The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches
Abstract
1. Introduction
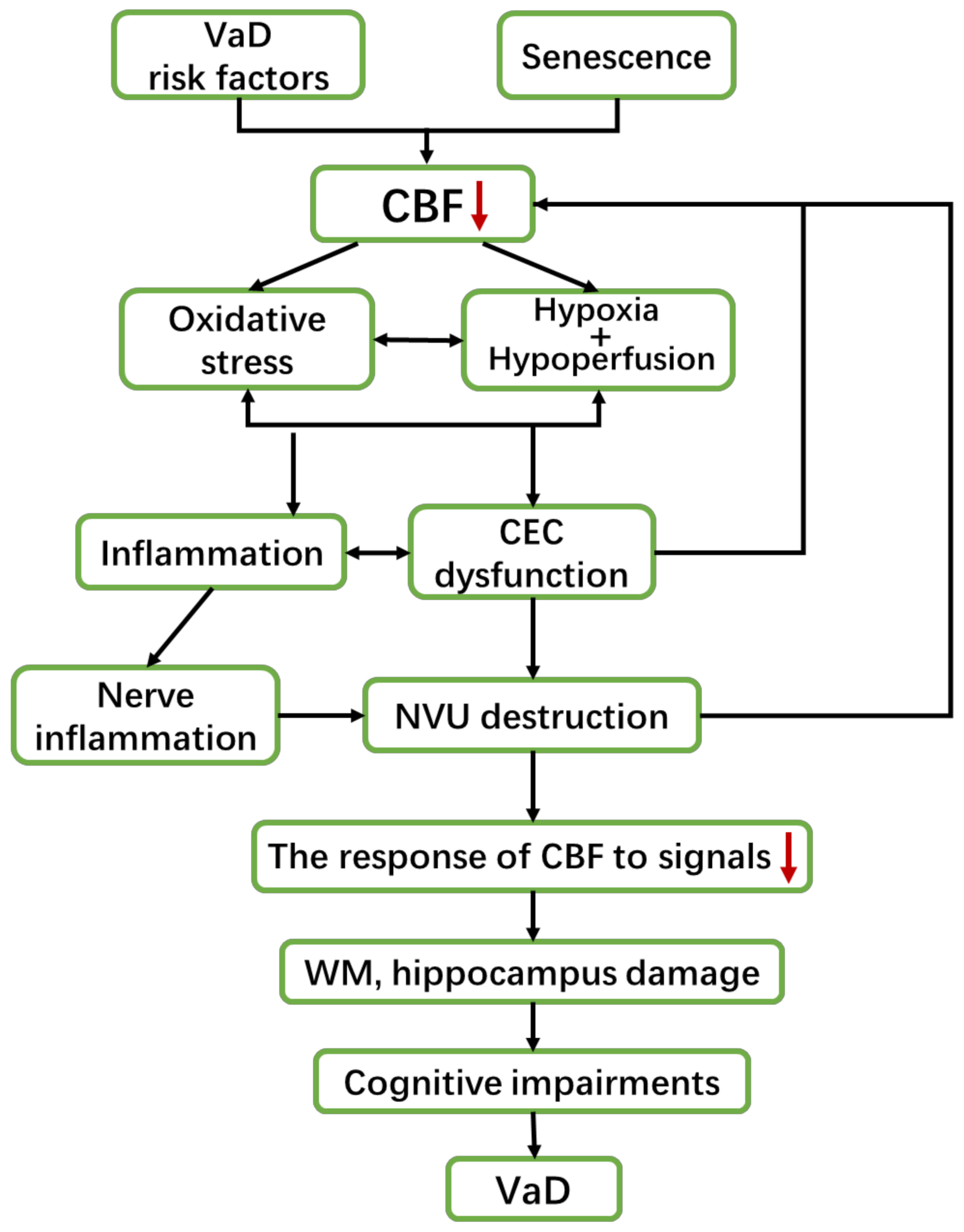
2. The Pathogenesis of VaD
2.1. VaD and Neurovascular Dysfunction (CEC Dysfunction and Nervous Impairment)
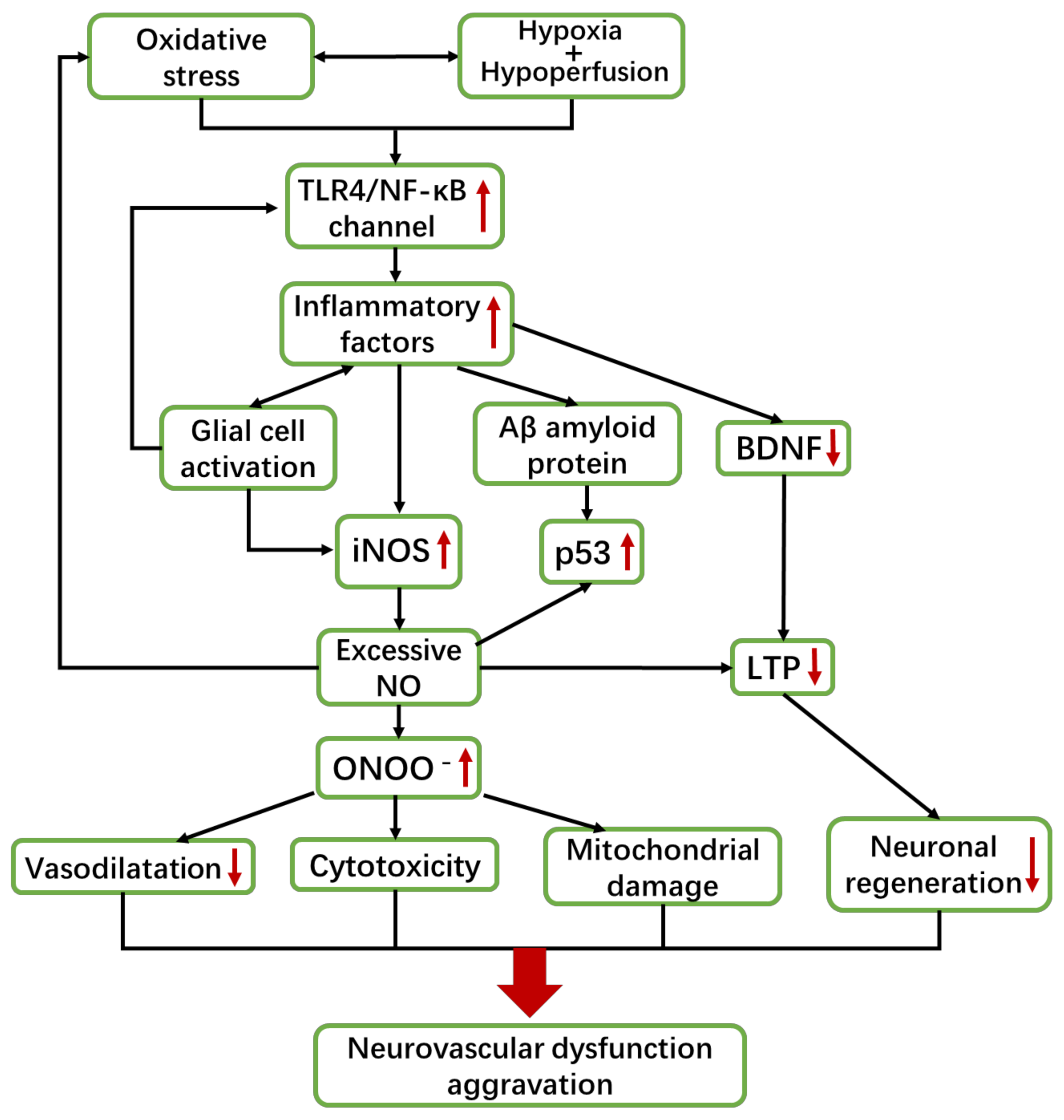
2.2. VaD and Inflammation
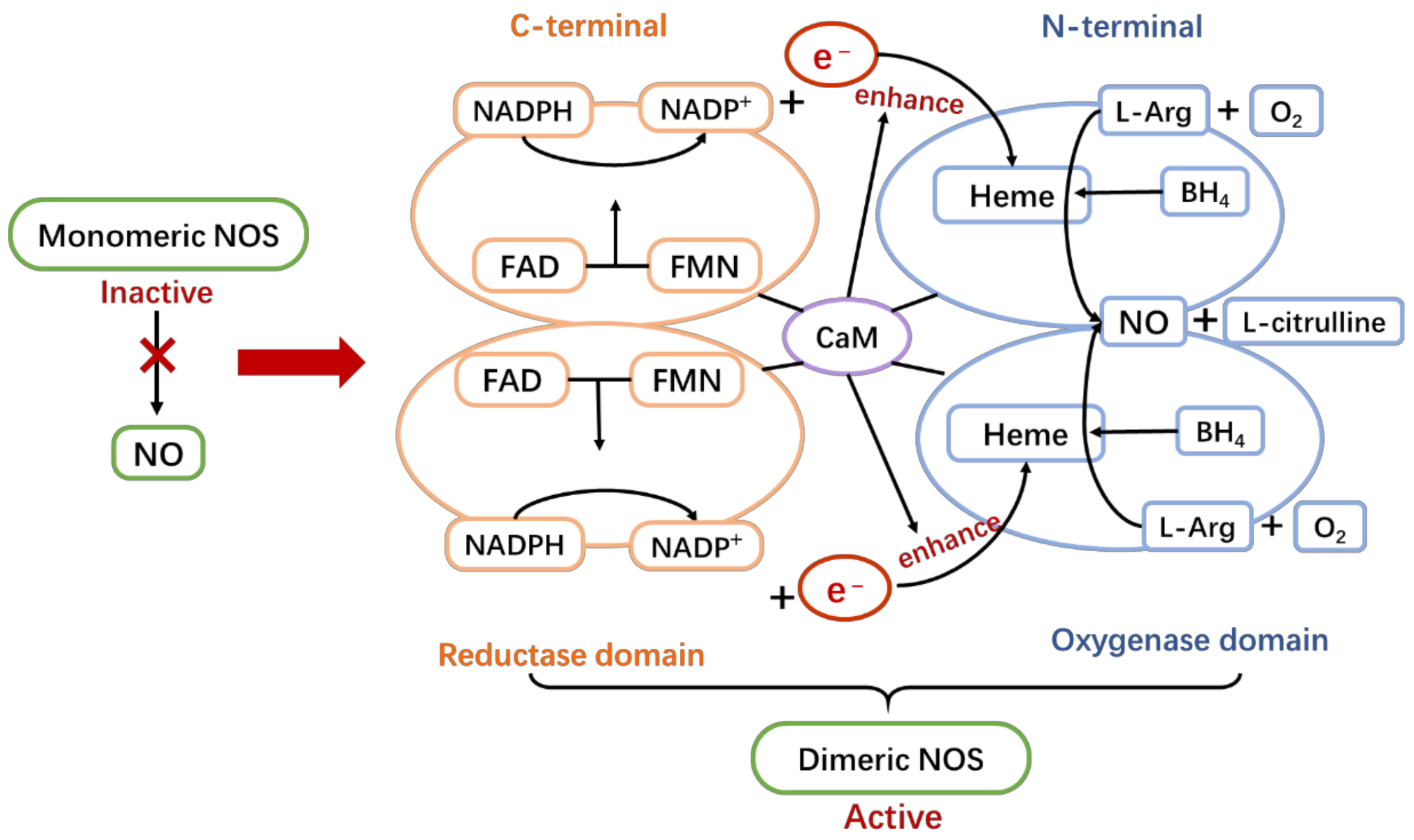
3. NOS/NO Pathway Overview
4. NOS/NO Pathway Plays a Key Role in Various Pathogenesis of VaD
4.1. NOS/NO Pathway Exhibits Dual Functions of Protection and Damage in the Brain
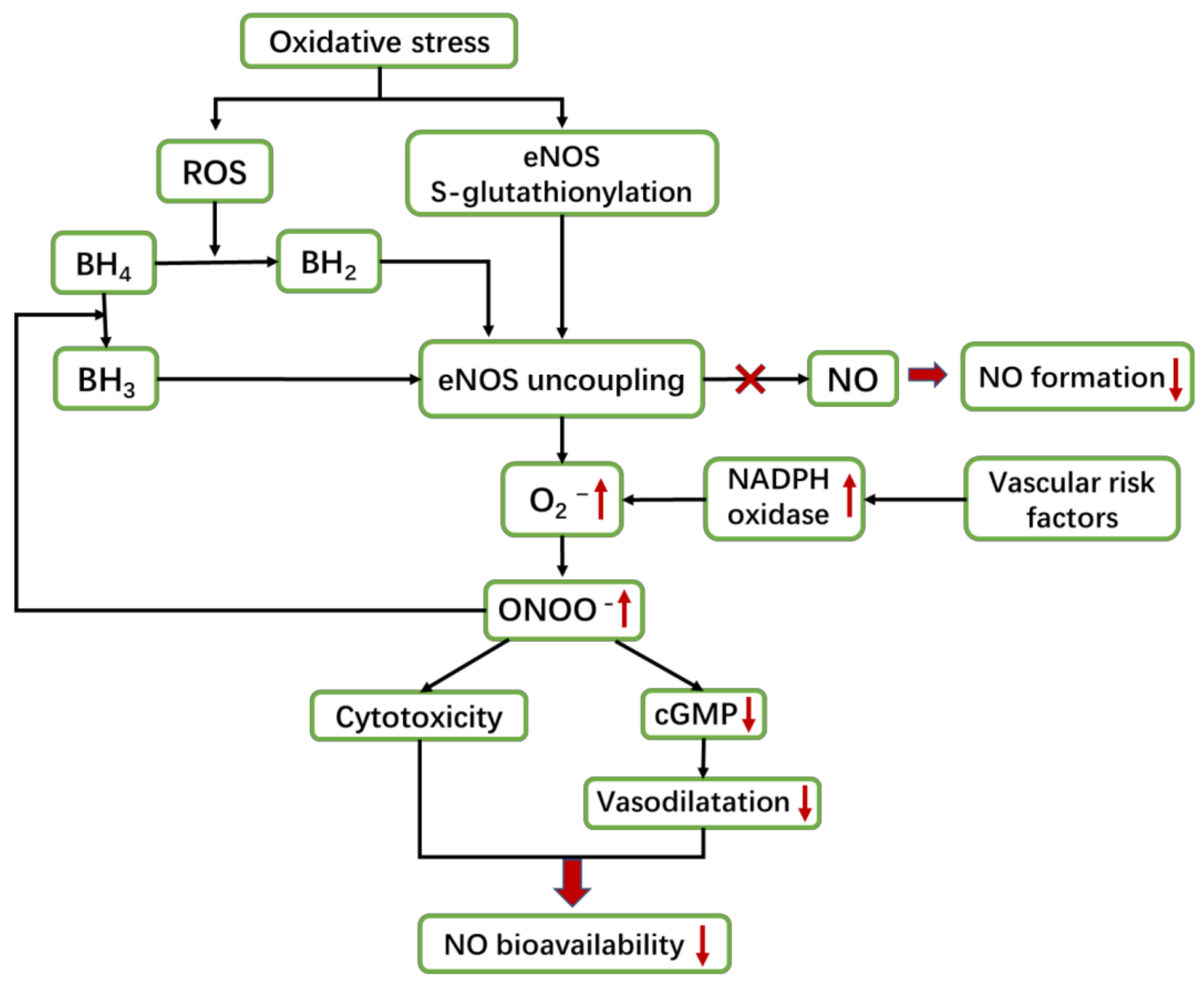
4.2. Oxidative Stress Is a Vital Mechanism of NOS/NO Pathway to Cause VaD
5. NOS/NO Pathway and Neurovascular Dysfunction
5.1. CEC Dysfunction (eNOS-Dominated)
5.1.1. eNOS Uncoupling
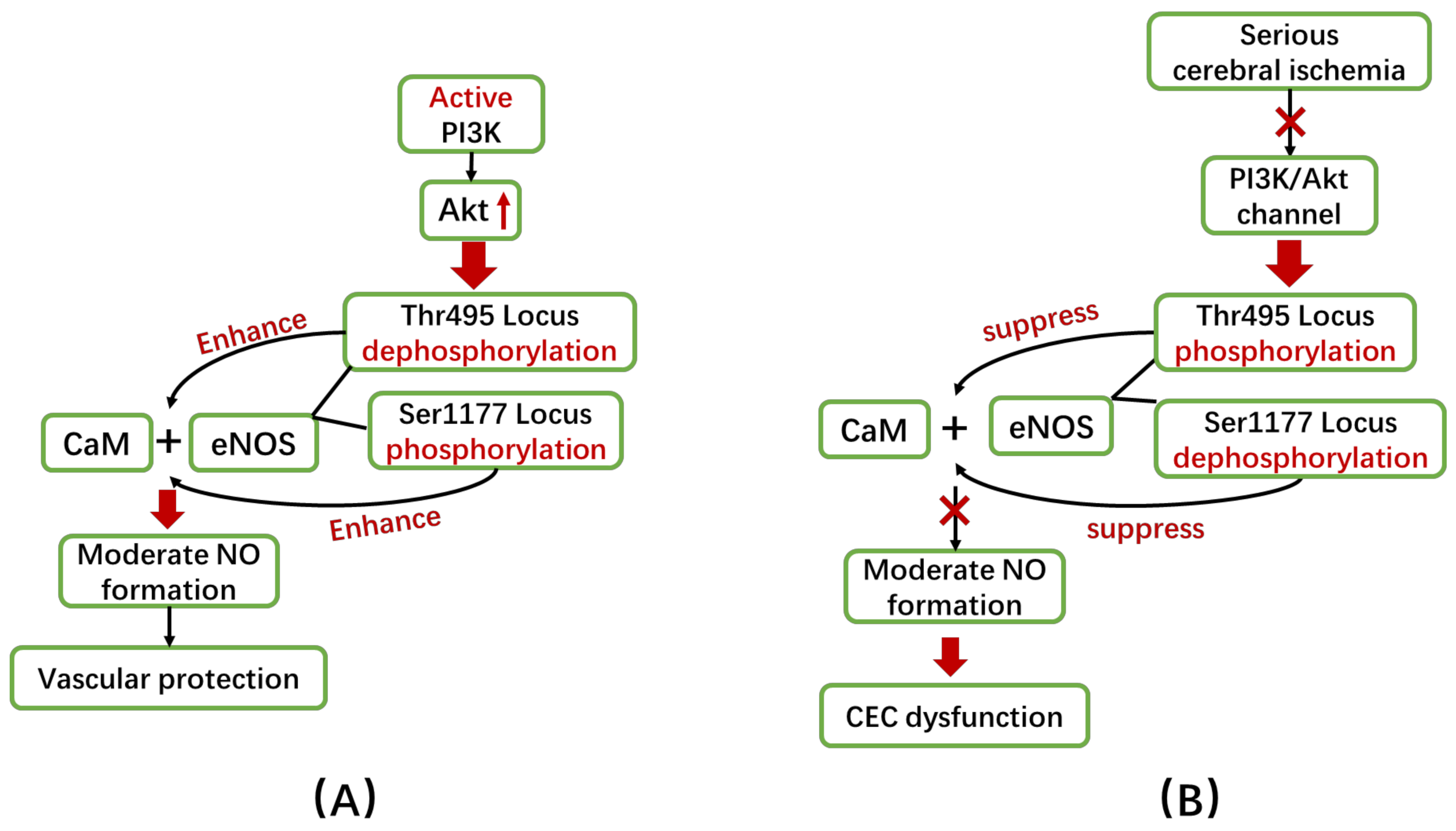
5.1.2. Changes in Phosphorylation of eNOS
5.1.3. eNOS and Angiogenesis
5.2. Nervous Impairment
5.2.1. nNOS and Neurovascular Coupling
5.2.2. eNOS and Neurotrophic Factors
6. NOS/NO Pathway and Inflammation (iNOS-Dominated)
7. NOS/NO Pathway-Involved VaD Therapies
7.1. Physical Exercise
7.2. Statins, Minocycline and NOS Inhibitors
7.3. Substrates and Cofactors Required for NO Synthesis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gulland, A. Global life expectancy increases by five years. BMJ 2016, 353, i2883. [Google Scholar] [CrossRef] [PubMed]
- Turana, Y.; Tengkawan, J.; Chia, Y.C.; Hoshide, S.; Shin, J.; Chen, C.H.; Buranakitjaroen, P.; Nailes, J.; Park, S.; Siddique, S.; et al. Hypertension and Dementia: A comprehensive review from the HOPE Asia Network. J. Clin. Hypertens. 2019, 21, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Wolters, F.J.; Arfan Ikram, M. Epidemiology of Vascular Dementia: Nosology in a Time of Epiomics. Arter. Thromb. Vasc. Biol. 2019, 39, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S. WHO’s global action plan on the public health response to dementia: Some challenges and opportunities. Aging Ment. Health 2020, 24, 197–1999. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Pathobiology of Vascular Dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Rizzi, L.; Rosset, I.; Roriz-Cruz, M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed. Res. Int. 2014, 2014, 908–915. [Google Scholar] [CrossRef]
- Kua, E.H.; Ho, E.; Tan, H.H.; Tsoi, C.; Thng, C.; Mahendran, R. The natural history of dementia. Psychogeriatrics 2014, 14, 196–201. [Google Scholar] [CrossRef]
- Staszewski, J.; Piusińska-Macoch, R.; Brodacki, B.; Skrobowska, E.; Macek, K.; Stępień, A. Vascular parkinsonism and vascular dementia are associated with an increased risk of vascular events or death. Arch. Med. Sci. Atheroscler. Dis. 2017, 2, 16–23. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef]
- Gupta, M.; Dasgupta, A.; Khwaja, G.A.; Chowdhury, D.; Patidar, Y.; Batra, A. Behavioural and psychological symptoms in poststroke vascular cognitive impairment. Behav. Neurol. 2014, 2014. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Attems, J. Challenges of multimorbidity of the aging brain: A critical update. J. Neural. Transm. 2015, 122, 505–521. [Google Scholar] [CrossRef]
- Wimo, A.; Jönsson, L.; Bond, J.; Prince, M.; Winblad, B. The worldwide economic impact of dementia 2010. Alzheimer’s Dement. 2013, 9, 1–11.e3. [Google Scholar] [CrossRef]
- Skrobot, O.A.; Black, S.E.; Chen, C.; DeCarli, C.; Erkinjuntti, T.; Ford, G.A.; Kalaria, R.N.; O’Brien, J.; Pantoni, L.; Pasquier, F.; et al. Progress toward standardized diagnosis of vascular cognitive impairment: Guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimer’s Dement. 2018, 14, 280–292. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Shorter, E. The history of nosology and the rise of the diagnostic and statistical manual of mental disorders. Dialogues Clin. Neurosci. 2015, 17, 59–67. [Google Scholar] [PubMed]
- Sachdev, P.; Kalaria, R.; O’Brien, J.; Skoog, I.; Alladi, S.; Black, S.E.; Blacker, D.; Blazer, D.; Chen, C.; Chui, H.; et al. Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Dis. Assoc. Disord. 2014, 28, 206–218. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Pani, A.; Ricci, M.; Locuratolo, N.; Fattapposta, F.; De Vincentis, G. Neuroimaging in Vascular Cognitive Impairment and Dementia: A Systematic Review. J. Alzheimer’s Dis. 2020, 73, 1279–1294. [Google Scholar] [CrossRef]
- Azarpazhooh, M.R.; Avan, A.; Cipriano, L.E.; Munoz, D.G.; Sposato, L.A.; Hachinski, V. Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimer’s Dement. 2018, 14, 148–156. [Google Scholar] [CrossRef]
- Venkat, P.; Chopp, M.; Chen, J. Models and mechanisms of vascular dementia. Exp. Neurol. 2015, 272, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front. Aging Neurosci. 2013, 5, 1–19. [Google Scholar] [CrossRef]
- Kalaria, R.N. The pathology and pathophysiology of vascular dementia. Neuropharmacology 2018, 134, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.T.; Iadecola, C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007, 102, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Barber, P.A. Dementia risk and prevention by targeting modifiable vascular risk factors. J. Neurochem. 2018, 44, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Aronow, W.S. Hypertension and cognitive impairment. Ann. Transl. Med. 2017, 5, 5–7. [Google Scholar] [CrossRef]
