Histone Deacetylase 1 Reduces Lipogenesis by Suppressing SREBP1 Transcription in Human Sebocyte Cell Line SZ95
Abstract
1. Introduction
2. Results
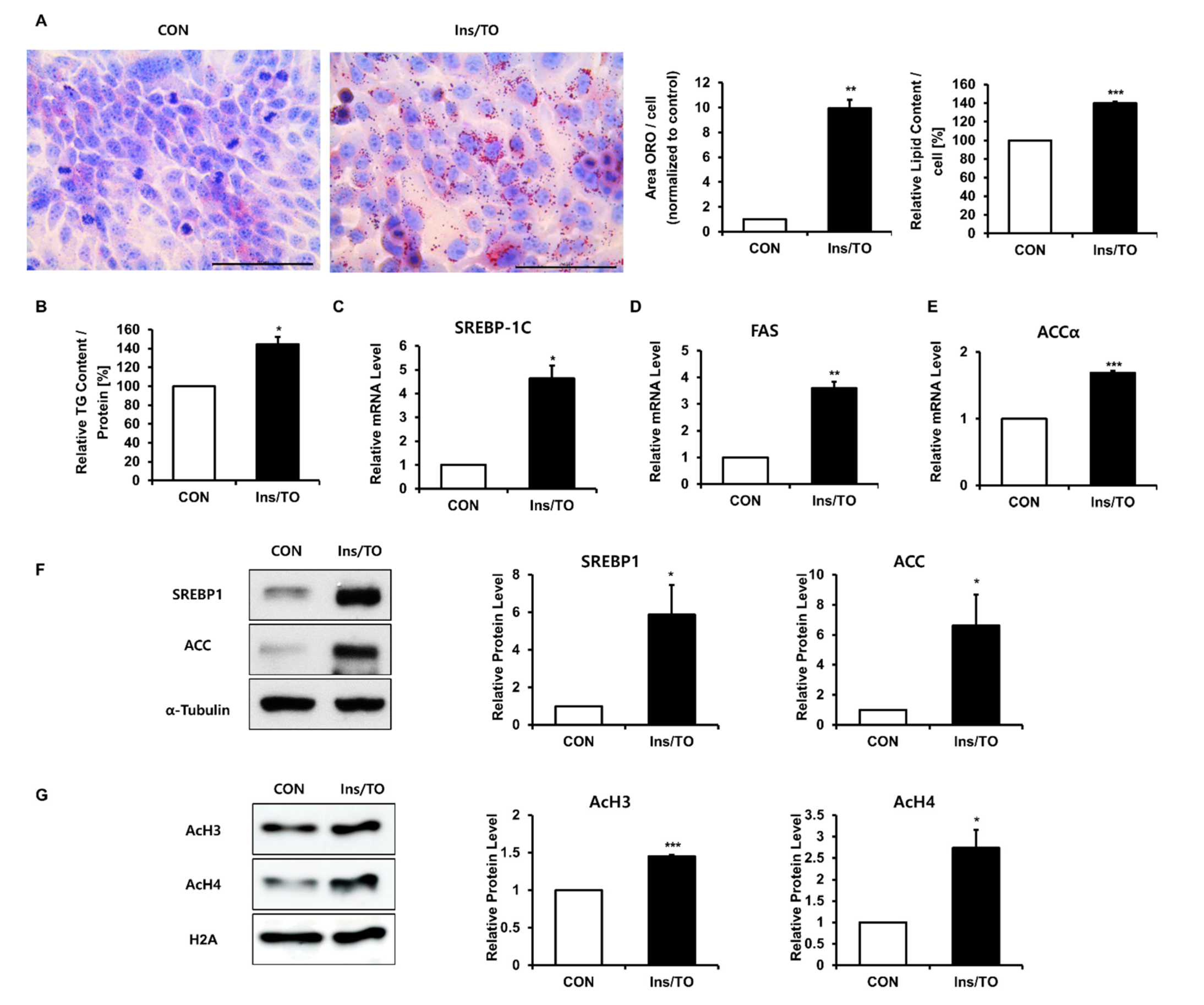
2.1. Insulin and LXR Agonist Treatment Enhanced Lipid Accumulation and Histone Acetylation in SZ95 Sebocytes
2.2. Increased Histone Acetylation Promoted Lipogenesis through SREBP1 Induction
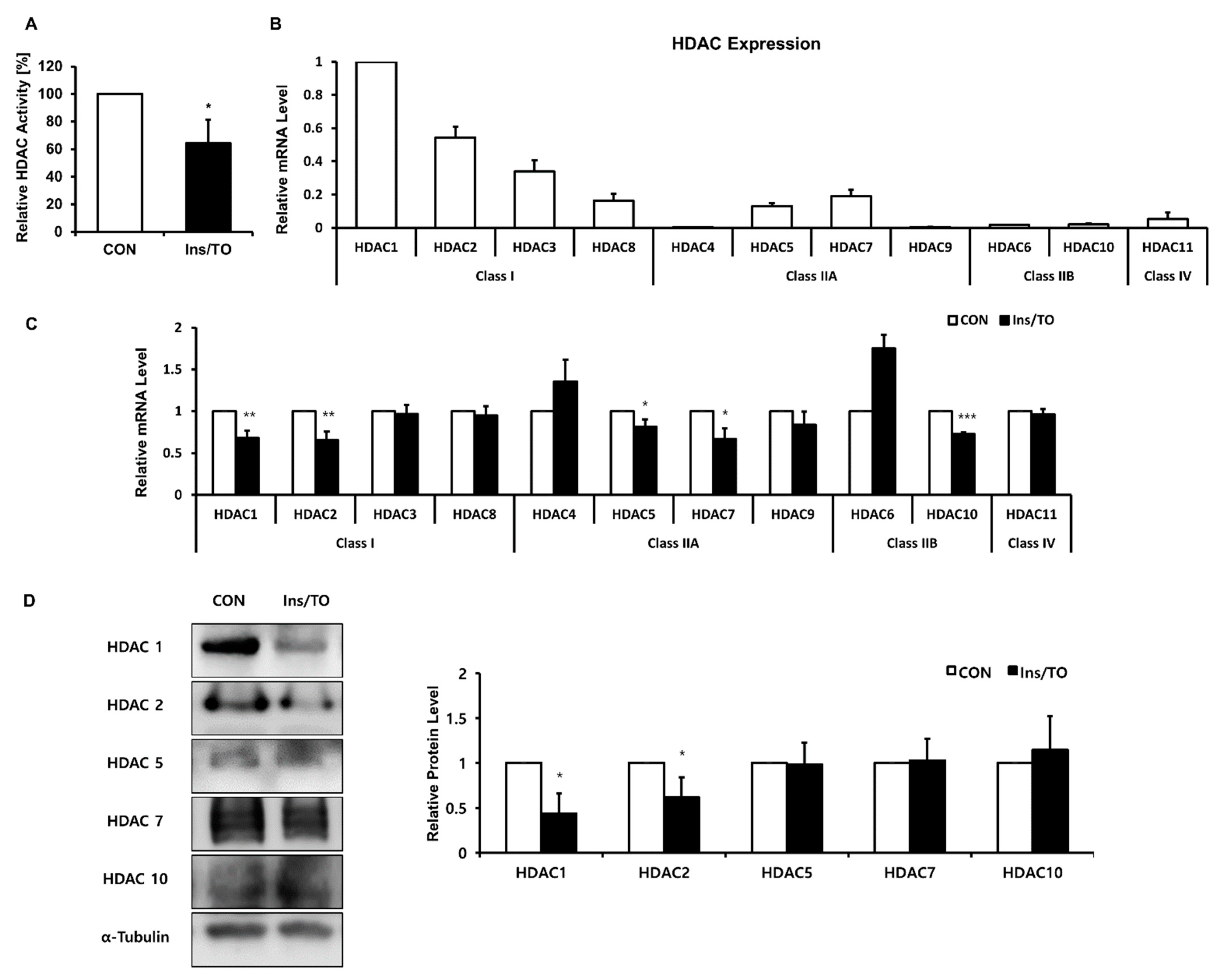
2.3. Insulin and LXR Agonist Treatment Decreased Overall HDAC Activity, and Specifically HDAC1 and HDAC2 Protein Expression
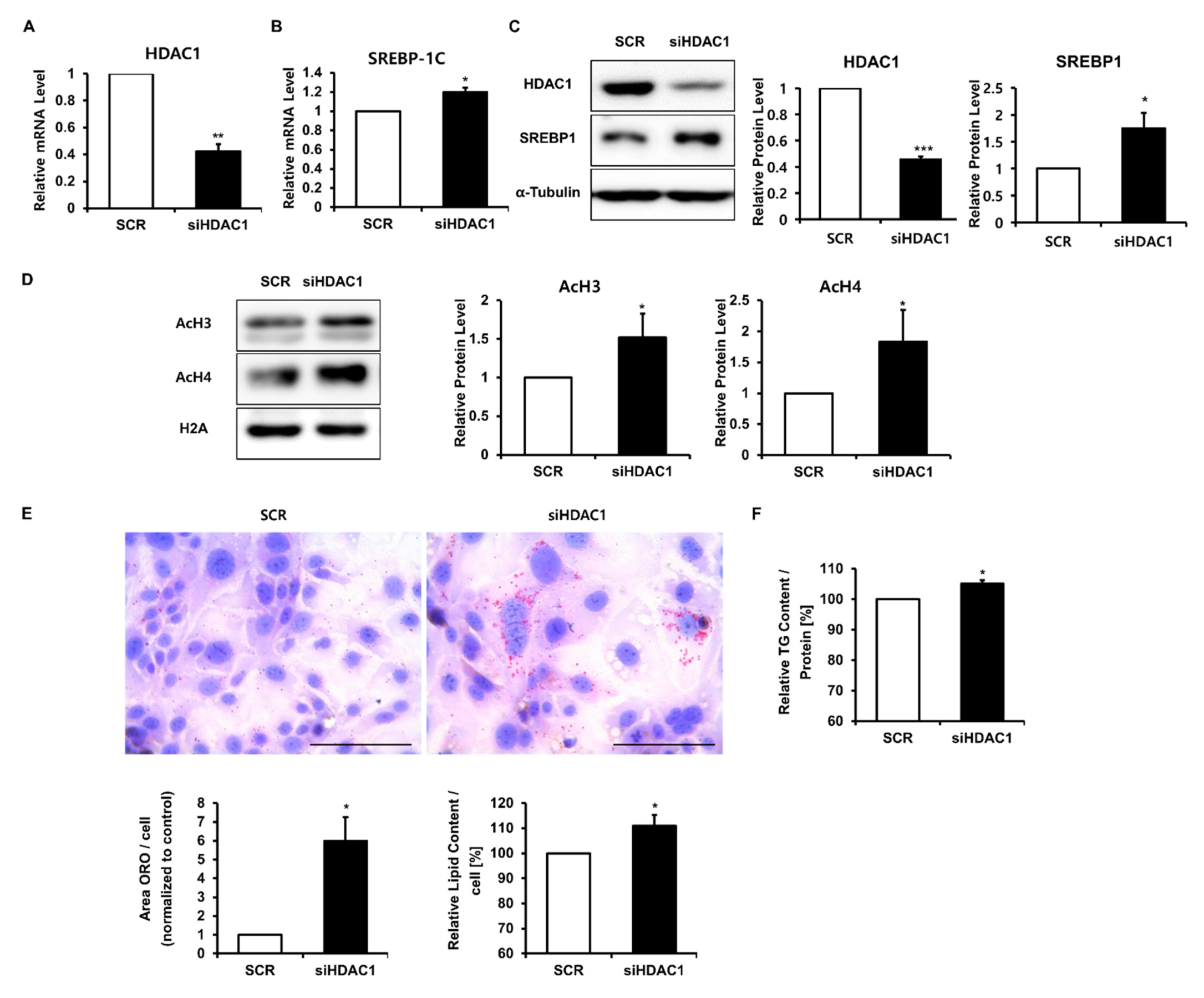
2.4. Decreased HDAC1 Is Crucial for Insulin and LXR Agonist-Induced Lipid Synthesis in SZ95 Sebocytes
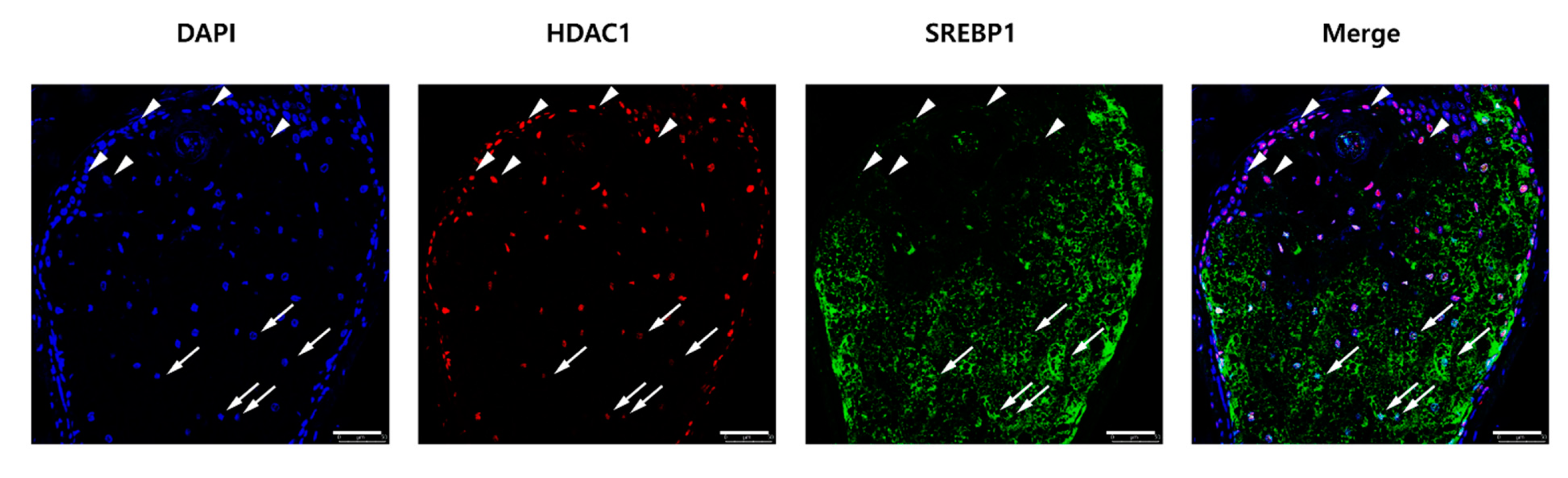
2.5. The HDAC1 and SREBP1 Expression Levels Were Inversely Correlated in Human Sebaceous Glands
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals and Reagents
4.3. Oil Red O Staining
4.4. Nile Red Staining
4.5. Determination of the TG Content
4.6. siRNA Transfection
4.7. Plasmid Constructs, Transient Transfection, and Luciferase Reporter Assay
4.8. Histone Extraction and Western Blotting
4.9. Immunofluorescence Staining
4.10. Quantitative Real-Time Polymerase Chain Reaction (PCR)
4.11. Histone Deacetylase Activity Assay
4.12. Human Skin Samples
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramasastry, P.; Downing, D.T.; Pochi, P.E.; Strauss, J.S. Chemical composition of human skin surface lipids from birth to puberty. J. Investig. Dermatol. 1970, 54, 139–144. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Acne and sebaceous gland function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef]
