BMP Signaling Interferes with Optic Chiasm Formation and Retinal Ganglion Cell Pathfinding in Zebrafish
Abstract
1. Introduction
2. Results
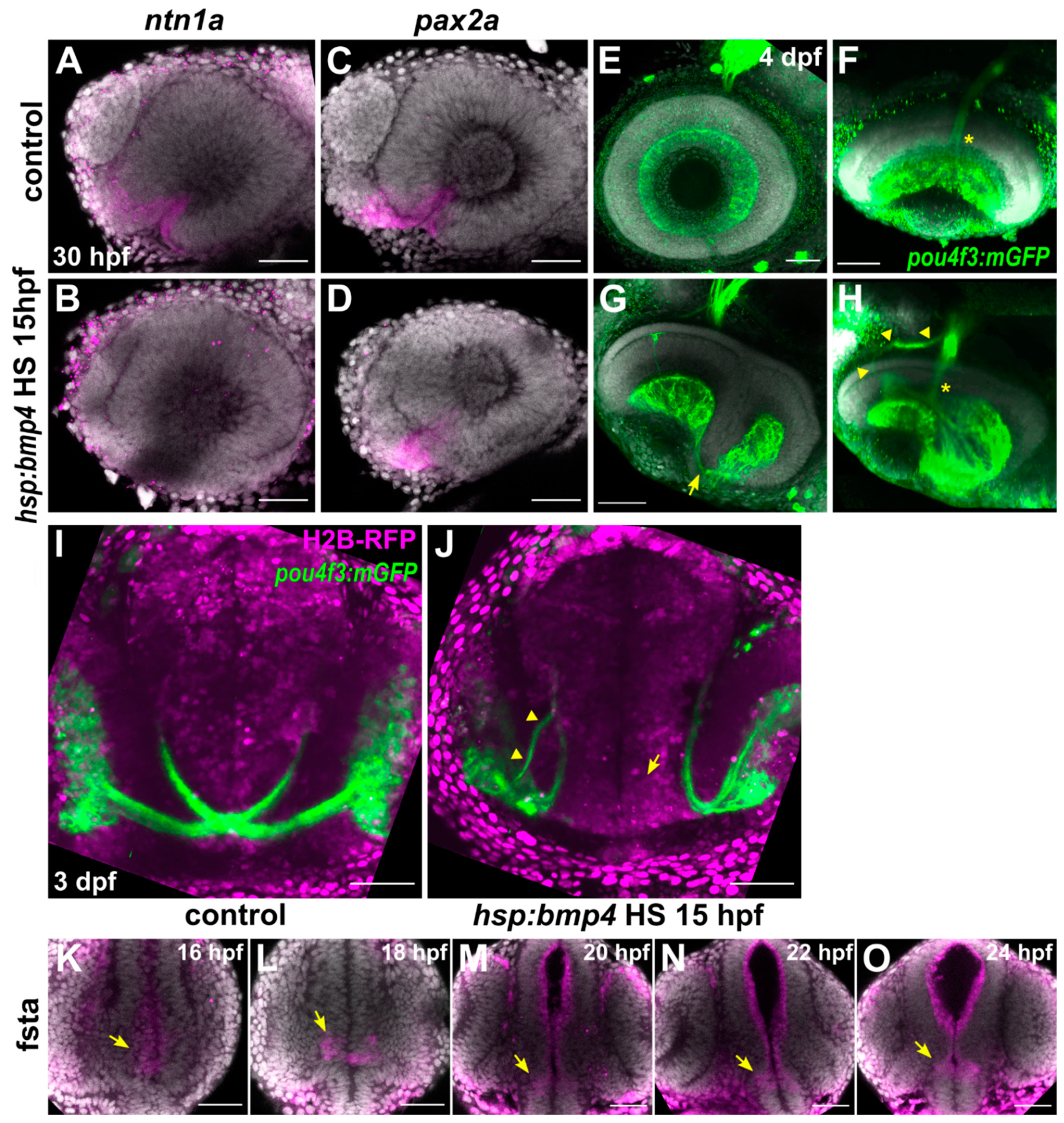
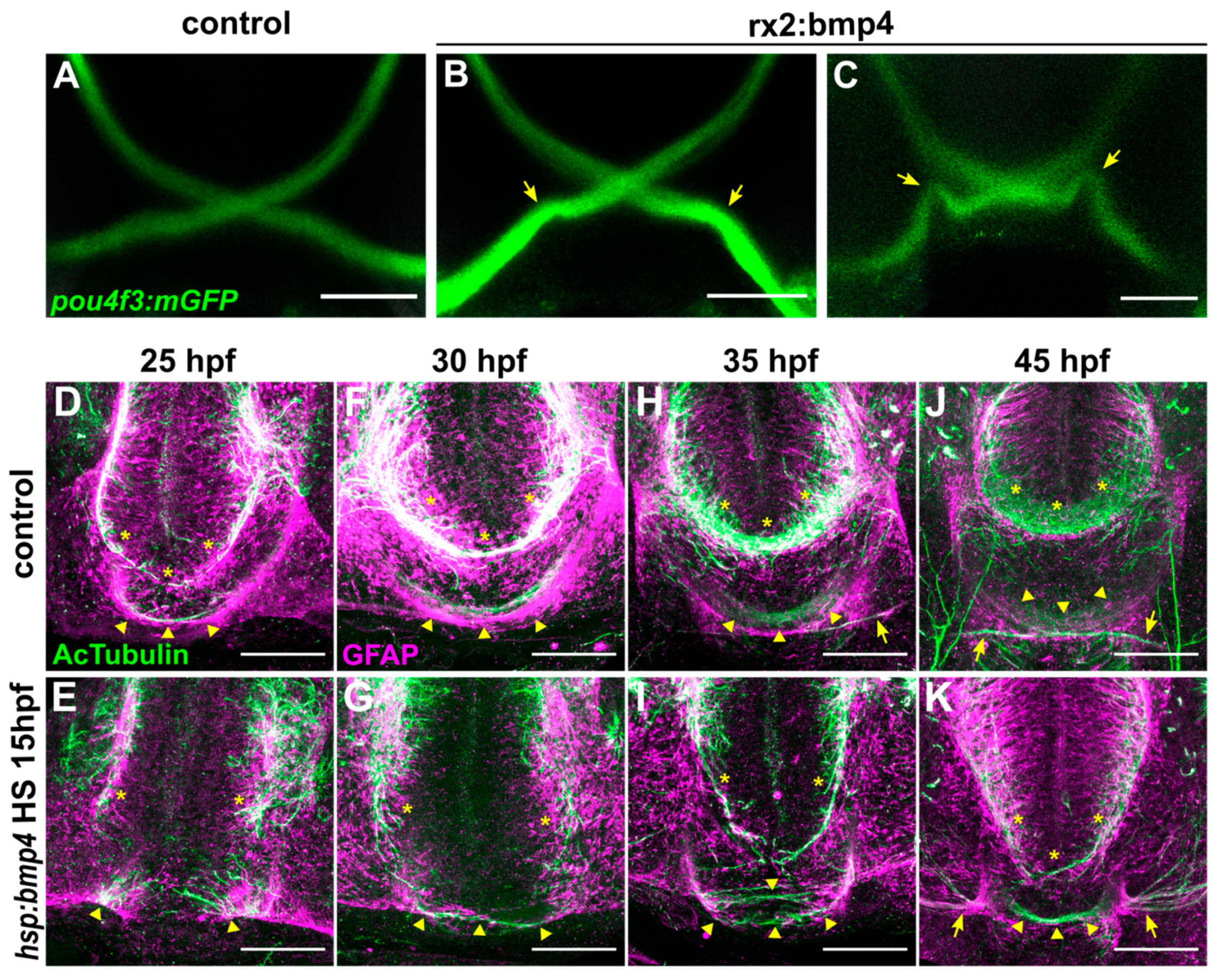
2.1. BMP Signaling-Induced Eye Malformations Do Not Affect Optic Nerve Formation But Disrupt Optic Chiasm Development
2.2. Ipsilateral Retinotectal Projections Are Part of a Larger Midline Defect in bmp4-Overexpressing Embryos
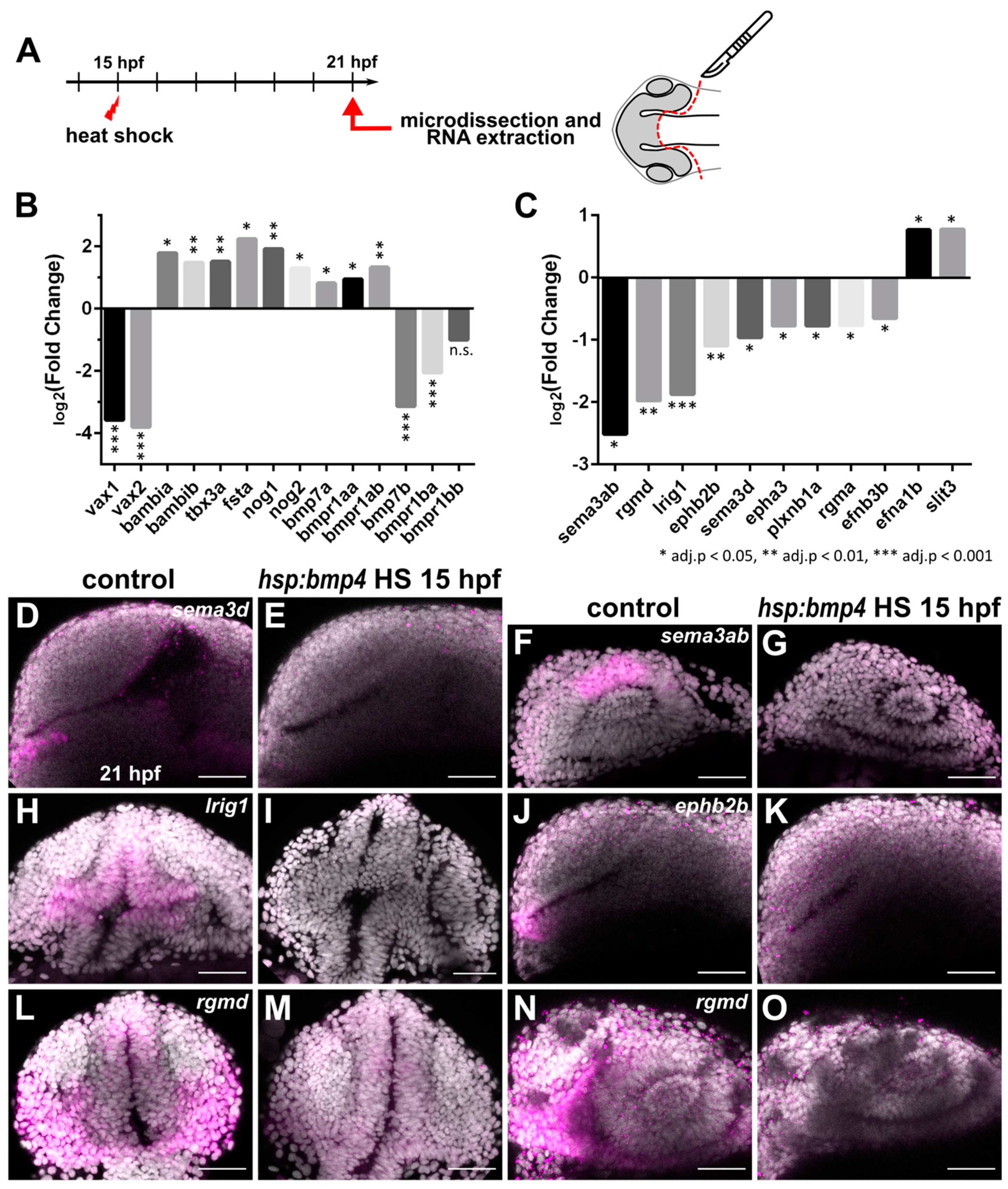
2.3. Transcriptional Analysis after BMP Induction Reveals Misregulation of Numerous Axon Guidance Factors
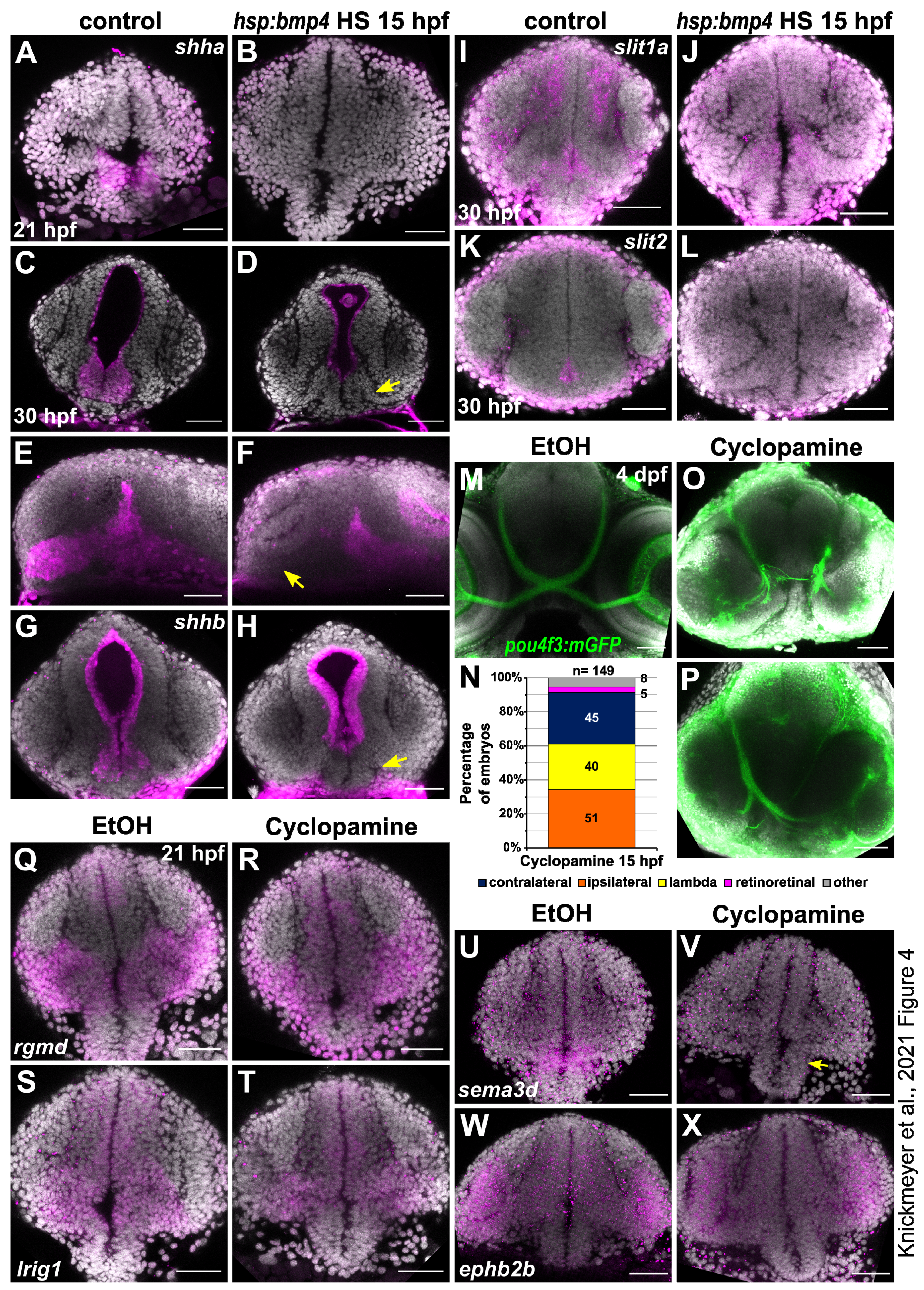
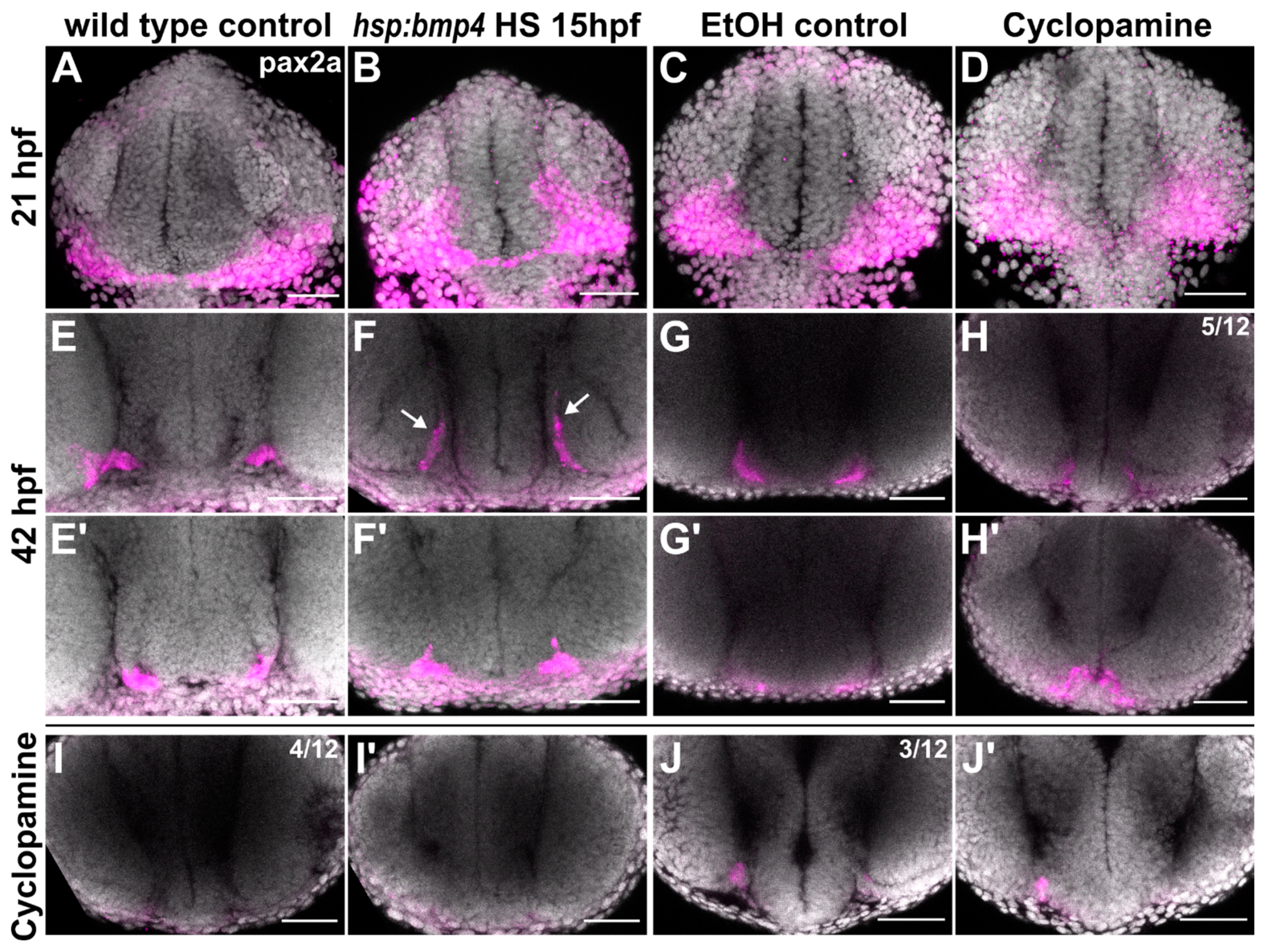
2.4. Sonic Hedgehog Ligand Expression Is Changed after bmp4 Induction
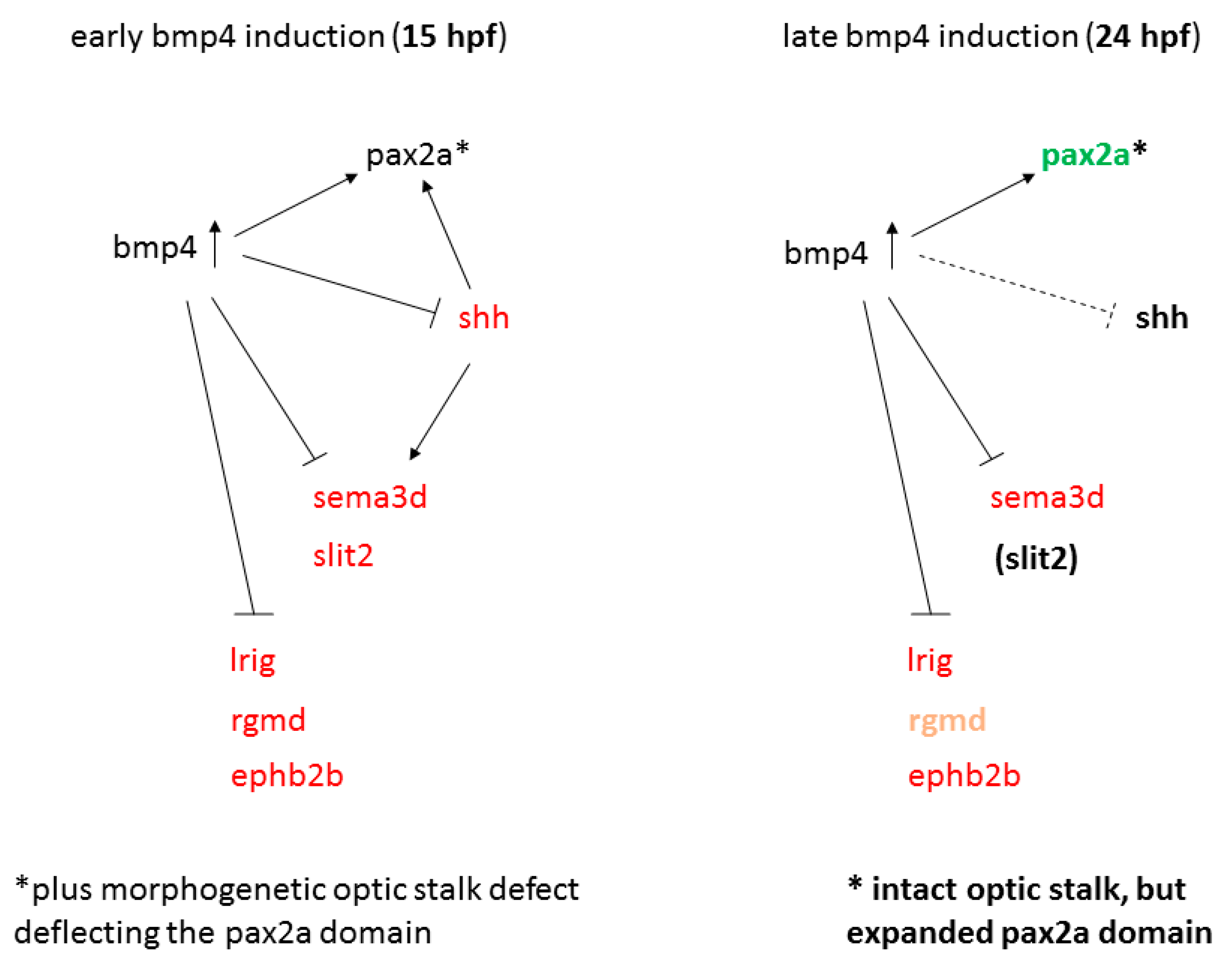
2.5. Late BMP Induction at 24 hpf Results in Different Projection Defects
2.6. RGC Axons Incorrectly Fasciculate with Contralateral Counterparts
3. Discussion
4. Materials and Methods
4.1. Zebrafish Care
4.2. Heat Shock Procedures
4.3. Analysis of Retinotectal Projections
4.4. Confocal Laser Scanning Microscopy
4.5. Image Processing
4.6. In Situ Hybridization
4.7. Microarray Sample Preparation
4.8. Microarray Processing and Analysis
4.9. Lipophilic Tracer Dye Injection
4.10. Immunohistochemistry
- Mouse anti-acetylated α-tubulin (6-11B-1, Millipore, Burlington, MA, USA) 1:200
- Chicken anti-GFP (A10262, Invitrogen, Carlsbad, CA, USA) 1:500
- Rabbit anti-GFAP (Z0334, Agilent Dako, Santa Clara CA, USA) 1:200
- Goat anti-rabbit Alexa Fluor 647 (Cell Signaling Technologies, Danvers, MA, USA) 1:500
- Donkey anti-mouse Alexa Fluor 488 (R37114, ThermoFisher) 1:250
- Goat anti-rabbit Alexa Fluor 555 (A-21428, ThermoFisher) 1:250
- Anti-chicken Alexa Fluor 488 (Invitrogen) 1:500
4.11. Compound Inhibitor Treatment
4.12. Promotor Motif Search
4.13. CRISPR/Cas9 F0 Analysis (Crispants)
4.14. Enucleation of Optic Cups
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walls, G.L. The Vertebrate Eye and Its Adaptive Radiation; Cranbrook Institute of Science: Bloomfield Hills, MI, USA, 1942. [Google Scholar]
