2020, 353, e2000082. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Hu, X.M.; Yi, Y.M.; Wan, J. Preparation and body distribution of freeze-dried powder of ursolic acid phospholipid nanoparticles. Drug Dev. Ind. Pharm. 2009, 35, 305–310. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, S.; Zhang, Y.; Chen, Z. Development of a liquid chromatography—Mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: Application to the pharmacokinetic and tissue distribution study. Anal. Bioanal. Chem. 2011, 399, 2877–2884. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Ong, T.H.; Kumar, A.P.; Lun, C.K.; Ho, P.C.; Wong, P.T.H.; Hui, K.M.; Sethi, G. Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS ONE 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Ge, Z.Q.; Du, X.Y.; Huang, X.N.; Qiao, B. Enhanced oral bioavailability of ursolic acid nanoparticles via antisolvent precipitation with TPGS1000 as a stabilizer. J. Drug Deliv. Sci. Technol. 2015, 29, 210–217. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug. Dispos. 2007, 28, 135–143. [Google Scholar] [CrossRef]
- Xi, J.; Chang, Q.; Chan, C.K.; Meng, Z.Y.; Wang, G.N.; Sun, J.B. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech 2009, 10, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Gao, Y.; Wang, M.; Fang, L.; Ping, Q. Propylene glycol-linked amino acid/dipeptide diester prodrugs of oleanolic acid for PepT1-mediated transport: Synthesis, intestinal permeability, and pharmacokinetics. Mol. Pharm. 2013, 10, 1378–1387. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Guo, B.; Li, Y.; Geng, Y.; Zhao, F.; Zhang, T. Enhancement of dissolution rate and oral bioavailability in beagle dogs of oleanolic acid by adsorbing onto porous silica using supercritical carbon dioxide. J. Drug Deliv. Sci. Technol. 2014, 24, 380–385. [Google Scholar] [CrossRef]
- Song, M.; Hang, T.J.; Wang, Y.; Jiang, L.; Wu, X.L.; Zhang, Z.; Shen, J.; Zhang, Y. Determination of oleanolic acid in human plasma and study of its pharmacokinetics in Chinese healthy male volunteers by HPLC tandem mass spectrometry. J. Pharm. Biomed. Anal. 2006, 40, 190–196. [Google Scholar] [CrossRef]
- Rada, M.; Castellano, J.M.; Perona, J.S.; Guinda, Á. GC-FID determination and pharmacokinetic studies of oleanolic acid in human serum. Biomed. Chromatogr. 2015, 29, 1687–1692. [Google Scholar] [CrossRef]
- De la Torre, R.; Carbó, M.; Pujadas, M.; Biel, S.; Mesa, M.D.; Covas, M.I.; Exposito, M.; Espejo, J.A.; Sanchez-Rodrigues, E.; Diaz-Pellicer, P.; et al. Pharmacokinetics of maslinic and oleanolic acids from olive oil—Effects on endothelial function in healthy adults. A randomized, controlled, dose–response study. Food Chem. 2020, 322, 126676. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Lin, M.C.; Mong, M.C.; Lin, C.Y. Bioavailability, distribution, and antioxidative effects of selected triterpenes in mice. J. Agric. Food Chem. 2012, 60, 7697–7701. [Google Scholar] [CrossRef]
- Tsai, S.J.; Yin, M.C. Anti-oxidative, anti-glycative and anti-apoptotic effects of oleanolic acid in brain of mice treated by d-galactose. Eur. J. Pharmacol. 2012, 689, 81–88. [Google Scholar] [CrossRef]
- Lu, Y.F.; Wan, X.L.; Xu, Y.; Liu, J. Repeated oral administration of oleanolic acid produces cholestatic liver injury in mice. Molecules 2013, 18, 3060–3071. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhou, S.Y.; Qian, Z.Z.; Zhang, H.L.; Qiu, L.H.; Song, Z.; Zhao, J.; Wang, P.; Hao, X.S.; Wang, H.Q. Evaluation of toxicity and single-dose pharmacokinetics of intravenous ursolic acid liposomes in healthy adult volunteers and patients with advanced solid tumors. Expert Opin. Drug Metab. Toxicol. 