- Esler, W.P.; Tesz, G.J.; Hellerstein, M.K.; Beysen, C.; Sivamani, R.; Turner, S.M.; Watkins, S.M.; Amor, P.A.; Carvajal-Gonzalez, S.; Geoly, F.J. Human sebum requires de novo lipogenesis, which is increased in acne vulgaris and suppressed by acetyl-CoA carboxylase inhibition. Sci. Transl. Med. 2019, 11, eaau8465. [Google Scholar] [CrossRef]
- Tóth, B.I.; Oláh, A.; Szöllősi, A.G.; Czifra, G.; Bíró, T. “Sebocytes’ makeup”-Novel mechanisms and concepts in the physiology of the human sebaceous glands. Pflügers Arch. Eur. J. Physiol. 2011, 461, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Deplewski, D.; Rosenfield, R.L. Growth hormone and insulin-like growth factors have different effects on sebaceous cell growth and differentiation. Endocrinology 1999, 140, 4089–4094. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulven, S.M.; Dalen, K.T.; Gustafsson, J.-Å.; Nebb, H.I. LXR is crucial in lipid metabolism. Prostagland. Leukot. Essent. Fat. Acids 2005, 73, 59–63. [Google Scholar] [CrossRef]
- Hong, I.; Lee, M.H.; Na, T.Y.; Zouboulis, C.C.; Lee, M.O. LXRalpha enhances lipid synthesis in SZ95 sebocytes. J. Investig. Derm. 2008, 128, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.E.; Harrison, W.J.; Bahta, A.W.; Zouboulis, C.C.; Burrin, J.M.; Philpott, M.P. Characterization of liver X receptor expression and function in human skin and the pilosebaceous unit. Exp. Dermatol. 2007, 16, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; DeBose-Boyd, R.A.; Brown, M.S. Protein sensors for membrane sterols. Cell 2006, 124, 35–46. [Google Scholar] [CrossRef]
