Abstract
The implication of ‘theranostic’ refers to targeting an identical receptor for diagnostic and therapeutic purposes, by the same radioligand, simultaneously or separately. In regard to extensive efforts, many considerable theranostic tracers have been developed in recent years. Emerging evidence strongly demonstrates the tendency of nuclear medicine towards therapies based on a diagnosis. This review is focused on the examples of targeted radiopharmaceuticals for the imaging and therapy of breast cancer.
1. Introduction
Breast cancer (BC) is the most frequent malignancy among women worldwide [1,2,3]. It is demonstrated that nearly about 70–80% of primary BC can be cured. Treatments of advanced species with distant metastasis by using prevalent procedures are almost impossible [4]. Effective and impressive role of nuclear medicine in direct detection of BC initiated first in the early 1990’s when technetium-99m-methoxyisobutylisonitrile ([99mTc]Tc-MIBI) was used for diagnosis of lesions of dense breasts which were not detectable by mammography [5]. Imaging facilities in nuclear medicine have made the diagnosis, staging, response and follow-up process evaluations convenient in a patient with BC [6]. Lately profitable progresses in therapeutic purposes of BC have been achieved. Based on molecular classification of BC, which can be categorised as luminal (A/B), HER2, basal like and breast like subtypes and also their prognosis, selective and specific radiopharmaceuticals can be designed and formulated [7].
In metastatic cases, severe pain can be palliated with vast series of radiopharmaceuticals including strontium-89 (89Sr), samarium-153 (153Sm), phosphorus-32 (32P) and rhenium-186/188 (186/188Re) [5,8]. A relatively recent huge progress in nuclear medicine is the application of imaging agents for the evaluation of uptake and localisation, biodistribution, the related dose of therapeutic tracer and response to treatment. This remarkable concept is named ‘theranostic’ (Figure 1) [9]. This unique application (therapy + diagnostics) means combination of radiolabelled ligands with gamma-(γ) or positron-(β+) emitting radionuclides for diagnostic purposes with the same ligand incorporating therapeutic radionuclides such as alpha-(α) or beta-(β−) emitters to perform a personalised targeted treatment based on the preliminary diagnostic procedure [10,11,12]. Several successful theranostic approaches based on peptidomimetics, antibodies and small molecules have presented significant responses in clinical trials. Based on the accumulation mechanism of these radiopharmaceuticals, they can be useful for specific or extensive types of tumours [13].
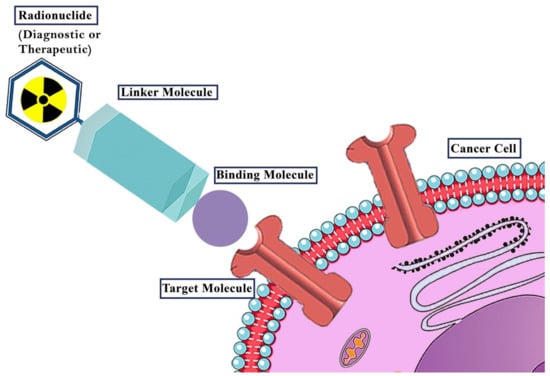
Figure 1.
The general concept of theranostic radiopharmaceuticals. A radionuclide is combined with a targeting vector (Binding molecule). The choice of radionuclide defines the purpose of the radiopharmaceutical. γ− or β+-emitters are used for diagnostics while β−- and α-emitters are applied in therapy. The targeting vector guides the radiopharmaceutical to its specific target (e.g., receptors). To combine the radionuclide with a target vector without reducing its affinity to its target, normally, depending on the type of radionuclide (e.g., metal, non-metal), linking structures (linker molecules) are necessary.
The aim of this review article is to discuss the concept of theranostic radiopharmaceuticals with application in BC.
2. Recent Progresses for Diagnosis and Treatment of BC
BC is one of the major problems leading to death in women worldwide [14]. Each year American Cancer Society estimates new cases and deaths in United States. Evidence demonstrate that the greatest number of deaths are related to lung, prostate and colorectal cancers in men and lung, breast and colorectal cancers in women [14]. It is strongly believed that distant metastases are responsible for more than 90% deaths caused by BC [15]. However very impressive progressions in diagnosis and treatment of BC, have been done recently, but metastatic recurrences would be inevitable in 20–30% of patients [16,17]. Depending on the pathological source of the tumours, liver, lung, bone and brain should be considered as metastatic tissues [18,19]. Against definite opinions of many reports based on that, distant metastases known as secondary or late symptoms of BC, there are evidences that prove that distant metastases could be also an early symptom in some cases [20]. Early diagnosis may include some contemporary methods such as digital mammography (DM), magnetic resonance imaging (MRI) and molecular breast imaging (MBI) [21]. Through mammogram studies, in addition to x-ray exposure to the patients, numerous false positive results in many cases will require further evaluations via imaging or pathological assays [22,23]. Due to the poor selectivity against high sensitivity of MRI, and also its dependency to contrast agents, this method does not use as a routine diagnostic procedure. However according to the considerations of national comprehensive cancer network (NCC), MRI can be authorised in some specific cases [22,24]. MBI uses radioactive tracers for diagnosis of BC [21]. In mid 2000 s [99mTc]Tc-sestamibi was used as MBI agent with gamma cameras as a reliable and selective method for functional imaging [25]. Nuclear medicine by providing of physiological patterns, plays a fundamental role in prognostic, staging and therapy of BC [26]. Molecular imaging with single photon emission computed tomography (SPECT/CT) and positron emission tomography/computed tomography (PET/CT) incorporation with radiolabelled molecules can be beneficial for staging, response evaluation, restaging, detection of recurrence and follow-up during or after cytostatic therapy for cancer management [27]. Besides that, therapeutic radiopharmaceuticals in nuclear medicine, compared to other conventional methods such as surgery, radiation therapy (RT), chemotherapy (CT) and endocrine (hormone) therapy (ET) are non-invasive and includes fewer side effects [21,28,29].
3. Theranostic Approaches for Cancer Management in Nuclear Medicine
The concept of radiotheranostics refers to 1941, when Saul Hertz at Massachusetts General Hospital (Boston, Mass) used radioiodine for thyrotoxicosis treatment [30]. This was the turning point in nuclear medicine and shortly thereafter, in 1942, the first publication of treatment of similar patients was published [31,32]. In order to evaluate effectiveness of the therapeutic procedure, the first diagnostic imaging with radioiodine was performed in 1950 at the University of California, Los Angeles (UCLA) [33]. Pre-targeting verification includes sensitivity, specificity and quantification imaging studies to prove the therapeutic feasibility of a specific radiotracer [34].
Nowadays, this exclusive integration of diagnosis and therapy are common. The most recent progress in this precedent is the administration of those radiotheranostic agents which target somatostatin receptors (SSTRs) in neuroendocrine tumours (NETs), human epidermal growth factor receptor 2 (HER2) antigens in BC and the prostate specific membrane antigen (PSMA) in prostate cancer (PC) [30,34,35]. The two prevalent ligands for NET are DOTA-Phe1-Tyr3-octreotide (DOTA-TOC) and DOTA-DPhe1,Tyr3-octreotate (DOTA-TATE) widely used worldwide [36,37,38,39]. Aside from the encouraging efficacy and safety of PSMA-617, PSMA-11 and PSMA-I&T for clinical assessment of PC, efforts are ongoing towards finding unpresented radiotheranostics with better capabilities [40,41,42,43]. Gene expression profiling for prognostic and predictive issues in BC has been received considerable attention in clinic recently [7].
Choosing the patients who can benefit from radioligand therapy with these implications resulted to worldwide demand towards radiotheranostic applications for oncology management in nuclear medicine.
4. Targeting HER2 Receptors by Theranostics
The most striking procedure in detection and treatment was about management of BC. Since this global cancer burden affects about 49.5% of the women population and almost more than >60 years of age [4]. ERBB2 overexpression in almost all types of BC leads to the proceeding of the human epidermal growth factor receptor-2 (HER2, one subtype of HER1-4 family) [4]. It is demonstrated that the external section of the HER2 receptor has no identifiable ligand unless in dimerisation with other growth factors. The most remarkable dimer for targeting diagnostic and therapeutic purposes is the HER2-HER3 dimer [44]. Activating of these receptors motivates complicated signal transduction pathways that conduce tumorigenic proses [45]. Nowadays HER2 is a key oncogene in BC [45]. Commonly in systemic therapy approaches anti-HER2 therapy is done for HER2 positive cases [4]. Advanced methods for diagnosis and therapy of BC have been done based on HER2 as a major identified factor whose amplification leads to uncontrolled cell proliferation in breast cancer [46]. Application of anti-HER2 monoclonal antibody ‘trastuzumab’ is the most common procedure in treatment of BC [47]. Considering that this therapeutic protocol imposes patient high costs and despite that may be ineffective, assessment pre-treatment physiologic manner of the trastuzumab would be noteworthy. Also, this is demonstrated that some negative HER2 patients can benefit from therapeutic trastuzumab as an anti-HER2 agent [48]. In this regard biodistribution and accumulation of trastuzumab radiolabelled with Indium-111, [111In]In-trastuzumab, as an imaging agent for single photon computed emission tomography (SPECT) has been evaluated [48]. In preclinical studies, significant and specific accumulation of [111In]In-trastuzumab was proved in human HER2 tumour-bearing mice [49]. So, it was supposed that this tracer can be a promising agent in humans. In clinical studies of [111In]In-trastuzumab in women patients confirmed with HER2 positive BC and eligible for treatment with trastuzumab or paclitaxel remarkable results were obtained [50]. In 12 final cases, 25 tumour lesions were detected. Diagnostic studies were accomplished in 24, 72, 96 and 168 h after injection through scintigraphy scans. Since blood vessels are clearly visible until 72 h, for accumulation and study investigations at least 96 h interval is strongly recommended in this paper [50]. Obviously, this is evidence of a high plasma level of [111In]In-trastuzumab within the first 72 h of the study. Despite the competition between radiolabelled trastuzumab and therapeutic trastuzumab, the saturation effect in diagnosis is negligible. In accordance with this, it can be concluded that radiolabelled trastuzumab can be used as a diagnostic agent during the prevalent therapeutic procedures [50].
Since PET provides higher resolution and detection sensitivity so many efforts toward preparation of PET derivatives for detection of metastatic lesions of breast cancer have been devoted. Cooper-64 (64Cu) is a positron emitting radionuclide (β+, 0.653 MeV [17.8%]; β−, 0.579 MeV [38.4%]) and has a half-life of 12.701 ± 0.002 h [50]. These characterisations make copper-64 a qualified PET radionuclide for high-quality detection purposes. A longer half-life of copper-64 compared to other PET radionuclides makes the transportation of the final radiopharmaceutical feasible, also will be appropriate for radiolabelling of compounds with longer biological half-life such as monoclonal antibodies [50].
In an effort, [64Cu]Cu-trastuzumab was used clinically in 6 patients with primary or metastatic HER2 positive breast cancer [51]. All patients received intravenous (IV) injection of 130 MBq of [64Cu]Cu-trastuzumab and diagnostic investigations during 1, 24 and 48 h after injections were done. In this study, it was demonstrated that the sensitivity of [64Cu]Cu-trastuzumab in brain metastases detection can be parallel to MR imaging and even superior to CT modality in some studied cases [51]. Generally, based on this clinical trial, it can be concluded that the [64Cu]Cu-trastuzumab diagnostic procedure is practicable, repeatable and safe. Sensitivity of the diagnostic scan in this study was low that it can be related to trastuzumab therapy during the procedure [51].
Immuno-PET imaging can be possible using Zirconium-89 (89Zr). This radiometal with a 78.4 h half-life, which is the longest half-life in the group of PET radionuclides, is completely opportune for radiolabelling of antibodies [52]. In a clinical trial performed between March 2006 and December 2008, [89Zr]Zr-trastuzumab (Figure 2) has been applied in 14 patients in order to assess HER2 positive lesions in metastatic BC [52]. It is supposed that 38.4 ± 1.6 MBq [89Zr]Zr-trastuzumab injected dose would be adequate for the evaluation of tumour uptake even 4–5 days after injection. Diagnostic procedures were done at early stages (1–3 days), also delayed imaging (4–7 days). According to this clinical trial, it is demonstrated that tumour uptake of [89Zr]Zr-trastuzumab is dose-dependent and tumour to non-target accumulation increases over time [52].
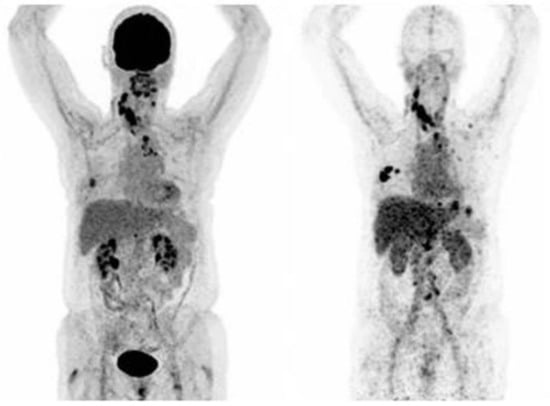
Figure 2.
PET-Scan of [18F]FDG (left) and [89Zr]Zr-trastuzumab (right) of a patient with a [89Zr]Zr-trastuzumab scan considered HER2-positive (Figure adapted from Bensch et al. [53] Creative Commons Attribution 4.0 International License).
Significant uptakes in the liver, bone and brain lesions were reported, so based on these findings PET scan for visualisation and quantitative evaluations in HER2 positive breast cancer patients would be feasible [52]. A pilot study of [68Ga]Ga-DOTA-F(ab′)2-trastuzumab was investigated in 16 patients with BC [54]. In the final study 15 patients enrolled with HER2 positive (8 patients) and HER2 negative (7 patients) characterisation. Among the HER2 positive patients, all but one received anti-HER2 trastuzumab therapy. This tracer was well tolerated and the kidney was reported as a critical organ with a mean dose of 0.383 Gy/37 MBq in this study. All 15 patients had undergone [18F]FDG PET scan and all showed [18F]FDG avid abnormalities. Despite this none of seven patients with the specification of HER2 negative showed accumulation of [68Ga]Ga-DOTA-F(ab′)2-trastuzumab. In all studied HER2 positive cases (8 patients whom three of them had received trastuzumab) only seven lesions were found admirably purposeful and correlated with FDG avid lesions.
Based on these findings, it is supposed that a high concentration of trastuzumab in blood circulation may compete with the radiolabelled compound. On the other hand, it may refer to the half-life of radionuclides used for radiolabelling. As it takes time for intact antibodies for accumulating in tumour tissues, radionuclides with higher half-life are preferred. In this regard radiolabelling with indium-111, iodine-131, zirconium-89 and iodine-124 was evaluated [54]. [124I]I-trastuzumab and [131I]I-herceptin in preclinical phase studies showed optimistic results in imaging and cell culture investigations [55,56]. Also, [99mTc]Tc-trastuzumab biodistribution study in tumour-bearing BALB/C female mouse whose tumour cells were established from a murine mammary carcinoma has been assayed [57]. Gamma camera imaging results showed a significant accumulation of [99mTc]Tc-trastuzumab in tumours [57].
In order to develop the therapeutic counterparts of diagnostic radiolabelled trastuzumab, therapeutic radiolabelled trastuzumab derivatives are under investigation. In a pilot study feasibility of [177Lu]Lu-trastuzumab for therapy of HER2 positive breast cancer patients has been proven [58]. In a pilot study, 10 women patients were enrolled to investigate [177Lu]Lu-trastuzumab effectiveness. Each patient was injected 0.18–0.44 GBq of the radiotracer. Diagnostic evaluations were done through the administered day, also 5 and 7 days after injection. 6 HER2 positive and 4 HER2 negative patients were enrolled by [18F]FDG PET scan and all diagnosed with metastatic disease [58]. In a biodistribution study of [177Lu]Lu-trastuzumab by SPECT/CT HER2 negative cases imaging represented no tracer accumulation in the tumour sites. In contrast, imaging of HER2 positive patients was associated with significant uptake in primary and metastatic tumours. The tumour to a non-target ratio of the radiotracer uptake increased considerably during the study interval. So, the T/N was initially 2.4 on the first day of study and increased to 3.9 on the 7th day. Supported by this remarkable information it can be concluded that 177Lu-radiolabeled trastuzumab derivatives would be feasible in HER2 positive primary and metastatic BC patients as a palliative tracer simultaneous to other treatments [58].
Another study employing [67Ga]Ga-THP-trastuzumab, proved a decrease of the viability of HER2 positive specified cells in vitro. Destruction of HER2 expressive cells benefits from Auger electron irradiation by gallium-67, and based on this fact [67Ga]Ga-THP-trastuzumab can also be considered as a therapeutic radiotracer [59].
5. Targeting Gastrin-Releasing Peptide Receptor (GRPR) by Theranostic Radiopharmaceuticals
One of the considerable aspects in nuclear medicine is about the peptides, as they are related to specific receptors which are expressed/overexpressed in various type of cancers [60]. There are widespread series of peptide-based theranostic radiopharmaceuticals with common use for oncology application in nuclear medicine [13,61,62,63]. There is a lot of interest to develop radiolabelled bombesin analogues. Bombesin is a specific ligand for bombesin receptors (BnR) [64]. However, bombesin receptors include three subtypes (BB1, BB2 and BB3) [65], with BB2 (formerly known as GRPR) being the specific receptor for the gastrin-releasing peptide (GRP) and a very promising targeting vector for the diagnosis and therapy of PC and BC [60].
MacDonald et al. [66] demonstrated that bombesin is a 27 amino acid peptide and has the functional c-terminal group just as GRP. Also, GRP is known as a growth factor in normal cells as well as cancerous cells. There is evidence that BB2 is overexpressed in various cancer types including breast, lung, prostate, ovarian or pancreatic cancer, as well as some of the central nervous system especially glioma, meningioma and neuroblastoma [67,68,69]. Evidence has shown that in BC GRP is overexpressed in 38–96% of cases [70,71], especially in oestrogen receptor (ER)-expressing patients [72,73,74]. Based on these results bombesin moved into the focus of investigations. Until today, extensive efforts for radiolabelling of bombesin analogues with SPECT (indium-111, technetium-99m) and PET radionuclides (copper-64, gallium-68, fluorine-18) were accomplished in order to develop specific diagnostic agents for BC [60]. These radiopharmaceuticals showed optimistic results for specific accumulation in BC in preclinical studies including BC cell lines as well as animal studies [60,75,76]. Based on these promising results, many clinical trials have been done with radiolabelled bombesin receptor (BnR) agonists.
Different research groups have been conceded comparative in vivo studies using 68Ga- and fluorine-18 labelled BnR-agonists as PET imaging agents. These revealed higher tumour uptake in comparison to [18F]FDG alone in ER-positive breast cancer-bearing nude-mice [77]. Conjugation of RGD peptide for targeting of αvβ3-integrins and BnR-agonist as heterodimeric PET imaging probes showed promising potential in imaging of BC. In a preclinical study, Liu Z et al. developed the 68Ga-, 64Cu- and 18F-labelled RGD- and BnR-targeting radiotracers that provide great images in xenografted mice due to the high accumulation in αvβ3-integrin and BnR expressing tumour sites [78].
In a limited clinical study, Stoykow et al. assessed the role of [68Ga]Ga-RM2 as a PET imaging tracer in 15 BC patients (Figure 3) [79]. The [68Ga]Ga-RM2 PET showed strongly increased uptake in 13/18 tumours compared to normal tissue. Moreover, progesterone receptor (PR) and ER expression were identified by all PET-positive primary tumours (13/13) compared to 1/5 PET-negative tumour. Which was identified as ER-positive. The others were not detected by PET. In a similar clinical study, Zang et al. discovered 29/34 primary tumours using [68Ga]Ga-NOTA-RM26. PET imaging proved the existence of lymph node metastases in 18 patients and positive correlations with the level of GRPR expressions were found. Moreover, the SUVmax in ER-positive tumours significantly increased in comparison to the negative tumours. It was also observed that the specificity and sensitivity of radiotracer increased in patients with ER-negative tumours, therefore these patients were excluded from this trial [80].
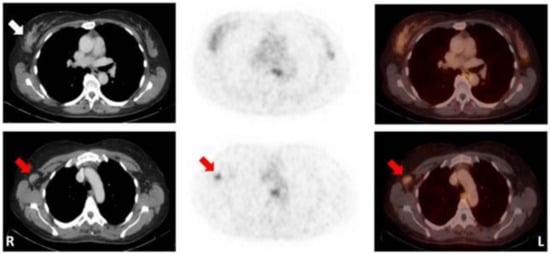
Figure 3.
Patient with a mucinous carcinoma of the right breast with low ER/PR-expression. CT (left); 68Ga-RM2-PET (middle); fusion images (right); primary tumour indicated by white arrow; lymph node metastasis indicated by red arrows. (Figure unmodified from Stykow et al. [79] Creative Commons Attribution 4.0 International License).
In another study, [18F]FDG was assessed in comparison with [68Ga]Ga-RM2 in 14 breast tumour-bearing patients by tissue micro-imaging technique. Also, Morgat et al. preformed GRPR, HER2 and Ki-67 immunohistochemistry studies for PR and ER for all cases. Results confirmed the lower [18F]FDG uptake in the PR-positive and specific binding of [68Ga]Ga-RM2 in ER-positive cancer groups. Moreover, a correlation was detected among GRPR expression in BC cells showing ER-positive tumours and indicated the diagnostic and therapeutic potential of GRPR antagonist in BC [81].
99mTC-based radio-biomarkers have a great potential for targeting ER/BnR. For example, 99mTc-BnR agonist indicated increased uptake in breast cancer cells and enhanced accumulations in studied tumours that overexpressed receptors of GRPR in animal models [82]. Various clinical trials were conducted using 99mTc-labeled BnR agonists for imaging breast lesions. Scopinaro F et al. comparatively evaluated technetium pertechnetate and 99mTc-labeled Bn in three BC patients. The imaging results revealed higher uptake of 99mTc-Bn tracer in the breast tumour and metastatic lymph node sites more than technetium pertechnetate [83].
Ji et al. conducted a study to investigate the use of [99mTc]Tc-RGD-Bn in comparison with the ultrasound for monitoring of the tumours. Similar sensitivity and specificity for SPECT/CT and ultrasound were observed because of dual receptor targeting of the [99mTc]Tc-RGD-Bn. In the case of metastatic sites the SPECT/CT was more sensitive than ultrasound. Therefore, [99mTc]Tc-RGD-Bn would be considered as dual-modality imaging agent for the diagnosis of BC [84]. Similarly, [99mTc]Tc-RGD-Bn, as well as [99mTc]Tc-3P4-RGD2, were proven for imaging of breast lesions in six patients. Among them, five malignant lesions had distinctive uptake, and four cases have shown αvβ3-integrin and GRPR expressions [85]. In another clinical study, the physiologic distribution of [99mTc]Tc-HYNIC-Lys3-Bn on 7 healthy subjects and 4 BC patients was investigated. The specific uptake was detected in the tumour site while bone marrow showed negligible uptake. Kidneys were the predominant excretion route of the radiotracer [86]. In a study, the [99mTc]Tc-RP527 as a BnR-agonist biomarker was evaluated in imaging of 6 BC patients and indicated the specific tumour accumulation in 4 patients along with 6 patients [87]. Also, this research group investigated that [99mTc]Tc-RP527 has a negligible uptake in tamoxifen-resistant patients.
A relatively new approach of tumour-targeting is taking into account the tumour stroma, which is representing a remarkable part of the tumoral microenvironment (TME) [88], as it affects patient prognosis and survival for several solid tumours like BC [89,90,91,92,93]. Studies could demonstrate that the tumour-stroma ratio stands in correlation with the risk to develop distant metastases and the overall patient survival [94,95]. The new focus on the TME led to further investigations that compounds of the TME may be useful targets. In this context, tumour-associated fibroblasts (CAFs) moved into the spotlight as they may be the dominating stromal cell type, depending on the cancer type [96]. Targeting CAFs can be achieved on different ways, among other things by targeting the fibroblast activation protein (FAP) which is significantly overexpressed by CAFs of numerous cancer types compared to healthy tissue [97]. The cancer-related overexpression of FAP and the significant role of CAFs in tumoral progression development of FAP inhibitors (FAPI) for cancer treatment and successively for theranostic application were logical conclusions. Recently, several preclinical as well as clinical studies with quinoline-based FAP inhibitors revealed their potential as radiotracers for tumour diagnosis [97,98,99,100,101,102]. First retrospective studies exhibited the potential of 68Ga-FAPI-PET/CT in comparison to 18F-FDG-PET/CT for a variety of tumour entities [98,103,104,105]. These results, even though further investigations are required, implicating its feasibility and advantage over 18F-FDG-PET/CT even though it still may not solve every of its limitations [97].
A first retrospective analysis was performed to determine the 68Ga-FAPI uptake in a variety of primary, metastatic or recurring cancers revealing the great potential of 68Ga-FAPI-PET/CT in breast cancer [106]. The highest average SUVmax was found in a group of five different cancers, amongst others breast cancer, where it outperformed 18F-FDG-PET/CT. Nevertheless, beside these promising results, the data on breast cancer with these new compounds are still limited [107]. Today (April 2021) there are 17 recruiting or not yet recruiting prospective trials, using FAP-specific PET, listed at clinicaltrials.gov of which at a fraction has breast cancer as condition (2/17).
Recently, in another clinical trial [108], another new designed FAPI derivative named DOTA.SA.FAPI (Figure 4) was applied for diagnosis of BC in a 31 years old female patient. This new FAPI is designed as a diagnostic and therapeutic agent after radiolabelling with gallium-68, lutetium-177 (177Lu) or actinium-225 (225Ac) just like FAPI-02 and FAPI-04 [109]. Interestingly, the results indicated correlations between accumulation of [68Ga]Ga-DOTA.SA.FAPI and [18F]FDG (Figure 4) [109]. An initial patient treatment with [177Lu]Lu-DOTA.SA.FAPI administering 3.2 GBq was performed and post treatment imaging was accomplished 24 h after the dose injection. Conformity of dose absorption in the lesions of [68Ga]Ga-DOTA.SA.FAPI and [177Lu]Lu-DOTA.SA.FAPI is obvious and depicted in Figure 4 [109].
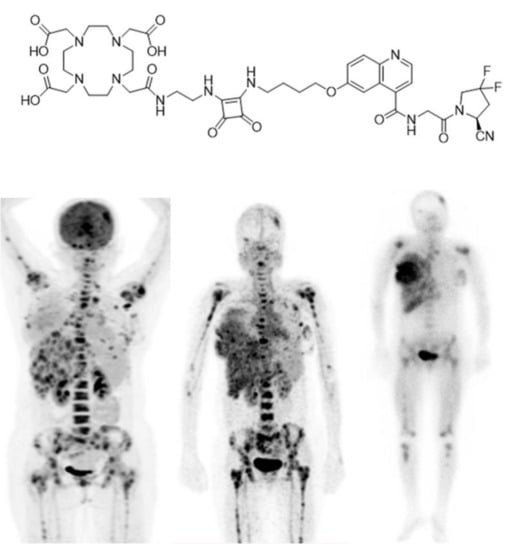
Figure 4.
Chemical structure of DOTA.SA.FAPI. PET-Scan of [18F]FDG (left), [68Ga]Ga-DOTA.SA.FAPI (middle) and [177Lu]Lu- DOTA.SA.FAPI (right) of a patient with HER2-positive, (ER and PR)-negative histopathology (Figure adapted from Ballal et al. [109] Creative Commons Attribution 4.0 International License).
We tried to summarise the clinical studies listed by clinicaltrials.gov through a brief report (Table 1). A query for ‘breast cancer’ AND ‘radiopharmaceuticals’ resulted in 76 studies. For the table, only completed studies were considered while terminated, active, recruiting and not recruiting studies were excluded. Overall, 35 out of 76 listed studies were summed up. All patients included in these clinical trials were over 18 years old. Pregnant or breast-feeding patients were excluded from all trials.

Table 1.
An overview of completed clinical trials performed on breast cancer conducted by: clinicaltrials.gov (accessed on 20 April 2021).
Even though theranostic applications in nuclear medicine with radionuclides like gallium-68 and lutetium-177 were booming in the past decade, due to the striking development of [68Ga]Ga-PSMA-11 which led to the introduction of several other PSMA-targeting radiolabelled ligands for diagnosis and therapy of PC, new compounds targeting BC are rare and mostly intended for improving diagnosis. This is reflected in clinical study overview, where no therapeutic intervention can be found.
6. Conclusions
Undoubtedly, the use of theranostic radiopharmaceuticals has very impressive benefits in nuclear medicine which cannot be denied. Planning for targeted treatment as well as ability of simultaneous evaluation of response to treatment is a great advantage that resulted by this methodology. In the review our effort was to provide a useful summary of efforts made in the field of BC. Based on the importance of BC it is expected that more efficient theranostic agents will be developed soon. The most considerable aspect of this field is the feasibility of early detection of BC and based on that improved cancer prognosis.
Author Contributions
All authers contributed equally in this study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.O.; Shyyan, R.; Eniu, A.; Smith, R.A.; Yip, C.H.; Bese, N.S.; Chow, L.W.; Masood, S.; Ramsey, S.D.; Carlson, R.W. Breast cancer in limited-resource countries: An overview of the Breast Health Global Initiative 2005 guidelines. Breast J. 2006, 12, S3–S15. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer (Primer). Nat. Rev. Dis. Primers 2019, 66. [Google Scholar] [CrossRef] [PubMed]
- Munro, P. Breast Cancer: An Overview of Nuclear Medicine Imaging & Treatment. Can. J. Med. Radiat. Technol. 2005, 36, 30–36. [Google Scholar]
- Vatsa, R.; Singh, S.S.; Ashwathanarayana, A.G.; Kumar, R.; Rana, N.; Shukla, J.; Mittal, B.R. Breast Cancer Imaging With PET Based Radiopharmaceuticals Other Than 18F-FDG. Clin. Nucl. Med. 2020, 45, e72–e76. [Google Scholar] [CrossRef]
- Cornejo, K.M.; Kandil, D.; Khan, A.; Cosar, E.F. Theranostic and molecular classification of breast cancer. Arch. Pathol. Lab. Med. 2014, 138, 44–56. [Google Scholar] [CrossRef]
- Bauman, G.; Charette, M.; Reid, R.; Sathya, J.; Therapeutic Radiopharmaceutical Guidelines Group of Cancer Care Ontario’s Program in Evidence-based Care. Radiopharmaceuticals for the palliation of painful bone metastases—A systematic review. Radiother. Oncol. 2005, 75, E251–E258. [Google Scholar] [CrossRef]
- Ballinger, J.R. Theranostics and precision medicine special feature: Review Article Theranostic radiopharmaceuticals: Established agents in current use. Br. J. Radiol. 2018, 91, 20170969. [Google Scholar] [CrossRef]
- Mango, L. Theranostics: A unique concept to nuclear medicine. Arch. Cancer Sci. 2017, 1, 001–004. [Google Scholar] [CrossRef][Green Version]
- Shrivastava, S.; Jain, S.; Kumar, D.; Soni, S.L.; Sharma, M. A review on theranostics: An approach to targeted diagnosis and therapy. Asian J. Pharm. Res. Dev. 2019, 7, 63–69. [Google Scholar] [CrossRef]
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Cianni, R.; Bagni, O. Theranostic approaches in nuclear medicine: Current status and future prospects. Expert Rev. Med. Devices 2020, 17, 331–343. [Google Scholar] [CrossRef]
- Langbein, T.; Weber, W.A.; Eiber, M. Future of theranostics: An outlook on precision oncology in nuclear medicine. J. Nucl. Med. 2019, 60, 13S–19S. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA A Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18. [Google Scholar] [CrossRef]
- Group, E.B.C.T.C. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar]
- Jin, X.; Mu, P. Targeting breast cancer metastasis. Breast Cancer Basic Clin. Res. 2015, 9, BCBCR-S25460. [Google Scholar] [CrossRef]
- Largillier, R.; Ferrero, J.-M.; Doyen, J.; Barriere, J.; Namer, M.; Mari, V.; Courdi, A.; Hannoun-Levi, J.; Ettore, F.; Birtwisle-Peyrottes, I. Prognostic factors in 1038 women with metastatic breast cancer. Ann. Oncol. 2008, 19, 2012–2019. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget 2017, 8, 27990. [Google Scholar] [CrossRef]
- Montemagno, C. Metastatic Heterogeneity of Breast Cancer: Companion and Theranostic Approach in Nuclear Medicine. Cancers 2020, 12, 821. [Google Scholar] [CrossRef]
- Nounou, M.I.; ElAmrawy, F.; Ahmed, N.; Abdelraouf, K.; Goda, S.; Syed-Sha-Qhattal, H. Breast cancer: Conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer: Basic Clin. Res. 2015, 9, BCBCR-S29420. [Google Scholar]
- Kerlikowske, K.; Hubbard, R.A.; Miglioretti, D.L.; Geller, B.M.; Yankaskas, B.C.; Lehman, C.D.; Taplin, S.H.; Sickles, E.A. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: A cohort study. Ann. Intern. Med. 2011, 155, 493–502. [Google Scholar] [CrossRef]
- Greenwood, H.I.; Dodelzon, K.; Katzen, J.T. Impact of advancing technology on diagnosis and treatment of breast cancer. Surg. Clin. 2018, 98, 703–724. [Google Scholar] [CrossRef]
- Van Goethem, M.; Tjalma, W.; Schelfout, K.; Verslegers, I.; Biltjes, I.; Parizel, P. Magnetic resonance imaging in breast cancer. Eur. J. Surg. Oncol. 2006, 32, 901–910. [Google Scholar] [CrossRef]
- Shermis, R.B.; Redfern, R.E.; Burns, J.; Kudrolli, H. Molecular breast imaging in breast cancer screening and problem solving. Radiographics 2017, 37, 1309–1606. [Google Scholar] [CrossRef]
- Greene, L.R.; Wilkinson, D. The role of general nuclear medicine in breast cancer. J. Med. Radiat. Sci. 2015, 62, 54–65. [Google Scholar] [CrossRef]
- Annex, I. Recent developments in nuclear medicine for cancer management: From nuclear medicine to molecular imaging. Nucl. Technol. Rev. 2010 2010, 2010, 57. [Google Scholar]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Gill, M.R.; Falzone, N.; Du, Y.; Vallis, K.A. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 2017, 18, e414–e423. [Google Scholar] [CrossRef]
- Jadvar, H.; Chen, X.; Cai, W.; Mahmood, U. Radiotheranostics in cancer diagnosis and management. Radiology 2018, 286, 388–400. [Google Scholar] [CrossRef]
- Hertz, S.; Roberts, A. Application of radioactive iodine in therapy of Graves’ disease. J. Clin. Investig. 1942, 21, 624. [Google Scholar]
- Hamilton, J.; Lawrence, J. Recent clinical developments in the therapeutic application of radio-phosphorus and radio-iodine. J. Clin. Investig. 1942, 21, 624. [Google Scholar]
- Ahn, B.-C. Personalized medicine based on theranostic radioiodine molecular imaging for differentiated thyroid cancer. Biomed Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Hapuarachchige, S.; Artemov, D. Theranostic pretargeting drug delivery and imaging platforms in cancer precision medicine. Front. Oncol. 2020, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- Redfern, J. Theranostics: Cancer imaging and therapy using injectable radionuclide-labeled ligands. Pharm. Pharm. Int. J. 2020, 8, 325–331. [Google Scholar] [CrossRef]
- Virgolini, I.; Ambrosini, V.; Bomanji, J.B.; Baum, R.P.; Fanti, S.; Gabriel, M.; Papathanasiou, N.D.; Pepe, G.; Oyen, W.; De Cristoforo, C. Procedure guidelines for pet/ct tumour imaging with 68 Ga-dota-conjugated peptides: 68 Ga-dota-toc, 68 Ga-dota-noc, 68 Ga-dota-tate. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2004–2010. [Google Scholar] [CrossRef]
- Werner, R.A.; Weich, A.; Kircher, M.; Solnes, L.B.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; Pomper, M.G.; Rowe, S.P.; Lapa, C. The theranostic promise for Neuroendocrine Tumors in the late 2010s-Where do we stand, where do we go? Theranostics 2018, 8, 6088. [Google Scholar] [CrossRef]
- Refardt, J.; Hofland, J.; Kwadwo, A.; Nicolas, G.P.; Rottenburger, C.; Fani, M.; Wild, D.; Christ, E. Theranostics in neuroendocrine tumors: An overview of current approaches and future challenges. Rev. Endocr. Metab. Disord. 2020, 1–14. [Google Scholar] [CrossRef]
- Hennrich, U.; Benešová, M. [68Ga] Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Vahidfar, N.; Fallahpoor, M.; Farzanehfar, S.; Divband, G.; Ahmadzadehfar, H. Historical review of pharmacological development and dosimetry of PSMA-based theranostics for prostate cancer. J. Radioanal. Nucl. Chem. 2019, 322, 237–248. [Google Scholar] [CrossRef]
- Jones, W.; Griffiths, K.; Barata, P.C.; Paller, C.J. PSMA theranostics: Review of the current status of PSMA-targeted imaging and radioligand therapy. Cancers 2020, 12, 1367. [Google Scholar] [CrossRef]
- Rahbar, K.; Afshar-Oromieh, A.; Jadvar, H.; Ahmadzadehfar, H. PSMA theranostics: Current status and future directions. Mol. Imaging 2018, 17. [Google Scholar] [CrossRef]
- Mayor, N.; Sathianathen, N.J.; Buteau, J.; Koschel, S.; Antón Juanilla, M.; Kapoor, J.; Azad, A.; Hofman, M.S.; Murphy, D.G. Prostate-specific membrane antigen theranostics in advanced prostate cancer: An evolving option. Bju Int. 2020, 126, 525–535. [Google Scholar] [CrossRef]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemother. Res. Pract. 2012, 2012. [Google Scholar] [CrossRef]
- Koutras, A.K.; Evans, T.J. The epidermal growth factor receptor family in breast cancer. Oncotargets Ther. 2008, 1, 5. [Google Scholar] [CrossRef]
- Cava, C.; Novello, C.; Martelli, C.; Lodico, A.; Ottobrini, L.; Piccotti, F.; Truffi, M.; Corsi, F.; Bertoli, G.; Castiglioni, I. Theranostic application of miR-429 in HER2+ breast cancer. Theranostics 2020, 10, 50. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Lauri, C.; Auletta, S.; Varani, M.; Onofrio, L.; Glaudemans, A.W.; Panzuto, F.; Marchetti, P. Radiopharmaceuticals for Breast Cancer and Neuroendocrine Tumors: Two Examples of How Tissue Characterization May Influence the Choice of Therapy. Cancers 2020, 12, 781. [Google Scholar] [CrossRef]
- Lub-de Hooge, M.N.; Kosterink, J.G.; Perik, P.J.; Nijnuis, H.; Tran, L.; Bart, J.; Suurmeijer, A.J.; de Jong, S.; Jager, P.L.; de Vries, E.G. Preclinical characterisation of 111In-DTPA-trastuzumab. Br. J. Pharmacol. 2004, 143, 99–106. [Google Scholar] [CrossRef]
- Gaykema, S.B.; de Jong, J.R.; Perik, P.J.; Brouwers, A.H.; Schröder, C.P.; Munnink, T.H.O.; Bongaerts, A.H.; de Vries, E.G.; Hooge, M.N.L.-d. 111In-trastuzumab scintigraphy in HER2-positive metastatic breast cancer patients remains feasible during trastuzumab treatment. Mol. Imaging 2014, 13. [Google Scholar] [CrossRef]
- Carrasquillo, J.A.; Morris, P.G.; Humm, J.L.; Smith-Jones, P.M.; Beylergil, V.; Akhurst, T.; O’donoghue, J.A.; Ruan, S.; Modi, S.; Hudis, C.A. Copper-64 trastuzumab PET imaging: A reproducibility study. Q. J. Nucl. Med. Mol. Imaging 2019, 63. [Google Scholar] [CrossRef]
- Dijkers, E.; Oude Munnink, T.; Kosterink, J.; Brouwers, A.; Jager, P.; De Jong, J.; Van Dongen, G.; Schröder, C.; Lub-de Hooge, M.; De Vries, E. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.G.; Schröder, C.P. 89 Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef]
- Beylergil, V.; Morris, P.G.; Smith-Jones, P.M.; Modi, S.; Solit, D.; Hudis, C.A.; Lu, Y.; O’Donoghue, J.; Lyashchenko, S.K.; Carrasquillo, J.A. Pilot study of 68Ga-DOTA-F (ab′) 2-trastuzumab in patients with breast cancer. Nucl. Med. Commun. 2013, 34, 1157. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, N.; Chen, Z.; Liu, T.; Xu, X.; Lei, X.; Shen, L.; Gao, J.; Yang, Z.; Zhu, H. Construction of 124 I-trastuzumab for noninvasive PET imaging of HER2 expression: From patient-derived xenograft models to gastric cancer patients. Gastric Cancer 2020, 23, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cao, H.; Xing, C.; Wei, S.; Jiang, G.; Liu, Z. Visualization and body distribution of [131I]-herceptin in nude mice with bt-474 breast carcinoma. Genet. Mol. Res. 2014, 13, 6804–6812. [Google Scholar] [CrossRef]
- Heydari, S.; Rajabi, H.; Rasaneh, S.; Daha, F.J. Radiolabeling of Herceptin with 99mTc as a Her2 tracer. Nov. Biomed. 2014, 2, 73–78. [Google Scholar]
- Bhusari, P.; Vatsa, R.; Singh, G.; Parmar, M.; Bal, A.; Dhawan, D.K.; Mittal, B.R.; Shukla, J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int. J. Cancer 2017, 140, 938–947. [Google Scholar] [CrossRef]
- bin Othman, M.F.; Verger, E.; Costa, I.; Tanapirakgul, M.; Cooper, M.S.; Imberti, C.; Lewington, V.J.; Blower, P.J.; Terry, S.Y. In vitro cytotoxicity of Auger electron-emitting [67Ga] Ga-trastuzumab. Nucl. Med. Biol. 2020, 80, 57–64. [Google Scholar] [CrossRef]
- Varshney, R.; Chitkara, A.; Saklani, M.; Kaur, A.; Mathur, R.; Tiwari, A.; Singh, B.; Mishra, A.K. Targeting Bombesin Peptide Receptors for Cancer Imaging: Perspective in Prostate, Lung and Breast Cancer. Nov. Approaches Cancer Study 2020, 5, 483–491. [Google Scholar]
- Navalkissoor, S.; Gnanasegaran, G.; Baum, R. Theranostics and precision medicine special feature. Br. J. Radiol. 2018, 91. [Google Scholar] [CrossRef]
- Farolfi, A.; Fendler, W.; Iravani, A.; Haberkorn, U.; Hicks, R.; Herrmann, K.; Walz, J.; Fanti, S. Theranostics for advanced prostate cancer: Current indications and future developments. Eur. Urol. Oncol. 2019, 2, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K. Molecular imaging radiotherapy: Theranostics for personalized patient management of neuroendocrine tumors (NETs). Theranostics 2012, 2, 448. [Google Scholar] [CrossRef]
- Jensen, R.; Battey, J.; Spindel, E.; Benya, R. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef]
- Ramos-Álvarez, I.; Moreno, P.; Mantey, S.A.; Nakamura, T.; Nuche-Berenguer, B.; Moody, T.W.; Coy, D.H.; Jensen, R.T. Insights into bombesin receptors and ligands: Highlighting recent advances. Peptides 2015, 72, 128–144. [Google Scholar] [CrossRef]
- McDonald, T.; Jörnvall, H.; Nilsson, G.; Vagne, M.; Ghatei, M.; Bloom, S.; Mutt, V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem. Biophys. Res. Commun. 1979, 90, 227–233. [Google Scholar] [CrossRef]
- Moreno, P.; Ramos-Álvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073. [Google Scholar] [CrossRef]
- Moody, T.W.; Mantey, S.A.; Pradhan, T.K.; Schumann, M.; Nakagawa, T.; Martinez, A.; Fuselier, J.; Coy, D.H.; Jensen, R.T. Development of high affinity camptothecin-bombesin conjugates that have targeted cytotoxicity for bombesin receptor-containing tumor cells. J. Biol. Chem. 2004, 279, 23580–23589. [Google Scholar] [CrossRef]
- Pu, F.; Qiao, J.; Xue, S.; Yang, H.; Patel, A.; Wei, L.; Hekmatyar, K.; Salarian, M.; Grossniklaus, H.E.; Liu, Z.-R. GRPR-targeted protein contrast agents for molecular imaging of receptor expression in cancers by MRI. Sci. Rep. 2015, 5, 16214. [Google Scholar] [CrossRef]
- Miah, S.; Bagu, E.; Goel, R.; Ogunbolude, Y.; Dai, C.; Ward, A.; Vizeacoumar, F.S.; Davies, G.; Vizeacoumar, F.J.; Anderson, D.; et al. Estrogen receptor signaling regulates the expression of the breast tumor kinase in breast cancer cells. BMC Cancer 2019, 19, 78. [Google Scholar] [CrossRef]
- Morgat, C.; Macgrogan, G.; Brouste, V.; Valérie, V.; Sevenet, N.; Bonnefoi, H.; Fernandez, P.; Debled, M.; Hindié, E. Expression of Gastrin-Releasing Peptide Receptor (GRPR) in Breast Cancer and its Association with Pathological, Biological and Clinical Parameters: A Study of 1432 Primary Tumors. J. Nucl. Med. 2017, 58. [Google Scholar] [CrossRef]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.-C.; Gugger, M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand 125I-[D-TYR6, β-ALA11, PHE13, NLE14] bombesin (6–14). Clin. Cancer Res. 2002, 8, 1139–1146. [Google Scholar]
- Dalm, S.U.; Martens, J.W.; Sieuwerts, A.M.; van Deurzen, C.H.; Koelewijn, S.J.; de Blois, E.; Maina, T.; Nock, B.A.; Brunel, L.; Fehrentz, J.-A. In vitro and in vivo application of radiolabeled gastrin-releasing peptide receptor ligands in breast cancer. J. Nucl. Med. 2015, 56, 752–757. [Google Scholar] [CrossRef]
- Morgat, C.; Schollhammer, R.; Macgrogan, G.; Barthe, N.; Vélasco, V.; Vimont, D.; Cazeau, A.-L.; Fernandez, P.; Hindié, E. Comparison of the binding of the gastrin-releasing peptide receptor (GRP-R) antagonist 68Ga-RM2 and 18F-FDG in breast cancer samples. PLoS ONE 2019, 14, e0210905. [Google Scholar] [CrossRef] [PubMed]
- Aghanejad, A.; Jalilian, A.R.; Maus, S.; Yousefnia, H.; Geramifar, P.; Beiki, D. Optimized production and quality control of 68Ga-DOTATATE. Iran. J. Nucl. Med. 2016, 24, 29–36. [Google Scholar]
- Hoppenz, P.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide-drug conjugates and their targets in advanced cancer therapies. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Pujatti, P.; Foster, J.; Finucane, C.; Hudson, C.; Burnet, J.; Pasqualoto, K.; Mengatti, J.; Mather, S.; Araújo, E.; Sosabowski, J. Evaluation and comparison of a new DOTA and DTPA-bombesin agonist in vitro and in vivo in low and high GRPR expressing prostate and breast tumor models. Appl. Radiat. Isot. 2014, 96C, 91–101. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, Y.; Liu, S.; Wang, F.; Chen, X. 18F, 64Cu, and 68Ga Labeled RGD-Bombesin Heterodimeric Peptides for PET Imaging of Breast Cancer. Bioconjugate Chem. 2009, 20, 1016–1025. [Google Scholar] [CrossRef]
- Stoykow, C.; Erbes, T.; Maecke, H.; Bulla, S.; Bartholomä, M.; Mayer, S.; Drendel, V.; Bronsert, P.; Werner, M.; Gitsch, G.; et al. Gastrin-releasing Peptide Receptor Imaging in Breast Cancer Using the Receptor Antagonist 68 Ga-RM2 and PET. Theranostics 2016, 6, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Mao, F.; Wang, H.; Zhang, J.; Liu, Q.; Peng, L.; Li, F.; Lang, L.; Chen, X.; Zhu, Z. 68Ga-NOTA-RM26 PET/CT in the Evaluation of Breast Cancer: A Pilot Prospective Study. Clin. Nucl. Med. 2018, 43, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Wiele, C.; Dumont, F.; Broecke, R.; Oosterlinck, W.; Cocquyt, V.; Serreyn, R.; Peers, S.; Thornback, J.; Slegers, G.; Dierckx, R. Technetium-99 m RP527, a GRP analogue for visualisation of GRP receptor-expressing malignancies: A feasibility study. Eur. J. Nucl. Med. 2000, 27, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Lara, L.; Ferro-Flores, G.; Ramírez, F.; Ocampo-García, B.; Santos-Cuevas, C.; Díaz, L.; Isaac-Olive, K. Improved radiopharmaceutical based on 99mTc-Bombesin–folate for breast tumour imaging. Nucl. Med. Commun. 2015, 37, 1. [Google Scholar] [CrossRef]
- Scopinaro, F.; Varvarigou, A.; Ussof, W.; De Vincentis, G.; Archimandritis, S.; Evangelatos, G.; Corleto, V.; Pulcini, A.; Capoccetti, F.; Remediani, S.; et al. Breast Cancer Takes up 99mTc Bombesin. A Preliminary Report. Tumori J. 2002, 88, S25–S28. [Google Scholar] [CrossRef]
- Ji, T.; Sun, Y.; Chen, B.; Ji, B.; Gao, S.; Ma, Q.; Cheng, G.; Zhang, H. The diagnostic role of 99mTc-dual receptor targeted probe and targeted peptide bombesin (RGD-BBN) SPET/CT in the detection of malignant and benign breast tumors and axillary lymph nodes compared to ultrasound. Hell. J. Nucl. Med. 2015, 18, 108–113. [Google Scholar] [CrossRef]
- Chen, Q.; ma, Q.; Chen, M.; Chen, B.; Wen, Q.; Jia, B.; Wang, F.; Sun, B.; Gao, S. An Exploratory Study on 99mTc-RGD-BBN Peptide Scintimammography in the Assessment of Breast Malignant Lesions Compared to 99mTc-3P4-RGD2. PLoS ONE 2015, 10, e0123401. [Google Scholar] [CrossRef] [PubMed]
- Santos-Cuevas, C.L.; Ferro-Flores, G.; de Murphy, C.A.; Pichardo-Romero, P.A. Targeted imaging of gastrin-releasing peptide receptors with 99mTc-EDDA/HYNIC-[Lys3]-bombesin: Biokinetics and dosimetry in women. Nucl. Med. Commun. 2008, 29, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Wiele, C.; Phonteyne, P.; Pauwels, P.; Goethals, I.; Broecke, R.; Cocquyt, V.; Dierckx, R. Gastrin-Releasing Peptide Receptor Imaging in Human Breast Carcinoma Versus Immunohistochemistry. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2008, 49, 260–264. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The chemistry, physiology and pathology of pH in cancer. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef]
- Annala, M.; Struss, W.J.; Warner, E.W.; Beja, K.; Vandekerkhove, G.; Wong, A.; Khalaf, D.; Seppälä, I.-L.; So, A.; Lo, G. Treatment outcomes and tumor loss of heterozygosity in germline DNA repair–deficient prostate cancer. Eur. Urol. 2017, 72, 34–42. [Google Scholar] [CrossRef]
- Calon, A.; Lonardo, E.; Berenguer-Llergo, A.; Espinet, E.; Hernando-Momblona, X.; Iglesias, M.; Sevillano, M.; Palomo-Ponce, S.; Tauriello, D.V.; Byrom, D. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015, 47, 320–329. [Google Scholar] [CrossRef]
- Pitcher, C.J.; Quittner, C.; Peterson, D.M.; Connors, M.; Koup, R.A.; Maino, V.C.; Picker, L.J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 1999, 5, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, J.; Micke, P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin. Cancer Biol. 2014, 25, 61–68. [Google Scholar] [CrossRef]
- Winslow, S.; Leandersson, K.; Edsjö, A.; Larsson, C. Prognostic stromal gene signatures in breast cancer. Breast Cancer Res. 2015, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liang, C.; Chen, M.; Su, W. Association between tumor-stroma ratio and prognosis in solid tumor patients: A systematic review and meta-analysis. Oncotarget 2016, 7, 68954. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, A.; Tollenaar, R.; v Pelt, G.; Zeestraten, E.; Dutton, S.; McConkey, C.; Domingo, E.; Smit, V.; Midgley, R.; Warren, B. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: Validation in the VICTOR trial. Ann. Oncol. 2013, 24, 179–185. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The dark side of fibroblasts: Cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef] [PubMed]
- Naya, M.; Murthy, V.L.; Taqueti, V.R.; Foster, C.R.; Klein, J.; Garber, M.; Dorbala, S.; Hainer, J.; Blankstein, R.; Resnic, F. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J. Nucl. Med. 2014, 55, 248–255. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Lindner, T.; Marschalek, M.M.; Loktev, A.; Lehnert, W.; Debus, J.; Jäger, D.; Flechsig, P.; Altmann, A. 68Ga-FAPI PET/CT: Biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J. Nucl. Med. 2019, 60, 386–392. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.-M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W. Development of fibroblast activation protein–targeted radiotracers with improved tumor retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C. A tumor-imaging method targeting cancer-associated fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.; Heirbaut, L.; Verkerk, R.; Cheng, J.D.; Joossens, J.; Cos, P.; Maes, L.; Lambeir, A.-M.; De Meester, I.; Augustyns, K. Extended structure–activity relationship and pharmacokinetic investigation of (4-quinolinoyl) glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J. Med. Chem. 2014, 57, 3053–3074. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pan, Q.; Yang, H.; Peng, L.; Zhang, W.; Li, F. Fibroblast Activation Protein–Targeted PET/CT with 68Ga-FAPI for Imaging IgG4-Related Disease: Comparison to 18F-FDG PET/CT. J. Nucl. Med. 2021, 62, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Hao, B.; Shang, Q.; Sun, L.; Chen, H. Comparison of 68Ga-FAPI and 18F-FDG PET/CT in a patient with cholangiocellular carcinoma: A case report. Clin. Nucl. Med. 2020, 45, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Koerber, S.A.; Staudinger, F.; Kratochwil, C.; Adeberg, S.; Haefner, M.F.; Ungerechts, G.; Rathke, H.; Winter, E.; Lindner, T.; Syed, M. The role of 68Ga-FAPI PET/CT for patients with malignancies of the lower gastrointestinal tract: First clinical experience. J. Nucl. Med. 2020, 61, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H. 68Ga-FAPI PET/CT: Tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Windisch, P.; Zwahlen, D.R.; Koerber, S.A.; Giesel, F.L.; Debus, J.; Haberkorn, U.; Adeberg, S. Clinical results of fibroblast activation protein (FAP) specific PET and implications for radiotherapy planning: Systematic review. Cancers 2020, 12, 2629. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A theranostic approach of [68 Ga] Ga-DOTA. SA. FAPi PET/CT-guided [177 Lu] Lu-DOTA. SA. FAPi radionuclide therapy in an end-stage breast cancer patient: New frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2020, 1–3. [Google Scholar] [CrossRef]
- Moon, E.S.; Elvas, F.; Vliegen, G.; De Lombaerde, S.; Vangestel, C.; De Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA 5m chelators. Ejnmmi Radiopharm. Chem. 2020, 5, 1–20. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).