- Mok, V.C.T.; Lam, B.Y.K.; Wong, A.; Ko, H.; Markus, H.S.; Wong, L.K.S. Early-onset and delayed-onset poststroke dementia-revisiting the mechanisms. Nat. Rev. Neurol. 2017, 13, 148–159. [Google Scholar] [CrossRef]
- Chen, J.; Cui, X.; Zacharek, A.; Cui, Y.; Roberts, C.; Chopp, M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke 2011, 42, 445–452. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W.S. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Giuffrida Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharm. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 1–11. [Google Scholar] [CrossRef]
- Zhu, J.; Song, W.; Li, L.; Fan, X. Endothelial nitric oxide synthase: A potential therapeutic target for cerebrovascular diseases. Mol. Brain 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Shu, X.; Keller, T.C.S.; Begandt, D.; Butcher, J.T.; Biwer, L.; Keller, A.S.; Columbus, L.; Isakson, B.E. Endothelial nitric oxide synthase in the microcirculation. Cell Mol. Life Sci. 2015, 72, 4561–4575. [Google Scholar] [CrossRef]
- Ally, A.; Powell, I.; Ally, M.M.; Chaitoff, K.; Nauli, S.M. Role of neuronal nitric oxide synthase on cardiovascular functions in physiological and pathophysiological states. Nitric Oxide Biol. Chem. 2020, 102, 52–73. [Google Scholar] [CrossRef]
- Lee, J.; Bae, E.H.; Ma, S.K.; Kim, S.W. Altered Nitric Oxide System in Cardiovascular and Renal Diseases. Chonnam Med. J. 2016, 52, 81. [Google Scholar] [CrossRef]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible nitric oxide synthase: Good or bad? Biomed. Pharm. 2017, 93, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Chen, H.J.C.; Bourgognon, J.M.; Steinert, J.R. Dysregulation of stress systems and nitric oxide signaling underlies neuronal dysfunction in Alzheimer’s disease. Free Radic. Biol. Med. 2019, 134, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Narne, P.; Pandey, V.; Phanithi, P.B. Role of Nitric Oxide and Hydrogen Sulfide in Ischemic Stroke and the Emergent Epigenetic Underpinnings. Mol. Neurobiol. 2019, 56, 1749–1769. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Buckley, J.S.; Salpeter, S.R. A Risk-Benefit Assessment of Dementia Medications: Systematic Review of the Evidence. Drugs Aging 2015, 32, 453–467. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fujishiro, H.; Takechi, H. Efficacy and Safety of Cholinesterase Inhibitors for Mild Cognitive Impairment:A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2019, 71, 513–523. [Google Scholar] [CrossRef]
- Witter, D.; McCord, M.; Suryadevara, U. Delirium associated with memantine use in a patient with vascular dementia. J. Clin. Psychopharmacol. 2015, 35, 736–738. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Sullivan, N.; Esiri, M.M. White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke 2001, 32, 1162–1167. [Google Scholar] [CrossRef]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit—concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Buga, A.M.; Popescu, B.; Muresanu, D. Vascular cognitive impairment, dementia, aging and energy demand. A vicious cycle. J. Neural Transm. 2015, 122, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Dormanns, K.; Brown, R.G.; David, T. The role of nitric oxide in neurovascular coupling. J. Theor. Biol. 2016, 394, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Andreone, B.J.; Lacoste, B.; Gu, C. Neuronal and Vascular Interactions. Annu. Rev. Neurosci. 2015, 38, 25–46. [Google Scholar] [CrossRef]
- Graves, S.I.; Baker, D.J. Implicating endothelial cell senescence to dysfunction in the ageing and diseased brain. Basic Clin. Pharmacol. Toxicol. 2020, 127, 102–110. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Carresi, C.; Scarano, F.; Bosco, F.; Nucera, S.; Ruga, S.; Zito, M.C.; et al. The “frail” brain blood barrier in neurodegenerative diseases: Role of early disruption of endothelial cell-to-cell connections. Int. J. Mol. Sci. 2018, 19, 2693. [Google Scholar] [CrossRef]
- Doulias, P.T.; Tenopoulou, M. Endothelial nitric oxide synthase-derived nitric oxide in the regulation of metabolism. F1000Research 2020, 9, 1–10. [Google Scholar]
- Wang, F.; Cao, Y.; Ma, L.; Pei, H.; Rausch, W.D.; Li, H. Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia. Front. Aging Neurosci. 2018, 10, 1–23. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Ledo, A.; Caetano, M.; Barbosa, R.M.; Laranjinha, J. Age-dependent impairment of neurovascular and neurometabolic coupling in the hippocampus. Front. Physiol. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Rouhl, R.P.W.; Damoiseaux, J.G.M.C.; Lodder, J.; Theunissen, R.O.M.F.I.H.; Knottnerus, I.L.H.; Staals, J.; Henskens, L.H.; Kroon, A.A.; de Leeuw, P.W.; Tervaert, J.W.C.; et al. Vascular inflammation in cerebral small vessel disease. Neurobiol. Aging 2012, 33, 1800–1806. [Google Scholar] [CrossRef]
- Baker, D.J.; Petersen, R.C. Cellular senescence in brain aging and neurodegenerative diseases: Evidence and perspectives. J. Clin. Investig. 2018, 128, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Elahy, M.; Jackaman, C.; Mamo, J.C.L.; Lam, V.; Dhaliwal, S.S.; Giles, C.; Nelson, D.; Takechi, R. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun. Ageing 2015, 12, 1–9. [Google Scholar] [CrossRef]