- Smith, T.M.; Cong, Z.; Gilliland, K.L.; Clawson, G.A.; Thiboutot, D.M. Insulin-like growth factor-1 induces lipid production in human SEB-1 sebocytes via sterol response element-binding protein-1. J. Investig. Dermatol. 2006, 126, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, C.; Wang, X.; Briggs, M.R.; Admon, A.; Wu, J.; Hua, X.; Goldstein, J.L.; Brown, M.S. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell 1993, 75, 187–197. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Shimomura, I.; Shimano, H.; Horton, J.D.; Goldstein, J.L.; Brown, M.S. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J. Clin. Investig. 1997, 99, 838–845. [Google Scholar] [CrossRef]
- Gelato, K.A.; Fischle, W. Role of histone modifications in defining chromatin structure and function. Biol. Chem. 2008, 389, 353–363. [Google Scholar] [CrossRef]
- Xu, W.; Wang, F.; Yu, Z.; Xin, F. Epigenetics and cellular metabolism. Genet. Epigenet. 2016, 8, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone deacetylases and mechanisms of regulation of gene expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, E.J.; Kim, J.E.; Lee, D.H.; Chung, J.H. Anacardic acid reduces lipogenesis in human differentiated adipocytes via inhibition of histone acetylation. J. Dermatol. Sci 2018, 89, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Bricambert, J.; Miranda, J.; Benhamed, F.; Girard, J.; Postic, C.; Dentin, R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J. Clin. Investig. 2010, 120, 4316–4331. [Google Scholar] [CrossRef]
- Yoo, E.J.; Chung, J.J.; Choe, S.S.; Kim, K.H.; Kim, J.B. Down-regulation of histone deacetylases stimulates adipocyte differentiation. J. Biol. Chem. 2006, 281, 6608–6615. [Google Scholar] [CrossRef]
- Ruijter, A.J.; GENNIP, A.H.; Caron, H.N.; Kemp, S.; Kuilenburg, A.B. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef]
- Imai, S.-I.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. Metabolic reprogramming by class I and II histone deacetylases. Trends Endocrinol. Metab. 2013, 24, 48–57. [Google Scholar] [CrossRef]
- Smith, T.M.; Gilliland, K.; Clawson, G.A.; Thiboutot, D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J. Investig. Dermatol. 2008, 128, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Snykers, S.; Henkens, T.; De Rop, E.; Vinken, M.; Fraczek, J.; De Kock, J.; De Prins, E.; Geerts, A.; Rogiers, V.; Vanhaecke, T. Role of epigenetics in liver-specific gene transcription, hepatocyte differentiation and stem cell reprogrammation. J. Hepatol. 2009, 51, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Viscarra, J.; Sul, H.S. Epigenetic Regulation of Hepatic Lipogenesis: Role in Hepatosteatosis and Diabetes. Diabetes 2020, 69, 525–531. [Google Scholar] [CrossRef]
- Trivedi, N.R.; Cong, Z.; Nelson, A.M.; Albert, A.J.; Rosamilia, L.L.; Sivarajah, S.; Gilliland, K.L.; Liu, W.; Mauger, D.T.; Gabbay, R.A. Peroxisome proliferator-activated receptors increase human sebum production. J. Investig. Dermatol. 2006, 126, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Harrison, W.J.; Bull, J.J.; Seltmann, H.; Zouboulis, C.C.; Philpott, M.P. Expression of lipogenic factors galectin-12, resistin, SREBP-1, and SCD in human sebaceous glands and cultured sebocytes. J. Investig. Dermatol. 2007, 127, 1309–1317. [Google Scholar] [CrossRef]
- Sanford, J.A.; O’Neill, A.M.; Zouboulis, C.C.; Gallo, R.L. Short-Chain Fatty Acids from Cutibacterium acnes Activate Both a Canonical and Epigenetic Inflammatory Response in Human Sebocytes. J. Immunol. 2019, 202, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.Y.; Li, Q.Z.; Lv, L.F. HDAC5 Inhibits Hepatic Lipogenic Genes Expression by Attenuating the Transcriptional Activity of Liver X Receptor. Cell Physiol. Biochem. 2016, 39, 1561–1567. [Google Scholar] [CrossRef]
- Kelly, R.D.; Cowley, S.M. The physiological roles of histone deacetylase (HDAC) 1 and 2: Complex co-stars with multiple leading parts. Biochem. Soc. Trans. 2013, 41, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Cubizolles, F.; Zhang, Y.; Reichert, N.; Kohler, H.; Seiser, C.; Matthias, P. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev. 2010, 24, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Seltmann, H.; Orfanos, C.E.; Neitzel, H. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95) 1. J. Investig. Dermatol. 1999, 113, 1011–1020. [Google Scholar] [CrossRef] [PubMed]


| Gene | Primers (5′ - 3′) | |
|---|---|---|
| Forward | Reverse | |
| 36B4 | TCGACAATGGCAGCATCTAC | TGATGCAACAGTTGGGTAGC |
| SREBP-1c | GCCATGGATTGCACTTT | CAAGAGAGGAGCTCAATG |
| ACCα | ATGCTGACCGAGAAAGCA | TGCGGATTTGCTTGAGGA |
| FAS | CCGAGGAACTCCCCTCAT | GCCAGCGTCTTCCACACT |
| HDAC1 | ATCTATCGCCCTCACAAAGC | AATCTCTGCATCTGCTTGCT |
| HDAC2 | TGCTACTACTACGACGGTGA | AGTGGCTTTATGGGGCCTA |
| HDAC3 | GAGAGTCAGCCCCACCAATA | GTTGTTCAGCTGGGTTGCTC |
| HDAC4 | GAG AGA CTC ACC CTT CCC G | CCG GTC TGC ACC AAC CAA G |
| HDAC5 | ACAGCATGACCCCTGACAAGG | GCT CCT GCT GCC GCT TGG |