- Chow, R.L.; Lang, R.A. Early Eye Development in Vertebrates. Annu. Rev. Cell Dev. Biol. 2001, 17, 255–296. [Google Scholar] [CrossRef]
- Sinn, R.; Wittbrodt, J. An Eye on Eye Development. Mech. Dev. 2013, 130, 347–358. [Google Scholar] [CrossRef]
- Heermann, S.; Schütz, L.; Lemke, S.; Krieglstein, K.; Wittbrodt, J. Eye Morphogenesis Driven by Epithelial Flow into the Optic Cup Facilitated by Modulation of Bone Morphogenetic Protein. eLife 2015, 4, e05216. [Google Scholar] [CrossRef]
- Kwan, K.M.; Otsuna, H.; Kidokoro, H.; Carney, K.R.; Saijoh, Y.; Chien, C.-B. A Complex Choreography of Cell Movements Shapes the Vertebrate Eye. Dev. Camb. Engl. 2012, 139, 359–372. [Google Scholar] [CrossRef]
- Picker, A.; Cavodeassi, F.; Machate, A.; Bernauer, S.; Hans, S.; Abe, G.; Kawakami, K.; Wilson, S.W.; Brand, M. Dynamic Coupling of Pattern Formation and Morphogenesis in the Developing Vertebrate Retina. PLoS Biol. 2009, 7. [Google Scholar] [CrossRef]
- Sidhaye, J.; Norden, C. Concerted Action of Neuroepithelial Basal Shrinkage and Active Epithelial Migration Ensures Efficient Optic Cup Morphogenesis. eLife 2017, 6, e22689. [Google Scholar] [CrossRef] [PubMed]
- Eckert, P.; Knickmeyer, M.D.; Schütz, L.; Wittbrodt, J.; Heermann, S. Morphogenesis and Axis Specification Occur in Parallel during Optic Cup and Optic Fissure Formation, Differentially Modulated by BMP and Wnt. Open Biol. 2019, 9, 180179. [Google Scholar] [CrossRef]
- Gordon, H.B.; Lusk, S.; Carney, K.R.; Wirick, E.O.; Murray, B.F.; Kwan, K.M. Hedgehog Signaling Regulates Cell Motility and Optic Fissure and Stalk Formation during Vertebrate Eye Morphogenesis. Development 2018, 145, dev165068. [Google Scholar] [CrossRef] [PubMed]
- Eckert, P.; Knickmeyer, M.D.; Heermann, S. In Vivo Analysis of Optic Fissure Fusion in Zebrafish: Pioneer Cells, Basal Lamina, Hyaloid Vessels, and How Fissure Fusion Is Affected by BMP. Int. J. Mol. Sci. 2020, 21, 2760. [Google Scholar] [CrossRef]
- Murcia-Belmonte, V.; Erskine, L. Wiring the Binocular Visual Pathways. Int. J. Mol. Sci. 2019, 20, 3282. [Google Scholar] [CrossRef] [PubMed]
- Burrill, J.D.; Easter, S.S. Development of the Retinofugal Projections in the Embryonic and Larval Zebrafish (Brachydanio Rerio). J. Comp. Neurol. 1994, 346, 583–600. [Google Scholar] [CrossRef]
- Deiner, M.S.; Kennedy, T.E.; Fazeli, A.; Serafini, T.; Tessier-Lavigne, M.; Sretavan, D.W. Netrin-1 and DCC Mediate Axon Guidance Locally at the Optic Disc: Loss of Function Leads to Optic Nerve Hypoplasia. Neuron 1997, 19, 575–589. [Google Scholar] [CrossRef]
- Bourikas, D.; Pekarik, V.; Baeriswyl, T.; Grunditz, Å.; Sadhu, R.; Nardó, M.; Stoeckli, E.T. Sonic Hedgehog Guides Commissural Axons along the Longitudinal Axis of the Spinal Cord. Nat. Neurosci. 2005, 8, 297–304. [Google Scholar] [CrossRef]
- Charron, F.; Stein, E.; Jeong, J.; McMahon, A.P.; Tessier-Lavigne, M. The Morphogen Sonic Hedgehog Is an Axonal Chemoattractant That Collaborates with Netrin-1 in Midline Axon Guidance. Cell 2003, 113, 11–23. [Google Scholar] [CrossRef]
- Karlstrom, R.O.; Trowe, T.; Klostermann, S.; Baier, H.; Brand, M.; Crawford, A.D.; Grunewald, B.; Haffter, P.; Hoffmann, H.; Meyer, S.U.; et al. Zebrafish Mutations Affecting Retinotectal Axon Pathfinding. Development 1996, 123, 427–438. [Google Scholar] [CrossRef]
- Karlstrom, R.O.; Talbot, W.S.; Schier, A.F. Comparative Synteny Cloning of Zebrafish You-Too: Mutations in the Hedgehog Target Gli2 Affect Ventral Forebrain Patterning. Genes Dev. 1999, 13, 388–393. [Google Scholar] [CrossRef]
- Karlstrom, R.O.; Tyurina, O.V.; Kawakami, A.; Nishioka, N.; Talbot, W.S.; Sasaki, H.; Schier, A.F. Genetic Analysis of Zebrafish Gli1 and Gli2 Reveals Divergent Requirements for Gli Genes in Vertebrate Development. Development 2003, 130, 1549–1564. [Google Scholar] [CrossRef]
- Torres, M.; Gómez-Pardo, E.; Gruss, P. Pax2 Contributes to Inner Ear Patterning and Optic Nerve Trajectory. Development 1996, 122, 3381–3391. [Google Scholar] [CrossRef]
- Trousse, F.; Martí, E.; Gruss, P.; Torres, M.; Bovolenta, P. Control of Retinal Ganglion Cell Axon Growth: A New Role for Sonic Hedgehog. Development 2001, 128, 3927–3936. [Google Scholar] [CrossRef]
- Macdonald, R.; Scholes, J.; Strähle, U.; Brennan, C.; Holder, N.; Brand, M.; Wilson, S.W. The Pax Protein Noi Is Required for Commissural Axon Pathway Formation in the Rostral Forebrain. Development 1997, 124, 2397–2408. [Google Scholar] [CrossRef]
- Fabre, P.J.; Shimogori, T.; Charron, F. Segregation of Ipsilateral Retinal Ganglion Cell Axons at the Optic Chiasm Requires the Shh Receptor Boc. J. Neurosci. 2010, 30, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camacho, C.; Bovolenta, P. Emerging Mechanisms in Morphogen-Mediated Axon Guidance. BioEssays 2009, 31, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Traiffort, E.; Moya, K.L.; Faure, H.; Hässig, R.; Ruat, M. High Expression and Anterograde Axonal Transport of Aminoterminal Sonic Hedgehog in the Adult Hamster Brain. Eur. J. Neurosci. 2001, 14, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.A.; Raff, M.C. A Role for Sonic Hedgehog in Axon-to-Astrocyte Signalling in the Rodent Optic Nerve. Development 1999, 126, 2901–2909. [Google Scholar] [CrossRef]
- Peng, J.; Fabre, P.J.; Dolique, T.; Swikert, S.M.; Kermasson, L.; Shimogori, T.; Charron, F. Sonic Hedgehog Is a Remotely Produced Cue That Controls Axon Guidance Trans-Axonally at a Midline Choice Point. Neuron 2018, 97, 326–340.e4. [Google Scholar] [CrossRef] [PubMed]
- Dell, A.L.; Fried-Cassorla, E.; Xu, H.; Raper, J.A. CAMP-Induced Expression of Neuropilin1 Promotes Retinal Axon Crossing in the Zebrafish Optic Chiasm. J. Neurosci. 2013, 33, 11076–11088. [Google Scholar] [CrossRef]
- Sakai, J.A.; Halloran, M.C. Semaphorin 3d Guides Laterality of Retinal Ganglion Cell Projections in Zebrafish. Development 2006, 133, 1035–1044. [Google Scholar] [CrossRef][Green Version]
- Hutson, L.D.; Chien, C.-B. Pathfinding and Error Correction by Retinal Axons: The Role of Astray/Robo2. Neuron 2002, 33, 205–217. [Google Scholar] [CrossRef]
- Monnier, P.P.; Sierra, A.; Macchi, P.; Deitinghoff, L.; Andersen, J.S.; Mann, M.; Flad, M.; Hornberger, M.R.; Stahl, B.; Bonhoeffer, F.; et al. RGM Is a Repulsive Guidance Molecule for Retinal Axons. Nature 2002, 419, 392–395. [Google Scholar] [CrossRef]
- Knickmeyer, M.D.; Mateo, J.L.; Eckert, P.; Roussa, E.; Rahhal, B.; Zuniga, A.; Krieglstein, K.; Wittbrodt, J.; Heermann, S. TGFb-Facilitated Optic Fissure Fusion and the Role of Bone Morphogenetic Protein Antagonism. Open Biol. 2018, 8, 170134. [Google Scholar] [CrossRef]
- Xiao, T.; Roeser, T.; Staub, W.; Baier, H. A GFP-Based Genetic Screen Reveals Mutations That Disrupt the Architecture of the Zebrafish Retinotectal Projection. Development 2005, 132, 2955–2967. [Google Scholar] [CrossRef]
- Miyake, A.; Mekata, Y.; Fujibayashi, H.; Nakanishi, K.; Konishi, M.; Itoh, N. Brorin Is Required for Neurogenesis, Gliogenesis, and Commissural Axon Guidance in the Zebrafish Forebrain. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Fürthauer, M.; Thisse, B.; Thisse, C. Three Different Noggin Genes Antagonize the Activity of Bone Morphogenetic Proteins in the Zebrafish Embryo. Dev. Biol. 1999, 214, 181–196. [Google Scholar] [CrossRef]
- French, C.R.; Erickson, T.; French, D.V.; Pilgrim, D.B.; Waskiewicz, A.J. Gdf6a Is Required for the Initiation of Dorsal–Ventral Retinal Patterning and Lens Development. Dev. Biol. 2009, 333, 37–47. [Google Scholar] [CrossRef]
- Veien, E.S.; Rosenthal, J.S.; Kruse-Bend, R.C.; Chien, C.-B.; Dorsky, R.I. Canonical Wnt Signaling Is Required for the Maintenance of Dorsal Retinal Identity. Development 2008, 135, 4101–4111. [Google Scholar] [CrossRef]
- Schmid, B.; Furthauer, M.; Connors, S.A.; Trout, J.; Thisse, B.; Thisse, C.; Mullins, M.C. Equivalent Genetic Roles for Bmp7/Snailhouse and Bmp2b/Swirl in Dorsoventral Pattern Formation. Development 2000, 127, 957–967. [Google Scholar] [CrossRef]
- Miyake, A.; Itoh, N. Fgf22 Regulated by Fgf3/Fgf8 Signaling Is Required for Zebrafish Midbrain Development. Biol. Open 2013, 2, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Collery, R.F.; Link, B.A. Dynamic Smad-Mediated BMP Signaling Revealed through Transgenic Zebrafish. Dev. Dyn. 2011, 240, 712–722. [Google Scholar] [CrossRef]
- Chocron, S.; Verhoeven, M.C.; Rentzsch, F.; Hammerschmidt, M.; Bakkers, J. Zebrafish Bmp4 Regulates Left–Right Asymmetry at Two Distinct Developmental Time Points. Dev. Biol. 2007, 305, 577–588. [Google Scholar] [CrossRef]
- Hao, J.; Ho, J.N.; Lewis, J.A.; Karim, K.A.; Daniels, R.N.; Gentry, P.R.; Hopkins, C.R.; Lindsley, C.W.; Hong, C.C. In Vivo Structure−Activity Relationship Study of Dorsomorphin Analogues Identifies Selective VEGF and BMP Inhibitors. ACS Chem. Biol. 2010, 5, 245–253. [Google Scholar] [CrossRef]
- Barresi, M.J.F.; Hutson, L.D.; Chien, C.-B.; Karlstrom, R.O. Hedgehog Regulated Slit Expression Determines Commissure and Glial Cell Position in the Zebrafish Forebrain. Development 2005, 132, 3643–3656. [Google Scholar] [CrossRef]
- Camus, L.M.; Lambert, L.A. Molecular Evolution of Hemojuvelin and the Repulsive Guidance Molecule Family. J. Mol. Evol. 2007, 65, 68–81. [Google Scholar] [CrossRef] [PubMed]
- van Erp, S.; van den Heuvel, D.M.A.; Fujita, Y.; Robinson, R.A.; Hellemons, A.J.C.G.M.; Adolfs, Y.; Van Battum, E.Y.; Blokhuis, A.M.; Kuijpers, M.; Demmers, J.A.A.; et al. Lrig2 Negatively Regulates Ectodomain Shedding of Axon Guidance Receptors by ADAM Proteases. Dev. Cell 2015, 35, 537–552. [Google Scholar] [CrossRef]
- Panza, P.; Sitko, A.A.; Maischein, H.-M.; Koch, I.; Flötenmeyer, M.; Wright, G.J.; Mandai, K.; Mason, C.A.; Söllner, C. The LRR Receptor Islr2 Is Required for Retinal Axon Routing at the Vertebrate Optic Chiasm. Neural Develop. 2015, 10. [Google Scholar] [CrossRef]
- Wu, R.S.; Lam, I.I.; Clay, H.; Duong, D.N.; Deo, R.C.; Coughlin, S.R. A Rapid Method for Directed Gene Knockout for Screening in G0 Zebrafish. Dev. Cell 2018, 46, 112–125.e4. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Znosko, W.; Tsang, M. The Characterization of a Zebrafish Mid-Hindbrain Mutant, Mid-Hindbrain Gone (Mgo). Dev. Dyn. 2009, 238, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Culverwell, J.; Walkowicz, M.; Toro, S.; Rick, J.M.; Neuhauss, S.C.F.; Varga, Z.M.; Karlstrom, R.O. Belladonna/(Lhx2) Is Required for Neural Patterning and Midline Axon Guidance in the Zebrafish Forebrain. Development 2006, 133, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Willer, J.R.; Willer, G.B.; Smith, K.; Gregg, R.G.; Gross, J.M. Zebrafish Blowout Provides Genetic Evidence for Patched1-Mediated Negative Regulation of