- Back, S.A.; Kroenke, C.D.; Sherman, L.S.; Lawrence, G.; Gong, X.; Taber, E.N.; Sonnen, J.A.; Larson, E.B.; Montine, T.J. White matter lesions defined by diffusion tensor imaging in older adults. Ann. Neurol. 2011, 70, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Hase, Y.; Horsburgh, K.; Ihara, M.; Kalaria, R.N. White matter degeneration in vascular and other ageing-related dementias. J. Neurochem. 2018, 144, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Niizuma, K.; Rashad, S.; Sumiyoshi, A.; Ryoke, R.; Endo, H.; Endo, T.; Sato, K.; Kawashima, R.; Tominaga, T.; et al. A refined model of chronic cerebral hypoperfusion resulting in cognitive impairment and a low mortality rate in rats. J. Neurosurg. 2019, 131, 892–902. [Google Scholar] [CrossRef]
- Al Ahmad, A.; Gassmann, M.; Ogunshola, O.O. Involvement of oxidative stress in hypoxia-induced blood-brain barrier breakdown. Microvasc. Res. 2012, 84, 222–225. [Google Scholar] [CrossRef]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef]
- Li, A.; Lv, S.; Yu, Z.; Zhang, Y.; Ma, H.; Zhao, H.; Piao, H.; Li, S.; Zhang, N.; Sun, C.; et al. Simvastatin attenuates hypomyelination induced by hypoxia-ischemia in neonatal rats. Neurol. Res. 2010, 32, 945–952. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A novel anti-inflammatory role of omega-3 PUFAs in prevention and treatment of atherosclerosis and vascular cognitive impairment and dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef] [PubMed]
- Du, S.Q.; Wang, X.R.; Zhu, W.; Ye, Y.; Yang, J.W.; Ma, S.M.; Ji, C.S.; Liu, C.Z. Acupuncture inhibits TXNIP-associated oxidative stress and inflammation to attenuate cognitive impairment in vascular dementia rats. CNS Neurosci. Ther. 2018, 24, 39–46. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Michel, T.; Vanhoutte, P.M. Cellular signaling and NO production. Pflugers Arch. Eur. J. Physiol. 2010, 459, 807–816. [Google Scholar] [CrossRef]
- Daff, S. NO synthase: Structures and mechanisms. Nitric Oxide Biol. Chem. 2010, 23, 1–11. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Fleming, I.; Busse, R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.P.; Schmidt, P.M.; Nedvetsky, P.I.; Nedvetskaya, T.Y.; Arun Kumar, H.S.; Meurer, S.; Deile, M.; Taye, A.; Knorr, A.; Lapp, H.; et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J. Clin. Investig. 2006, 116, 2552–2561. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Casadei, B. Sub-cellular targeting of constitutive NOS in health and disease. J. Mol. Cell Cardiol. 2012, 52, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Regulation of lymphocytes by nitric oxide. Methods Mol. Biol. 2011, 677, 375–393. [Google Scholar] [PubMed]
- Cau, S.B.A.; Carneiro, F.S.; Tostes, R.C. Differential modulation of nitric oxide synthases in aging: Therapeutic opportunities. Front. Physiol. 2012, 3, 1–11. [Google Scholar] [CrossRef]
- Dawson, T.M.; Dawson, V.L. Nitric Oxide Signaling in Neurodegeneration and Cell Death. Adv. Pharmacol. 2018, 82, 57–83. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Ledo, A.; Barbosa, R.M.; Laranjinha, J. Neurovascular-neuroenergetic coupling axis in the brain: Master regulation by nitric oxide and consequences in aging and neurodegeneration. Free Radic. Biol. Med. 2017, 108, 668–682. [Google Scholar] [CrossRef]
- Zaciragic, A. New Insights into Possible Role of NOS-NO-ADMA Pathway Dysfunction in the Development of Cognitive Decline and Dementia: Exploring the Vascular Features of Alzheimer’s Disease. Int. J. Neurol. Res. 2015, 1, 191–195. [Google Scholar] [CrossRef][Green Version]
- Tohgi, H.; Abe, T.; Yamazaki, K.; Murata, T.; Isobe, C.; Ishizaki, E. The cerebrospinal fluid oxidized NO metabolites, nitrite and nitrate, in Alzheimer’s disease and vascular dementia of Binswanger type and multiple small infarct type. J. Neural. Transm. 1998, 105, 1283–1291. [Google Scholar] [CrossRef]
- Tan, X.L.; Xue, Y.Q.; Ma, T.; Wang, X.; Li, J.J.; Lan, L.; Malik, K.U.; McDonald, M.P.; Dopico, A.M.; Liao, F.-F. Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Mol. Neurodegener. 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Willmot, M.; Gray, L.; Gibson, C.; Murphy, S.; Bath, P.M.W. A systematic review of nitric oxide donors and L-arginine in experimental stroke; effects on infarct size and cerebral blood flow. Nitric Oxide Biol. Chem. 2005, 12, 141–149. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, P.L.; Ma, J.; Meng, W.; Ayata, C.; Fishman, M.C.; Moskowitz, M.A. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J. Cereb. Blood Flow Metab. 1996, 16, 981–987. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, P.L.; Panahian, N.; Dalkara, T.; Fishman, M.C.; Moskowitz, M.A. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 1994, 265, 1883–1885. [Google Scholar] [CrossRef]
- Iadecola, C.; Zhang, F.; Casey, R.; Nagayama, M.; Ross, M.E. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 1997, 17, 9157–9164. [Google Scholar] [CrossRef] [PubMed]
- Zarkovic, N. Roles and Functions of ROS and RNS in Cellular Physiology and Pathology. Cells 2020, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Grant, M.M.; Aldred, S. Oxidative stress in vascular dementia and alzheimer’s disease: A common pathology. J. Alzheimer’s Dis. 2009, 17, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef]
