Leptin Receptor Compound Heterozygosity in Humans and Animal Models
Abstract
1. Introduction
2. Leptin Receptor
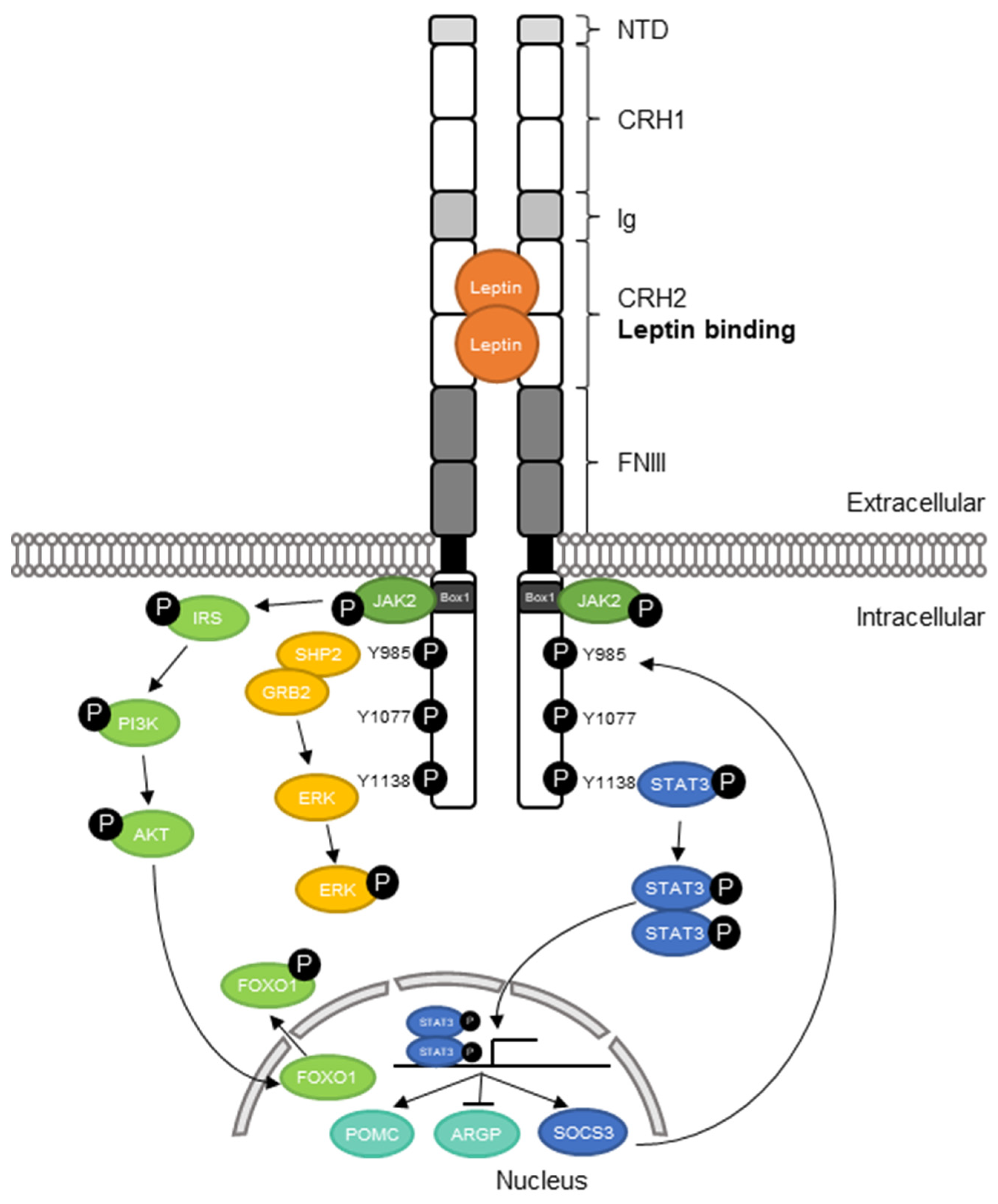
2.1. Structure of the Leptin Receptor
2.2. Isoforms
2.3. Leptin Receptor Signaling
2.4. Function
2.5. Inheritance Pattern of Human LEPR Mutation and Functional Analysis
3. Pathogenesis of Leptin Receptor Mutations
4. Therapy Options
5. Animal Models of Compound Heterozygous Lepr Mutations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- King, R.C.; Stansfield, W.D.; Mulligan, P.K. A Dictionary of Genetics; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Cordeiro, J.M.; Barajas-Martinez, H.; Hong, K.; Burashnikov, E.; Pfeiffer, R.; Orsino, A.M.; Wu, Y.S.; Hu, D.; Brugada, J.; Brugada, P.; et al. Compound Heterozygous Mutations P336L and I1660V in the Human Cardiac Sodium Channel Associated with the Brugada Syndrome. Circulation 2006, 114, 2026–2033. [Google Scholar] [CrossRef]
- Moulson, C.L.; Go, G.; Gardner, J.M.; Van Der Wal, A.C.; Smitt, J.H.S.; Van Hagen, J.M.; Miner, J.H. Homozygous and Compound Heterozygous Mutations in ZMPSTE24 Cause the Laminopathy Restrictive Dermopathy. J. Investig. Dermatol. 2005, 125, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.A.; Mohler, K.P.; Hopkins, K.W.; Oakley, D.H.; Sweetser, D.A.; Ibba, M.; Frosch, M.P.; Thibert, R.L. Novel Compound Heterozygous Mutations Expand the Recognized Phenotypes of FARS2 -Linked Disease. J. Child Neurol. 2016, 31, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Huvenne, H.; Dubern, B.; Clément, K.; Poitou, C. Rare Genetic Forms of Obesity: Clinical Approach and Current Treatments in 2016. Obes. Facts 2016, 9, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, L.; Glorie-Docter, M.; Van Den Akker, E.; Delemarre-Van De Waal, H.A. Obesitas Door Melanocortine-4-Receptormutaties. Ned. Tijdschr. Geneeskd. 2012, 156, 1–7. [Google Scholar]
- Khosropour, S.; Hamidi, M.; Fattahi, A.; Khodadadi, I.; Karami, M.; Fazilati, M.; Vaisi-Raygani, A.; Tavilani, H. Leptin and Leptin-Receptor Polymorphisms in Fertile and Infertile Men. Syst. Biol. Reprod. Med. 2017, 63, 7–14. [Google Scholar] [CrossRef]