2013, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Aqil, M.; Imam, S.S.; Ahad, A.; Moolakkadath, T.; Sultana, Y.; Mujeeb, M. Ursolic acid loaded intra nasal nano lipid vesicles for brain tumour: Formulation, optimization, in-vivo brain/plasma distribution study and histopathological assessment. Biomed. Pharmacother. 2018, 106, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shen, Y.; Yang, S.; Lei, W.; Luo, C.; Hou, Y.; Bai, G. Metabolite identification of ursolic acid in mouse plasma and urine after oral administration by ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry. RSC Adv. 2018, 8, 6532–6539. [Google Scholar] [CrossRef]
- Pozo, O.J.; Pujadas, M.; Gleeson, S.B.; Mesa-García, M.D.; Pastor, A.; Kotronoulas, A.; Fito, M.; Covas, M.I.; Navarro, J.R.F.; Espejo, J.A.; et al. Liquid chromatography tandem mass spectrometric determination of triterpenes in human fluids: Evaluation of markers of dietary intake of olive oil and metabolic disposition of oleanolic acid and maslinic acid in humans. Anal. Chim. Acta 2017, 990, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qi, C.; Huang, J.; Tang, X.; Li, C.; Huang, K.; Xu, J.; Guo, G. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Srinivasan, R.; Aruna, A.; Lee, J.S.; Kim, M.; Shivakumar, M.S.; Natarajan, D. Antioxidant and antiproliferative potential of bioactive molecules ursolic acid and thujone isolated from memecylon edule and elaeagnus indica and their inhibitory effect on topoisomerase II by molecular docking approach. BioMed Res. Int. 2020, 2020, 8716927. [Google Scholar] [CrossRef]
- Shihab, Y.H.; Cheinc, Y.C.; Wangd, J.Y.; Fue, Y.S. Ursolic acid protects hippocampal neurons against kainate-induced excitotoxicity in rats. Neurosci. Lett. 2004, 362, 136–140. [Google Scholar]
- Zhang, T.; Su, J.; Wang, K.; Zhu, T.; Li, X. Ursolic acid reduces oxidative stress to alleviate early brain injury following experimental subarachnoid hemorrhage. Neurosci. Lett. 2014, 579, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Peshattiwara, V.; Mukea, S.; Kaikinia, A.; Baglea, S.; Dighe, V.; Sathaye, S. Mechanistic evaluation of ursolic acid against rotenone induced Parkinson’s disease—Emphasizing the role of mitochondrial biogenesis. Brain Res. Bull. 2020, 160, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Zahra, W.; Singh, S.S.; Birla, H.; Keswani, C.; Dilnashin, H.; Rathore, A.S.; Singh, R.; Singh, R.K.; Singh, S.P. Anti-inflammatory activity of ursolic acid in MPTP-induced parkinsonian mouse model. Neurotox. Res. 2019, 36, 452–462. [Google Scholar] [CrossRef]
- Ding, H.; Wang, H.; Zhu, L.; Wei, W. Ursolic acid ameliorates early brain injury after experimental traumatic brain injury in mice by activating the Nrf2 pathway. Neurochem. Res. 2017, 42, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Lia, L.; Zhanga, X.; Cuia, L.; Wanga, L.; Liua, H.; Jia, H.; Dua, Y. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res. Bull. 2013, 1497, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Msibi, Z.N.P.; Mabandla, M.V. Oleanolic acid mitigates 6-hydroxydopamine neurotoxicity by attenuating intracellular ROS in PC12 cells and striatal microglial activation in rat brains. Front. Physiol. 2019, 10, 1059. [Google Scholar] [CrossRef]
- Rong, Z.T.; Gong, X.J.; Sun, H.B.; Li, J.M.; Ji, H. Protective effects of oleanolic acid on cerebral ischemic damage in vivo and H2O2-induced injury in vitro. Pharm. Biol. 2011, 49, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal cell death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, D.M.; Zheng, Y.L.; Hu, B.; Zhang, Z.F.; Ye, Q.; Liu, C.M.; Shan, Q.; Wang, Y.J. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cereb. Cortex 2010, 20, 2540–2548. [Google Scholar] [CrossRef]