- Li, P.; Stetler, R.A.; Leak, R.K.; Shi, Y.; Li, Y.; Yu, W.; Bennett, M.V.; Chen, J. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology 2018, 134, 13. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Sheen, J.M.; Hu, W.L.; Hung, Y.C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, Y.; Wang, G.; Mao, L.; Zhang, D.; Wang, J. Effects of resveratrol on learning and memory in rats with vascular dementia. Mol. Med. Rep. 2019, 20, 4587–4593. [Google Scholar] [CrossRef]
- Gocmez, S.S.; Şahin, T.D.; Yazir, Y.; Duruksu, G.; Eraldemir, F.C.; Polat, S.; Utikan, T. Resveratrol prevents cognitive deficits by attenuating oxidative damage and inflammation in rat model of streptozotocin diabetes induced vascular dementia. Physiol. Behav. 2019, 201, 198–207. [Google Scholar] [CrossRef]
- Casado, Á.; Encarnación López-Fernández, M.; Concepción Casado, M.; De La Torre, R. Lipid peroxidation and antioxidant enzyme activities in vascular and alzheimer dementias. Neurochem. Res. 2008, 33, 450–458. [Google Scholar] [CrossRef]
- Gackowski, D.; Rozalski, R.; Siomek, A.; Dziaman, T.; Nicpon, K.; Klimarczyk, M.; Araszkiewicz, A.; Olinski, R. Oxidative stress and oxidative DNA damage is characteristic for mixed Alzheimer disease/vascular dementia. J. Neurol. Sci. 2008, 266, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wolin, M.S. Reactive oxygen species and the control of vascular function. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H539–H549. [Google Scholar] [CrossRef]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bhayadia, R.; Schmidt, B.M.W.; Melk, A.; Hömme, M. Senescence-Induced Oxidative Stress Causes Endothelial Dysfunction. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Nitric oxide and neuronal death. Nitric Oxide Biol. Chem. 2010, 23, 153–165. [Google Scholar] [CrossRef]
- Atochin, D.N.; Huang, P.L. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflugers Arch. Eur. J. Physiol. 2010, 460, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y. The multiple actions of NO. Pflugers Arch. Eur. J. Physiol. 2010, 459, 829–839. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Katusic, Z.S.; Austin, S.A. Neurovascular protective function of endothelial nitric oxide—Recent advances. Circ. J. 2016, 80, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Katusic, Z.S.; Austin, S.A. Endothelial nitric oxide: Protector of a healthy mind. Eur. Heart J. 2014, 35, 888–894. [Google Scholar] [CrossRef]
- Fujita, Y.; Ihara, M.; Ushiki, T.; Hirai, H.; Kizaka-Kondoh, S.; Hiraoka, M.; Ito, H.; Takahashi, R. Early protective effect of bone marrow mononuclear cells against ischemic white matter damage through augmentation of cerebral blood flow. Stroke 2010, 41, 2938–2943. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. Eur. J. Physiol. 2010, 459, 923–939. [Google Scholar] [CrossRef]
- Yang, Y.M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, 1829–1836. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A.; Ullrich, V.; Mülsch, A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the CGMP-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Wang, T.Y.; Varadharaj, S.; Reyes, L.A.; Hemann, C.; Talukder, M.A.H.; Chen, Y.R.; Druhan, L.J.; Zweier, J.L. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 2010, 468, 1115–1118. [Google Scholar] [CrossRef]
- Zweier, J.L.; Chen, C.A.; Druhan, L.J. S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid. Redox Signal. 2011, 14, 1769–1775. [Google Scholar] [CrossRef]
- Chang, F.; Flavahan, S.; Flavahan, N.A. Potential pitfalls in analyzing structural uncoupling of enos: Aging is not associated with increased enzyme monomerization. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H80–H88. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- McMillan, K.; Adler, M.; Auld, D.S.; Baldwin, J.J.; Blasko, E.; Browne, L.J.; Chelsky, D.; Davey, D.; Dolle, R.E.; Eagen, K.A.; et al. Allosteric inhibitors of inducible nitric oxide synthase dimerization discovered via combinatorial chemistry. Proc. Natl. Acad. Sci. USA 2000, 97, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Dessy, C.; Feron, O.; Balligand, J.L. The regulation of endothelial nitric oxide synthase by caveolin: A paradigm validated in vivo and shared by the “endothelium-derived hyperpolarizing factor. Pflugers Arch. Eur. J. Physiol. 2010, 459, 817–827. [Google Scholar] [CrossRef]
- Bir, S.C.; Xiong, Y.; Kevil, C.G.; Luo, J. Emerging role of PKA/eNOS pathway in therapeutic angiogenesis for ischaemic tissue diseases. Cardiovasc. Res. 2012, 95, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.T.; Sullivan, J.C.; Spradley, F.T.; d’Uscio, L.V.; Katusic, Z.S.; Pollock, J.S. Antihypertensive therapy increases tetrahydrobiopterin levels and NO/cGMP signaling in small arteries of angiotensin II-infused hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, 718–724. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Siamwala, J.H.; Chatterjee, S. ENOS phosphorylation in health and disease. Biochimie 2010, 92, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Gielis, J.F.; Lin, J.Y.; Wingler, K.; Van Schil, P.E.Y.; Schmidt, H.H.; Moens, A.L. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free Radic. Biol. Med. 2011, 50, 765–776. [Google Scholar] [CrossRef]
- Yagita, Y.; Kitagawa, K.; Oyama, N.; Yukami, T.; Watanabe, A.; Sasaki, T.; Mochizuki, H. Functional deterioration of endothelial nitric oxide synthase after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2013, 33, 1532–1539. [Google Scholar] [CrossRef]
- Natarajan, M.; Konopinski, R.; Krishnan, M.; Roman, L.; Bera, A.; Hongying, Z.; Habib, S.L.; Mohan, S. Inhibitor-κB kinase attenuates HSP90-Dependent endothelial nitric oxide synthase function in vascular endothelial cells. Am. J. Physiol. Cell Physiol. 2015, 308, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.R.; Liu, L. HDL slowing down endothelial progenitor cells senescence: A novel anti-atherogenic property of HDL. Med. Hypotheses 2008, 70, 338–342. [Google Scholar] [CrossRef]
- Atochin, D.N.; Wang, A.; Liu, V.W.T.; Critchlow, J.D.; Dantas, A.P.V.; Looft-Wilson, R.; Murata, T.; Salomone, S.; Shin, H.K.; Ayata, C.; et al. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J. Clin. Investig. 2007, 117, 1961–1967. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, S. Beneficial Effect of Protein Tyrosine Phosphatase Inhibitor and Phytoestrogen in Dyslipidemia-Induced Vascular Dementia in Ovariectomized Rats. J. Stroke Cerebrovasc. Dis. 2015, 24, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Geary, G.G.; Mcneill, A.M.; Ospina, J.A.; Krause, D.N.; Korach, K.S.; Duckles, S.P. Genome and hormones: Gender differences in physiology selected contribution: Cerebrovascular NOS and cyclooxygenase are unaffected by estrogen in mice lacking estrogen receptor-α. J. Appl. Physiol. 2001, 91, 2391–2399. [Google Scholar] [CrossRef]
- Umegaki, H. Type 2 diabetes as a risk factor for cognitive impairment: Current insights. Clin. Interv. Aging 2014, 9, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Yates, K.F.; Sweat, V.; Yau, P.L.; Turchiano, M.M.; Convit, A. Impact of metabolic syndrome on cognition and brain: A selected review of the literature. Arter. Thromb. Vasc. Biol. 2012, 32, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Ungvari, Z.; Zhang, C. Resveratrol Improves Endothelial Function: Role of TNFα and Vascular Oxidative Stress. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1164–1171. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, C.S.; Ok, S.H.; Kim, D.; Kim, K.N.; Hong, J.M.; Kim, J.Y.; Bae, S.I.; An, S.; Sohn, J.T. Bupivacaine-induced contraction is attenuated by endothelial nitric oxide release modulated by activation of both stimulatory and inhibitory phosphorylation (Ser1177 and Thr495) of endothelial nitric oxide synthase. Eur. J. Pharmacol. 2019, 853, 121–128. [Google Scholar] [CrossRef]
- Wu, P.R.; Chen, B.R.; Hsieh, C.C.; Lin, W.C.; Wu, K.K.; Hwu, Y.; Chen, P.F. The N-terminal portion of autoinhibitory element modulates human endothelial nitric-oxide synthase activity through coordinated controls of phosphorylation at Thr495and Ser1177. Biosci. Rep. 2014, 34, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Hagensen, M.K.; Vanhoutte, P.M.; Bentzon, J.F. Arterial endothelial cells: Still the craftsmen of regenerated endothelium. Cardiovasc. Res. 2012, 95, 281–289. [Google Scholar] [CrossRef]
- Monnier, A.; Prigent-Tessier, A.; Quirié, A.; Bertrand, N.; Savary, S.; Gondcaille, C.; Garnier, P.; Demougeot, C.; Marie, C. Brain-derived neurotrophic factor of the cerebral microvasculature: A forgotten and nitric oxide-dependent contributor of brain-derived neurotrophic factor in the brain. Acta Physiol. 2017, 219, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Descamps, B.; Saif, J.; Benest, A.V.; Biglino, G.; Bates, D.O.; Chamorro-Jorganes, A.; Emanueli, C. BDNF (Brain-Derived Neurotrophic Factor) promotes embryonic stem cells differentiation to endothelial cells via a molecular pathway, including MicroRNA-214, EZH2 (Enhancer of Zeste Homolog 2), and eNOS (Endothelial Nitric Oxide Synthase). Arter. Thromb. Vasc. Biol. 2018, 38, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, N.; Li, S.; Han, R.; Huang, Q.; Hu, J.; Jin, K.; Ji, X. Limb ischemic conditioning improved cognitive deficits via eNOS-dependent augmentation of angiogenesis after chronic cerebral hypoperfusion in rats. Aging Dis. 2018, 9, 869–879. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, D.Y. Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide Biol. Chem. 2009, 20, 223–230. [Google Scholar] [CrossRef]
- Balbi, M.; Ghosh, M.; Longden, T.A.; Jativa Vega, M.; Gesierich, B.; Hellal, F.; Lourbopoulos, A.; Nelson, M.T.; Plesnila, N. Dysfunction of mouse cerebral arteries during early aging. J. Cereb. Blood Flow Metab. 2015, 35, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, C.F.; Santos, R.M.; Barbosa, R.M.; Cadenas, E.; Radi, R.; Laranjinha, J. Neurovascular coupling in hippocampus is mediated via diffusion by neuronal-derived nitric oxide. Free Radic. Biol. Med. 2014, 73, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Mishra, R.; Ashraf, Q.M.; Delivoria-Papadopoulos, M. Nitric oxide-mediated mechanism of neuronal nitric oxide synthase and inducible nitric oxide synthase expression during hypoxia in the cerebral cortex of newborn piglets. Neuroscience 2006, 140, 857–863. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Ferreira, N.R.; Santos, R.M.; Lukacova, N.; Barbosa, R.M.; Laranjinha, J. The pattern of glutamate-induced nitric oxide dynamics in vivo and its correlation with nNOS expression in rat hippocampus, cerebral cortex and striatum. Brain Res. 2014, 1554, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gerónimo-Olvera, C.; Tristán-López, L.; Martínez-Lazcano, J.C.; García-Lara, L.; Sánchez-Mendoza, A.; Morales-Martínez, A.; Hernández-Melesio, M.A.; Arregui, L.; Ríos, C.; Pérez-Severiano, F. Striatal Protection in nNOS Knock-Out Mice After Quinolinic Acid-Induced Oxidative Damage. Neurochem. Res. 2019, 44, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Snapyan, M.; Lemasson, M.; Brill, M.S.; Blais, M.; Massouh, M.; Ninkovic, J.; Gravel, C.; Berthod, F.; Götz, M.; Barker, P.A.; et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J. Neurosci. 2009, 29, 4172–4188. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zacharek, A.; Zhang, C.; Jiang, H.; Li, Y.; Roberts, C.; Lu, M.; Kapke, A.; Chopp, M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J. Neurosci. 2005, 25, 2366–2375. [Google Scholar] [CrossRef]
- Hayakawa, K.; Pham, L.D.D.; Katusic, Z.S.; Arai, K.; Lo, E.H. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc. Natl. Acad. Sci. USA 2012, 109, 7505–7510. [Google Scholar] [CrossRef]
- Wolburg, H.; Noell, S.; Mack, A.; Wolburg-Buchholz, K.; Fallier-Becker, P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009, 335, 75–96. [Google Scholar] [CrossRef]
- Cui, X.; Chopp, M.; Zacharek, A.; Ning, R.; Ding, X.; Roberts, C.; Chen, J. Endothelial nitric oxide synthase regulates white matter changes via the BDNF/TrKB pathway after stroke in mice. PLoS ONE 2013, 8, e80358. [Google Scholar] [CrossRef]
- Guo, S.; Kim, W.J.; Lok, J.; Lee, S.R.; Besancon, E.; Luom, B.H.; Stins, M.F.; Wang, X.; Dedhar, S.; Lo, E.H. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 7582–7587. [Google Scholar] [CrossRef]
- Hariharan, A.; Jing, Y.; Collie, N.D.; Zhang, H.; Liu, P. Altered neurovascular coupling and brain arginine metabolism in endothelial nitric oxide synthase deficient mice. Nitric Oxide Biol. Chem. 2019, 87, 60–72. [Google Scholar] [CrossRef]
- Eguchi, K.; Shindo, T.; Ito, K.; Ogata, T.; Kurosawa, R.; Kagaya, Y.; Monma, Y.; Ichijo, S.; Kasukabe, S.; Miyata, S.; et al. Whole-brain low-intensity pulsed ultrasound therapy markedly improves cognitive dysfunctions in mouse models of dementia—Crucial roles of endothelial nitric oxide synthase. Brain Stimul. 2018, 11, 959–973. [Google Scholar] [CrossRef]
- Roy, A.; Jana, M.; Kundu, M.; Corbett, G.T.; Rangaswamy, S.B.; Mishra, R.K.; Luan, C.H.; Gonzalez, F.J.; Pahan, K. HMG-CoA Reductase Inhibitors Bind to PPARα to Upregulate Neurotrophin Expression in the Brain and Improve Memory in Mice. Cell Metab. 2015, 22, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xing, M.; Wang, Y.; Tao, H.; Cheng, Y. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience 2015, 311, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, S.; Eliasson, M.J.L.; Sawa, A.; Watkins, C.C.; Krug, D.; Gupta, A.; Arai, T.; Ferrante, R.J.; Snyder, S.H. Species, strain and developmental variations in hippocampal neuronal and endothelial nitric oxide synthase clarify discrepancies in nitric oxide-dependent synaptic plasticity. Neuroscience 2003, 119, 979–990. [Google Scholar] [CrossRef]
- Doreulee, N.; Sergeeva, O.A.; Yanovsky, Y.; Chepkova, A.N.; Selbach, O.; Gödecke, A.; Schrader, J.; Haas, H.L. Cortico-striatal synaptic plasticity in endothelial nitric oxide synthase deficient mice. Brain Res. 2003, 964, 159–163. [Google Scholar] [CrossRef]
- Hopper, R.A.; Garthwaite, J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J. Neurosci. 2006, 26, 11513–11521. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 2015, 36, 161–178. [Google Scholar] [CrossRef]
- Zheng, B.; Zheng, T.; Wang, L.; Chen, X.; Shi, C.; Zhao, S. Aminoguanidine inhibition of iNOS activity ameliorates cerebral vasospasm after subarachnoid hemorrhage in rabbits via restoration of dysfunctional endothelial cells. J. Neurol. Sci. 2010, 295, 97–103. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, N. Pharmacological inhibition of inducible nitric oxide synthase (iNOS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, convalesce behavior and biochemistry of hypertension induced vascular dementia in rats. Pharmacol. Biochem. Behav. 2013, 103, 821–830. [Google Scholar] [CrossRef]
- Mori, K.; Togashi, H.; Ueno, K.; Matsumoto, M.; Yoshioka, M. Aminoguanidine prevented the impairment of learning behavior and hippocampal long-term potentiation following transient cerebral ischemia. Behav. Brain Res. 2001, 120, 159–168. [Google Scholar] [CrossRef]
- Lu, Z.; Li, Y.; Jin, J.; Zhang, X.; Lopes-Virella, M.F.; Huang, Y. Toll-like receptor 4 activation in microvascular endothelial cells triggers a robust inflammatory response and cross talk with mononuclear cells via interleukin-6. Arter. Thromb. Vasc. Biol. 2012, 32, 1696–1706. [Google Scholar] [CrossRef]
- Tu, X.K.; Yang, W.Z.; Chen, J.P.; Chen, Y.; Ouyang, L.Q.; Xu, Y.C.; Shi, C.C. Curcumin Inhibits TLR2/4-NF-κB Signaling Pathway and Attenuates Brain Damage in Permanent Focal Cerebral Ischemia in Rats. Inflammation 2014, 37, 1544–15551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Lin, S.; Yang, Q.W. Toll-like receptors in cerebral ischemic inflammatory injury. J. Neuroinflammation 2011, 8, 134. [Google Scholar] [CrossRef]
- Lee, M.; Rey, K.; Besler, K.; Wang, C.; Choy, J. Immunobiology of nitric oxide and regulation of inducible nitric oxide synthase. Macrophages 2017, 62, 181–207. [Google Scholar]