- Ramos-Lobo, A.M.; Donato, J. The Role of Leptin in Health and Disease. Temperature 2017, 4, 258–291. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Ahlma, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of Leptin in the Neuroendocrine Response to Fasting. Nature 1996, 382, 250–252. [Google Scholar]
- Frederich, R.C.; Hamann, A.; Anderson, S.; Löllmann, B.; Lowell, B.B.; Flier, J.S. Leptin Levels Reflect Body Lipid Content in Mice: Evidence for Diet-Induced Resistance to Leptin Action. Nat. Med. 1995, 1, 1311–1314. [Google Scholar] [CrossRef]
- Knight, Z.A.; Hannan, K.S.; Greenberg, M.L.; Friedman, J.M. Hyperleptinemia Is Required for the Development of Leptin Resistance. PLoS ONE 2010, 5, e11376. [Google Scholar] [CrossRef]
- Münzberg, H.; Morrison, C.D. Structure, Production and Signaling of Leptin. Metabolism 2015, 64, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; Jebb, S.A.; Langmack, G.; Lawrence, E.; Cheetham, C.H.; Prentice, A.M.; Hughes, I.A.; McCamish, M.A.; O’Rahilly, S. Effects of Recombinant Leptin Therapy in a Child with Congenital Leptin Deficiency. N. Engl. J. Med. 1999, 341, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Montague, C.T.; Farooqi, I.S.; Whitehead, J.P.; Soos, M.A.; Rau, H.; Wareham, N.J.; Sewter, C.P.; Digby, J.E.; Mohammed, S.N.; Hurst, J.A.; et al. Congenital Leptin Deficiency Is Associated with Severe Early-Onset Obesity in Humans. Nature 1997, 387, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Hummel, K.P.; Dickie, M.M.; Coleman, D.L. Diabetes, a New Mutation in the Mouse. Science 1966, 153, 1127–1128. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, L.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.; Campfield, L.; Clark, F.; Deeds, J.; et al. Identification and Expression Cloning of a Leptin Receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Zabeau, L.; Defeau, D.; Van Der Heyden, J.; Iserentant, H.; Vandekerckhove, J.; Tavernier, J. Functional Analysis of Leptin Receptor Activation Using a Janus Kinase/Signal Transducer and Activator of Transcription Complementation Assay. Mol. Endocrinol. 2004, 18, 150–161. [Google Scholar] [CrossRef]
- Nunziata, A.; Funcke, J.B.; Borck, G.; Von Schnurbein, J.; Brandt, S.; Lennerz, B.; Moepps, B.; Gierschik, P.; Fischer-Posovszky, P.; Wabitsch, M. Functional and Phenotypic Characteristics of Human Leptin Receptor Mutations. J. Endocr. Soc. 2019, 3, 27–41. [Google Scholar] [CrossRef]
- Peelman, F.; Iserentant, H.; De Smet, A.S.; Vandekerckhove, J.; Zabeau, L.; Tavernier, J. Mapping of Binding Site III in the Leptin Receptor and Modeling of a Hexameric Leptin·leptin Receptor Complex. J. Biol. Chem. 2006, 281, 15496–15504. [Google Scholar] [CrossRef] [PubMed]
- Peelman, F.; Zabeau, L.; Moharana, K.; Savvides, S.N.; Tavernier, J. 20 Years of Leptin: Insights into Signaling Assemblies of the Leptin Receptor. J. Endocrinol. 2014, 223, T9–T23. [Google Scholar] [CrossRef]
- Bahrenberg, G.; Behrmann, I.; Barthel, A.; Hekerman, P.; Heinrich, P.C.; Joost, H.G.; Becker, W. Identification of the Critical Sequence Elements in the Cytoplasmic Domain of Leptin Receptor Isoforms Required for Janus Kinase/Signal Transducer and Activator of Transcription Activation by Receptor Heterodimers. Mol. Endocrinol. 2002, 16, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Vaisse, C.; Halaas, J.L.; Horvath, C.M.; Dernell, J.; Stoffel, M.; Friedman, J.M. Leptin Activation of Stat3 in the Hypothalamus of Wild-Type and Ob/Ob Mice but Not Db/Db Mice. Nat. Genet. 1996, 14, 95–97. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, K.W.; Kim, M.S. Leptin Signalling Pathways in Hypothalamic Neurons. Cell. Mol. Life Sci. 2016, 73, 1457–1477. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Seeley, R.J.; Campfield, L.A.; Burn, P.; Baskin, D.G. Identification of Targets of Leptin Action in Rat Hypothalamus. J. Clin. Investig. 1996, 98, 1101–1106. [Google Scholar] [CrossRef]
- LEPR. Leptin Receptor—Mus Musculus (Mouse). Available online: https://www.uniprot.org/uniprot/P48356 (accessed on 17 March 2021).
- Hileman, S.M.; Tornøe, J.; Flier, J.S.; Bjørbæk, C. Transcellular Transport of Leptin by the Short Leptin Receptor Isoform ObRa in Madin-Darby Canine Kidney Cells. Endocrinology 2000, 141, 1955–1961. [Google Scholar] [CrossRef]
- LEPR. Leptin Receptor Precursor—Homo Sapiens (Human)—LEPR Gene & Protein. Available online: https://www.uniprot.org/uniprot/P48357#expression (accessed on 17 March 2021).