Hedgehog Signaling within the Proximal Optic Vesicle of the Vertebrate Eye. Dev. Biol. 2008, 319, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, S.A.; Tyurina, O.V.; Miller, E.; Bagas, A.; Karlstrom, R.O. Brother of Cdo (Umleitung) Is Cell-Autonomously Required for Hedgehog-Mediated Ventral CNS Patterning in the Zebrafish. Dev. Camb. Engl. 2011, 138, 75–85. [Google Scholar] [CrossRef]
- Sekimizu, K.; Nishioka, N.; Sasaki, H.; Takeda, H.; Karlstrom, R.O.; Kawakami, A. The Zebrafish Iguana Locus Encodes Dzip1, a Novel Zinc-Finger Protein Required for Proper Regulation of Hedgehog Signaling. Development 2004, 131, 2521–2532. [Google Scholar] [CrossRef]
- Zhao, L.; Saitsu, H.; Sun, X.; Shiota, K.; Ishibashi, M. Sonic Hedgehog Is Involved in Formation of the Ventral Optic Cup by Limiting Bmp4 Expression to the Dorsal Domain. Mech. Dev. 2010, 127, 62–72. [Google Scholar] [CrossRef]
- Patten, I.; Placzek, M. Opponent Activities of Shh and BMP Signaling during Floor Plate Induction In Vivo. Curr. Biol. 2002, 12, 47–52. [Google Scholar] [CrossRef]
- Pittman, A.J.; Law, M.-Y.; Chien, C.-B. Pathfinding in a Large Vertebrate Axon Tract: Isotypic Interactions Guide Retinotectal Axons at Multiple Choice Points. Development 2008, 135, 2865–2871. [Google Scholar] [CrossRef]
- Xu, H.; Leinwand, S.G.; Dell, A.L.; Fried-Cassorla, E.; Raper, J.A. The Calmodulin-Stimulated Adenylate Cyclase ADCY8 Sets the Sensitivity of Zebrafish Retinal Axons to Midline Repellents and Is Required for Normal Midline Crossing. J. Neurosci. 2010, 30, 7423–7433. [Google Scholar] [CrossRef]
- Liu, Z.-Z.; Zhu, J.; Wang, C.-L.; Wang, X.; Han, Y.-Y.; Liu, L.-Y.; Xu, H.A. CRMP2 and CRMP4 Are Differentially Required for Axon Guidance and Growth in Zebrafish Retinal Neurons. Neural Plast. 2018, 2018. [Google Scholar] [CrossRef]
- Plump, A.S.; Erskine, L.; Sabatier, C.; Brose, K.; Epstein, C.J.; Goodman, C.S.; Mason, C.A.; Tessier-Lavigne, M. Slit1 and Slit2 Cooperate to Prevent Premature Midline Crossing of Retinal Axons in the Mouse Visual System. Neuron 2002, 33, 219–232. [Google Scholar] [CrossRef]
- Rick, J.M.; Horschke, I.; Neuhauss, S.C.F. Optokinetic Behavior Is Reversed in Achiasmatic Mutant Zebrafish Larvae. Curr. Biol. 2000, 10, 595–598. [Google Scholar] [CrossRef]
- Raper, J.; Mason, C. Cellular Strategies of Axonal Pathfinding. Cold Spring Harb. Perspect. Biol. 2010, 2, a001933. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Marquardt, T. What Axons Tell Each Other: Axon–Axon Signaling in Nerve and Circuit Assembly. Curr. Opin. Neurobiol. 2013, 23, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Quiring, R.; Wittbrodt, B.; Henrich, T.; Ramialison, M.; Burgtorf, C.; Lehrach, H.; Wittbrodt, J. Large-Scale Expression Screening by Automated Whole-Mount in Situ Hybridization. Mech. Dev. 2004, 121, 971–976. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Grant, C.E.; Bailey, T.L.; Noble, W.S. FIMO: Scanning for Occurrences of a given Motif. Bioinformatics 2011, 27, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, M.; Thumberger, T.; del Sol Keyer, M.; Wittbrodt, J.; Mateo, J.L. CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PLoS ONE 2015, 10, e012. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knickmeyer, M.D.; Mateo, J.L.; Heermann, S. BMP Signaling Interferes with Optic Chiasm Formation and Retinal Ganglion Cell Pathfinding in Zebrafish. Int. J. Mol. Sci. 2021, 22, 4560. https://doi.org/10.3390/ijms22094560
Knickmeyer MD, Mateo JL, Heermann S. BMP Signaling Interferes with Optic Chiasm Formation and Retinal Ganglion Cell Pathfinding in Zebrafish. International Journal of Molecular Sciences. 2021; 22(9):4560. https://doi.org/10.3390/ijms22094560
Chicago/Turabian StyleKnickmeyer, Max D., Juan L. Mateo, and Stephan Heermann. 2021. "BMP Signaling Interferes with Optic Chiasm Formation and Retinal Ganglion Cell Pathfinding in Zebrafish" International Journal of Molecular Sciences 22, no. 9: 4560. https://doi.org/10.3390/ijms22094560
APA StyleKnickmeyer, M. D., Mateo, J. L., & Heermann, S. (2021). BMP Signaling Interferes with Optic Chiasm Formation and Retinal Ganglion Cell Pathfinding in Zebrafish. International Journal of Molecular Sciences, 22(9), 4560. https://doi.org/10.3390/ijms22094560