- De Reuck, J.L. Histopathological stainings and definitions of vascular disruptions in the elderly brain. Exp. Gerontol. 2012, 47, 834–837. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, B.; Xia, R.; Jia, Q.Y. Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9601–9614. [Google Scholar]
- Rosenberg, G.A.; Bjerke, M.; Wallin, A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke 2014, 45, 1531–1538. [Google Scholar] [CrossRef]
- Belkhelfa, M.; Beder, N.; Mouhoub, D.; Amri, M.; Hayet, R.; Tighilt, N.; Bakheti, S.; Laimouche, S.; Azzouz, D.; Belhadj, R.; et al. The involvement of neuroinflammation and necroptosis in the hippocampus during vascular dementia. J. Neuroimmunol. 2018, 320, 48–57. [Google Scholar] [CrossRef]
- Thal, D.R.; Grinberg, L.T.; Attems, J. Vascular dementia: Different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp. Gerontol. 2012, 47, 816–824. [Google Scholar] [CrossRef]
- Dai, C.; Gu, W. P53 post-translational modification: Deregulated in tumorigenesis. Trends Mol. Med. 2010, 16, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Forrester, K.; Ambs, S.; Lupold, S.E.; Kapust, R.B.; Spillare, E.A.; Weinberg, W.C.; Felley-Bosco, E.; Wang, X.W.; Geller, D.A.; Tzeng, E. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc. Natl. Acad. Sci. USA 1996, 93, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Koizumi, K.; El Jamal, S.; Zhou, P.; Previti, M.L.; Van Nostrand, W.E.; Carlson, G.; Iadecola, C. Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke 2014, 45, 1815–1821. [Google Scholar] [CrossRef]
- Schmitz, T.; Chew, L.J. Cytokines and myelination in the central nervous system. Sci. World J. 2008, 8, 1119–1147. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; MacVicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef]
- Wang, B.; Han, S. Inhibition of Inducible Nitric Oxide Synthase Attenuates Deficits in Synaptic Plasticity and Brain Functions Following Traumatic Brain Injury. Cerebellum 2018, 17, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Covacu, R.; Danilov, A.L.; Rasmussen, B.S.; Hallén, K.; Moe, M.C.; Lobell, A.; Johansson, C.B.; Svensson, M.A.; Olsson, T.; Brundin, L. Nitric Oxide Exposure Diverts Neural Stem Cell Fate from Neurogenesis Towards Astrogliogenesis. Stem Cells 2006, 24, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Czerwinski, P.; Xia, N.; Förstermann, U.; Li, H. Downregulation of BDNF expression by PKC and by TNF-in human endothelial cells. Pharmacology 2015, 96, 1–10. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Iadecola, C. Hypertension, dietary salt and cognitive impairment. J. Cereb. Blood Flow Metab. 2018, 38, 2112–2128. [Google Scholar] [CrossRef]
- Faraco, G.; Brea, D.; Garcia-Bonilla, L.; Wang, G.; Racchumi, G.; Chang, H.; Buendia, I.; Santisteban, M.M.; Segarra, S.G.; Koizumi, K.; et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 2018, 21, 240–249. [Google Scholar] [CrossRef]
- Nguyen, H.; Chiasson, V.L.; Chatterjee, P.; Kopriva, S.E.; Young, K.J.; Mitchell, B.M. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc. Res. 2013, 97, 696–704. [Google Scholar] [CrossRef]
- Gertz, K.; Priller, J.; Kronenberg, G.; Fink, K.B.; Winter, B.; Schröck, H.; Ji, S.; Milosevic, M.; Harms, C.; Böhm, M.; et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ. Res. 2006, 99, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, S.; Luo, Y.; Huang, W.; Chen, L.; Zhang, Y.; Zhang, L.; Chao, F. Exercise-induced Nitric Oxide Contributes to Spatial Memory and Hippocampal Capillaries in Rats. Int. J. Sports Med. 2020, 41, 951–961. [Google Scholar]
- Koloverou, E.; Tambalis, K.; Panagiotakos, D.B.; Georgousopoulou, E.; Chrysohoou, C.; Skoumas, I.; Tousoulis, D.; Stefanadis, C.; Pitsavos, C. Moderate physical activity reduces 10-year diabetes incidence: The mediating role of oxidative stress biomarkers. Int. J. Public Health 2018, 63, 297–305. [Google Scholar] [CrossRef]
- Trigiani, L.J.; Hamel, E. An endothelial link between the benefits of physical exercise in dementia. J. Cereb. Blood Flow Metab. 2017, 37, 2649–2664. [Google Scholar] [CrossRef]
- Padilla, J.; Simmons, G.H.; Bender, S.B.; Arce-Esquivel, A.A.; Whyte, J.J.; Laughlin, M.H. Vascular effects of exercise: Endothelial adaptations beyond active muscle beds. Physiology 2011, 26, 132–145. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Eng, J.J.; Boyd, L.A.; Jacova, C.; Davis, J.C.; Bryan, S.; Lee, P.; Brasher, P.; Hsiung, G.Y.R. Promotion of the mind through exercise (PROMoTE): A proof-of-concept randomized controlled trial of aerobic exercise training in older adults with vascular cognitive impairment. BMC Neurol. 2010, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu-Ambrose, T.; Best, J.R.; Davis, J.C.; Eng, J.J.; Lee, P.E.; Jacova, C.; Boyd, L.A.; Brasher, P.M.; Munkacsy, M.; Cheung, W. Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology 2016, 87, 2082–2090. [Google Scholar] [CrossRef]
- Tong, X.K.; Hamel, E. Simvastatin restored vascular reactivity, endothelial function and reduced string vessel pathology in a mouse model of cerebrovascular disease. J. Perinatol. 2015, 35, 512–520. [Google Scholar] [CrossRef]
- Saha, R.N.; Pahan, K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid. Redox Signal. 2006, 8, 929–947. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Yan, Y.; Sun, S.Q.; Zhang, J.; Huang, L.G.; Yan, N.; Wu, F.; Li, J.Y. Minocycline attenuates cognitive impairment and restrains oxidative stress in the hippocampus of rats with chronic cerebral hypoperfusion. Neurosci. Bull. 2008, 24, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, T.; Lian, Y.J.; Ma, R.; Yang, S.; Cho, J.Y. Nitric oxide synthase inhibitors: A review of patents from 2011 to the present. Expert Opin. Ther. Pat. 2015, 25, 49–68. [Google Scholar] [CrossRef]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid. Redox Signal. 2014, 20, 3040–3077. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, V.; Balzan, S. LOX-1 and ROS, inseparable factors in the process of endothelial damage. Free Radic. Res. 2014, 48, 841–848. [Google Scholar] [CrossRef]
- Armengou, A.; Hurtado, O.; Leira, R.; Obón, M.; Pascual, C.; Moro, M.A.; Lizasoain, I.; Castillo, J.; Dávalos, A. L-arginine levels in blood as a marker of nitric oxide-mediated brain damage in acute stroke: A clinical and experimental study. J. Cereb. Blood Flow Metab. 2003, 23, 978–984. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Tahmasebinejad, Z.; Azizi, F. Dietary L-arginine intake and the incidence of coronary heart disease: Tehran lipid and glucose study. Nutr. Metab. 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Siervo, M. Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure in adults: A systematic review and meta-analysis. J. Hypertens. 2017, 35, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.; Ashor, A.W.; Oggioni, C.; Ahluwalia, A.; Mathers, J.C.; Siervo, M. Effects of inorganic nitrate and beetroot supplementation on endothelial function: A systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Vitamin C for Type 2 Diabetes Mellitus and Hypertension. Arch. Med. Res. 2019, 50, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Anjum, I.; Fayyaz, M.; Wajid, A.; Sohail, W.; Ali, A. Does Obesity Increase the Risk of Dementia: A Literature Review. Cureus 2018, 10, e2660. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Hodgson, J.M. Dietary Nitrate, Nitric Oxide, and Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 2036–2052. [Google Scholar] [CrossRef]
- Bond, V.; Curry, B.H.; Adams, R.G.; Asadi, M.S.; Millis, R.M.; Haddad, G.E. Effects of dietary nitrates on systemic and cerebrovascular hemodynamics. Cardiol. Res. Pract. 2013, 1. [Google Scholar] [CrossRef]
- Wightman, E.L.; Haskell-ramsay, C.F.; Thompson, K.; Blackwell, J.R.; Winyard, P.G.; Forster, J.; Jones, A.M.; Kennedy, D.O. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiol. Behav. 2015, 149, 149–158. [Google Scholar] [CrossRef]
- Li, T.; Lu, X.; Sun, Y.; Yang, X. Effects of spinach nitrate on insulin resistance, endothelial dysfunction markers and inflammation in mice with high-fat and high-fructose consumption. Food Nutr. Res. 2016, 60, 32010. [Google Scholar] [CrossRef]
- Scalera, F.; Borlak, J.; Beckmann, B.; Martens-Lobenhoffer, J.; Thum, T.; Täger, M.; Bode-Böger, S.M. Endogenous nitric oxide synthesis inhibitor asymmetric dimethyl L-arginine accelerates endothelial cell senescence. Arter. Thromb. Vasc. Biol. 2004, 24, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Fleszar, M.G.; Wiśniew, J.; Zboch, M.; Diakowska, D. Targeted metabolomic analysis of nitric oxide/L-arginine pathway metabolites in dementia: Association with pathology, severity, and structural brain changes. Sci. Rep. 2019, 9, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Worthmann, H.; Deb, M.; Chen, S.; Weissenborn, K. Nitric oxide (NO) and asymmetric dimethylarginine (ADMA): Their pathophysiological role and involvement in intracerebral hemorrhage. Neurol. Res. 2011, 33, 541–548. [Google Scholar] [CrossRef]
- Jacobi, J.; Maas, R.; Cardounel, A.J.; Arend, M.; Pope, A.J.; Cordasic, N.; Heusinger-Ribeiro, J.; Atzler, D.; Strobel, J.; Schwedhelm, E.; et al. Dimethylarginine Dimethylaminohydrolase Overexpression Ameliorates Atherosclerosis in Apolipoprotein E-Deficient Mice by Lowering Asymmetric Dimethylarginine. Am. J. Pathol. 2010, 176, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M. Arginine metabolism revisited. J. Nutr. 2016, 146, 2579S–2586S. [Google Scholar] [CrossRef] [PubMed]
- Atzler, D.; Schwedhelm, E.; Nauck, M.; Ittermann, T.; Böger, R.H. Serum reference intervals of homoarginine, ADMA, and SDMA in the Study of Health in Pomerania. Clin. Chem. Lab. Med. 2014, 52, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.-Y.; Hong, F.-F.; Yang, S.-L. The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4540. https://doi.org/10.3390/ijms22094540
Zhu H-Y, Hong F-F, Yang S-L. The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches. International Journal of Molecular Sciences. 2021; 22(9):4540. https://doi.org/10.3390/ijms22094540
Chicago/Turabian StyleZhu, Han-Yan, Fen-Fang Hong, and Shu-Long Yang. 2021. "The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches" International Journal of Molecular Sciences 22, no. 9: 4540. https://doi.org/10.3390/ijms22094540
APA StyleZhu, H.-Y., Hong, F.-F., & Yang, S.-L. (2021). The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches. International Journal of Molecular Sciences, 22(9), 4540. https://doi.org/10.3390/ijms22094540