- Maamra, M.; Bidlingmaier, M.; Postel-Vinay, M.-C.C.; Wu, Z.; Strasburger, C.J.; Ross, R.J.M. Generation of Human Soluble Leptin Receptor by Proteolytic Cleavage of Membrane-Anchored Receptors. Endocrinology 2001, 142, 4389–4393. [Google Scholar] [CrossRef] [PubMed]
- Schaab, M.; Kratzsch, J. The Soluble Leptin Receptor. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 661–670. [Google Scholar] [CrossRef]
- Håkansson, M.L.; Brown, H.; Ghilardi, N.; Skoda, R.C.; Meister, B. Leptin Receptor Immunoreactivity in Chemically Defined Target Neurons of the Hypothalamus. J. Neurosci. 1998, 18, 559–572. [Google Scholar] [CrossRef]
- Devos, R.; Guisez, Y.; Van Der Heyden, J.; White, D.W.; Kalai, M.; Fountoulakis, M.; Plaetinck, G. Ligand-Independent Dimerization of the Extracellular Domain of the Leptin Receptor and Determination of the Stoichiometry of Leptin Binding. J. Biol. Chem. 1997, 272, 18304–18310. [Google Scholar] [CrossRef]
- Mancour, L.V.; Daghestani, H.N.; Dutta, S.; Westfield, G.H.; Schilling, J.; Oleskie, A.N.; Herbstman, J.F.; Chou, S.Z.; Skiniotis, G. Ligand-Induced Architecture of the Leptin Receptor Signaling Complex. Mol. Cell 2012, 48, 655–661. [Google Scholar] [CrossRef]
- Banks, A.S.; Davis, S.M.; Bates, S.H.; Myers, M.G. Activation of Downstream Signals by the Long Form of the Leptin Receptor. J. Biol. Chem. 2000, 275, 14563–14572. [Google Scholar] [CrossRef]
- Bjørbæk, C.; Uotani, S.; Da Silva, B.; Flier, J.S. Divergent Signaling Capacities of the Long and Short Isoforms of the Leptin Receptor. J. Biol. Chem. 1997, 272, 32686–32695. [Google Scholar] [CrossRef]
- Carpenter, L.R.; Farruggella, T.J.; Symes, A.; Karow, M.L.; Yancopoulos, G.D.; Stahl, N. Enhancing Leptin Response by Preventing SH2-Containing Phosphatase 2 Interaction with Ob Receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 6061–6066. [Google Scholar] [CrossRef] [PubMed]
- Niswender, K.D.; Morton, G.J.; Stearns, W.H.; Rhodes, C.J.; Myers, M.G.; Schwartz, M.W. Intracellular Signalling: Key Enzyme in Leptin-Induced Anorexia. Nature 2001, 413, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Bjørbæk, C.; Elmquist, J.K.; Frantz, J.D.; Shoelson, S.E.; Flier, J.S. Identification of SOCS-3 as a Potential Mediator of Central Leptin Resistance. Mol. Cell 1998, 1, 619–625. [Google Scholar] [CrossRef]
- Joly-Amado, A.; Denis, R.G.P.; Castel, J.; Lacombe, A.; Cansell, C.; Rouch, C.; Kassis, N.; Dairou, J.; Cani, P.D.; Ventura-Clapier, R.; et al. Hypothalamic AgRP-Neurons Control Peripheral Substrate Utilization and Nutrient Partitioning. EMBO J. 2012, 31, 4276–4288. [Google Scholar] [CrossRef]
- Münzberg, H.; Huo, L.; Nillni, E.A.; Hollenberg, A.N.; Bjørbæk, C. Role of Signal Transducer and Activator of Transcription 3 in Regulation of Hypothalamic Proopiomelanocortin Gene Expression by Leptin. Endocrinology 2003, 144, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.K.; Wang, Y.; Banks, A.S.; Lavery, H.J.; Haq, A.K.; Maratos-Flier, E.; et al. STAT3 Signalling Is Required for Leptin Regulation of Energy Balance but Not Reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Pak, Y.K.; Jang, P.G.; Namkoong, C.; Choi, Y.S.; Won, J.C.; Kim, K.S.; Kim, S.W.; Kim, H.S.; Park, J.Y.; et al. Role of Hypothalamic Foxo1 in the Regulation of Food Intake and Energy Homeostasis. Nat. Neurosci. 2006, 9, 901–906. [Google Scholar] [CrossRef]
