Small Bowel Epithelial Precursor Lesions: A Focus on Molecular Alterations
Abstract
1. Introduction
2. Sporadic Small Bowel Dysplastic Glandular Lesions
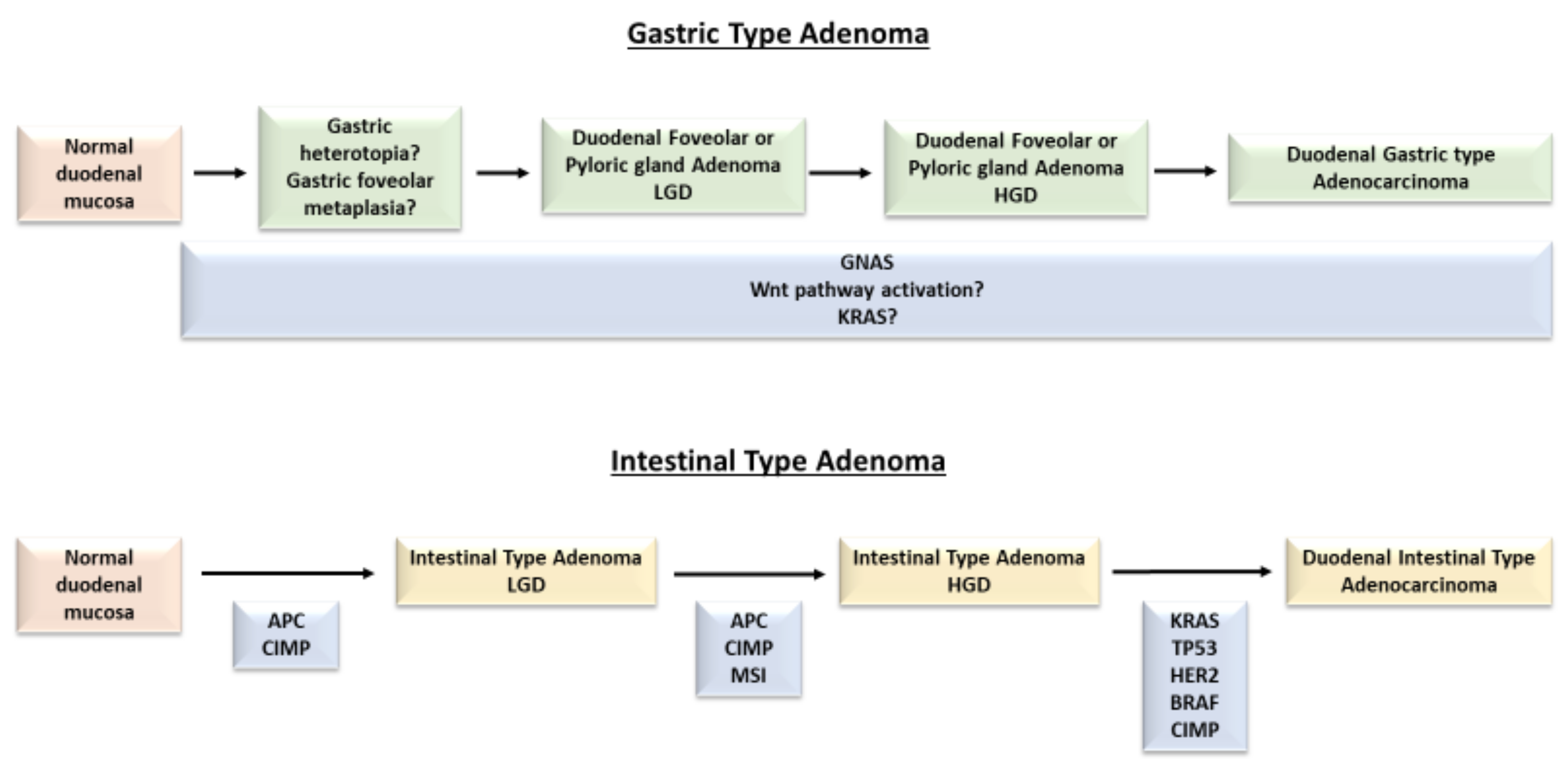
2.1. Non-Ampullary Duodenal Adenomas
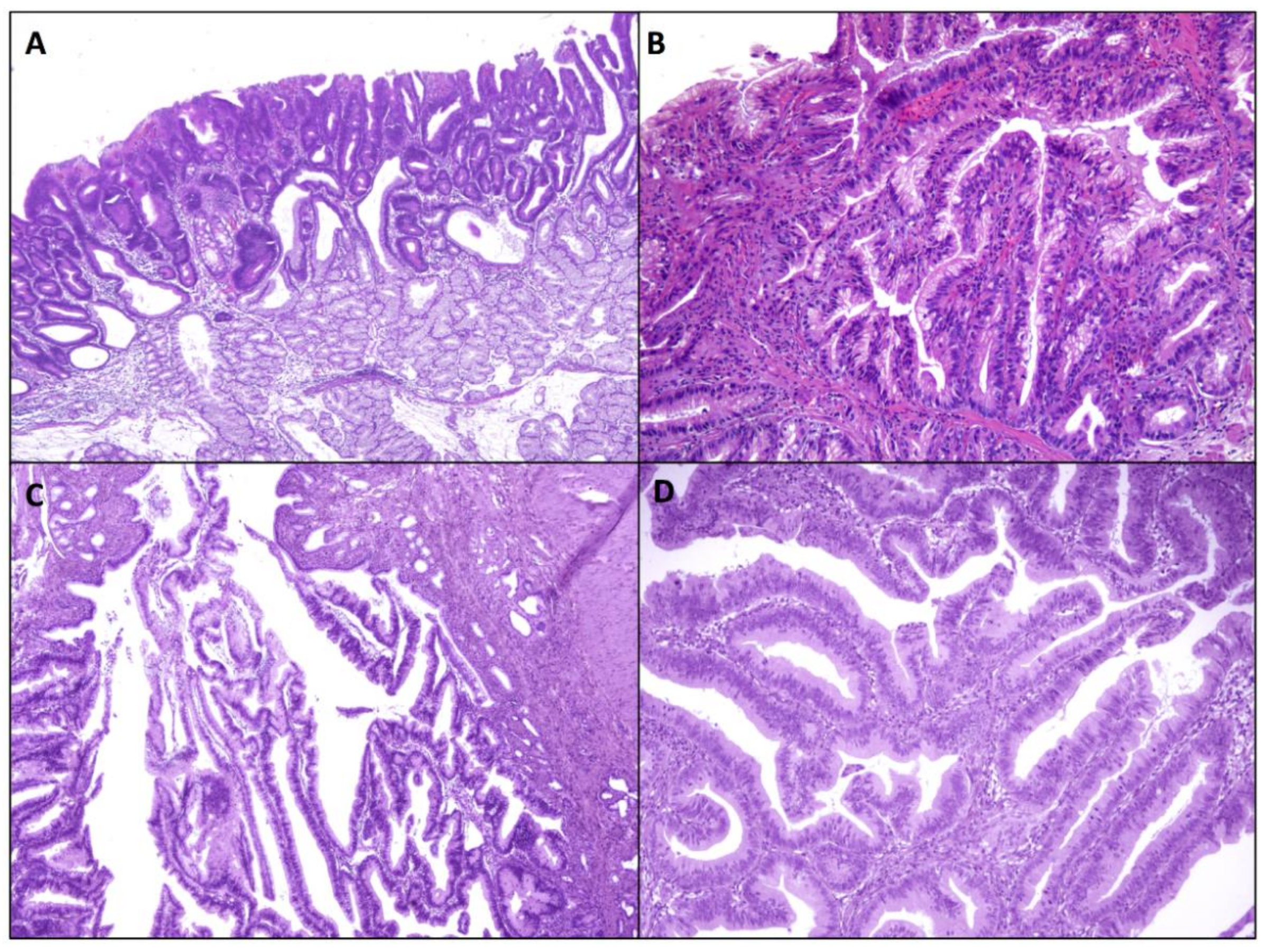
2.1.1. Intestinal-Type Adenomas
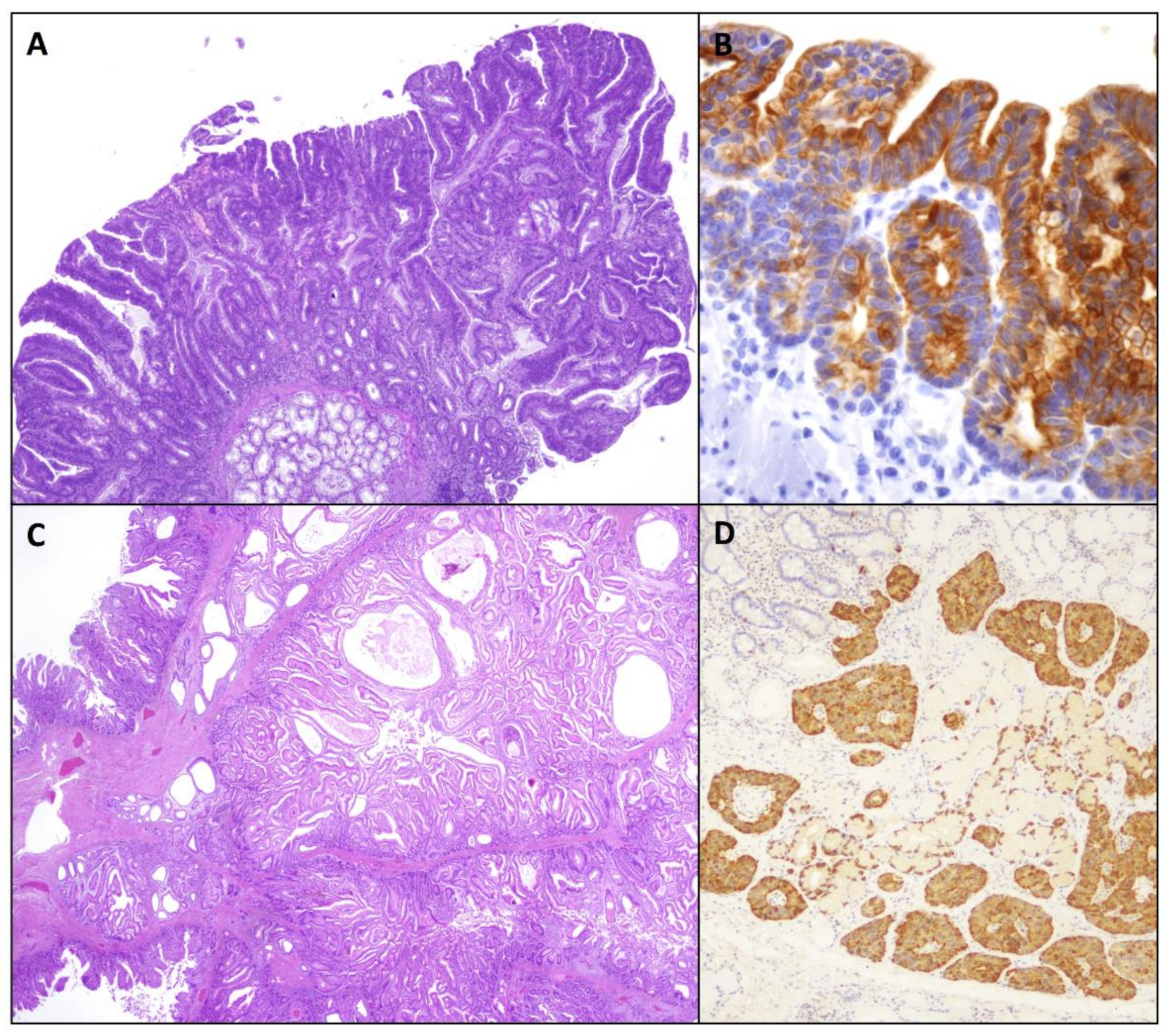
2.1.2. Gastric-Type Adenomas
2.2. Ampullary Preinvasive Neoplasms
2.2.1. Ampullary Duodenal Adenomas
2.2.2. Intra-Ampullary Papillary-Tubular Neoplasms
3. Small Bowel Adenomas in Hereditary Syndromes
4. Premalignant Epithelial Lesions in Celiac Disease and Crohn’s Disease
4.1. Celiac Disease
4.2. Crohn’s Disease
5. Serrated Lesions
6. Hamartomatous Lesions
7. Duodenal Neuroendocrine Lesions in Multiple Endocrine Neoplasia Type 1 (MEN1)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kővári, B.; Kim, B.H.; Lauwers, G.Y. The pathology of gastric and duodenal polyps: Current concepts. Histopathology 2021, 78, 106–124. [Google Scholar] [CrossRef]
- Collins, K.; Ligato, S. Duodenal Epithelial Polyps: A Clinicopathologic Review. Arch. Pathol. Lab. Med. 2019, 143, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Chung, W.C.; Kim, E.J.; Kim, S.H.; Paik, C.N.; Lee, B.I.; Cho, Y.S.; Lee, K.M. Evaluation of non-ampullary duodenal polyps: Comparison of non-neoplastic and neoplastic lesions. World J. Gastroenterol. 2010, 16, 5474–5480. [Google Scholar] [CrossRef] [PubMed]
- Sekine, S.; Shia, J. Non-ampullary adenoma. In WHO Classification of Tumours Editorial Board. Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 118–120. [Google Scholar]
- Okada, K.; Fujisaki, J.; Kasuga, A.; Omae, M.; Kubota, M.; Hirasawa, T.; Ishiyama, A.; Inamori, M.; Chino, A.; Yamamoto, Y.; et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: A study of follow-up surveillance. Am. J. Gastroenterol. 2011, 106, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Banck, M.S.; Kanwar, R.; Kulkarni, A.A.; Boora, G.K.; Metge, F.; Kipp, B.R.; Zhang, L.; Thorland, E.C.; Minn, K.T.; Tentu, R.; et al. The genomic landscape of small intestine neuroendocrine tumors. J. Clin. Invest. 2013, 123, 2502–2508. [Google Scholar] [CrossRef]
- Francis, J.M.; Kiezun, A.; Ramos, A.H.; Serra, S.; Pedamallu, C.S.; Qian, Z.R.; Banck, M.S.; Kanwar, R.; Kulkarni, A.A.; Karpathakis, A.; et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat. Genet. 2013, 45, 1483–1486. [Google Scholar] [CrossRef]
- Schrock, A.B.; Devoe, C.E.; McWilliams, R.; Sun, J.; Aparicio, T.; Stephens, P.J.; Ross, J.S.; Wilson, R.; Miller, V.A.; Ali, S.M.; et al. Genomic profiling of small-bowel adenocarcinoma. JAMA Oncol. 2017, 3, 1546–1553. [Google Scholar] [CrossRef]
- Hänninen, U.A.; Katainen, R.; Tanskanen, T.; Plaketti, R.M.; Laine, R.; Hamberg, J.; Ristimäki, A.; Pukkala, E.; Taipale, M.; Mecklin, J.P.; et al. Exome-wide somatic mutation characterization of small bowel adenocarcinoma. PLoS Genet. 2018, 14, e1007200. [Google Scholar] [CrossRef]
- Adam, L.; San Lucas, F.A.; Fowler, R.; Yu, Y.; Wu, W.; Liu, Y.; Wang, H.; Menter, D.; Tetzlaff, M.T.; Ensor, J., Jr.; et al. DNA Sequencing of Small Bowel Adenocarcinomas Identifies Targetable Recurrent Mutations in the ERBB2 Signaling Pathway. Clin. Cancer Res. 2019, 25, 641–651. [Google Scholar] [CrossRef]
- Aparicio, T.; Svrcek, M.; Henriques, J.; Afchain, P.; Lièvre, A.; Tougeron, D.; Gagniere, J.; Terrebonne, E.; Piessen, G.; Legoux, J.L.; et al. Panel gene profiling of small bowel adenocarcinoma: Results from the NADEGE prospective cohort. Int. J. Cancer 2021, 148, 1731–1742. [Google Scholar] [CrossRef]
- Hijikata, K.; Nemoto, T.; Igarashi, Y.; Shibuya, K. Extra-ampullary duodenal adenoma: A clinicopathological study. Histopathology 2017, 71, 200–207. [Google Scholar] [CrossRef]
- Yoshida, M.; Shimoda, T.; Abe, M.; Kakushima, N.; Kawata, N.; Takizawa, K.; Ono, H.; Sugino, T. Clinicopathological characteristics of non-ampullary duodenal tumors and their phenotypic classification. Pathol. Int. 2019, 69, 398–406. [Google Scholar] [CrossRef]
- Mitsuishi, T.; Hamatani, S.; Hirooka, S.; Fukasawa, N.; Aizawa, D.; Hara, Y.; Dobashi, A.; Goda, K.; Fukuda, T.; Saruta, M.; et al. Clinicopathological characteristics of duodenal epithelial neoplasms: Focus on tumors with a gastric mucin phenotype (pyloric gland-type tumors). PLoS ONE 2017, 12, e0174985. [Google Scholar] [CrossRef]
- Wagner, P.L.; Chen, Y.T.; Yantiss, R.K. Immunohistochemical and molecular features of sporadic and FAP-associated duodenal adenomas of the ampullary and nonampullary mucosa. Am. J. Surg. Pathol. 2008, 32, 1388–1395. [Google Scholar] [CrossRef]
- Ota, R.; Sawada, T.; Tsuyama, S.; Sasaki, Y.; Suzuki, H.; Kaizaki, Y.; Hasatani, K.; Yamamoto, E.; Nakanishi, H.; Inagaki, S.; et al. Integrated genetic and epigenetic analysis of cancer-related genes in non-ampullary duodenal adenomas and intramucosal adenocarcinomas. J. Pathol. 2020, 252, 330–342. [Google Scholar] [CrossRef]
- Niwa, A.; Kuwano, S.; Tomita, H.; Kimura, K.; Orihara, Y.; Kanayama, T.; Noguchi, K.; Hisamatsu, K.; Nakashima, T.; Hatano, Y.; et al. The different pathogeneses of sporadic adenoma and adenocarcinoma in non-ampullary lesions of the proximal and distal duodenum. Oncotarget 2017, 8, 41078–41090. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Yamamichi, N.; Tomida, S.; Takeuchi, C.; Kageyama-Yahara, N.; Takahashi, Y.; Shiogama, K.; Inada, K.I.; Ichinose, M.; Fujishiro, M.; et al. Identification of marker genes and pathways specific to precancerous duodenal adenomas and early stage adenocarcinomas. J. Gastroenterol. 2019, 54, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Ohtsuka, K.; Ohnishi, H.; Abe, N.; Furuse, J.; Watanabe, T.; Sugiyama, M. APC:T1556fs and STK11 mutations in duodenal adenomas and adenocarcinomas. Surg. Today 2018, 48, 765–772. [Google Scholar] [CrossRef]
- Laforest, A.; Aparicio, T.; Zaanan, A.; Silva, F.P.; Didelot, A.; Desbeaux, A.; Le Corre, D.; Benhaim, L.; Pallier, K.; Aust, D.; et al. ERBB2 gene as a potential therapeutic target in small bowel adenocarcinoma. Eur. J. Cancer 2014, 50, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, A.; Sekine, S.; Kushima, R.; Ogawa, R.; Taniguchi, H.; Tsuda, H.; Kanai, Y. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J. Pathol. 2013, 229, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Vanoli, A.; Di Sabatino, A.; Furlan, D.; Klersy, C.; Grillo, F.; Fiocca, R.; Mescoli, C.; Rugge, M.; Nesi, G.; Fociani, P.; et al. Small Bowel Carcinomas in Coeliac or Crohn’s Disease: Clinico-pathological, Molecular, and Prognostic Features. A Study from the Small Bowel Cancer Italian Consortium. J. Crohns Colitis 2017, 11, 942–953. [Google Scholar] [CrossRef]
- Fu, T.; Pappou, E.P.; Guzzetta, A.A.; Jeschke, J.; Kwak, R.; Dave, P.; Hooker, C.M.; Morgan, R.; Baylin, S.B.; Iacobuzio-Donahue, C.A.; et al. CpG island methylator phenotype-positive tumors in the absence of MLH1 methylation constitute a distinct subset of duodenal adenocarcinomas and are associated with poor prognosis. Clin. Cancer Res. 2012, 18, 4743–4752. [Google Scholar] [CrossRef]
- Sun, L.; Guzzetta, A.A.; Fu, T.; Chen, J.; Jeschke, J.; Kwak, R.; Vatapalli, R.; Baylin, S.B.; Iacobuzio-Donahue, C.A.; Wolfgang, C.L.; et al. CpG island methylator phenotype and its association with malignancy in sporadic duodenal adenomas. Epigenetics 2014, 9, 738–746. [Google Scholar] [CrossRef]
- Chen, Z.M.; Scudiere, J.R.; Abraham, S.C.; Montgomery, E. Pyloric gland adenoma: An entity distinct from gastric foveolar type adenoma. Am. J. Surg. Pathol. 2009, 33, 186–193. [Google Scholar] [CrossRef]
- Miller, G.C.; Kumarasinghe, M.P.; Borowsky, J.; Choi, W.T.; Setia, N.; Clauditz, T.; Gidwani, R.; Sufiyan, W.; Lauwers, G.Y.; Brown, I.S. Clinicopathological features of pyloric gland adenomas of the duodenum: A multicentre study of 57 cases. Histopathology 2020, 76, 404–410. [Google Scholar] [CrossRef]
- Hida, R.; Yamamoto, H.; Hirahashi, M.; Kumagai, R.; Nishiyama, K.; Gi, T.; Esaki, M.; Kitazono, T.; Oda, Y. Duodenal Neoplasms of Gastric Phenotype: An Immunohistochemical and Genetic Study with a Practical Approach to the Classification. Am. J. Surg. Pathol. 2017, 41, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Chlumská, A.; Waloschek, T.; Mukenšnabl, P.; Martínek, P.; Kašpírková, J.; Zámečník, M. Pyloric gland adenoma: A histologic, immunohistochemical and molecular genetic study of 23 cases. Cesk. Patol. 2015, 51, 137–143. [Google Scholar]
- O’Hayre, M.; Vázquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, G.; Sekine, S.; Ogawa, R.; Matsubara, A.; Mori, T.; Taniguchi, H.; Kushima, R.; Hiraoka, N.; Tsuta, K.; Tsuda, H.; et al. Frequent GNAS mutations in low-grade appendiceal mucinous neoplasms. Br. J. Cancer 2013, 108, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Kuboki, Y.; Tanji, E.; Yoshida, S.; Hatori, T.; Yamamoto, M.; Shibata, N.; Shimizu, K.; Kamatani, N.; Shiratori, K. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci. Rep. 2011, 1, 161. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Basturk, O.; Brannon, A.R.; Bhanot, U.; Scott, S.N.; Bouvier, N.; LaFemina, J.; Jarnagin, W.R.; Berger, M.F.; Klimstra, D.; et al. GNAS and KRAS Mutations Define Separate Progression Pathways in Intraductal Papillary Mucinous Neoplasm-Associated Carcinoma. J. Am. Coll. Surg. 2015, 220, 845–854. [Google Scholar] [CrossRef]
- Bazzichetto, C.; Luchini, C.; Conciatori, F.; Vaccaro, V.; Di Cello, I.; Mattiolo, P.; Falcone, I.; Ferretti, G.; Scarpa, A.; Cognetti, F.; et al. Morphologic and Molecular Landscape of Pancreatic Cancer Variants as the Basis of New Therapeutic Strategies for Precision Oncology. Int. J. Mol. Sci. 2020, 21, 8841. [Google Scholar] [CrossRef]
- Yamada, M.; Sekine, S.; Ogawa, R.; Taniguchi, H.; Kushima, R.; Tsuda, H.; Kanai, Y. Frequent activating GNAS mutations in villous adenoma of the colorectum. J. Pathol. 2012, 228, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, A.; Ogawa, R.; Suzuki, H.; Oda, I.; Taniguchi, H.; Kanai, Y.; Kushima, R.; Sekine, S. Activating GNAS and KRAS mutations in gastric foveolar metaplasia, gastric heterotopia, and adenocarcinoma of the duodenum. Br. J. Cancer 2015, 112, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Tanji, C.; Nakayama, K.I.; Kikuchi, A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol. Cell. Biol. 2005, 25, 9063–9072. [Google Scholar] [CrossRef]
- Kushima, R.; Rüthlein, H.J.; Stolte, M.; Bamba, M.; Hattori, T.; Borchard, F. ‘Pyloric gland-type adenoma’ arising in heterotopic gastric mucosa of the duodenum, with dysplastic progression of the gastric type. Virchows Arch. 1999, 435, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Vieth, M.; Kushima, R.; Borchard, F.; Stolte, M. Pyloric gland adenoma: A clinico-pathological analysis of 90 cases. Virchows Arch. 2003, 442, 317–321. [Google Scholar] [CrossRef]
- Sakurai, T.; Sakashita, H.; Honjo, G.; Kasyu, I.; Manabe, T. Gastric foveolar metaplasia with dysplastic changes in Brunner gland hyperplasia: Possible precursor lesions for Brunner gland adenocarcinoma. Am. J. Surg. Pathol. 2005, 29, 1442–1448. [Google Scholar] [CrossRef]
- Ushiku, T.; Arnason, T.; Fukayama, M.; Lauwers, G.Y. Extra-ampullary duodenal adenocarcinoma. Am. J. Surg. Pathol. 2014, 38, 1484–1493. [Google Scholar] [CrossRef]
- Xue, Y.; Vanoli, A.; Balci, S.; Reid, M.M.; Saka, B.; Bagci, P.; Memis, B.; Choi, H.; Ohike, N.; Tajiri, T.; et al. Non-ampullary-duodenal carcinomas: Clinicopathologic analysis of 47 cases and comparison with ampullary and pancreatic adenocarcinomas. Mod. Pathol. 2017, 30, 255–266. [Google Scholar] [CrossRef]
- Emilsson, L.; Semrad, C.; Lebwohl, B.; Green, P.; Ludvigsson, J.F. Risk of Small Bowel Adenocarcinoma, Adenomas, and Carcinoids in a Nationwide Cohort of Individuals with Celiac Disease. Gastroenterology 2020, 159, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Ang, D.C.; Shia, J.; Tang, L.H.; Katabi, N.; Klimstra, D.S. The utility of immunohistochemistry in subtyping adenocarcinoma of the ampulla of vater. Am. J. Surg. Pathol. 2014, 38, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Schaefer, N.; Wolff, M.; Fischer, H.P. Carcinoma of the ampulla of Vater: Comparative histologic/immunohistochemical classification and follow-up. Am. J. Surg. Pathol. 2004, 28, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.K.; Jamieson, N.B.; Johns, A.L.; Scarlett, C.J.; Pajic, M.; Chou, A.; Pinese, M.; Humphris, J.L.; Jones, M.D.; Toon, C.; et al. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J. Clin. Oncol. 2013, 31, 1348–1356. [Google Scholar] [CrossRef]
- Overman, M.J.; Soifer, H.S.; Schueneman, A.J.; Ensor, J., Jr.; Adsay, V.; Saka, B.; Neishaboori, N.; