Molecular Hydrogen as a Potential Clinically Applicable Radioprotective Agent
Abstract
1. Introduction
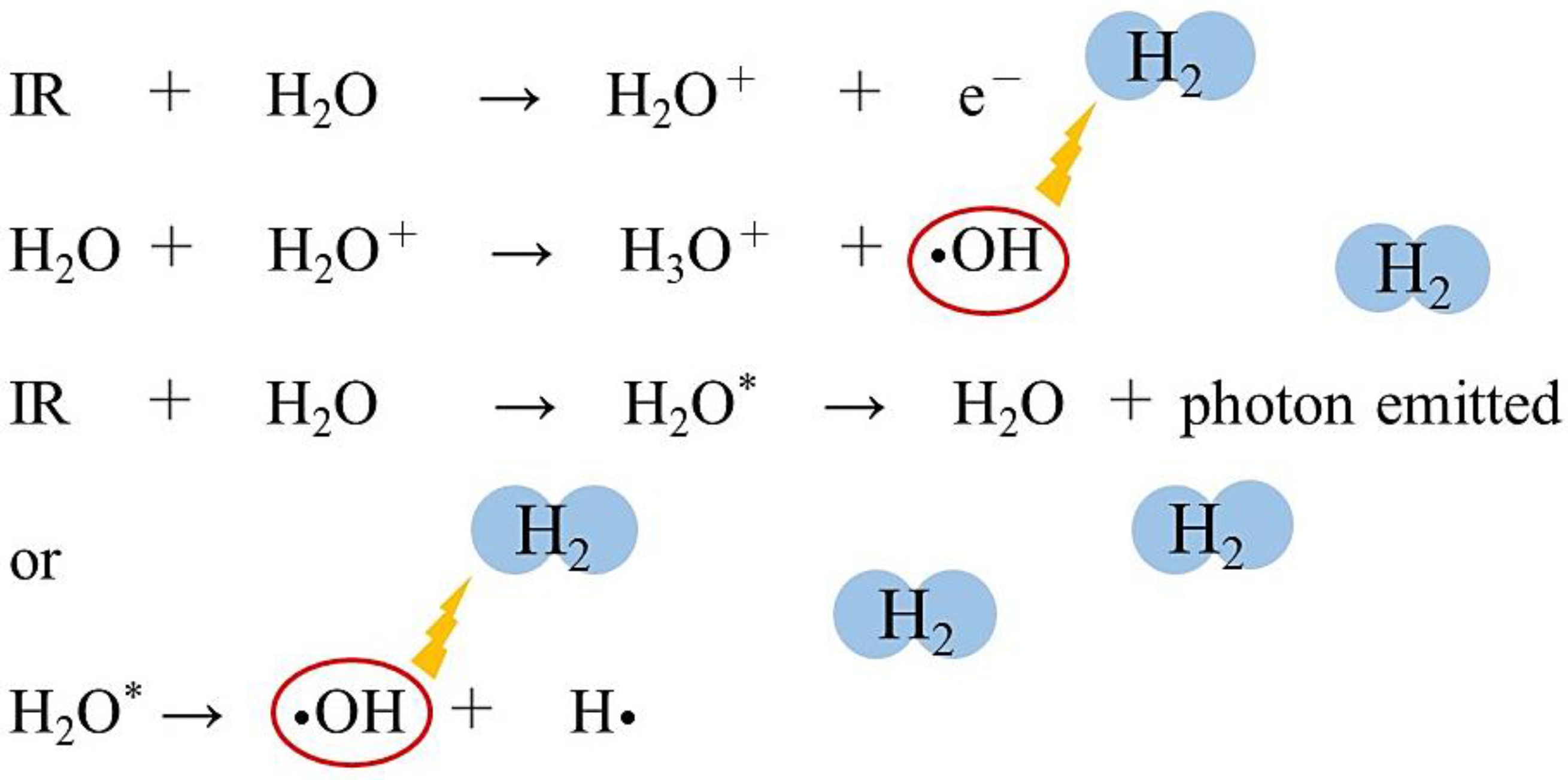
2. Biological Effects of Radiation
3. Radioprotective Effects of H2 in Animal Models
3.1. Protective Effects on Cognitive Impairment
3.2. Protective Effects on the Immune System
3.3. Protective Effects against Lung Injury
3.4. Protective Effects on Myocardial Injury
3.5. Protective Effects against Gastrointestinal Disorders
3.6. Protective Effects against Hematopoietic Cell Injury
3.7. Protective Effects on Sperm Dysfunction
3.8. Protective Effects against Skin Damage
3.9. Protective Effects against Cartilage Damage
3.10. Inhibitory Effects on Carcinogenesis (Thymic Lymphoma)
4. Radioprotective Effects of H2 in Humans
4.1. Improvement of Decreased QOL in Cancer Treatment
4.2. Improvement of Bone Marrow Damage in Cancer Treatment
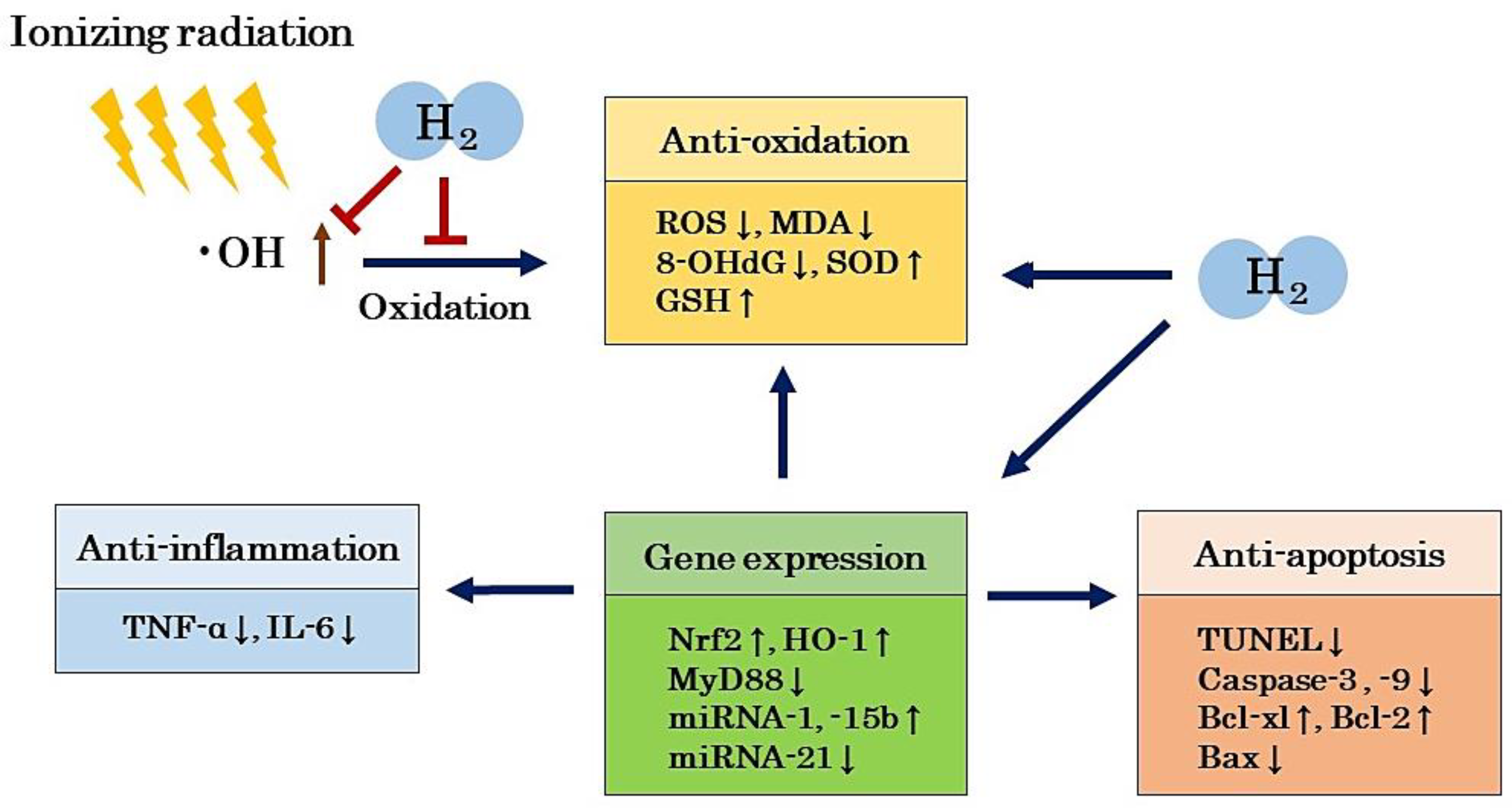
5. Mechanism of the Radioprotective Effects of H2
5.1. Antioxidant Effects
5.2. Anti-Inflammatory Effects
5.3. Anti-Apoptotic Effects
5.4. Regulation of Gene Expression
6. Prospects of H2 as a Radioprotective Agent
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer-Valuck, B.W.; Rao, Y.J.; Michalski, J.M. Intensity-modulated radiotherapy for prostate cancer. Transl. Androl. Urol. 2018, 7, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Luo, Y.; Zhou, D. Hematopoietic stem cell injury induced by ionizing radiation. Antioxid. Redox Signal. 2014, 20, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog. Nucleic Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [PubMed]
- Caer, S.L. Water radiolysis: Influence of oxide surfaces on H2 production under ionizing radiation. Water 2011, 3, 235–253. [Google Scholar]
- Nickoloff, J.A.; Sharma, N.; Taylor, L. Clustered DNA double-strand breaks: Biological effects and relevance to cancer radiotherapy. Gene 2020, 11, 99. [Google Scholar] [CrossRef]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Yanagihara, T.; Arai, K.; Miyamae, K.; Sato, B.; Shudo, T.; Yamada, M.; Aoyama, M. Electrolyzed hydrogen-saturated water for drinking use elicits an antioxidative effect; a feeding test with rats. Biosci. Biotrechnol. Biochem. 2005, 69, 1985–1987. [Google Scholar] [CrossRef]
- Hirano, S.I.; Ichikawa, Y.; Kurokawa, R.; Takefuji, Y.; Satoh, F. A “philosophical molecule,” hydrogen may overcome senescence and intractable diseases. Med. Gas Res. 2020, 10, 47–49. [Google Scholar] [CrossRef]
- Hirano, S.i.; Ichikawa, Y.; Sato, B.; Satoh, F.; Takefuji, Y. Hydrogen is promising for medical applications. Clean. Technol. 2020, 2, 33. [Google Scholar] [CrossRef]
- Sato, T.; Kinoshita, M.; Yamamoto, T.; Ito, M.; Nishida, T.; Takeuchi, M.; Saitoh, D.; Seki, S.; Mukai, Y. Treatment of irradiated mice with high-dose ascorbic acid reduced lethality. PLoS ONE 2015, 10, e0117020. [Google Scholar] [CrossRef]
- Drouet, M.; Mourcin, F.; Grenier, N.; Leroux, V.; Denis, J.; Mayol, J.F.; Thullier, P.; Lataillade, J.J.; Herodin, F. Single administration of stem cell factor, FLT-3 ligand, megakaryocyte growth and development factor, and ineterleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: Long-term follow-up of hematopoiesis. Blood 2004, 103, 878–885. [Google Scholar] [CrossRef]
- Farese, A.M.; Casey, D.B.; Smith, W.G.; Vigneulle, R.M.; McKearn, J.P.; MacVittie, T. Leridistim, a chimeric dual G-CSF and IL-3 receptor agonist, enhances multilineage hematopoietic recovery in a nonhuman primate model of radiation-induced myelosuppression: Effect of schedule, dose, and route of administration. Stem Cells 2001, 19, 522–533. [Google Scholar] [CrossRef]
- Herodin, F.; Bourin, P.; Mayol, J.F.; Lataillade, J.J.; Drouet, M. Short-term injection of antiapoptotic cytokine combinations soon after lethal gamma-irradiation promotes survival. Blood 2003, 101, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- MacVittie, T.J.; Farese, A.M.; Smith, W.G.; Baum, C.M.; Burton, E.; McKearn, J.P. Myelopoietin, an engineered chimeric IL-3 and G-CSF receptor agonist, stimulates multilineage hematopoietic recovery in a nonhuman primate model of radiation-induced myelosuppression. Blood 2000, 95, 837–845. [Google Scholar] [CrossRef]
- Nuszkiewicz, J.; Wanzniak, A.; Szewezyk-Golec, K. Inonizing radiation as a source of oxidative stress-The protective role of melatonin and Vitamin D. Int. J. Mol. Sci. 2020, 21, 5804. [Google Scholar] [CrossRef] [PubMed]
- Seed, T.M.; Fry, S.A.; Neta, R.; Weiss, J.W.; Jarrett, D.G.; Thomassen, D. Prevention and treatments: Summary statement. Milit. Med. 2002, 167, 87–93. [Google Scholar]
- Thorstad, W.I.; Haughey, B.; Chao, K.S.-C. Pilot study of subcutaneous amifostine in patients undergoing postoperative intensity modulated radiation therapy for head and neck cancer: Preliminary data. Semin. Oncol. 2003, 30, 96–100. [Google Scholar] [CrossRef]
- Seed, T.M.; Inal, C.E.; Singh, V.K. Radioprotection of hematopoietic progenitors by low dose amifostine prophylaxis. Int. J. Radiat. Biol. 2014, 90, 594–604. [Google Scholar] [CrossRef][Green Version]
- Mishra, K.; Alsbeih, G. Appraised of biochemical classes of radioprotectors: Evidence, current status and guidelines for future development. 3 Biotech 2017, 7, 292. [Google Scholar] [CrossRef]
- Huang, B.; He, T.; Yao, Q.; Zhang, L.; Yao, Y.; Tang, H.; Gong, P. Amifostine suppresses the side effects of radiation on BMSCs by promoting cell proliferation and reducing ROS production. Stem Cells Int. 2019, 2019, 8749090. [Google Scholar] [CrossRef] [PubMed]
- Mertsch, K.; Grune, T.; Kunstmann, S.; Wiesner, B.; Ladhoff, A.M.; Siems, W.G.; Haseloff, R.F.; Blasig, I.E. Protective effects of the thiophosphate amifostine (WR2721) and a lazaroid (U83836E) on lipid peroxidation in endothelial cells during hypoxia/reoxygenation. Biochem. Pharmacol. 1998, 56, 945–954. [Google Scholar] [CrossRef]
- Kang, K.M.; Kang, Y.N.; Choi, I.B.; Gu, Y.; Kawamura, T.; Toyoda, Y.; Nakao, A. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med. Gas Res. 2011, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.i.; Aoki, Y.; Li, X.K.; Ichimaru, N.; Takahara, S.; Takefuji, Y. Protective Effects of Hydrogen Gas Inhalation on Radiation-Induced Bone Marrow Damage in Cancer Patients: A Retrospective Observational Study. 2020. Available online: https://www.researchsquare.com/article/rs-16275/v1 (accessed on 22 March 2021).
- Hirano, S.i.; Aoki, Y.; Li, X.K.; Ichimaru, N.; Takahara, S.; Takefuji, Y. Protective effects of hydrogen gas inhalation on radiation-induced bone marrow damage in cancer patients: A retrospective observational study. Med. Gas Res. 2021, 11, in press. [Google Scholar]
- Qian, L.; Shen, J.; Chuai, Y.; Cai, J. Hydrogen as a new class of radioprotective agent. Int. J. Biol. Sci. 2013, 9, 887–894. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, Y.; Wu, S.; Wu, W.; Deng, Y.; Shao, A. Molecular hydrogen: A potential radioprotective agent. Biomed. Pharmacother. 2020, 130, 110589. [Google Scholar] [CrossRef]
- Kasai, H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res. 1997, 387, 147–163. [Google Scholar] [CrossRef]
- Floyd, R.A. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis 1990, 11, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Pohl, L.R. An immunochemical approach of identifying and characterizing protein targets of toxic reactive metabolites. Chem. Res. Toxicol. 1993, 6, 786–793. [Google Scholar] [CrossRef]
- Dubner, D.; Gisone, P.; Jaitovich, I.; Perez, M. Free radicals production and estimation of oxidative stress related to gamma irradiation. Biol. Trace Elem. Res. 1995, 47, 265–270. [Google Scholar] [CrossRef]
- Verma, S.P.; Sonwalkar, N. Structural changes in plasma membranes prepared from irradiated Chinese hamster V79 cells as revealed by Raman spectroscopy. Radiat. Res. 1991, 126, 27–35. [Google Scholar] [CrossRef]
- Xu, W.L.; Aikeremu, D.; Sun, J.G.; Zhang, Y.J.; Xu, J.B.; Zhou, W.Z.; Zhao, X.B.; Wang, H.; Yuan, H. Effect of intensity-modulated radiation therapy on sciatic nerve injury caused by echinococcosis. Neural Regen. Res. 2021, 16, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Tesei, A.; Arienti, C.; Bossi, G.; Santi, S.; Santis, I.D.; Bevilacqua, A.; Zanoni, M.; Pignatta, S.; Cortesi, M.; Zamagni, A.; et al. TP53 drives abscopal effects by secretion of senescence-associated molecular signals in non-small cell lung cancer. Int. Exp. Clin. Cancer Res. 2021, 40, 89. [Google Scholar] [CrossRef]
- Peng, Q.; Weng, K.; Li, S.; Xu, R.; Wang, Y.; Wu, Y. A perspective of epigenetic regulation in radiotherapy. Front. Cell Dev. Biol. 2021, 9, 624312. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, H.; Yin, J.; Wang, R.; Song, J.; Hu, B.; Li, J.; Qin, X. Effect of hydrogen rich water on radiation-induced cognitive dysfunction in rats. Radiat. Res. 2020, 193, 16–23. [Google Scholar] [CrossRef]
- Qian, L.; Li, B.; Cao, F.; Huang, Y.; Liu, S.; Cai, J.; Gao, F. Hydrogen-rich PBS protects cultured human cells from ionizing radiation-induced cellular damage. Nucl. Technol. Radiat. Prot. 2010, 25, 23–29. [Google Scholar] [CrossRef]
- Qian, L.; Cao, F.; Cui, J.; Huang, Y.; Zhou, X.; Liu, S.; Cai, J. Radioprotective effect of hydrogen in cultured cells and mice. Free Radic. Res. 2010, 44, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, B.; Liu, C.; Chuai, Y.; Lei, J.; Gao, F.; Cui, J.; Sun, D.; Cheng, Y.; Zhou, C.; et al. Hydrogen-rich saline protects immunocytes from radiation-induced apoptosis. Med. Sci. Monit. 2012, 18, BR144–BR148. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, Y.; Liu, W.; Xuan, Z.; Wu, S.; Yu, S.; Mei, K.; Huang, Y.; Zhang, P.; Cai, J.; et al. Protective effect of hydrogen-rich saline against radiation-induced immune dysfunction. J. Cell Mol. Med. 2014, 18, 938–946. [Google Scholar] [CrossRef]
- Terasaki, Y.; Ohsawa, I.; Terasaki, M.; Takahashi, M.; Kunugi, S.; Dedong, K.; Urushiyama, H.; Anemori, S.; Kaneko-Togashi, M.; Kuwahara, N.; et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 301, L415–L426. [Google Scholar] [CrossRef]
- Qian, L.; Cao, F.; Cui, J.; Wang, Y.; Huang, Y.; Chuai, Y.; Zaho, L.; Jiang, H.; Cai, J. The potential cardioprotective effects of hydrogen in irradiated mice. J. Radiat. Res. 2010, 51, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Kura, B.; Kalocayova, B.; LeBaron, T.W.; Frimmel, K.; Buday, J.; Surovy, J.; Slezak, J. Regulation of microRNAs by molecular hydrogen contributes to the prevention of radiation-induced damage in the rat myocardium. Mol. Cell Biochem. 2019, 457, 61–72. [Google Scholar] [CrossRef]
- Xiao, H.W.; Li, Y.; Dan, L.; Dong, J.L.; Zhou, L.X.; Zhao, S.Y.; Zheng, Q.S.; Wang, H.C.; Cui, M.; Fan, S.J. Hydrogen water ameliorates radiation-induced gastrointestinal toxicity via MyD88′s effects on the gut microbiota. Exp. Mol. Med. 2018, 50, e433. [Google Scholar] [CrossRef]
- Qiu, X.; Dong, K.; Guan, J.; He, J. Hydrogen attenuates radiation-induced intestinal damage by reducing oxidative stress and inflammatory response. Int. Immunopharmacol. 2020, 84, 106517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, X.; Han, X.; Li, Y.; Lu, L.; Li, D.; Fan, S. Hydrogen-rich water ameliorates total body irradiation-induced hematopoietic stem cell injury by reducing hydroxyl radical. Oxid. Med. Cell. Longev. 2017, 3, 8241678. [Google Scholar] [CrossRef]
- Cauai, Y.; Shen, J.; Qian, L.; Wang, Y.; Huang, Y.; Gao, F.; Cui, J.; Ni, J.; Zhao, L.; Liu, S.; et al. Hydrogen-rich saline protects spermatogenesis and hematopoiesis in irradiated BALB/c mice. Med. Sci. Monit. 2012, 18, BR89–BR94. [Google Scholar]
- Chuai, Y.; Gao, F.; Li, B.; Zhao, L.; Qian, L.; Cao, F.; Wang, L.; Sun, X.; Cui, J. Hydrogen-rich saline attenuates radiation-induced male germ cell loss in mice through reducing hydroxyl radicals. Biochem. J. 2012, 442, 49–56. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, B.; Yang, M.; Li, Z.; Zhang, Y.; Jiang, D. Protection by hydrogen against gamma ray-induced testicular damage in rats. Basic Clin. Pharmacol. Toxicol. 2013, 112, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.; Zhao, S.; Qian, L.; Li, B.; Ni, J.; Cai, J. Hydrogen protects rats from dermatitis caused by local radiation. J. Dermatol. Treat. 2014, 25, 182–188. [Google Scholar] [CrossRef]
- Watanabe, S.; Fujita, M.; Ishihara, M.; Tachibana, S.; Yamamoto, Y.; Kaji, T.; Kawauchi, T.; Kanatani, Y. Protective effect of inhalation of hydrogen gas on radiation-induced dermatitis and skin injury in rats. J. Radiat. Res. 2014, 55, 1107–1113. [Google Scholar] [CrossRef]
- Zhou, P.; Lin, B.; Wang, P.; Pan, T.; Wang, S.; Chen, W.; Cheng, S.; Liu, S. The healing effect of hydrogen-rich water on acute radiation-induced skin injury in rats. J. Radiat. Res. 2019, 60, 17–22. [Google Scholar] [CrossRef]
- Chen, Y.; Zong, C.; Jia, J.; Liu, Y.; Zhang, Z.; Cai, B.; Tian, L. A study on the protective effect of molecular hydrogen on osteoradionecrosis of the jaw in rats. Int. J. Oral Maxillofac. Surg. 2020, 49, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, C.; Zhang, J.; Gao, F.; Li, B.; Chuai, Y.; Liu, C.; Cai, J. Hydrogen protects mice from radiation induced thymic lymphoma in BALB/c mice. Int. J. Biol. Sci. 2011, 7, 297–300. [Google Scholar] [CrossRef] [PubMed]
- NERL Data. Radiation Chemistry Data Center, Notre Dame Radiation Laboratory (n.d.). 2011. Available online: http://kinetics.nist.gov/solution/ (accessed on 12 April 2021).
- Yahyapour, R.; Amini, R.; Rezapour, S.; Cheki, M.; Rezaeyan, A.; Farhood, B.; Shabeeb, D.; Musa, A.E.; Faiiah, H.; Najafi, M. Radiation-induced inflammation and autoimmune disease. Millit. Med. Res. 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.i.; Ichikawa, Y.; Sato, B.; Yamamoto, H.; Takefuji, Y.; Satoh, F. Potential therapeutic application of hydrogen in chronic inflammatory diseases: Possible inhibiting role on mitochondrial stress. Int. J. Mol. Sci. 2021, 22, 2549. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Takahara, T.; Que, W.; Fujino, M.; Guo, W.Z.; Hirano, S.I.; Ye, L.P.; Li, X.K. Hydrogen-rich water protects liver injury in nonalcoholic steatohepatitis through HO-1 enhancement via IL-10 and Sirt 1 signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G450–G463. [Google Scholar] [CrossRef]
- Xie, K.; Zhang, Y.; Wang, Y.; Meng, X.; Wang, Y.; Yu, Y.; Chen, H. Hydrogen attenuates sepsis-associated encephalopathy by NRF2 mediated NLRP3 pathway inactivation. Inflamm. Res. 2015, 69, 697–710. [Google Scholar] [CrossRef]
- Xie, K.; Wang, Y.; Yin, L.; Wang, Y.; Chen, H.; Mao, X.; Wang, G. Hydrogen gas alleviates sepsis-induced brain injury by improving mitochondrial biogenesis through the activation of PGC-α in mice. Shock 2021, 55, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishijima, Y.; Adachi, N.; Tachibana, S.; Chitoku, S.; Mukaihara, S.; Sakamoto, M.; Kudo, Y.; Nakazawa, J.; Kaneko, K.; et al. Improved brain MRI indices in the acute brain stem infarct sires treated with hydroxy radical scavengers, Edaravone and hydrogen, as compared to Edaravone alone. A non-controlled study. Med. Gas Res. 2011, 1, 12. [Google Scholar] [CrossRef]
- Sasano, N.; Enomoto, A.; Hosoi, Y.; Katsumura, Y.; Matsumoto, Y.; Shiraishi, K.; Miyagawa, K.; Igaki, H.; Nakagawa, K. Free radical scavenger edaravone suppresses X-ray-induced apoptosis through p53 inhibition in MOLT-4 cells. J. Radiat. Res. 2007, 48, 495–503. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Akagi, J.; Baba, H. Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol. Rep. 2018, 41, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Shimizu, K.; Ogura, H.; Kurokawa, T.; Umemoto, E.; Motooka, D.; Nakamura, S.; Ichimaru, N.; Takeda, K.; Takahara, S.; et al. Hydrogen-rich saline regulates intestinal barrier dysfunction, dysbiosis and bacterial translocation in a murine model of sepsis. Shock 2018, 50, 640–647. [Google Scholar] [CrossRef]
- Katsumata, Y.; Sano, F.; Abe, T.; Tamura, T.; Fujisawa, T.; Shiraishi, Y.; Khosaka, S.; Ueda, I.; Honmma, K.; Suzuki, M.; et al. The effects of hydrogen gas inhalation on adverse left ventricular remodeling after percutaneous coronary intervention for ST-elevated myocardial infraction. First pilot study in humans. Circ. J. 2017, 81, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Nagatani, K.; Otani, N.; Nawashiro, H.; Sugawara, T.; Wada, K.; Mori, K. Hydrogen improves neurological function through attenuation of blood-brain barrier disruption in spontaneously hypertensive stroke-prone rats. BMC Neurosci. 2015, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Jin, K.; Xu, H.; Wang, C.; Zhang, Z. Subcutaneous injection of hydrogen gas is a novel effective treatment for type 2 diabetes. J. Diabetes Investig. 2018, 9, 83–90. [Google Scholar] [CrossRef]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome: An open label pilot study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef]
- Liu, C.; Kurokawa, R.; Fujino, M.; Hirano, S.i.; Sato, B.; Li, X.K. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4, 5485. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Homma, K.; Suzuki, S.; Sano, M.; Sasaki, J. Hydrogen gas distribution in organs after inhalation: Real-time monitoring of tissue hydrogen concentration in rat. Sci. Rep. 2019, 9, 1255. [Google Scholar] [CrossRef]
- Yoritaka, A.; Takanashi, M.; Hirayama, M.; Nakahara, T.; Ohta, S.; Hattori, N. Pilot study of H2 therapy in Parkinson’s disease. A randomized double-blind placebo-controlled trial. Mov. Disord. 2013, 28, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Nishijima, Y.; Ohta, S.; Sakamoto, M.; Kinone, K.; Horikoshi, T.; Tamaki, M.; Takeshita, H.; Futatuki, T.; Ohishi, W.; et al. Hydrogen gas inhalation treatment in acute cerebral infarction: A randomized clinical study on safety and neuroprotection. J. Stroke Cerebrovasc. 2017, 26, 2587–2594. [Google Scholar] [CrossRef]
- Ishibashi, T.; Sato, B.; Rikitake, M.; Seo, T.; Kurokawa, R.; Hara, Y.; Naritomi, Y.; Hara, H.; Nagao, T. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: An open-label pilot study. Med. Gas Res. 2012, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Sato, B.; Shibata, S.; Sakai, T.; Hara, Y.; Naritomi, Y. Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: A randomized, double-blind placebo-controlled pilot study. Int. Immunopharmacol. 2014, 21, 468–473. [Google Scholar] [CrossRef]
- Nishimaki, K.; Asada, T.; Ohsawa, I.; Nakajima, E.; Ikejima, C.; Yokota, T.; Kamimura, N.; Ohta, S. Effects of molecular hydrogen assessed by an animal model and a randomized clinical study on mild cognitive impairment. Curr. Alzheimer Res. 2017, 15, 482–492. [Google Scholar] [CrossRef]
- Chen, J.; Mu, F.; Lu, T.; Ma, Y.; Du, D.; Xu, K. A gallbladder carcinoma patient with pseudo-progressive remission after hydrogen inhalation. Onco Targets Ther. 2019, 12, 8645–8651. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.R.; Raza, A.; Ahmed, H.; Polizzotti, B.D.; Padera, R.F.; Andrews, N.; Kheir, J. Safety of inhaled hydrogen gas in healthy mice. Med. Gas Res. 2019, 9, 133–138. [Google Scholar]
- Levitt, M.D. Production and excretion of hydrogen gas in man. N. Engl. J. Med. 1969, 281, 122–127. [Google Scholar] [CrossRef]
- Shin, W. Medical applications of breath hydrogen measurements. Anal. Bioanal. Chem. 2014, 406, 3931–3939. [Google Scholar] [CrossRef]
- Aoki, Y. Increased concentrations of breath hydrogen gas in Japanese centenarians. Anti-Aging Med. 2013, 10, 101–105. [Google Scholar]
- Shimouchi, A.; Nose, K.; Shirai, M.; Kondo, T. Estimation of molecular hydrogen consumption in the human whole body after the ingestion of hydrogen-rich water. Adv. Exp. Med. Biol. 2012, 737, 245–250. [Google Scholar] [PubMed]
| Damages/Damage Models | Species/Cells | Effects of H2 | Ref. No. |
|---|---|---|---|
| Cell-free system | •OH is produced by the Fenton reaction and water radiolysis, and it was reduced by H2. | [48] | |
| Cognitive impairment | Rats | Radiation-induced cognitive dysfunction was protected by H2-rich water. | [36] |
| Immune dysfunction | AHH-1 cells | Pretreatment with H2-rich PBS prior to radiation reduced the levels of MDA and 8-OHdG. | [37] |
| AHH-1 cells | Pretreatment with H2-rich saline increased the viability of AHH-1 cells and inhibited apoptosis. | [38] | |
| AHH-1 cells | Pretreatment with H2-rich medium reduced •OH induced by radiation. | [39] | |
| Mice | H2-rich saline protected immunocytes from radiation-induced apoptosis. | [39] | |
| Mice | H2-rich saline protected against radiation-induced immune dysfunction. | [40] | |
| Lung damage | A549 cells | H2-rich PBS suppressed ROS production, and improved oxidative stress and apoptosis markers. | [41] |
| Mice | H2 gas inhibited not only acute lung damage, but also chronic lung damage. | [41] | |
| Myocardial damage | Mice | H2-rich water protected against radiation-induced myocardium damage. | [42] |
| Rats | H2-rich water protected against radiation-induced myocardium damage. | [43] | |
| Gastrointestinal damage | HIEC | H2-rich PBS inhibited apoptosis and increased the cell viability of HIEC. | [37] |
| Mice | H2-rich saline protected against radiation-induced gastrointestinal disorders. | [38] | |
| Mice | H2 water ameliorated radiation-induced gastrointestinal toxicity. | [44] | |
| IEC-6 cells | H2-rich medium improved survival and inhibited ROS production. | [45] | |
| Mice | H2-rich saline improved mouse survival and intestinal mucosal damage and function. | [45] | |
| Hematopoietic cell injury | Mice | H2-rich water ameliorated radiation-induced hematopoietic stem cell injury. | [46] |
| Spermatogenesis and hematopoiesis disorders | Mice | H2-rich saline protected spermatogenesis and hematopoietic functions of irradiated mice. | [47] |
| Testicular damage | Rats | H2-rich saline protected against radiation-induced testicular damage. | [49] |
| Skin damage | HaCaT cells | H2-rich medium protected HaCaT cells from radiation injury by improving the survival rate. | [50] |
| Rats | H2-rich saline reduced the severity of dermatitis, accelerated tissue recovery, and inhibited weight loss. | [50] | |
| Rats | Prior inhalation of H2 gas mitigated radiation-induced skin damage. | [51] | |
| Rats | H2-rich water promoted wound healing in radiation-induced skin lesions. | [52] | |
| Cartilage damage | BMSC | H2-rich medium increased cell viability and differentiation potential. | [53] |
| Rats | H2-rich saline protected against the osteonecrosis of jaw cartilage induced by radiation. | [53] | |
| Thymic lymphoma | Mice | H2-rich saline protected against radiation-induced thymic lymphoma. | [54] |
| Impaired QOL | Humans | H2-rich water improved side effects of poor QOL by radiation therapy. | [23] |
| Bone marrow damage | Humans | H2 gas inhalation protected bone marrow damage in cancer patients receiving IMRT. | [24,25] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, S.-i.; Ichikawa, Y.; Sato, B.; Yamamoto, H.; Takefuji, Y.; Satoh, F. Molecular Hydrogen as a Potential Clinically Applicable Radioprotective Agent. Int. J. Mol. Sci. 2021, 22, 4566. https://doi.org/10.3390/ijms22094566
Hirano S-i, Ichikawa Y, Sato B, Yamamoto H, Takefuji Y, Satoh F. Molecular Hydrogen as a Potential Clinically Applicable Radioprotective Agent. International Journal of Molecular Sciences. 2021; 22(9):4566. https://doi.org/10.3390/ijms22094566
Chicago/Turabian StyleHirano, Shin-ichi, Yusuke Ichikawa, Bunpei Sato, Haru Yamamoto, Yoshiyasu Takefuji, and Fumitake Satoh. 2021. "Molecular Hydrogen as a Potential Clinically Applicable Radioprotective Agent" International Journal of Molecular Sciences 22, no. 9: 4566. https://doi.org/10.3390/ijms22094566
APA StyleHirano, S.-i., Ichikawa, Y., Sato, B., Yamamoto, H., Takefuji, Y., & Satoh, F. (2021). Molecular Hydrogen as a Potential Clinically Applicable Radioprotective Agent. International Journal of Molecular Sciences, 22(9), 4566. https://doi.org/10.3390/ijms22094566