| HDAC6 | ATCTGGCGGAGTGGAAGAA | AAGTGACACTGGAGTCCTGA |
| HDAC7 | CTCACTGTCAGCCCCAGAG | CTGGTGCTTCAGCATGACC |
| HDAC8 | AAACGGGCCAGTATGGTG | CTGACCTTCTGGAGATGCTG |
| HDAC9 | CAACAAAACCCTAGCAGCCT | GCCCACAGGAACTTCTGACT |
| HDAC10 | CACTAGCGAGGGCGTTTG | GGGTCGTCCCAGAGCA |
| HDAC11 | GGATGCTACACACAACCCA | CCCATTTTCCGGCATCAAAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.S.; Lee, Y.; Shin, M.H.; Cho, S.I.; Zouboulis, C.C.; Kim, M.K.; Lee, D.H.; Chung, J.H. Histone Deacetylase 1 Reduces Lipogenesis by Suppressing SREBP1 Transcription in Human Sebocyte Cell Line SZ95. Int. J. Mol. Sci. 2021, 22, 4477. https://doi.org/10.3390/ijms22094477
Shin HS, Lee Y, Shin MH, Cho SI, Zouboulis CC, Kim MK, Lee DH, Chung JH. Histone Deacetylase 1 Reduces Lipogenesis by Suppressing SREBP1 Transcription in Human Sebocyte Cell Line SZ95. International Journal of Molecular Sciences. 2021; 22(9):4477. https://doi.org/10.3390/ijms22094477
Chicago/Turabian StyleShin, Hye Sun, Yuri Lee, Mi Hee Shin, Soo Ick Cho, Christos C. Zouboulis, Min Kyoung Kim, Dong Hun Lee, and Jin Ho Chung. 2021. "Histone Deacetylase 1 Reduces Lipogenesis by Suppressing SREBP1 Transcription in Human Sebocyte Cell Line SZ95" International Journal of Molecular Sciences 22, no. 9: 4477. https://doi.org/10.3390/ijms22094477
APA StyleShin, H. S., Lee, Y., Shin, M. H., Cho, S. I., Zouboulis, C. C., Kim, M. K., Lee, D. H., & Chung, J. H. (2021). Histone Deacetylase 1 Reduces Lipogenesis by Suppressing SREBP1 Transcription in Human Sebocyte Cell Line SZ95. International Journal of Molecular Sciences, 22(9), 4477. https://doi.org/10.3390/ijms22094477