- Kitamura, T.; Feng, Y.; Kitamura, Y.I.; Chua, S.C.; Xu, A.W.; Barsh, G.S.; Rossetti, L.; Accili, D. Forkhead Protein FoxO1 Mediates Agrp-Dependent Effects of Leptin on Food Intake. Nat. Med. 2006, 12, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.P.; Çakir, I.; Carrington, S.J.; Cone, R.D.; Ghamari-Langroudi, M.; Gillyard, T.; Gimenez, L.E.; Litt, M.J. 60 YEARS OF POMC: Regulation of Feeding and Energy Homeostasis by α-MSH. J. Mol. Endocrinol. 2016, 56, T157–T174. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S.; Creemers, J.W.M.; Ohagi, S.; Raffin-Sanson, M.L.; Sanders, L.; Montague, C.T.; Hutton, J.C.; O’Rahilly, S. Obesity and Impaired Prohormone Processing Associated with Mutations in the Human Prohormone Convertase 1 Gene. Nat. Genet. 1997, 16, 303–306. [Google Scholar] [CrossRef]
- Bjørbæk, C.; Buchholz, R.M.; Davis, S.M.; Bates, S.H.; Pierroz, D.D.; Gu, H.; Neel, B.G.; Myers, M.G.; Flier, J.S. Divergent Roles of SHP-2 in ERK Activation by Leptin Receptors. J. Biol. Chem. 2001, 276, 4747–4755. [Google Scholar] [CrossRef]
- Le Beyec, J.; Cugnet-Anceau, C.; Pépin, D.; Alili, R.; Cotillard, A.; Lacorte, J.-M.; Basdevant, A.; Laville, M.; Clément, K. Homozygous Leptin Receptor Mutation Due to Uniparental Disomy of Chromosome 1: Response to Bariatric Surgery. J. Clin. Endocrinol. Metab. 2013, 98, E397–E402. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.S.; Wangensteen, T.; Collins, S.; Kimber, W.; Matarese, G.; Keogh, J.M.; Lank, E.; Bottomley, B.; Lopez-Fernandez, J.; Ferraz-Amaro, I.; et al. Clinical and Molecular Genetic Spectrum of Congenital Deficiency of the Leptin Receptor. N. Engl. J. Med. 2007, 356, 237–247. [Google Scholar] [CrossRef] [PubMed]
- DePaoli, A.M. 20 Years of Leptin: Leptin in Common Obesity and Associated Disorders of Metabolism. J. Endocrinol. 2014, 223, T71–T81. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.; Légrádi, G.; Mihály, E.; Huang, Q.H.; Tatro, J.B.; Rand, W.M.; Emerson, C.H.; Lechan, R.M. α-Melanocyte-Stimulating Hormone Is Contained in Nerve Terminals Innervating Thyrotropin-Releasing Hormone-Synthesizing Neurons in the Hypothalamic Paraventricular Nucleus and Prevents Fasting-Induced Suppression of Prothyrotropin-Releasing Hormone Gene E. J. Neurosci. 2000, 20, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Nillni, E.A.; Vaslet, C.; Harris, M.; Hollenberg, A.; Bjorbaek, C.; Flier, J.S. Leptin Regulates Prothyrotropin-Releasing Hormone Biosynthesis. J. Biol. Chem. 2000, 275, 36124–36133. [Google Scholar] [CrossRef]
- Clément, K.; Vaisse, C.; Lahlou, N.; Cabrol, S.; Pelloux, V.; Cassuto, D.; Gourmelen, M.; Dina, C.; Chambaz, J.; Lacorte, J.-M.M.; et al. A Mutation in the Human Leptin Receptor Gene Causes Obesity and Pituitary Dysfunction. Nature 1998, 392, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.R.; Mehrjardi, M.Y.V.; Dilaver, N.; Tajamolian, M.; Enayati, S.; Ebrahimi, P.; Amoli, M.M.; Farooqi, S.; Maroofian, R. Potential Role of Gender Specific Effect of Leptin Receptor Deficiency in an Extended Consanguineous Family with Severe Early-Onset Obesity. Eur. J. Med. Genet. 2018, 61, 465–467. [Google Scholar] [CrossRef]
- Singireddy, A.V.; Inglis, M.A.; Zuure, W.A.; Kim, J.S.; Anderson, G.M. Neither Signal Transducer and Activator of Transcription 3 (STAT3) or STAT5 Signaling Pathways Are Required for Leptin’s Effects on Fertility in Mice. Endocrinology 2013, 154, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.L.; Unger, E.K.; Myers, M.G.; Xu, A.W. Specific Physiological Roles for Signal Transducer and Activator of Transcription 3 in Leptin Receptor-Expressing Neurons. Mol. Endocrinol. 2008, 22, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the Obese Gene Product on Body Weight Regulation in Ob/Ob Mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Coppari, R.; Ichinose, M.; Lee, C.E.; Pullen, A.E.; Kenny, C.D.; McGovern, R.A.; Tang, V.; Liu, S.M.; Ludwig, T.; Chua, S.C.; et al. The Hypothalamic Arcuate Nucleus: A Key Site for Mediating Leptin’s Effects on Glucose Homeostasis and Locomotor Activity. Cell Metab. 2005, 1, 63–72. [Google Scholar] [CrossRef]
- Morton, G.J.; Gelling, R.W.; Niswender, K.D.; Morrison, C.D.; Rhodes, C.J.; Schwartz, M.W. Leptin Regulates Insulin Sensitivity via Phosphatidylinositol-3-OH Kinase Signaling in Mediobasal Hypothalamic Neurons. Cell Metab. 2005, 2, 411–420. [Google Scholar] [CrossRef]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin Modulates the T-Cell Immune Response and Reverses Starvation-Induced Immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Matarese, G.; Lord, G.M.; Keogh, J.M.; Lawrence, E.; Agwu, C.; Sanna, V.; Jebb, S.A.; Perna, F.; Fontana, S.; et al. Beneficial Effects of Leptin on Obesity, T Cell Hyporesponsiveness, and Neuroendocrine/Metabolic Dysfunction of Human Congenital Leptin Deficiency. J. Clin. Investig. 2002, 110, 1093–1103. [Google Scholar] [CrossRef]
- Mazen, I.; El-Gammal, M.; Abdel-Hamid, M.; Farooqi, I.S.; Amr, K. Homozygosity for a Novel Missense Mutation in the Leptin Receptor Gene (P316T) in Two Egyptian Cousins with Severe Early Onset Obesity. Mol. Genet. Metab. 2011, 102, 461–464. [Google Scholar] [CrossRef]
- Shackelford, T.K.; Vonk, J. The Oxford Handbook of Comparative Evolutionary Psychology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Gandhi Muruganandhan, S.; Manian, R. Computational and Artificial Neural Network Based Study of Functional SNPs of Human LEPR Protein Associated with Reproductive Function. J. Cell. Biochem. 2019, 120, 18910–18926. [Google Scholar] [CrossRef]
- Olza, J.; Rupérez, A.I.; Gil-Campos, M.; Leis, R.; Cañete, R.; Tojo, R.; Gil, Á.; Aguilera, C.M. Leptin Receptor Gene Variant Rs11804091 Is Associated with BMI and Insulin Resistance in Spanish Female Obese Children: A Case-Control Study. Int. J. Mol. Sci. 2017, 18, 1690. [Google Scholar] [CrossRef]
- Park, K.S.; Shin, H.D.; Park, B.L.; Cheong, H.S.; Cho, Y.M.; Lee, H.K.; Lee, J.-Y.; Lee, J.-K.; Oh, B.; Kimm, K. Polymorphisms in the Leptin Receptor (LEPR)-Putative Association with Obesity and T2DM. J. Hum. Genet. 2006, 51, 85–91. [Google Scholar] [CrossRef]
- Nesrine, Z.; Haithem, H.; Imen, B.; Fadoua, N.; Asma, O.; Fadhel, N.M.; Ali, B. Leptin and Leptin Receptor Polymorphisms, Plasma Leptin Levels and Obesity in Tunisian Volunteers. Int. J. Exp. Pathol. 2018, 99, 121–130. [Google Scholar] [CrossRef]
- Shastry, B.S. SNPs in Disease Gene Mapping, Medicinal Drug Development and Evolution. J. Hum. Genet. 2007, 52, 871–880. [Google Scholar] [CrossRef]
- Voigtmann, F.; Wolf, P.; Landgraf, K.; Stein, R.; Kratzsch, J.; Schmitz, S.; Abou Jamra, R.; Blüher, M.; Meiler, J.; Beck-Sickinger, A.G.; et al. Identification of a Novel Leptin Receptor (LEPR) Variant and Proof of Functional Relevance Directing Treatment Decisions in Patients with Morbid Obesity. Metabolism 2021, 116, 154438. [Google Scholar] [CrossRef]
- Andiran, N.; Çelik, N.; Andiran, F. Homozygosity for Two Missense Mutations in the Leptin Receptor Gene (P316T;W646C) in a Turkmenian Girl with Severe Early-Onset Obesity. J. Pediatr. Endocrinol. Metab. 2011, 24, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Armağan, C.; Yılmaz, C.; Koç, A.; Abacı, A.; Ülgenalp, A.; Böber, E.; Erçal, D.; Demir, K. A Toddler with a Novel LEPR Mutation. Hormones 2019, 18, 237–240. [Google Scholar] [CrossRef]
- Huvenne, H.; Le Beyec, J.; Pépin, D.; Alili, R.; Kherchiche, P.P.; Jeannic, E.; Frelut, M.; Lacorte, J.; Nicolino, M.; Viard, A.; et al. Seven Novel Deleterious LEPR Mutations Found in Early-Onset Obesity: A ΔExon6–8 Shared by Subjects From Reunion Island, France, Suggests a Founder Effect. J. Clin. Endocrinol. Metab. 2015, 100, 757–766. [Google Scholar] [CrossRef]
- Kakar, N.; Ahmad, J.; Kubisch, C.; Borck, G. Exon Skipping and Severe Childhood-Onset Obesity Caused by a Leptin Receptor Mutation. Am. J. Med. Genet. Part A 2013, 161, 2672–2674. [Google Scholar] [CrossRef] [PubMed]
- Kleinendorst, L.; Van Haelst, M.M.; Van Den Akker, E.L.T. Young Girl with Severe Early-Onset Obesity and Hyperphagia. BMJ Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Bonnefond, A.; Manzoor, J.; Philippe, J.; Durand, E.; Arshad, M.; Sand, O.; Butt, T.A.; Falchi, M.; Arslan, M.; et al. Novel LEPR Mutations in Obese Pakistani Children Identified by PCR-Based Enrichment and next Generation Sequencing. Obesity 2014, 22, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Bonnefond, A.; Manzoor, J.; Shabir, F.; Ayesha, H.; Philippe, J.; Durand, E.; Crouch, H.; Sand, O.; Ali, M.; et al. Genetic Variants in LEP, LEPR, and MC4R Explain 30% of Severe Obesity in Children from a Consanguineous Population. Obesity 2015, 23, 1687–1695. [Google Scholar] [CrossRef]
- Vauthier, V.; Jaillard, S.; Journel, H.; Dubourg, C.; Jockers, R.; Dam, J. Homozygous Deletion of an 80 Kb Region Comprising Part of DNAJC6 and LEPR Genes on Chromosome 1P31.3 Is Associated with Early Onset Obesity, Mental Retardation and Epilepsy. Mol. Genet. Metab. 2012, 106, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Hannema, S.E.; Wit, J.M.; Houdijk, M.E.C.A.M.; Van Haeringen, A.; Bik, E.C.; Verkerk, A.J.M.H.; Uitterlinden, A.G.; Kant, S.G.; Oostdijk, W.; Bakker, E.; et al. Novel Leptin Receptor Mutations Identified in Two Girls with Severe Obesity Are Associated with Increased Bone Mineral Density. Horm. Res. Paediatr. 2016, 85, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kimber, W.; Peelman, F.; Prieur, X.; Wangensteen, T.; O’Rahilly, S.; Tavernier, J.; Farooqi, I.S. Functional Characterization of Naturally Occurring Pathogenic Mutations in the Human Leptin Receptor. Endocrinology 2008, 149, 6043–6052. [Google Scholar] [CrossRef]
- Zorn, S.; von Schnurbein, J.; Kohlsdorf, K.; Denzer, C.; Wabitsch, M. Diagnostic and Therapeutic Odyssey of Two Patients with Compound Heterozygous Leptin Receptor Deficiency. Mol. Cell. Pediatr. 2020, 7, 15. [Google Scholar] [CrossRef]
- Clément, K.; van den Akker, E.; Argente, J.; Bahm, A.; Chung, W.K.; Connors, H.; De Waele, K.; Farooqi, I.S.; Gonneau-Lejeune, J.; Gordon, G.; et al. Efficacy and Safety of Setmelanotide, an MC4R Agonist, in Individuals with Severe Obesity Due to LEPR or POMC Deficiency: Single-Arm, Open-Label, Multicentre, Phase 3 Trials. Lancet Diabetes Endocrinol. 2020, 8, 960–970. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves First Treatment for Weight Management for People with Certain Rare Genetic Conditions—FDA. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-first-treatment-weight-management-people-certain-rare-genetic-conditions (accessed on 22 February 2021).
- Berger, C.; Heyne, H.O.; Heiland, T.; Dommel, S.; Höfling, C.; Guiu-Jurado, E.; Roßner, S.; Dannemann, M.; Kelso, J.; Kovacs, P.; et al. A Compound Heterozygous Leptin Receptor Mouse Model Mimics Db/Db Mouse Characteristics but Displays More Severe Obesity. J. Lipid. Res. 2021. under review. [Google Scholar]
- Chung, W.K.; Belfi, K.; Chua, M.; Wiley, J.; Mackintosh, R.; Nicolson, M.; Boozer, C.N.; Leibel, R.L. Heterozygosity for Lep(Ob) or Lepr(Db) Affects Body Composition and Leptin Homeostasis in Adult Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 274, R985–R990. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, X.; Li, H.; Hu, Y.; Zhou, J.; He, D.; Feng, Y.; Lu, L.; Du, G.; Hu, Y.; et al. The 14th Ile Residue Is Essential for Leptin Function in Regulating Energy Homeostasis in Rat. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Wallis, N.; Raffan, E. The Genetic Basis of Obesity and Related Metabolic Diseases in Humans and Companion Animals. Genes 2020, 11, 1378. [Google Scholar] [CrossRef] [PubMed]

| Mutation Gene Level | Mutation Protein Level | Protein | Symptoms | Treatment | |
|---|---|---|---|---|---|
| Kimber 2008, Farooqi 2007 [48,78] | 1 bp deletion in codon 15 and Arg612His | - Arg612His | “Receptors with some residual ability to phosphorylate STAT3 in response to leptin” | -Early extreme obesity -Hyperphagia -Lep not elevated -Impaired growth | Not described |
| Huvenne 2015 [71] | c.1604–1G > A and c.Δexon6–8 | p.535–1G>A (probably exon 12 skipping) and p.Pro166CysfsX7 | Probably truncated protein and truncated protein (172 aa) | -Early extreme obesity -Hyperphagia -Elevated leptin level | Care models described |
| c.1264T > C and c.2131 dup | p.Tyr422His and p.Thr711AsnfsX18 | Probably damaged and truncated protein | -Early extreme obesity -Hyperphagia -Elevated Lep level -Type 2 diabetes (1 of sibling) -No Hypogonadotropic hypogonadism | Care models described | |
| Hannema 2016 [77] | c.1753–1dupG c.2168C > T | p.Met585Aspfs * 2 p.Ser723Phe | Probably truncated protein | -Early extreme obesity -Normal Lep for obese -Increased growth -No Hypogonadotropic hypogonadism | Not described |
| Zorn 2020 [79] | c.2598-3_2607delTA- GAATGAAAAAG c.2227 T > C | Intron p.Ser743Pro | Truncated protein | -Early extreme obesity -Hyperphagia -Hypogonadotropic hypogonadism -Dyslipidemia -Hyperinsulinemia -Hepatic steatosis -Hyperuricemia | -Behavioral treatments -Bariatric surgery -MC4R agonist Setmelanotide therapy |
| c.1874G > A c.2051A > C | p.His684Pro p.Trp625 * | Truncated protein | -Early extreme obesity -Hyperphagia -Hypogonadotropic hypogonadism -Growth hormone deficiency | -Conservative treatment -Gastric banding and removal -MC4R agonist Setmelanotide therapy | |
| Voigtmann 2020 [68] | c.1231_1233 c.1835G > A | p.Tyr411del p.Trp664Arg | Probably truncated protein | -Early severe obesity -Hyperphagia -Short statue -Hypothyroidism -Dyslipidemia -Hyperinsulinemia -Normal Lep for obese | -MC4R agonist Setmelanotide in clinical trail |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, C.; Klöting, N. Leptin Receptor Compound Heterozygosity in Humans and Animal Models. Int. J. Mol. Sci. 2021, 22, 4475. https://doi.org/10.3390/ijms22094475
Berger C, Klöting N. Leptin Receptor Compound Heterozygosity in Humans and Animal Models. International Journal of Molecular Sciences. 2021; 22(9):4475. https://doi.org/10.3390/ijms22094475
Chicago/Turabian StyleBerger, Claudia, and Nora Klöting. 2021. "Leptin Receptor Compound Heterozygosity in Humans and Animal Models" International Journal of Molecular Sciences 22, no. 9: 4475. https://doi.org/10.3390/ijms22094475
APA StyleBerger, C., & Klöting, N. (2021). Leptin Receptor Compound Heterozygosity in Humans and Animal Models. International Journal of Molecular Sciences, 22(9), 4475. https://doi.org/10.3390/ijms22094475