- Wang, Y.; He, Z.; Deng, S. Ursolic acid reduces the metalloprotease/anti-metalloprotease imbalance in cerebral ischemia and reperfusion injury. Drug Des. Devel. Ther. 2016, 10, 1663–1674. [Google Scholar] [CrossRef]
- Honarvara, F.; Hojatia, V.; Bakhtiarib, N.; Vaezia, G.; Myelin, M.J. Protection by ursolic acid in cuprizone-induced demyelination in mice. Iran. J. Pharm. Res. 2019, 18, 1978–1988. [Google Scholar]
- Zhanga, Y.; Lia, X.; Cirica, B.; Curtisc, M.T.; Chend, W.J.; Rostamia, A.; Zhanga, G.X. A dual effect of ursolic acid to the treatment of multiple sclerosis through both immunomodulation and direct remyelination. Proc. Natl. Acad. Sci. USA 2020, 117, 9082–9093. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Li, A.C.; Willson, T.M.; Kelly, C.J.; Glass, C.K. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998, 391, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Klockgether, T.; Feinstein, D.L. Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J. Neurosci. 2000, 20, 6862–6867. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, R.; Yi, J.H.; Vemuganti, R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front. Biosci. 2008, 13, 1813–1826. [Google Scholar] [CrossRef]
- Lenglet, S.; Montecucco, F.; Mach, F. Role of matrix metalloproteinases in animal models of ischemic stroke. Curr. Vasc. Pharmacol. 2015, 13, 161–166. [Google Scholar] [CrossRef]
- Villapol, S. Roles of peroxisome proliferator-activated receptor-gamma on brain and peripheral inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, R.; Zhang, Y.; Jia, R.; Zhao, K.; Zhang, S.; Liang, H. Protective effect of ursolic acid on ischemic brain injury by regulating hypoxia-inducible factor 1-alpha. Int. J. Clin. Exp. Med. 2019, 12, 3612–3621. [Google Scholar]
- Arandarcikaite, O.; (Lithuanian University of Health Sciences, Kaunas, Lithuania); Liobikas, J.; (Lithuanian University of Health Sciences, Kaunas, Lithuania). Personal Communication, 2021.
- Liobikas, J.; Majiene, D.; Trumbeckaite, S.; Kursvietiene, L.; Masteikova, R.; Kopustinskiene, D.M.; Savickas, A.; Bernatoniene, J. Uncoupling and antioxidant effects of ursolic acid in isolated rat heart mitochondria. J. Nat. Prod. 2011, 74, 1640–1644. [Google Scholar] [CrossRef]
- Caltana, L.; Rutolo, D.; Nieto, M.L.; Brusco, A. Further evidence for the neuroprotective role of oleanolic acid in a model of focal brain hypoxia in rats. Neurochem. Int. 2014, 79, 79–87. [Google Scholar] [CrossRef]
- Strickland, M.; Stoll, E.A. Metabolic reprogramming in glioma. Front. Cell Dev. Biol. 2017, 5, 43. [Google Scholar] [CrossRef]
- Gu, J.; Wang, J.; Liu, X.; Sai, K.; Mai, J.; Xing, F.; Chen, Z.; Yang, X.; Lu, W.; Guo, C.; et al. IL-6 derived from therapy-induced senescence facilitates the glycolytic phenotype in glioblastoma cells. Am. J. Cancer Res. 2021, 11, 458–478. [Google Scholar]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.J.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Umans, R.A.; Sontheimer, H. Combating malignant astrocytes: Strategies mitigating tumor invasion. Neurosci. Res. 2018, 126, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Khoshnevisan, A. Cancer stem cells and chemoresistance in glioblastoma multiform: A review article. J. Stem Cells 2015, 10, 271–285. [Google Scholar] [PubMed]
- Seystahl, K.; Wick, W.; Weller, M. Therapeutic options in recurrent glioblastoma—An update. Crit. Rev. Oncol. Hematol. 2016, 99, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic acid and its derivatives as bioactive agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed]
- Hodon, J.; Borkova, L.; Pokorny, J.; Kazakova, A.; Urban, M. Design and synthesis of pentacyclic triterpene conjugates and their use in medicinal research. Eur. J. Med. Chem. 2019, 182, 111653. [Google Scholar] [CrossRef]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduño-Ramirez, M.L.; García, M.L.; Calpena, A.C.; Souto, E.B. In vitro cytotoxicity of oleanolic/ursolic acids-loaded in plga nanoparticles in different cell lines. Pharmaceuticals 2019, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Borková, L.; Frydrych, I.; Jakubcová, N.; Adáme, R.; Lišková, B.; Gurská, S.; Medvedíková, M.; Hajdúch, M.; Urban, M. Synthesis and biological evaluation of triterpenoid thiazoles derived from betulonic acid, dihydrobetulonic acid, and ursonic acid. Eur. J. Med. Chem. 2020, 185, 111806. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic acid-based derivatives as potential anti-cancer agents: An update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Tan, Z.; Hu, Y.; Shu, Y.; Bao, R.; Jiang, L.; Wu, X. Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells. Cancer Cell Int. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.S.; Zhou, W.; Park, I.; Kang, K.; Lee, S.R.; Piao, X.; Park, J.B.; Kwon, T.K.; Na, M.; Hur, G.M. C-27-carboxylated oleanane triterpenoids up-regulate TRAIL DISC assembly via p38 MAPK and CHOP-mediated DR5 expression in human glioblastoma cells. Biochem. Pharmacol. 2018, 158, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Deeb, D.; Jian, H.; Liu, Y.; Dulchavsky, S.A.; Gautam, S.C. Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-kappaB and Notch1 signaling. J. Neurooncol. 2007, 84, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Jo, H.J.; Lee, K.J.; Choi, J.W.; An, J.H. Oleanolic acid induces p53-dependent apoptosis via the ERK/JNK/AKT pathway in cancer cell lines in prostatic cancer xenografts in mice. Oncotarget 2018, 9, 26370–26386. [Google Scholar] [CrossRef]
- Conway, G.E.; Zizyte, D.; Mondala, J.R.M.; He, Z.; Lynam, L.; Lecourt, M.; Barcia, C.; Howe, O.; Curtin, J.F. Ursolic acid inhibits collective cell migration and promotes JNK-dependent lysosomal associated cell death in glioblastoma multiforme cells. Pharmaceuticals 2021, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Wang, X.; Jiang, C. Ursolic acid inhibits proliferation and induces apoptosis in human glioblastoma cell lines U251 by suppressing TGF-β1/miR-21/PDCD4 pathway. Basic Clin. Pharmacol. Toxicol. 2012, 111, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Huang, B.R.; Liao, P.J.; Yen, G.C. Ursolic acid triggers nonprogrammed death (necrosis) in human glioblastoma multiforme DBTRG-05MG cells through MPT pore opening and ATP decline. Mol. Nutr. Food Res. 2014, 58, 2146–2156. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y.; Zhang, R.; Tu, X.; Gong, X. Ursolic acid induces autophagy in U87MG cells via ROS-dependent endoplasmic reticulum stress. Chem. Biol. Interact. 2014, 218, 28–41. [Google Scholar] [CrossRef]
- Frolova, T.S.; Lipeeva, A.V.; Baev, D.S.; Tsepilov, Y.A.; Sinitsyna, O.I. Apoptosis as the basic mechanism of cytotoxic action of ursolic and pomolic acids in glioma cells. Mol. Biol. 2017, 51, 809–816. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, S.; Ding, F.; Guo, S.; Ying, G.; Yan, Z. Ursolic acid attenuates temozolomide resistance in glioblastoma cells by downregulating O(6)-methylguanine-DNA methyltransferase (MGMT) expression. Am. J. Transl. Res. 2016, 8, 3299–3308. [Google Scholar] [PubMed]
- Huang, H.C.; Huang, C.Y.; Lin-Shiau, S.Y.; Lin, J.K. Ursolic acid inhibits IL-1beta or TNF-alpha-induced C6 glioma invasion through suppressing the association ZIP/p62 with PKC-zeta and downregulating the MMP-9 expression. Mol. Carcinog. 2009, 48, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, K.; Siwu, E.R.O.; Nozaki, S.; Watanabe, Y.; Tanaka, K.; Fukase, K. Ursolic acid derivatives from Bangladeshi medicinal plant, Saurauja roxburghii: Isolation and cytotoxic activity against A431 and C6 glioma cell lines. Phytochem. Lett. 2011, 4, 287–291. [Google Scholar] [CrossRef]
- Bergamin, L.S.; Figueiró, F.; Dietrich, F.; Manica, F.M.; Filippi-Chiela, E.C.; Mendes, F.B.; Jandrey, E.H.F.; Lopes, D.V.; Oliveira, F.H.; Nascimento, I.C.; et al. Interference of ursolic acid treatment with glioma growth: An in vitro and in vivo study. Eur. J. Pharmacol. 2017, 811, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Yu, W.; Xue, Y.; Xiao, L.; Xu, H. Hypoxia imaging and biological evaluation of the radiosensitizing effect of oleanolic acid. Biomed. Res. Int. 2018, 2018, 2694679. [Google Scholar] [CrossRef]
- Gudoityte, E. Effects of Ursolic Acid on Astroglial Cell Cultures. Master’s Thesis, Lithuanian University of Health Sciences, Kaunas, Lithuania, 2020. [Google Scholar]
| Plant Species | Family | Plant Part | OA mg/g DW | UA mg/g DW | Reference |
|---|---|---|---|---|---|
| Betula alba | Betulaceae | bark | 11.0 | not detected | [10] |
| Calendula officinalis | Compositae | flowers | not detected | 20.5 | [25] |
| Chaenomeles japonica | Rosaceae | fruit peel | not detected | 5.7 | [26] |
| Coffea arabica | Rubiaceae | leaves | not detected | 18.0 | [10] |
| Crataegus pinnatifida | Rosaceae | leaves | 1.0 | 5.2 | [10] |
| Eriobotrya japonica | Rosaceae | fruit peel | not detected | 8.0 | [26] |
| flowers | 0.9 | 3.6 | [34] | ||
| Lavandula angustifolia | Lamiaceae | herbs | 4.5 | 15.9 | [10] |
| Ligustrum lucidum | Oleaceae | leaves | 6.3 | 9.8 | [41] |
| Malus domestica | Rosaceae | fruit peel | 9.4 | [26] | |
| Melissa officinalis | Lamiaceae | herbs | 1.6 | 6.7 | [10] |
| Nerium oleander | Apocynaceae | leaves | 3.7 | 12.7 | [10] |
| Ocimum basilicum | Lamiaceae | herbs | not detected | 3.0 | [10] |
| Olea europaea | Oleaceae | leaves | 31.0 | 3.8 | [10] |
| fruits | 21.0 | not detected | [10] | ||
| bark | 9.8 | not detected | [10] | ||
| Origanum majorana | Lamiaceae | herbs | 1.9 | 6.6 | [10] |
| Origanum vulgare | Lamiaceae | herbs | not detected | 2.8 | [10] |
| Panax quinquefolium | Araliaceae | roots | 3.1 | not detected | [25] |
| Prunus persica | Rosaceae | fruit peel | not detected | 3.0 | [26] |
| Pyrus communis | Rosaceae | fruit peel | not detected | 7.2 | [26] |
| Salvia officinalis | Lamiaceae | herbs | 6.7 | 18 | [10] |
| Sambucus nigra | Adoxaceae | leaves | 1.2 | 5.8 | [10] |
| bark | 0.8 | 3.2 | [10] | ||
| Satureja montana | Lamiaceae | herbs | 1.4 | 4.9 | [10] |
| Silphium trifoliatum | Asteraceae | leaves | 22.0 | 15.5 | [25] |
| Thymus vulgaris | Lamiaceae | herbs | 3.7 | 9.4 | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudoityte, E.; Arandarcikaite, O.; Mazeikiene, I.; Bendokas, V.; Liobikas, J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int. J. Mol. Sci. 2021, 22, 4599. https://doi.org/10.3390/ijms22094599
Gudoityte E, Arandarcikaite O, Mazeikiene I, Bendokas V, Liobikas J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. International Journal of Molecular Sciences. 2021; 22(9):4599. https://doi.org/10.3390/ijms22094599
Chicago/Turabian StyleGudoityte, Evelina, Odeta Arandarcikaite, Ingrida Mazeikiene, Vidmantas Bendokas, and Julius Liobikas. 2021. "Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential" International Journal of Molecular Sciences 22, no. 9: 4599. https://doi.org/10.3390/ijms22094599
APA StyleGudoityte, E., Arandarcikaite, O., Mazeikiene, I., Bendokas, V., & Liobikas, J. (2021). Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. International Journal of Molecular Sciences, 22(9), 4599. https://doi.org/10.3390/ijms22094599








