What Are the Potential Roles of Nuclear Perlecan and Other Heparan Sulphate Proteoglycans in the Normal and Malignant Phenotype
Abstract
1. Introduction
1.1. Intracellular Perlecan—What Does It Do?
- (1)
- Is the structure of nuclear-associated perlecan similar to the perlecan that occurs in the pericellular and extracellular environment?
- (2)
- What are the specific functional roles of nuclear-associated and perinuclear perlecan?
- (3)
- What, if any, are perlecan’s interactive ligands within the nucleus and perinuclear regions of the cell?
- (4)
- How do (1)–(3) relate to disease processes such as those occurring in tumour development.
1.2. Biosynthesis of Perlecan
Heparan Sulphate Is a Highly Interactive Molecule
2. Perlecan’s Roles in Vascularised, Tensional and Weight Bearing Shear-Loaded Tissues
3. Mechanosensory Processes in Tensional and Weight Bearing Tissues Effect the Nucleus and Impact on Gene Expression
4. Membrane Polarization, Evolution of Membrane Energetics and Roles for GAGs in Motive Proton Gradients
Phosphatases/Kinases and HATs/HDACs in Cell Signaling and Gene Regulation
5. Inhibition of Histone Acetyl Transferases and the Use of HDACs as Therapeutic Agents
Histone acetylation/Deacetylation of Chromatin Effects Nucleosomal Structure and Impacts on Chondrocyte Regulation
6. Cytoskeleton Mediated Spatial Re-Organisation of Cellular Components in Pre-Motile Cells
7. Structural Organisation of the Nucleus
7.1. Nuclear HS-PGs
7.2. Nuclear Heparanase Regulates HSPG Structure and Modulates Gene Activity
7.3. HS-Proteoglycan Mediated Interactions with the Cytoskeleton
7.4. Nuclear Protein Interactions with HS-PGs Effects Cytoskeletal Organization
8. Control of Chromatin Structure and Gene Regulation by HS-PGs
9. Nuclear FGF-1, FGF-2 and FGFRs
Neuronal Cell-Repair Responses Initiated by FGF-2, FGFR-1 and HS-PGs
10. Investigations on Nuclear Interactomes Reveals the Roles of Nuclear HS-PGs
11. Changes in Chromatin Structure in the Nucleus—Can Perlecan Stabilise Chromatin?
12. Nuclear Mechanosensory Properties: Dynamic Interplay of Proteins in the Nucleoskeleton, Nuclear Envelope and Cytoskeleton Provide Mechanical Support to the Nucleus
13. Nuclear HS-PGs and Tumour Development
14. Perlecan’s Contributions to the Malignant Phenotype
14.1. Perlecan-FGF-FGFR Interactions
14.2. Perlecan-HS Interactions with Histones
14.3. Perlecan-VEGF-2 Interactions Promote Vascularisation of Tumours
14.4. Perlecan Contributes to Cancer Promoting Processes Proposed by Hanahan and Weinberg
14.5. Perlecan and Potential Antagonism with Histone Transport to the Nucleus
14.6. Electrostatic Interactions between Lysine and Arginine Histone Residues and Perlecan-HS
15. HS Is Involved in Regulatory Processes in Cancer
Histone Acetylation-Deacetylation, HS and Regulation of the Malignant Phenotype
16. Potential of HS as a Therapeutic Target in Cancer
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
Abbreviations
| ADAM | a disintegrin and metalloproteinase |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| BAF | barrier-to-autointegration factor |
| BMPs | bone morphogenetic proteins |
| CASK | calcium/calmodulin dependent serine protein kinase |
| DOCK8 | dedicator of cytokinesis 8 |
| E2F | a group of genes that encode a family of transcription factors involved in control of the G1/S transition |
| EGR-1 | early growth response 1 |
| EMT | epithelial-mesenchymal transition |
| FAK | focal adhesion kinase |
| FGF | fibroblast growth factor |
| GTPase | nucleotide guanosine triphosphatase |
| HAT | histone acetyl transferase |
| HDAC | histone deacetylase |
| HIF-1/HIF-2 | hypoxia inducible factor-1/2 |
| KASH | Klarsicht, ANC-1, Syne Homology |
| LAP2 | Thymopoetin |
| LDL | low density lipoprotein |
| LG4/LG5 | laminin G like domains 4 and 5 |
| LINC | a linker of nucleoskeleton-cytoskeleton complex |
| MAPK | mitogen-activated protein kinase |
| MEF2C | myocyte-specific enhancer factor 2C, MADS box transcription enhancer factor 2 |
| NEK11 | never-in-mitosis gene a-related kinase 11 |
| NF-κB/p65 | a NF-κB/Rel protein member encoded by the RelA gene |
| NFκβ | nuclear factor kappa-light-chain-enhancer of activated B cells |
| OCT-1 | octamer transcription factor-1 |
| OPG | Osteoprotegerin |
| pCAF HAT | p300/CBP-associated factor complex with HAT activity |
| p300 | histone acetylase p300 |
| PDGF | platelet derived growth factor |
| PDZ | PDZ, a combined term consisting of the first letter of Post synaptic density protein, Drosophila post synaptic density protein, and Zonula occludens-1 protein |
| PHDs | prolyl hydroxylases |
| PI3K-AKT | phosphatidylinositol-3-kinase/a serine/threonine protein kinase |
| PLC | phospholipase C |
| RAS | RAS term is derived from two rat sarcoma cancer causing viruses |
| Rho | Rho family of GTP’ases |
| RTK | receptor tyrosine kinase |
| RUNX2 | runt-related transcription factor 2 |
| Src | proto-oncogene tyrosine-protein kinase |
| SMCs | smooth muscle cells |
| STAT | signal transducer and activator of transcription proteins |
| SUN | Sad1 and UNC-84 |
| VEGF | vascular endothelial cell growth factor |
| Wnt | The term Wnt is derived from a mouse proto-oncogene (Int1, integration-1 protein) and the Wingless protein identified in Drosophila. The Wnt term is a condensation of the Int and Wg protein terms and stands for the Wingless-related integration site. |
References
- Hayes, A.; Melrose, J. 3D Distribution of Perlecan within Intervertebral Disc Chondrons Suggests Novel Regulatory Roles for this Multifunctional Modular Heparan Sulphate Proteoglycan. Eur. Cells Mater. 2021, 41, 73–89. [Google Scholar] [CrossRef]
- Chen, L.; Sanderson, R.D. Heparanase regulates levels of syndecan-1 in the nucleus. PLoS ONE 2009, 4, e4947. [Google Scholar] [CrossRef] [PubMed]
- Farfán, N.; Orellana-Serradell, O.; Herrera, D.; Chrzanowsky, D.; Cubillos, P.; Marín, G.; De Herreros, A.A.G.; Castellón, E.A.; Contreras, H.R. SNAIL expression correlates with the translocation of syndecan 1 intracellular domain into the nucleus in prostate cancer cell lines. Int. J. Mol. Med. 2020, 45, 1073–1080. [Google Scholar]
- Kovalszky, I.; Hjerpe, A.; Dobra, K. Nuclear translocation of heparan sulfate proteoglycans and their functional significance. Biochim. Biophys. Acta 2014, 1840, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Singh, A.; Shrinet, J.; Parniewska, M.M.; Fuxe, J.; Dobra, K.; Hjerpe, A. Mapping the Interactome of the Nuclear Heparan Sulfate Proteoglycan Syndecan-1 in Mesothelioma Cells. Biomolecules 2020, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Häring, M.; Roughley, P.J.; Margolis, R.K.; Margolis, R.U. Glypican and biglycan in the nuclei of neurons and glioma cells: Presence of functional nuclear localization signals and dynamic changes in glypican during the cell cycle. J. Cell Biol. 1997, 139, 851–864. [Google Scholar] [CrossRef]
- Multhaupt, H.; Yoneda, A.; Whiteford, J.R.; Oh, E.S.; Lee, W.; Couchman, J.R. Syndecan signaling: When, where and why? J. Physiol. Pharmacol. 2009, 60, 31–38. [Google Scholar]
- Stewart, M.; Sanderson, R.D. Heparan sulfate in the nucleus and its control of cellular functions. Matrix Biol. 2014, 35, 56–59. [Google Scholar] [CrossRef]
- Stewart, M.; Ramani, V.C.; Sanderson, R.D. Shed syndecan-1 translocates to the nucleus of cells delivering growth factors and inhibiting histone acetylation: A novel mechanism of tumor-host cross-talk. J. Biol. Chem. 2015, 290, 941–949. [Google Scholar] [CrossRef]
- Geetha-Habib, M.; Campbell, S.C.; Schwartz, N.B. Subcellular localization of the synthesis and glycosylation of chondroitin sulfate proteoglycan core protein. J. Biol. Chem. 1984, 259, 7300–7310. [Google Scholar] [CrossRef]
- Ratcliffe, A.; Fryer, P.R.; Hardingham, T.E. Proteoglycan biosynthesis in chondrocytes: Protein A-gold localization of proteoglycan protein core and chondroitin sulfate within Golgi subcompartments. J. Cell Biol. 1985, 101, 2355–2365. [Google Scholar] [CrossRef]
- Kimura, J.; Lohmander, L.S.; Hascall, V.C. Studies on the biosynthesis of cartilage proteoglycan in a model system of cultured chondrocytes from the Swarm rat chondrosarcoma. J. Cell. Biochem. 1984, 26, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Godman, G.; Lane, N. On the site of sulfation in the chondrocyte. J. Cell Biol. 1964, 21, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Kimata, K.; Okayama, M.; Suzuki, S.; Suzuki, I.; Hoshino, M. Nascent mucopolysaccharides attached to the Golgi membrane of chondrocytes. Biochim. Biophys. Acta 1971, 237, 606–610. [Google Scholar] [CrossRef]
- Pacifici, M.; Soltesz, R.; Thal, G.; Shanley, D.J.; Boettiger, D.; Holtzer, H. Immunological characterization of the major chick cartilage proteoglycan and its intracellular localization in cultured chondroblasts: A comparison with Type II procollagen. J. Cell Biol. 1983, 97, 1724–1736. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, F.; Linhardt, R.J. Analysis of the Glycosaminoglycan Chains of Proteoglycans. J. Histochem. Cytochem. 2021, 69, 121–135. [Google Scholar] [CrossRef]
- Vertel, B.; Dorfman, A. An immunohistochemical study of extracellular matrix formation during chondrogenesis. Dev. Biol. 1978, 62, 1–12. [Google Scholar] [CrossRef]
- Vertel, B.; Dorfman, A. Simultaneous localization of type II collagen and core protein of chondroitin sulfate proteoglycan in individual chondrocytes. Proc. Natl. Acad. Sci. USA 1979, 76, 1261–1264. [Google Scholar] [CrossRef]
- Vertel, B.; Barkman, L.L. Immunofluorescence studies of chondroitin sulfate proteoglycan biosynthesis: The use of monoclonal antibodies. Collagen Relat. Res. 1984, 4, 1–20. [Google Scholar] [CrossRef]
- Lohmander, L.; Hascall, V.C.; Yanagishita, M.; Kuettner, K.E.; Kimura, J.H. Post-translational events in proteoglycan synthesis: Kinetics of synthesis of chondroitin sulfate and oligosaccharides on the core protein. Arch. Biochem. Biophys. 1986, 250, 211–227. [Google Scholar] [CrossRef]
- Ethen, C.; Machacek, M.; Prather, B.; Tatge, T.; Yu, H.; Wu, Z.L. Nonradioactive glycosyltransferase and sulfotransferase assay to study glycosaminoglycan biosynthesis. Methods Mol. Biol. 2015, 1229, 431–441. [Google Scholar]
- Sasarman, F.; Maftei, C.; Campeau, P.M.; Brunel-Guitton, C.; Mitchell, G.A.; Allard, P. Biosynthesis of glycosaminoglycans: Associated disorders and biochemical tests. J. Inherit. Metab. Dis. 2016, 39, 173–188. [Google Scholar] [CrossRef]
- Mochizuki, H.; Yoshida, K.; Shibata, Y.; Kimata, K. Tetrasulphated disaccharide unit in heparin sulphate: Enzymatic formation and tissue distribution. J. Biol. Chem. 2008, 283, 31237–31245. [Google Scholar] [CrossRef] [PubMed]
- Whitelock, J.; Iozzo, R.V. Heparan sulfate: A complex polymer charged with biological activity. Chem. Rev. 2005, 105, 2745–2764. [Google Scholar] [CrossRef] [PubMed]
- Ori, A.; Wilkinson, M.C.; Fernig, D.G. A systems biology approach for the investigation of the heparin/heparan sulfate interactome. J. Biol. Chem. 2011, 286, 19892–19904. [Google Scholar] [CrossRef]
- Monneau, Y.; Arenzana-Seisdedos, F.; Lortat-Jacob, H. The sweet spot: How GAGs help chemokines guide migrating cells. J. Leukoc. Biol. 2016, 99, 935–953. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Q.; Mournetas, V.; Lane, B.; Sutton, R.; Fernig, D.G.; Vasieva, O. The Heparin-Binding Protein Interactome in Pancreatic Diseases. Pancreatology 2013, 13, 598–604. [Google Scholar] [CrossRef]
- Nunes, Q.; Su, D.; Brownridge, P.J.; Simpson, D.M.; Sun, C.; Li, Y.; Bui, T.P.; Zhang, X.; Huang, W.; Rigden, D.J.; et al. The Heparin-Binding Proteome in Normal Pancreas and Murine Experimental Acute Pancreatitis. PLoS ONE 2019, 14, e0217633. [Google Scholar] [CrossRef]
- Meneghetti, M.; Hughes, A.J.; Rudd, T.R.; Nader, H.B.; Powell, A.K.; Yates, E.A.; Lima, M.A. Heparan sulfate and heparin interactions with proteins. J. R. Soc. Interface 2015, 12, 20150589. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, W.; Kalus, I.; Padva, M.; Baldwin, R.J.; Merry, C.L.; Dierks, T. The heparanome—The enigma of encoding and decoding heparan sulfate sulfation. J. Biotechnol. 2007, 129, 290–307. [Google Scholar] [CrossRef]
- Simon Davis, D.; Parish, C.R. Heparan sulfate: A ubiquitous glycosaminoglycan with multiple roles in immunity. Front. Immunol. 2013, 4, 470. [Google Scholar] [CrossRef]
- Han, K.; Jeon, S.; Um, J.W.; Ko, J. Emergent Synapse Organizers: LAR-RPTPs and Their Companions. Int. Rev. Cell Mol. Biol. 2016, 324, 39–65. [Google Scholar] [PubMed]
- Han, K.; Kim, Y.J.; Yoon, T.H.; Kim, H.; Bae, S.; Um, J.W.; Choi, S.Y.; Ko, J. LAR-RPTPs Directly Interact with Neurexins to Coordinate Bidirectional Assembly of Molecular Machineries. J. Neurosci. 2020, 40, 8438–8462. [Google Scholar] [CrossRef]
- Long, F.; Zhou, J.; Peng, H. Visualization and analysis of 3D microscopic images. PLoS Comput. Biol. 2012, 8, e1002519. [Google Scholar] [CrossRef]
- Ori, A.; Wilkinson, M.C.; Fernig, D.G. The heparanome and regulation of cell function: Structures, functions and challenges. Front. Biosci. 2008, 13, 4309–4338. [Google Scholar] [CrossRef] [PubMed]
- Aviezer, D.; Yayon, A. Heparin-dependent binding and autophosphorylation of epidermal growth factor (EGF) receptor by heparin-binding EGF-like growth factor but not by EGF. Proc. Natl. Acad. Sci. USA 1994, 91, 12173–12177. [Google Scholar] [CrossRef] [PubMed]
- Higashiyama, S.; Abraham, J.A.; Klagsbrun, M. Heparin-binding EGF-like growth factor stimulation of smooth muscle cell migration: Dependence on interactions with cell surface heparan sulfate. J. Cell Biol. 1993, 122, 933–940. [Google Scholar] [CrossRef]
- Coombe, D. Biological implications of glycosaminoglycan interactions with haemopoietic cytokines. Immunol. Cell Biol. 2008, 86, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Harmer, N. Insights into the role of heparan sulphate in fibroblast growth factor signalling. Biochem. Soc. Trans. 2006, 34, 442–445. [Google Scholar] [CrossRef]
- Ornitz, D. FGFs, heparan sulfate and FGFRs: Complex interactions essential for development. Bioessays 2000, 22, 108–112. [Google Scholar] [CrossRef]
- Ornitz, D.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef]
- Ornitz, D.; Marie, P.J. Fibroblast growth factors in skeletal development. Curr. Top. Dev. Biol. 2019, 133, 195–234. [Google Scholar]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Wiedłocha, A.; Sørensen, V. Signaling, internalization, and intracellular activity of fibroblast growth factor. Curr. Top. Microbiol. Immunol. 2004, 286, 45–79. [Google Scholar] [PubMed]
- Lord, M.; Chuang, C.Y.; Melrose, J.; Davies, M.J.; Iozzo, R.V.; Whitelock, J.M. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol. 2014, 35, 112–122. [Google Scholar] [CrossRef]
- Stringer, S. The role of heparan sulphate proteoglycans in angiogenesis. Biochem. Soc. Trans. 2006, 34, 451–453. [Google Scholar] [CrossRef]
- Cecchi, F.; Pajalunga, D.; Fowler, C.A.; Uren, A.; Rabe, D.C.; Peruzzi, B.; Macdonald, N.J.; Blackman, D.K.; Stahl, S.J.; Byrd, R.A.; et al. Targeted disruption of heparan sulfate interaction with hepatocyte and vascular endothelial growth factors blocks normal and oncogenic signaling. Cancer Cell 2012, 22, 250–262. [Google Scholar] [CrossRef]
- Rubin, J.; Day, R.M.; Breckenridge, D.; Atabey, N.; Taylor, W.G.; Stahl, S.J.; Wingfield, P.T.; Kaufman, J.D.; Schwall, R.; Bottaro, D.P. Dissociation of heparan sulfate and receptor binding domains of hepatocyte growth factor reveals that heparan sulfate-c-met interaction facilitates signaling. J. Biol. Chem. 2001, 276, 32977–32983. [Google Scholar] [CrossRef] [PubMed]
- Silber, L.; Walenga, J.M.; Fareed, J.; Kovacs, E.J. Heparan sulphate inhibition of cell proliferation induced by TGFbeta and PDGF. Mediat. Inflamm. 1993, 2, 299–302. [Google Scholar] [CrossRef]
- Rider, C. Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochem. Soc. Trans. 2006, 34, 458–460. [Google Scholar] [CrossRef]
- Rider, C.; Mulloy, B. Heparin, Heparan Sulphate and the TGF-β Cytokine Superfamily. Molecules 2017, 22, 713. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B.; Rider, C.C. The Bone Morphogenetic Proteins and Their Antagonists. Vitam. Horm. 2015, 99, 63–90. [Google Scholar] [PubMed]
- Sheng, G.; Oh, Y.I.; Chang, S.K.; Hsieh-Wilson, L.C. Tunable heparan sulfate mimetics for modulating chemokine activity. J. Am. Chem. Soc. 2013, 135, 10898–10901. [Google Scholar] [CrossRef] [PubMed]
- Tyler, P.; Guimond, S.E.; Turnbull, J.E.; Zubkova, O.V. Single-entity heparan sulfate glycomimetic clusters for therapeutic applications. Angew. Chem. 2015, 54, 2718–2723. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.; Cheng, B.; Farrugia, B.L.; McCarthy, S.; Whitelock, J.M. Platelet Factor 4 Binds to Vascular Proteoglycans and Controls Both Growth Factor Activities and Platelet Activation. J. Biol. Chem. 2017, 292, 4054–4063. [Google Scholar] [CrossRef]
- Xie, M.; Li, J.P. Heparan sulfate proteoglycan—A common receptor for diverse cytokines. Cell. Signal. 2019, 54, 115–121. [Google Scholar] [CrossRef]
- Bartolini, B.; Caravà, E.; Caon, I.; Parnigoni, A.; Moretto, P.; Passi, A.; Vigetti, D.; Viola, M.; Karousou, E. Heparan Sulfate in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1245, 147–161. [Google Scholar]
- Hassan, N.; Greve, B.; Espinoza-Sánchez, N.A.; Götte, M. Cell-surface heparan sulfate proteoglycans as multifunctional integrators of signaling in cancer. Cell. Signal. 2021, 77, 109822. [Google Scholar] [CrossRef]
- Whitelock, J.M.; Melrose, J.; Iozzo, R.V. Diverse cell signaling events modulated by perlecan. Biochemistry 2008, 47, 11174–11183. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Hayes, A.J.; Whitelock, J.M.; Little, C.B. Perlecan, the “jack of all trades” proteoglycan of cartilaginous weight-bearing connective tissues. Bioessays 2008, 30, 457–469. [Google Scholar] [CrossRef]
- Siegel, G.; Malmsten, M.; Ermilov, E. Anionic biopolyelectrolytes of the syndecan/perlecan superfamily: Physicochemical properties and medical significance. Adv. Colloid Interface Sci. 2014, 205, 275–318. [Google Scholar] [CrossRef]
- Garl, P.; Wenzlau, J.M.; Walker, H.A.; Whitelock, J.M.; Costell, M.; Weiser-Evans, M.C. Perlecan-induced suppression of smooth muscle cell proliferation is mediated through increased activity of the tumor suppressor PTEN. Circ. Res. 2004, 94, 175–183. [Google Scholar] [CrossRef]
- Gotha, L.; Lim, S.Y.; Osherov, A.B.; Wolff, R.; Qiang, B.; Erlich, I.; Nili, N.; Pillarisetti, S.; Chang, Y.T.; Tran, P.K.; et al. Heparan sulfate side chains have a critical role in the inhibitory effects of perlecan on vascular smooth muscle cell response to arterial injury. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H337–H345. [Google Scholar] [CrossRef]
- Segev, A.; Nili, N.; Osherov, A.B.; Qiang, B.; Wong, A.J.; Pillarisetti, S.; Strauss, B.H. A perlecan-inducing compound significantly inhibits smooth muscle cell function and in-stent intimal hyperplasia: Novel insights into the diverse biological effects of perlecan. EuroIntervention 2010, 6, 134–140. [Google Scholar] [CrossRef]
- Segev, A.; Nili, N.; Strauss, B.H. The role of perlecan in arterial injury and angiogenesis. Cardiovasc. Res. 2004, 63, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Walker, H.; Whitelock, J.M.; Garl, P.J.; Nemenoff, R.A.; Stenmark, K.R.; Weiser-Evans, M.C. Perlecan up-regulation of FRNK suppresses smooth muscle cell proliferation via inhibition of FAK signaling. Mol. Biol. Cell 2003, 14, 1941–1952. [Google Scholar] [CrossRef]
- Pei, S.; Parthasarathy, S.; Parajuli, A.; Martinez, J.; Lv, M.; Jiang, S.; Wu, D.; Wei, S.; Lu, X.L.; Farach-Carson, M.C.; et al. Perlecan/Hspg2 deficiency impairs bone’s calcium signaling and associated transcriptome in response to mechanical loading. Bone 2020, 131, 115078. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lai, X.; Price, C.; Thompson, W.R.; Li, W.; Quabili, T.R.; Tseng, W.J.; Liu, X.S.; Zhang, H.; Pan, J.; et al. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J. Bone Miner. Res. 2014, 29, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Wilusz, R.E.; Defrate, L.E.; Guilak, F. A biomechanical role for perlecan in the pericellular matrix of articular cartilage. Matrix Biol. 2012, 31, 320–327. [Google Scholar] [CrossRef]
- Zoeller, J.; Whitelock, J.M.; Iozzo, R.V. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009, 28, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. Perlecan, a modular instructive proteoglycan with diverse functional properties. Int. J. Biochem. Cell Biol. 2020, 128, 105849. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Tellman, T.V.; Farach-Carson, M.C. Flipping the Molecular Switch: Influence of Perlecan and Its Modifiers in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1245, 133–146. [Google Scholar] [PubMed]
- Goyal, A.; Pal, N.; Concannon, M.; Paul, M.; Doran, M.; Poluzzi, C.; Sekiguchi, K.; Whitelock, J.M.; Neill, T.; Iozzo, R.V. Endorepellin, the angiostatic module of perlecan, interacts with both the alpha2beta1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2): A dual receptor antagonism. J. Biol. Chem. 2011, 286, 25947–25962. [Google Scholar] [CrossRef]
- Douglass, S.; Goyal, A.; Iozzo, R.V. The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy. Connect. Tissue Res. 2015, 56, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Elgundi, Z.; Papanicolaou, M.; Major, G.; Cox, T.R.; Melrose, J.; Whitelock, J.M.; Farrugia, B.L. Cancer Metastasis: The Role of the Extracellular Matrix and the Heparan Sulfate Proteoglycan Perlecan. Front. Oncol. 2020, 9, 1482. [Google Scholar] [CrossRef]
- Jiang, X.; Couchman, J.R. Perlecan and tumor angiogenesis. J. Histochem. Cytochem. 2003, 51, 1393–1410. [Google Scholar] [CrossRef]
- Ingber, D. Cellular tensegrity: Defining new rules of biological design that govern the cytoskeleton. J. Cell Sci. 1993, 104, 613–627. [Google Scholar]
- Ingber, D. Control of capillary growth and differentiation by extracellular matrix. Use of a tensegrity (tensional integrity) mechanism for signal processing. Chest 1991, 99, 34S–40S. [Google Scholar] [CrossRef]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef]
- Chen, C.; Ingber, D.E. Tensegrity and mechanoregulation: From skeleton to cytoskeleton. Osteoarthr. Cartil. 1999, 7, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 2008, 97, 163–179. [Google Scholar] [CrossRef]
- Ingber, D. Tensegrity and mechanotransduction. J. Bodyw. Mov. Ther. 2008, 12, 198–200. [Google Scholar] [CrossRef]
- Ingber, D.; Dike, L.; Hansen, L.; Karp, S.; Liley, H.; Maniotis, A.; McNamee, H.; Mooney, D.; Plopper, G.; Sims, J.; et al. Cellular tensegrity: Exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int. Rev. Cytol. 1994, 150, 173–224. [Google Scholar]
- Ingber, D. Mechanical control of tissue morphogenesis during embryological development. Int. J. Dev. Biol. 2006, 50, 255–266. [Google Scholar] [CrossRef]
- Wang, N.; Ingber, D.E. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys. J. 1994, 66, 2181–2189. [Google Scholar] [CrossRef]
- Athirasala, A.; Hirsch, N.; Buxboim, A. Nuclear mechanotransduction: Sensing the force from within. Curr. Opin. Cell Biol. 2017, 46, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kirby, T.; Lammerding, J. Emerging views of the nucleus as a cellular mechanosensor. Nat. Cell Biol. 2018, 20, 373–381. [Google Scholar] [CrossRef]
- Wang, N.; Naruse, K.; Stamenović, D.; Fredberg, J.J.; Mijailovich, S.M.; Tolić-Nørrelykke, I.M.; Polte, T.; Mannix, R.; Ingber, D.E. Mechanical behavior in living cells consistent with the tensegrity model. Proc. Natl. Acad. Sci. USA 2001, 98, 7765–7770. [Google Scholar] [CrossRef]
- Ingber, D. Tensegrity, I. Cell structure and hierarchical systems biology. J. Cell Sci. 2003, 116, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D. Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003, 116, 1397–1408. [Google Scholar] [CrossRef]
- Ingber, D.; Wang, N.; Stamenovic, D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep. Prog. Phys. 2014, 77, 046603. [Google Scholar] [CrossRef]
- Stamenović, D.; Mijailovich, S.M.; Tolić-Nørrelykke, I.M.; Chen, J.; Wang, N. Cell prestress. II. Contribution of microtubules. Am. J. Physiol. Cell Physiol. 2002, 282, C617–C624. [Google Scholar] [CrossRef]
- Wang, N.; Tolić-Nørrelykke, I.M.; Chen, J.; Mijailovich, S.M.; Butler, J.P.; Fredberg, J.J.; Stamenović, D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am. J. Physiol. Cell Physiol. 2002, 282, C606–C616. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, J.; Uzer, G.; van Wijnen, A.J.; Rubin, J. Gene regulation through dynamic actin control of nuclear structure. Exp. Biol. Med. 2019, 244, 1345–1353. [Google Scholar] [CrossRef]
- Ingber, D. The origin of cellular life. Bioessays 2000, 22, 1160–1170. [Google Scholar] [CrossRef]
- Ingber, D. Integrins, tensegrity, and mechanotransduction. Gravit. Space Biol. Bull. 1997, 10, 49–55. [Google Scholar]
- Ingber, D. Tensegrity: The architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997, 59, 575–599. [Google Scholar] [CrossRef]
- Guilak, F.; Hayes, A.J.; Melrose, J. Perlecan In Pericellular Mechanosensory Cell-Matrix Communication, Extracellular Matrix Stabilisation and Mechanoregulation of Load-Bearing Connective Tissues. Int. J. Mol. Sci. 2021, 22, 2716. [Google Scholar] [CrossRef]
- Chen, W.; Hsu, W.T.; Yen, M.H.; Changou, C.A.; Han, C.L.; Chen, Y.J.; Cheng, J.Y.; Chang, T.H.; Lee, O.K.; Ho, J.H. Alteration of mesenchymal stem cells polarity by laminar shear stimulation promoting β-catenin nuclear localization. Biomaterials 2019, 190–191, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Proton gradients at the origin of life. Bioessays 2017, 39, 6. [Google Scholar] [CrossRef] [PubMed]
- Lane, N. Why Are Cells Powered by Proton Gradients? Nat. Educ. 2010, 3, 18. [Google Scholar]
- Else, P. Mammals to membranes: A reductionist story. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2020, 253, 110552. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sugahara, K.; Ozbek, S. Evolution of glycosaminoglycans: Comparative biochemical study. Commun. Integr. Biol. 2011, 4, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Selberg, J.; Jia, M.; Rolandi, M. Proton conductivity of glycosaminoglycans. PLoS ONE 2019, 14, e0202713. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J. Functional Consequences of Keratan Sulfate Sulfation in Electrosensory Tissues and in Neuronal Regulation. Adv. Biosyst. 2019, 3, e1800327. [Google Scholar] [CrossRef]
- Lane, N.; Martin, W.F. The origin of membrane bioenergetics. Cell 2012, 151, 1406–1416. [Google Scholar] [CrossRef]
- Deamer, D. Membranes and the Origin of Life: A Century of Conjecture. J. Mol. Evol. 2016, 83, 159–168. [Google Scholar] [CrossRef]
- Strbak, O.; Kanuchova, Z.; Krafcik, A. Proton Gradients as a Key Physical Factor in the Evolution of the Forced Transport Mechanism Across the Lipid Membrane. Orig. Life Evol. Biosph. 2016, 46, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-C.; Qi, R.Z.; Paudel, H.; Zhu, H.-J. Regulation and function of protein kinases and phosphatases. Enzym. Res. 2011, 2011, 794089. [Google Scholar] [CrossRef]
- Ubersax, J.; Ferrell, J.E., Jr. Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol. 2007, 8, 530–541. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Braconi Quintaje, S.; Orchard, S. The annotation of both human and mouse kinomes in UniProtKB/Swiss-Prot: One small step in manual annotation, one giant leap for full comprehension of genomes. Mol. Cell. Proteomics 2008, 7, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Buczek-Thomas, J.A.; Hsia, E.; Rich, C.B.; Foster, J.A.; Nugent, M.A. Inhibition of histone acetyltransferase by glycosaminoglycans. J. Cell. Biochem. 2008, 105, 108–120. [Google Scholar] [CrossRef]
- Purushothaman, A.; Hurst, D.R.; Pisano, C.; Mizumoto, S.; Sugahara, K.; Sanderson, R.D. Heparanase-mediated loss of nuclear syndecan-1 enhances histone acetyltransferase (HAT) activity to promote expression of genes that drive an aggressive tumor phenotype. J. Biol. Chem. 2011, 286, 30377–30383. [Google Scholar] [CrossRef] [PubMed]
- Carpio, L.; Westendorf, J.J. Histone Deacetylases in Cartilage Homeostasis and Osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 52. [Google Scholar] [CrossRef]
- Im, G.; Choi, Y.J. Epigenetics in osteoarthritis and its implication for future therapeutics. Expert Opin. Biol. Ther. 2013, 13, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Haqqi, T.M. Epigenetics in osteoarthritis: Potential of HDAC inhibitors as therapeutics. Pharmacol. Res. 2018, 128, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ropero, S.; Esteller, M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Eberharter, A.; Becker, P.B. Histone acetylation: A switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002, 3, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Verdone, L.; Caserta, M.; Di Mauro, E. Role of histone acetylation in the control of gene expression. Biochem. Cell Biol. 2005, 83, 344–353. [Google Scholar] [CrossRef]
- Champoux, J.J. DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef]
- Wang, J.C. Cellular roles of DNA topoisomerases: A molecular perspective. Nat. Rev. Mol. Cell Biol. 2002, 3, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Z.; Guo, K.L.; Wei, X.; Zhang, Y.; Wang, X.; Wei, L. The role of histone deacetylase 4 during chondrocyte hypertrophy and endochondral bone development. Bone Jt. Res. 2020, 9, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Xiang, C.; Sang, X.; Wang, X.; Shi, Y.; Wang, N.; Wang, S.; Li, P.; Wei, X.; Zhang, M.; et al. Histone deacetylase 4 deletion results in abnormal chondrocyte hypertrophy and premature ossification from collagen type 2α1 expressing cells. Mol. Med. Rep. 2020, 22, 4031–4040. [Google Scholar] [PubMed]
- Gu, X.; Wei, L.; Li, P.C.; Che, X.D.; Zhao, R.P.; Han, P.F.; Lu, J.G.; Wei, X.C. Adenovirus-mediated transduction with Histone Deacetylase 4 ameliorates disease progression in an osteoarthritis rat model. Int. Immunopharmacol. 2019, 75, 105752. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Wei, L.; Zhang, Z.; Guo, L.; Zhang, C.; Li, Y.; Sun, C.; Sun, X.; Wang, S.; Li, P.; et al. Decreased histone deacetylase 4 is associated with human osteoarthritis cartilage degeneration by releasing histone deacetylase 4 inhibition of runt-related transcription factor-2 and increasing osteoarthritis-related genes: A novel mechanism of human osteoarthritis cartilage degeneration. Arthritis Res. Ther. 2014, 16, 491. [Google Scholar] [PubMed]
- Lu, J.; Sun, Y.; Ge, Q.; Teng, H.; Jiang, Q. Histone deacetylase 4 alters cartilage homeostasis in human osteoarthritis. BMC Musculoskelet. Disord. 2014, 15, 438. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Huang, Z.; Chen, W.; Huang, G.; Meng, F.; Zhang, Z.; Kang, Y. MicroRNA-92a-3p regulates the expression of cartilage-specific genes by directly targeting histone deacetylase 2 in chondrogenesis and degradation. Osteoarthr. Cartil. 2017, 25, 521–532. [Google Scholar] [CrossRef]
- Chen, W.; Chen, L.; Zhang, Z.; Meng, F.; Huang, G.; Sheng, P.; Zhang, Z.; Liao, W. MicroRNA-455-3p modulates cartilage development and degeneration through modification of histone H3 acetylation. Biochim. Biophys. Acta 2016, 1863, 2881–2891. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Zhang, Z.; Yang, Z.; Kang, Y.; Zhao, X.; Long, D.; Hu, S.; Gu, M.; He, S.; et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics 2018, 8, 2862–2883. [Google Scholar] [CrossRef]
- Gallinari, P.; Di Marco, S.; Jones, P.; Pallaoro, M.; Steinkühler, C. HDACs, histone deacetylation and gene transcription: From molecular biology to cancer therapeutics. Cell Res. 2007, 17, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, E.; Valkov, V.; Jung, M.; Jung, M.O. Characterization of novel inhibitors of histone acetyltransferases. Mol. Cancer Ther. 2007, 6, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Hontecillas-Prieto, L.; Flores-Campos, R.; Silver, A.; de Álava, E.; Hajji, N.; García-Domínguez, D.J. Synergistic Enhancement of Cancer Therapy Using HDAC Inhibitors: Opportunity for Clinical Trials. Front. Genet. 2020, 11, 578011. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef] [PubMed]
- Becsky, D.; Szabo, K.; Gyulai-Nagy, S.; Gajdos, T.; Bartos, Z.; Balind, A.; Dux, L.; Horvath, P.; Erdelyi, M.; Homolya, L.; et al. Syndecan-4 Modulates Cell Polarity and Migration by Influencing Centrosome Positioning and Intracellular Calcium Distribution. Front. Cell Dev. Biol. 2020, 8, 575227. [Google Scholar] [CrossRef]
- Maninová, M.; Vomastek, T. Dorsal stress fibers, transverse actin arcs, and perinuclear actin fibers form an interconnected network that induces nuclear movement in polarizing fibroblasts. FEBS J. 2016, 283, 3676–3693. [Google Scholar] [CrossRef]
- Fedarko, N.S.; Ishihara, M.; Conrad, H.E. Control of cell division in hepatoma cells by exogenous heparan sulfate proteoglycan. J. Cell. Physiol. 1989, 139, 287–294. [Google Scholar] [CrossRef]
- Hsia, E.; Richardson, T.P.; Nugent, M.A. Nuclear localization of basic fibroblast growth factor is mediated by heparan sulfate proteoglycans through protein kinase C signaling. J. Cell. Biochem. 2003, 88, 1214–1225. [Google Scholar] [CrossRef]
- Ishihara, M.; Conrad, H.E. Correlations between heparan sulfate metabolism and hepatoma growth. J. Cell. Physiol. 1989, 138, 467–476. [Google Scholar] [CrossRef]
- Ishihara, M.; Fedarko, N.S.; Conrad, H.E. Transport of heparan sulfate into the nuclei of hepatocytes. J. Biol. Chem. 1986, 261, 13575–13580. [Google Scholar] [CrossRef]
- Mathiesen, S.B.; Lunde, M.; Aronsen, J.M.; Romaine, A.; Kaupang, A.; Martinsen, M.; de Souza, G.A.; Nyman, T.A.; Sjaastad, I.; Christensen, G.; et al. The cardiac syndecan-4 interactome reveals a role for syndecan-4 in nuclear translocation of muscle LIM protein (MLP). J. Biol. Chem. 2019, 294, 8717–8731. [Google Scholar] [CrossRef]
- Szatmári, T.; Mundt, F.; Kumar-Singh, A.; Möbus, L.; Ötvös, R.; Hjerpe, A.; Dobra, K. Molecular targets and signaling pathways regulated by nuclear translocation of syndecan-1. BMC Cell Biol. 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Schrage, Y.M.; Hameetman, L.; Szuhai, K.; Cleton-Jansen, A.M.; Taminiau, A.H.; Hogendoorn, P.C.; Bovee, J.V. Aberrant heparan sulfate proteoglycan localization, despite normal exostosin, in central chondrosarcoma. Am. J. Pathol. 2009, 174, 979–988. [Google Scholar] [CrossRef]
- Li, J.; Yuan, W.; Jiang, S.; Ye, W.; Yang, H.; Shapiro, I.M.; Risbud, M.V. Prolyl-4-hydroxylase domain protein 2 controls NF-kappaB/p65 transactivation and enhances the catabolic effects of inflammatory cytokines on cells of the nucleus pulposus. J. Biol. Chem. 2015, 290, 7195–7207. [Google Scholar] [CrossRef]
- Fujita, N.; Markova, D.; Anderson, D.G.; Chiba, K.; Toyama, Y.; Shapiro, I.M.; Risbud, M.V. Expression of prolyl hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: Distinct roles of PHD2 and PHD3 proteins in controlling HIF-1alpha activity in hypoxia. J. Biol. Chem. 2012, 287, 16975–16986. [Google Scholar] [CrossRef] [PubMed]
- Couchman, J.; Gopal, S.; Lim, H.C.; Nørgaard, S.; Multhaupt, H.A. Fell-Muir Lecture: Syndecans: From peripheral coreceptors to mainstream regulators of cell behaviour. Int. J. Exp. Pathol. 2015, 96, 1–10. [Google Scholar] [CrossRef]
- Okina, E.; Manon-Jensen, T.; Whiteford, J.R.; Couchman, J.R. Syndecan proteoglycan contributions to cytoskeletal organization and contractility. Scand. J. Med. Sci. Sport. 2009, 19, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Okina, E.; Grossi, A.; Gopal, S.; Multhaupt, H.A.; Couchman, J.R. Alpha-actinin interactions with syndecan-4 are integral to fibroblast-matrix adhesion and regulate cytoskeletal architecture. Int. J. Biochem. Cell Biol. 2012, 44, 2161–2174. [Google Scholar] [CrossRef]
- Yoneda, A.; Couchman, J.R. Regulation of cytoskeletal organization by syndecan transmembrane proteoglycans. Matrix Biol. 2003, 22, 25–33. [Google Scholar] [CrossRef]
- Bass, M.; Humphries, M.J. Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signalling. Biochem. J. 2002, 368, 1–15. [Google Scholar] [CrossRef]
- Maninová, M.; Klímová, Z.; Parsons, J.T.; Weber, M.J.; Iwanicki, M.P.; Vomastek, T. The reorientation of cell nucleus promotes the establishment of front-rear polarity in migrating fibroblasts. J. Mol. Biol. 2013, 425, 2039–2055. [Google Scholar] [CrossRef]
- Yoo, J.; Jeong, M.J.; Cho, H.J.; Oh, E.S.; Han, M.Y. Dynamin II interacts with syndecan-4, a regulator of focal adhesion and stress-fiber formation. Biochem. Biophys. Res. Commun. 2005, 328, 424–431. [Google Scholar] [CrossRef]
- Schnoor, M.; Stradal, T.E.; Rottner, K. Cortactin: Cell Functions of A Multifaceted Actin-Binding Protein. Trends Cell Biol. 2018, 28, 79–98. [Google Scholar] [CrossRef]
- Chojnacka, K.; Mruk, D.D. The Src non-receptor tyrosine kinase paradigm: New insights into mammalian Sertoli cell biology. Mol. Cell. Endocrinol. 2015, 415, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Pataki, C.; Couchman, J.R.; Brábek, J. Wnt Signaling Cascades and the Roles of Syndecan Proteoglycans. J. Histochem. Cytochem. 2015, 63, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Baciu, P.; Saoncella, S.; Lee, S.H.; Denhez, F.; Leuthardt, D.; Goetinck, P.F. Syndesmos, a protein that interacts with the cytoplasmic domain of syndecan-4, mediates cell spreading and actin cytoskeletal organization. J. Cell Sci. 2000, 113, 315–324. [Google Scholar] [PubMed]
- Foley, K.; Young, P.W. The non-muscle functions of actinins: An update. Biochem. J. 2014, 459, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, M.; Chen, W.; Simons, M. Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration. J. Cell. Physiol. 2000, 184, 373–379. [Google Scholar] [CrossRef]
- Luyten, A.; Mortier, E.; Van Campenhout, C.; Taelman, V.; Degeest, G.; Wuytens, G.; Lambaerts, K.; David, G.; Bellefroid, E.J.; Zimmermann, P. The postsynaptic density 95/disc-large/zona occludens protein syntenin directly interacts with frizzled 7 and supports noncanonical Wnt signaling. Mol. Biol. Cell 2008, 19, 1594–1604. [Google Scholar] [CrossRef]
- Shimada, T.; Yasuda, S.; Sugiura, H.; Yamagata, K. Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions. Int. J. Mol. Sci. 2019, 20, 4171. [Google Scholar] [CrossRef]
- Ethell, I.; Hagihara, K.; Miura, Y.; Irie, F.; Yamaguchi, Y. Synbindin, A novel syndecan-2-binding protein in neuronal dendritic spines. J. Cell Biol. 2000, 151, 53–68. [Google Scholar] [CrossRef]
- Hsueh, Y. The role of the MAGUK protein CASK in neural development and synaptic function. Curr. Med. Chem. 2006, 13, 1915–1927. [Google Scholar] [CrossRef]
- Fan, S.; Feng, Y.; Wei, Z.; Xia, B.; Gong, W. Solution structure of synbindin atypical PDZ domain and interaction with syndecan-2. Protein Pept. Lett. 2009, 16, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.; Zhu, D.; Zhu, H. CD44 isoform-cytoskeleton interaction in oncogenic signaling and tumor progression. Front. Biosci. 1998, 3, d637–d649. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.; Sharma, R.; Nisakar, D.; Purohit, G.; Ganguli, M. Exogenous chondroitin sulfate glycosaminoglycan associate with arginine-rich peptide-DNA complexes to alter their intracellular processing and gene delivery efficiency. Biochim. Biophys. Acta 2015, 1848, 1053–1064. [Google Scholar] [CrossRef]
- Reilly, J.; Mizukoshi, E.; Maher, P.A. Ligand dependent and independent internalization and nuclear translocation of fibroblast growth factor (FGF) receptor 1. DNA Cell Biol. 2004, 23, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Belting, M.; Fransson, L.Å.; Mani, K. Nucleolin is a nuclear target of heparan sulfate derived from glypican-1. Exp. Cell Res. 2017, 354, 31–39. [Google Scholar] [CrossRef]
- Hiragami-Hamada, K.; Nakayama, J.I. Do the charges matter?-balancing the charges of the chromodomain proteins on the nucleosome. J. Biochem. 2019, 165, 455–458. [Google Scholar] [CrossRef]
- Lin, D.; Hoelz, A. The Structure of the Nuclear Pore Complex (An Update). Annu. Rev. Biochem. 2019, 88, 725–783. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Iwamoto, M.; Hiraoka, Y.; Haraguchi, T. Function of nuclear membrane proteins in shaping the nuclear envelope integrity during closed mitosis. J. Biochem. 2017, 161, 471–477. [Google Scholar] [CrossRef]
- Buchwalter, A.; Hetzer, M.W. Nuclear pores set the speed limit for mitosis. Cell 2014, 156, 868–869. [Google Scholar] [CrossRef][Green Version]
- Hetzer, M.; Walther, T.C.; Mattaj, I.W. Pushing the envelope: Structure, function, and dynamics of the nuclear periphery. Annu. Rev. Cell Dev. Biol. 2005, 21, 347–380. [Google Scholar] [CrossRef] [PubMed]
- Capelson, M.; Hetzer, M.W. The role of nuclear pores in gene regulation, development and disease. EMBO Rep. 2009, 10, 697–705. [Google Scholar] [CrossRef]
- D’Angelo, M.; Gomez-Cavazos, J.S.; Mei, A.; Lackner, D.H.; Hetzer, M.W. A change in nuclear pore complex composition regulates cell differentiation. Dev. Cell 2012, 22, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Buchwalter, A.; Kaneshiro, J.M.; Hetzer, M.W. Coaching from the sidelines: The nuclear periphery in genome regulation. Rev. Genet. 2019, 20, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Hampoelz, B.; Andres-Pons, A.; Kastritis, P.; Beck, M. Structure and Assembly of the Nuclear Pore Complex. Annu. Rev. Biophys. 2019, 48, 515–536. [Google Scholar] [CrossRef]
- du Preez, L.; Patterton, H.G. Secondary structures of the core histone N-terminal tails: Their role in regulating chromatin structure. Subcell. Biochem. 2013, 61, 37–55. [Google Scholar]
- Peng, Y.; Li, S.; Landsman, D.; Panchenko, A.R. Histone tails as signaling antennas of chromatin. Curr. Opin. Struct. Biol. 2020, 67, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.; Winston, F.; Berger, S.L. The biochemical and genetic discovery of the SAGA complex. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1864, 194669. [Google Scholar] [CrossRef]
- Dueva, R.; Akopyan, K.; Pederiva, C.; Trevisan, D.; Dhanjal, S.; Lindqvist, A.; Farnebo, M. Neutralization of the Positive Charges on Histone Tails by RNA Promotes an Open Chromatin Structure. Cell Chem. Biol. 2019, 26, 1436–1449.e5. [Google Scholar] [CrossRef]
- Lee, Y.; Tan, Y.J.; Falasca, M.; Oon, C.E. Cancer-Associated Fibroblasts: Epigenetic Regulation and Therapeutic Intervention in Breast Cancer. Cancers 2020, 12, 2949. [Google Scholar] [CrossRef]
- Li, G.; Tian, Y.; Zhu, W.G. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 576946. [Google Scholar] [CrossRef]
- Phimmachanh, M.; Han, J.Z.R.; O’Donnell, Y.E.I.; Latham, S.L.; Croucher, D.R. Histone Deacetylases and Histone Deacetylase Inhibitors in Neuroblastoma. Front. Cell Dev. Biol. 2020, 8, 578770. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, B.R. Structures of Native-like Nucleosomes: One Step Closer toward Understanding the Structure and Function of Chromatin. J. Mol. Biol. 2020, 433, 166648. [Google Scholar] [CrossRef]
- Maher, P. Nuclear Translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J. Cell Biol. 1996, 134, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Arese, M.; Chen, Y.; Florkiewicz, R.Z.; Gualandris, A.; Shen, B.; Rifkin, D.B. Nuclear activities of basic fibroblast growth factor: Potentiation of low-serum growth mediated by natural or chimeric nuclear localization signals. Mol. Biol. Cell 1999, 10, 1429–1444. [Google Scholar] [CrossRef]
- Arnaud, E.; Touriol, C.; Boutonnet, C.; Gensac, M.C.; Vagner, S.; Prats, H.; Prats, A.C. A new 34-kilodalton isoform of human fibroblast growth factor 2 is cap dependently synthesized by using a non-AUG start codon and behaves as a survival factor. Mol. Cell. Biol. 1999, 19, 505–514. [Google Scholar] [CrossRef]
- Chlebova, K.; Bryja, V.; Dvorak, P.; Kozubik, A.; Wilcox, W.R.; Krejci, P. High molecular weight FGF2: The biology of a nuclear growth factor. Cell. Mol. Life Sci. 2009, 66, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kole, D.; Grella, A.; Dolivo, D.; Shumaker, L.; Hermans, W.; Dominko, T. High molecular weight FGF2 isoforms demonstrate canonical receptor-mediated activity and support human embryonic stem cell self-renewal. Stem Cell Res. 2017, 21, 106–116. [Google Scholar] [CrossRef]
- Tuzon, C.T.; Rigueur, D.; Merrill, A.E. Nuclear Fibroblast Growth Factor Receptor Signaling in Skeletal Development and Disease. Curr. Osteoporos. Rep. 2019, 17, 138–146. [Google Scholar] [CrossRef]
- Levine, J.; Prystowsky, M.B. Polypeptide growth factors in the nucleus: A review of function and translocation. Neuroimmunomodulation 1995, 2, 290–298. [Google Scholar] [CrossRef]
- Pickles, J.; van Heumen, W.R. The expression of messenger RNAs coding for growth factors, their receptors, and eph-class receptor tyrosine kinases in normal and ototoxically damaged chick cochleae. Dev. Neurosci. 1997, 19, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Bastos, M.; Pignatelli, D.; Borges, T.; Aragüés, J.M.; Fonseca, F.; Pereira, B.D.; Socorro, S.; Lemos, M.C. Novel FGFR1 mutations in Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism: Evidence for the involvement of an alternatively spliced isoform. Fertil. Steril. 2015, 104, 1261–1267.e1. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Odagiri, H.; Nakatani, H.; Miyagawa, K.; Naito, K.; Sakamoto, H.; Katoh, O.; Yoshida, T.; Sugimura, T.; Terada, M. K-sam, an amplified gene in stomach cancer, is a member of the heparin-binding growth factor receptor genes. Proc. Natl. Acad. Sci. USA 1990, 87, 5983–5987. [Google Scholar] [CrossRef]
- Leadbeater, W.E.; Gonzalez, A.M.; Logaras, N.; Berry, M.; Turnbull, J.E.; Logan, A. Intracellular trafficking in neurones and glia of fibroblast growth factor-2, fibroblast growth factor receptor 1 and heparan sulphate proteoglycans in the injured adult rat cerebral cortex. J. Neurochem. 2006, 96, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Clarke, W.; Berry, M.; Smith, C.; Kent, A.; Logan, A. Coordination of fibroblast growth factor receptor 1 (FGFR1) and fibroblast growth factor-2 (FGF-2) trafficking to nuclei of reactive astrocytes around cerebral lesions in adult rats. Mol. Cell. Neurosci. 2001, 17, 17–30. [Google Scholar] [CrossRef]
- Gharbaran, R. Advances in the molecular functions of syndecan-1 (SDC1/CD138) in the pathogenesis of malignancies. Crit. Rev. Oncol. Hematol. 2015, 94, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, D.; Stoneley, M.; Ramakrishna, M.; Alexandrova, J.; Dezi, V.; Juke-Jones, R.; Lilley, K.S.; Cain, K.; Willis, A.E. Identification of the RNA polymerase I-RNA interactome. Nucleic Acids Res. 2018, 46, 11002–11013. [Google Scholar] [CrossRef]
- Dong, W.; Zhao, H.; Zhang, C.; Geng, P.; Sarengaowa; Li, Q.; Zhu, J.; Li, G.; Zhang, S.; Ye, M.; et al. Gene silencing of heparanase results in suppression of invasion and migration of hepatoma cells. World J. Surg. Oncol. 2014, 12, 85. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Leng, X.; Hu, Y.; Shen, H.; Song, X. Heparanase mediates vascular endothelial growth factor gene transcription in high-glucose human retinal microvascular endothelial cells. Mol. Vis. 2017, 23, 579–587. [Google Scholar]
- Parish, C.; Freeman, C.; Ziolkowski, A.F.; He, Y.Q.; Sutcliffe, E.L.; Zafar, A.; Rao, S.; Simeonovic, C.J. Unexpected new roles for heparanase in Type 1 diabetes and immune gene regulation. Matrix Biol. 2013, 32, 228–233. [Google Scholar] [CrossRef]
- Ramani, V.; Vlodavsky, I.; Ng, M.; Zhang, Y.; Barbieri, P.; Noseda, A.; Sanderson, R.D. Chemotherapy induces expression and release of heparanase leading to changes associated with an aggressive tumor phenotype. Matrix Biol. 2016, 55, 22–34. [Google Scholar] [CrossRef]
- Schubert, S.Y.; Ilan, N.; Shushy, M.; Ben-Izhak, O.; Vlodavsky, I.; Goldshmidt, O. Human heparanase nuclear localization and enzymatic activity. Lab. Investig. 2004, 84, 535–544. [Google Scholar]
- Allahverdi, A.; Chen, Q.; Korolev, N.; Nordenskiöld, L. Chromatin compaction under mixed salt conditions: Opposite effects of sodium and potassium ions on nucleosome array folding. Sci. Rep. 2015, 5, 8512. [Google Scholar] [CrossRef]
- Kaczmarczyk, A.; Meng, H.; Ordu, O.; Noort, J.V.; Dekker, N.H. Chromatin fibers stabilize nucleosomes under torsional stress. Nat. Commun. 2020, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Dröge, P. Chromatin Architectural Factors as Safeguards against Excessive Supercoiling during DNA Replication. Int. J. Mol. Sci. 2020, 21, 4504. [Google Scholar] [CrossRef]
- Agrawal, A.; Lele, T.P. Mechanics of nuclear membranes. J. Cell Sci. 2019, 132, jcs229245. [Google Scholar] [CrossRef]
- Fernández-Jiménez, N.; Pradillo, M. The role of the nuclear envelope in the regulation of chromatin dynamics during cell division. J. Exp. Bot. 2020, 71, 5148–5159. [Google Scholar] [CrossRef]
- Szczesny, S.; Mauck, R.L. The Nuclear Option: Evidence Implicating the Cell Nucleus in Mechanotransduction. J. Biomech. Eng. 2017, 139, 0210061–02100616. [Google Scholar] [CrossRef] [PubMed]
- Guilluy, C.; Burridge, K. Nuclear mechanotransduction: Forcing the nucleus to respond. Nucleus 2015, 6, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Skepper, J.N.; Yang, F.; Davies, J.D.; Hegyi, L.; Roberts, R.G.; Weissberg, P.L.; Ellis, J.A.; Shanahan, C.M. Nesprins: A novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J. Cell Sci. 2001, 114, 4485–4498. [Google Scholar] [PubMed]
- Lambert, M. Spectrin and its interacting partners in nuclear structure and function. Exp. Biol. Med. 2018, 243, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.; Vartiainen, M.K. Diverse functions for different forms of nuclear actin. Curr. Opin. Cell Biol. 2017, 46, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Meinke, P.; Nguyen, T.D.; Wehnert, M.S. The LINC complex and human disease. Biochem. Soc. Trans. 2011, 39, 1693–1697. [Google Scholar] [CrossRef]
- Tzur, Y.; Wilson, K.L.; Gruenbaum, Y. SUN-domain proteins: ‘Velcro’ that links the nucleoskeleton to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2006, 7, 782–788. [Google Scholar] [CrossRef]
- Tapley, E.; Starr, D.A. Connecting the nucleus to the cytoskeleton by SUN-KASH bridges across the nuclear envelope. Curr. Opin. Cell Biol. 2013, 25, 57–62. [Google Scholar] [CrossRef]
- Sears, R.; Roux, K.J. Diverse cellular functions of barrier-to-autointegration factor and its roles in disease. J. Cell Sci. 2020, 133, jcs246546. [Google Scholar] [CrossRef] [PubMed]
- Torras-Llort, M.; Medina-Giró, S.; Escudero-Ferruz, P.; Lipinszki, Z.; Moreno-Moreno, O.; Karman, Z.; Przewloka, M.R.; Azorín, F. A fraction of barrier-to-autointegration factor (BAF) associates with centromeres and controls mitosis progression. Commun. Biol. 2020, 3, 454. [Google Scholar] [CrossRef]
- Foisner, R.; Gerace, L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell 1993, 73, 1267–1279. [Google Scholar] [CrossRef]
- Pavin, N.; Tolić, I.M. Mechanobiology of the Mitotic Spindle. Dev. Cell 2021, 56, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Xu, N.; Wang, G.; Ren, H.; Li, S.; Lei, J.; Lin, Q.; Wang, L.; Gu, X.; Zhang, H.; et al. The lamin-A/C-LAP2α-BAF1 protein complex regulates mitotic spindle assembly and positioning. J. Cell Sci. 2015, 128, 2830–2841. [Google Scholar] [CrossRef] [PubMed]
- Burakov, A.; Nadezhdina, E.S. Centering and Shifting of Centrosomes in Cells. Cells 2020, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, W. Intermediate filaments and cellular mechanics. Cell Biol. Int. 2018, 42, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wiche, G.; Winter, L. Plectin isoforms as organizers of intermediate filament cytoarchitecture. Bioarchitecture 2011, 1, 14–20. [Google Scholar] [CrossRef]
- Koch, A.; Holaska, J.M. Emerin in health and disease. Semin. Cell Dev. Biol. 2014, 29, 95–106. [Google Scholar] [CrossRef]
- Berk, J.; Tifft, K.E.; Wilson, K.L. The nuclear envelope LEM-domain protein emerin. Nucleus 2013, 4, 298–314. [Google Scholar] [CrossRef]
- Dechat, T.; Korbei, B.; Vaughan, O.A.; Vlcek, S.; Hutchison, C.J.; Foisner, R. Lamina-associated polypeptide 2alpha binds intranuclear A-type lamins. J. Cell Sci. 2000, 113, 3743–3784. [Google Scholar]
- Smith, B.; Odero-Marah, V.A. The role of Snail in prostate cancer. Cell Adhes. Migr. 2012, 6, 433–441. [Google Scholar] [CrossRef]
- Bachy, S.; Letourneur, F.; Rousselle, P. Syndecan-1 interaction with the LG4/5 domain in laminin-332 is essential for keratinocyte migration. J. Cell. Physiol. 2008, 214, 238–249. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.; Choi, Y.; Choi, S.; Hong, E.; Oh, E.S. Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1. Biochem. Biophys. Res. Commun. 2011, 409, 148–153. [Google Scholar] [CrossRef]
- Iwamoto, K.; Takahashi, H.; Okuzaki, D.; Osawa, H.; Ogino, T.; Miyoshi, N.; Uemura, M.; Matsuda, C.; Yamamoto, H.; Mizushima, T.; et al. Syntenin-1 promotes colorectal cancer stem cell expansion and chemoresistance by regulating prostaglandin E2 receptor. Br. J. Cancer 2020, 123, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Kegelman, T.; Das, S.K.; Hu, B.; Bacolod, M.D.; Fuller, C.E.; Menezes, M.E.; Emdad, L.; Dasgupta, S.; Baldwin, A.S.; Bruce, J.N.; et al. MDA-9/syntenin is a key regulator of glioma pathogenesis. Neuro Oncol. 2014, 16, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Kashyap, R.; Zimmermann, P. Syntenin: Key player in cancer exosome biogenesis and uptake? Cell Adhes. Migr. 2017, 11, 124–126. [Google Scholar] [CrossRef]
- Tsoyi, K.; Osorio, J.C.; Chu, S.G.; Fernandez, I.E.; De Frias, S.P.; Sholl, L.; Cui, Y.; Tellez, C.S.; Siegfried, J.M.; Belinsky, S.A.; et al. Lung Adenocarcinoma Syndecan-2 Potentiates Cell Invasiveness. Am. J. Respir. Cell Mol. Biol. 2019, 60, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Beekman, J.; Coffer, P.J. The ins and outs of syntenin, a multifunctional intracellular adaptor protein. J. Cell Sci. 2008, 121, 1349–1355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nilsson, U.; Johnsson, R.; Fransson, L.A.; Ellervik, U.; Mani, K. Attenuation of tumor growth by formation of antiproliferative glycosaminoglycans correlates with low acetylation of histone H3. Cancer Res. 2010, 70, 3771–3779. [Google Scholar] [CrossRef] [PubMed]
- Brockstedt, U.; Dobra, K.; Nurminen, M.; Hjerpe, A. Immunoreactivity to cell surface syndecans in cytoplasm and nucleus: Tubulin-dependent rearrangements. Exp. Cell Res. 2002, 274, 235–245. [Google Scholar] [CrossRef]
- Dudas, J.; Ramadori, G.; Knittel, T.; Neubauer, K.; Raddatz, D.; Egedy, K.; Kovalszky, I. Effect of heparin and liver heparan sulphate on interaction of HepG2-derived transcription factors and their cis-acting elements: Altered potential of hepatocellular carcinoma heparan sulphate. Biochem. J. 2000, 350, 245–251. [Google Scholar] [CrossRef]
- Fedarko, N.S.; Conrad, H.E. A unique heparan sulfate in the nuclei of hepatocytes: Structural changes with the growth state of the cells. J. Cell Biol. 1986, 102, 587–599. [Google Scholar] [CrossRef]
- Ishihara, M.; Fedarko, N.S.; Conrad, H.E. Involvement of phosphatidylinositol and insulin in the coordinate regulation of proteoheparan sulfate metabolism and hepatocyte growth. J. Biol. Chem. 1987, 262, 4708–4716. [Google Scholar] [CrossRef]
- Bhavanandan, V.P.; Davidson, E.A. Mucopolysaccharides associated with nuclei of cultured mammalian cells. Proc. Natl. Acad. Sci. USA 1975, 72, 2032–2036. [Google Scholar] [CrossRef]
- Zong, F.; Fthenou, E.; Wolmer, N.; Hollosi, P.; Kovalszky, I.; Szilak, L.; Mogler, C.; Nilsonne, G.; Tzanakakis, G.; Dobra, K. Syndecan-1 and FGF-2, but not FGF receptor-1, share a common transport route and co-localize with heparanase in the nuclei of mesenchymal tumor cells. PLoS ONE 2009, 4, e7346. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Naomoto, Y.; Nobuhisa, T.; Okawa, T.; Takaoka, M.; Shirakawa, Y.; Yamatsuji, T.; Matsuoka, J.; Mizushima, T.; Matsuura, H.; et al. Heparanase regulates esophageal keratinocyte differentiation through nuclear translocation and heparan sulfate cleavage. Differentiation 2006, 74, 235–243. [Google Scholar] [CrossRef]
- Pathak, R.; Soujanya, M.; Mishra, R.K. Deterioration of nuclear morphology and architecture: A hallmark of senescence and aging. Ageing Res. Rev. 2021, 67, 101264. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.; Liao, H.J. Receptor tyrosine kinases in the nucleus. Cold Spring Harb. Perspect. Biol. 2013, 5, a008979. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, R.; Yang, Y.; Kelly, T.; MacLeod, V.; Dai, Y.; Theus, A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: Growth regulation and the prospect of new cancer therapies. J. Cell. Biochem. 2005, 96, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, M.; Maher, P.A.; Stachowiak, E.K. Integrative nuclear signaling in cell development—A role for FGF receptor-1. DNA Cell Biol. 2007, 26, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, J.; Casar, J.C.; Fuentealba, L.; Carey, D.J.; Brandan, E. Extracellular matrix histone H1 binds to perlecan, is present in regenerating skeletal muscle and stimulates myoblast proliferation. J. Cell Sci. 2002, 115, 2041–2051. [Google Scholar] [PubMed]
- Bernardes, N.; Chook, Y.M. Nuclear import of histones. Biochem. Soc. Trans. 2020, 48, 2753–2767. [Google Scholar] [CrossRef]
- Martire, S.; Banaszynski, L.A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef]
- Shteper, P.; Zcharia, E.; Ashhab, Y.; Peretz, T.; Vlodavsky, I.; Ben-Yehuda, D. Role of promoter methylation in regulation of the mammalian heparanase gene. Oncogene 2003, 22, 7737–7749. [Google Scholar] [CrossRef]
- Verza, F.; Das, U.; Fachin, A.L.; Dimmock, J.R.; Marins, M. Roles of Histone Deacetylases and Inhibitors in Anticancer Therapy. Cancers 2020, 12, 1664. [Google Scholar] [CrossRef]
- Mang, D.; Roy, S.R.; Zhang, Q.; Hu, X.; Zhang, Y. Heparan Sulfate-Instructed Self-Assembly Selectively Inhibits Cancer Cell Migration. ACS Appl. Mater. Interfaces 2021. [Google Scholar] [CrossRef]
- Afratis, N.; Nikitovic, D.; Multhaupt, H.A.; Theocharis, A.D.; Couchman, J.R.; Karamanos, N.K. Syndecans—Key regulators of cell signaling and biological functions. FEBS J. 2017, 284, 27–41. [Google Scholar] [CrossRef]
- Couchman, J.; Multhaupt, H.; Sanderson, R.D. Recent Insights into Cell Surface Heparan Sulphate Proteoglycans and Cancer. F1000Research 2016, 5, 1541. [Google Scholar] [CrossRef]
- Gopal, S.; Arokiasamy, S.; Pataki, C.; Whiteford, J.R.; Couchman, J.R. Syndecan receptors: Pericellular regulators in development and inflammatory disease. Open Biol. 2021, 11, 200377. [Google Scholar] [CrossRef] [PubMed]
- Lepedda, A.; Nieddu, G.; Piperigkou, Z.; Kyriakopoulou, K.; Karamanos, N.; Formato, M. Circulating Heparan Sulfate Proteoglycans as Biomarkers in Health and Disease. Semin. Thromb. Hemost. 2021, 47, 295–307. [Google Scholar] [PubMed]
- Jayatilleke, K.; Hulett, M.D. Heparanase and the hallmarks of cancer. J. Transl. Med. 2020, 18, 453. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Barash, U.; Nguyen, H.M.; Yang, S.M.; Ilan, N. Biology of the Heparanase-Heparan Sulfate Axis and Its Role in Disease Pathogenesis. Semin. Thromb. Hemost. 2021, 47, 240–253. [Google Scholar] [PubMed]
- Yang, Y.; Ahn, J.; Raghunathan, R.; Kallakury, B.V.; Davidson, B.; Kennedy, Z.B.; Zaia, J.; Goldman, R. Expression of the Extracellular Sulfatase SULF2 Affects Survival of Head and Neck Squamous Cell Carcinoma Patients. Front. Oncol. 2021, 10, 582827. [Google Scholar] [CrossRef] [PubMed]
- Faria-Ramos, I.; Poças, J.; Marques, C.; Santos-Antunes, J.; Macedo, G.; Reis, C.A.; Magalhães, A. Heparan Sulfate Glycosaminoglycans: (Un)Expected Allies in Cancer Clinical Management. Biomolecules 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
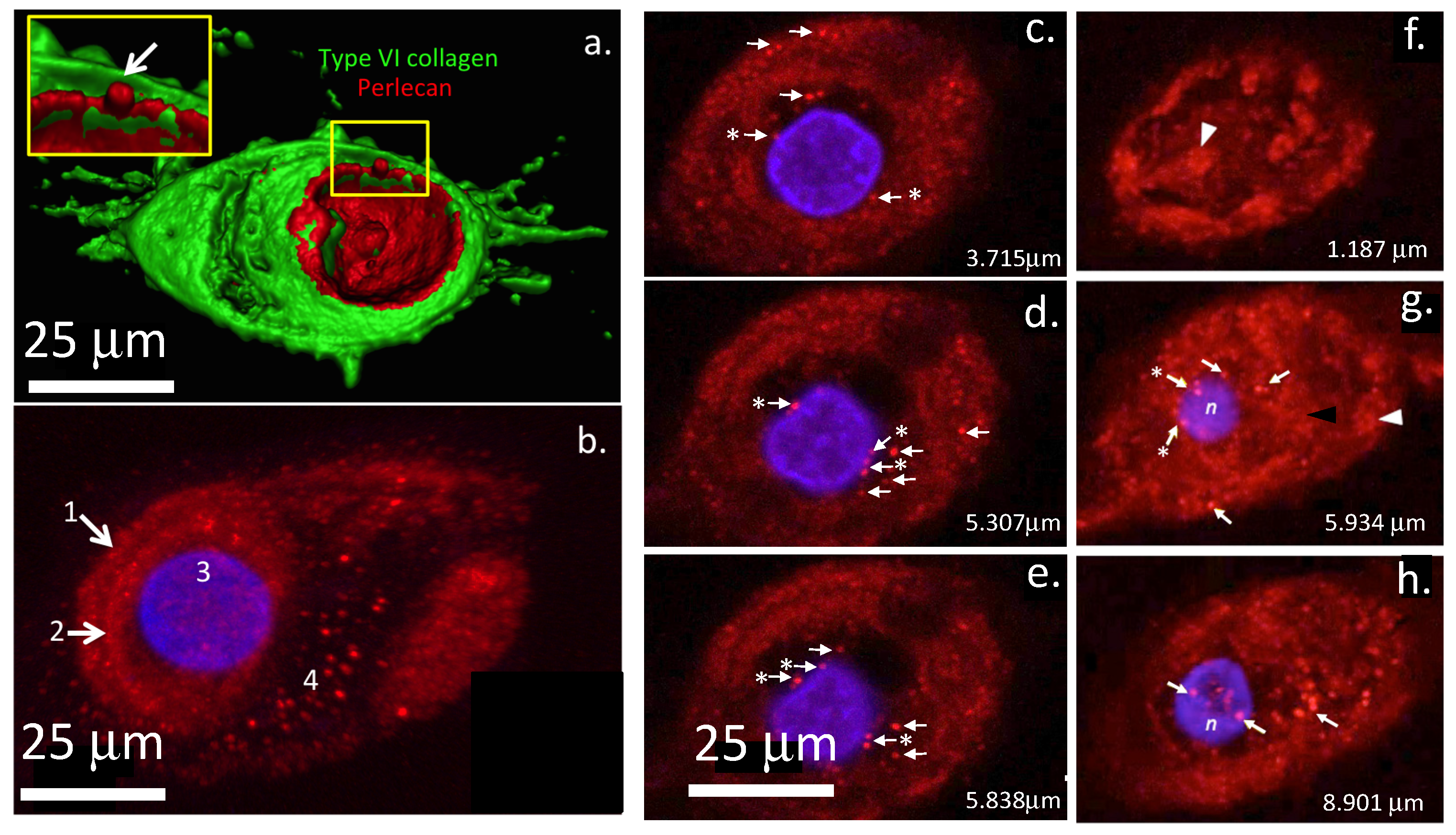
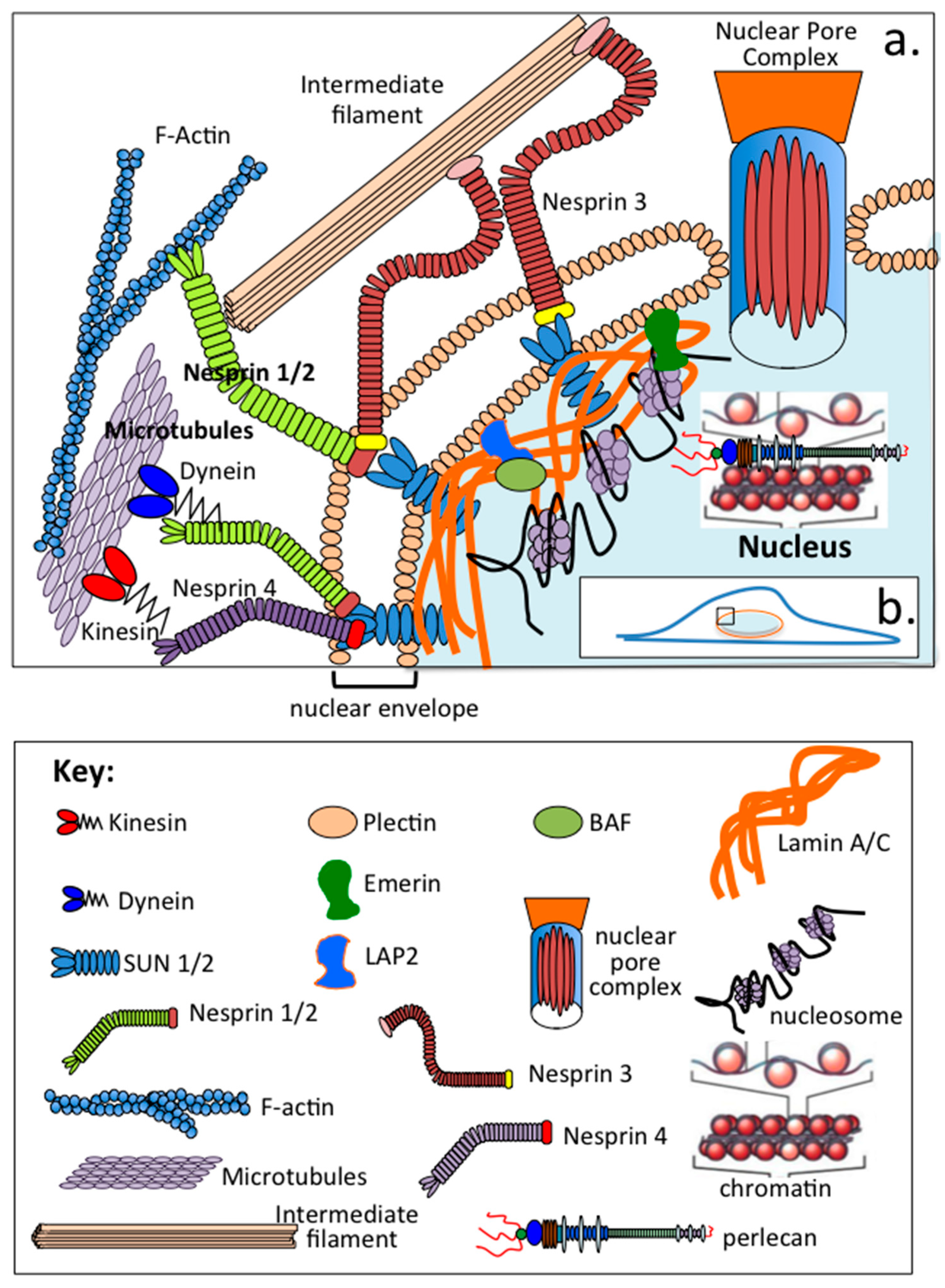
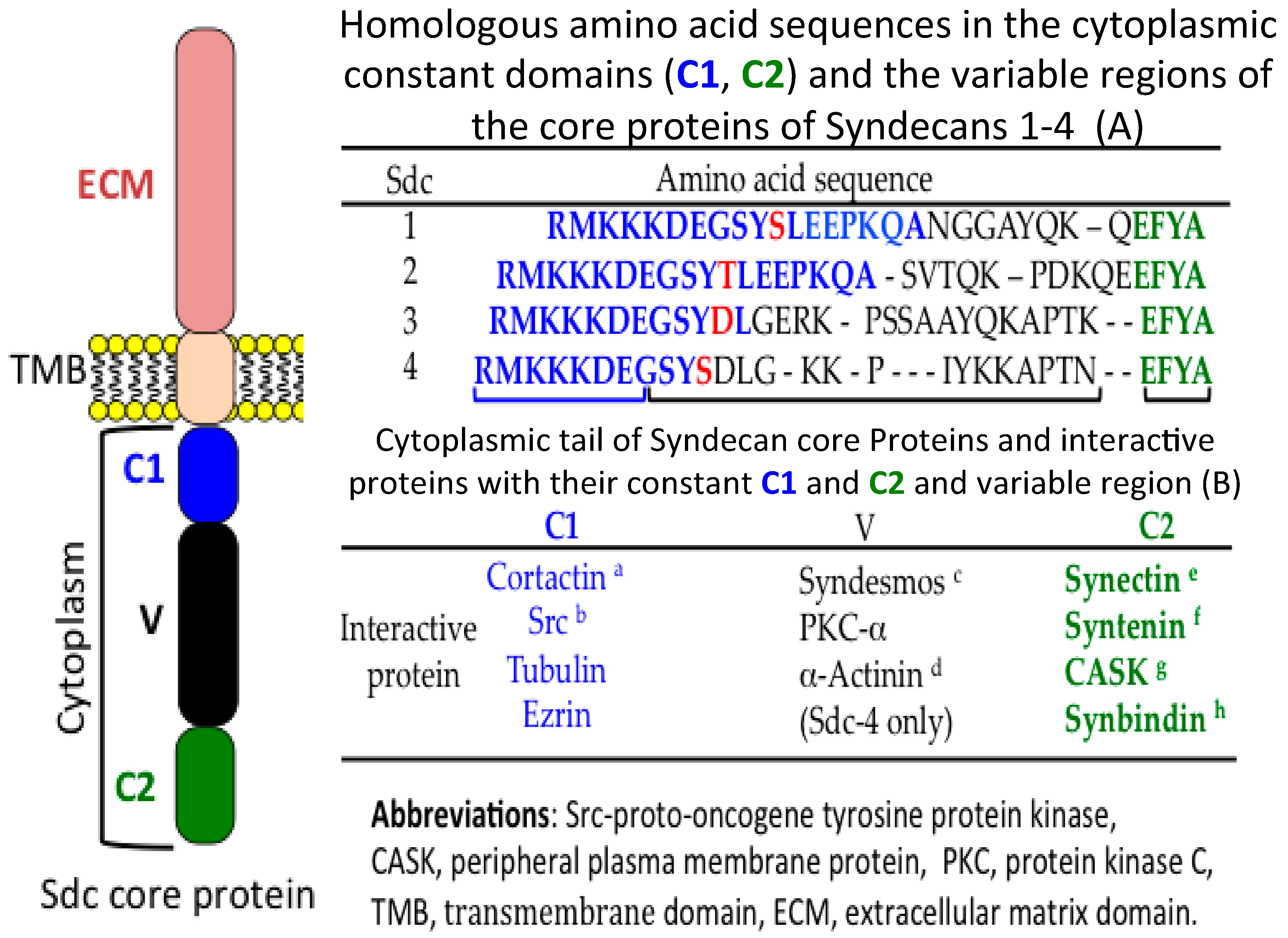
| Protein | Reference |
| Growth factors | |
| EGF family | [36,37] |
| FGF family | [38,39,40,41,42,43,44] |
| VEGF | [45,46] |
| HGF | [47,48] |
| PDGF | [49] |
| TGF-β superfamily | [50,51] |
| Cytokines/Chemokines/Morphogens | |
| BMPs | [52] |
| CCL2 | [53,54] |
| PF-4 | [55] |
| HH | [56] |
| Wnt | [57,58] |
| A. Go Biological Process Terms Enriched in the Heparin/HS Interactome. | |||
| Term | Name | Count * | % * |
| GO: 0009611 | Response to wounding | 120 | 27.8 |
| GO: 0042330 | Taxis | 55 | 12.8 |
| GO: 0006935 | Chemotaxis | 55 | 12.8 |
| GO: 0006954 | Inflammatory response | 73 | 16.9 |
| GO: 0006952 | Defence response | 91 | 21.1 |
| GO: 0007626 | Locomotory behavior | 62 | 14.4 |
| GO: 0006955 | Immune response | 91 | 21.1 |
| GO: 0042060 | Wound healing | 51 | 11.8 |
| GO: 0016477 | Cell migration | 57 | 13.2 |
| GO: 0007610 | Behavior | 71 | 16.5 |
| GO: 0051674 | Localisation of the cell | 58 | 13.5 |
| GO: 0048870 | Cell motility | 58 | 13.5 |
| GO: 0042127 | Regulation of cell proliferation | 90 | 20.9 |
| GO: 0006928 | Cell motion | 70 | 16.2 |
| GO: 0032101 | Regulation of response to external stimulus | 43 | 10.0 |
| GO: 0001568 | Blood vessel development | 51 | 11.8 |
| GO: 0001944 | Vascular development | 51 | 11.8 |
| GO: 0051605 | Protein maturation by peptide bond cleavage | 33 | 7.7 |
| GO: 0007267 | Cell–cell signaling | 76 | 17.6 |
| GO: 0016485 | Protein processing | 36 | 8.4 |
| B. KEGG Pathways Enriched in the Heparin/HS Interactome. | |||
| Term | Name | Count * | % * |
| hsa04610 | Complement and coagulation cascades | 42 | 9.7 |
| hsa04060 | Cytokine-cytokine receptor interaction | 63 | 14.6 |
| hsa04512 | ECM-receptor interaction | 35 | 8.1 |
| hsa04510 | Focal adhesion | 43 | 10.0 |
| hsa05200 | Pathways in cancer | 52 | 12.1 |
| hsa05218 | Melanoma | 22 | 5.1 |
| hsa04062 | Chemokine signaling pathway | 34 | 7.9 |
| hsa05020 | Prion diseases | 15 | 3.5 |
| hsa04810 | Regulation of actin cytoskeleton | 33 | 7.7 |
| hsa04350 | TGF-β signalling pathway | 18 | 4.2 |
| hsa04672 | Intestinal immune network for IgA production | 13 | 3.0 |
| hsa05322 | Systemic lupus erythematosus | 18 | 4.2 |
| hsa04010 | MAPK signalling pathway | 30 | 7.0 |
| hsa04640 | Hematopoietic cell lineage | 14 | 3.2 |
| hsa04621 | NOD-like receptor signaling pathway | 11 | 2.6 |
| hsa05219 | Bladder cancer | 9 | 2.1 |
| hsa05310 | Asthma | 7 | 1.6 |
| hsa05222 | Small cell lung cancer | 12 | 2.8 |
| HUGO/MGI Symbol | Receptor Name | Alternative Symbol | Explanation of Symbol |
|---|---|---|---|
| FGFR1/Fgfr1 | Fgf receptor 1 | Flg Flt2 Cek KAL2 K-sam | Fms-like gene, ambigiously named since the FLG gene encodes filaggrin a skin protein Fms-like tyrosine kinase 2, a gene on chromosome 8p12 encoding FGFR1 Chicken embryo kinase 1, chick cochlea eph class receptor tyrosine kinase [193] Kallman syndrome 2, mutated FGFR-1 found in Kallman syndrome 2 [194] KATO III cell-derived stomach cancer amplified gene, sharing homology with FGFR-1 [195] |
| FGFR2/Fgfr2 | Fgf receptor 2 | Bek Cek3 Kgfr | Bacterial kinase, alias for FGFR2 Chicken embryo kinase 3, chick fgfr2 Keratinocyte growth factor receptor |
| FGFR3/Fgfr3 | Fgf receptor 3 | Cek2 | Chicken embryo kinase 2, chick fgfr3 |
| FGFR4/Fgfr4 | Fgf receptor 4 | Tkf | Tyrosine kinase related to FGFR4 |
| Tumour Type | HS or Proteoglycan Identified | Reference(s) |
|---|---|---|
| Bladder carcinoma | HS | [234] |
| Breast carcinoma | SDC-1 | [235] |
| Glioma | HS, GPC-1 | [6] |
| Chondrosarcoma | SDC-2 | [144] |
| Hepatocyte carcinoma | HS | [236,237] |
| Lung cancer, Adenocarcinoma | HS, SDC-1 | [235] |
| Melanoma | HS | [238] |
| Mesothelioma | SDC-1 | [204,235] |
| Monocytic leukemia | HS | [239] |
| Myeloma | SDC-1 | [2,115] |
| Neuroblastoma | SDC-1 | [235] |
| Nuclear HS and HSPGs identified in normal mammalian cell nuclei | ||
| Cell type | HS or Proteoglycan | Reference |
| Astrocytes | HS, GPC-2, SDC-2, SDC-3 | [240] |
| Neurons | HS, GPC-1, SDC-2, SDC-3 | [6] |
| Corneal fibroblasts Corneal endothelial cells | HS, HSPG | [114,241,242,243] |
| Esophageal keratinocytes | HS | [244] |
| Intervertebral disc cell | Perlecan | [1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayes, A.J.; Melrose, J. What Are the Potential Roles of Nuclear Perlecan and Other Heparan Sulphate Proteoglycans in the Normal and Malignant Phenotype. Int. J. Mol. Sci. 2021, 22, 4415. https://doi.org/10.3390/ijms22094415
Hayes AJ, Melrose J. What Are the Potential Roles of Nuclear Perlecan and Other Heparan Sulphate Proteoglycans in the Normal and Malignant Phenotype. International Journal of Molecular Sciences. 2021; 22(9):4415. https://doi.org/10.3390/ijms22094415
Chicago/Turabian StyleHayes, Anthony J., and James Melrose. 2021. "What Are the Potential Roles of Nuclear Perlecan and Other Heparan Sulphate Proteoglycans in the Normal and Malignant Phenotype" International Journal of Molecular Sciences 22, no. 9: 4415. https://doi.org/10.3390/ijms22094415
APA StyleHayes, A. J., & Melrose, J. (2021). What Are the Potential Roles of Nuclear Perlecan and Other Heparan Sulphate Proteoglycans in the Normal and Malignant Phenotype. International Journal of Molecular Sciences, 22(9), 4415. https://doi.org/10.3390/ijms22094415






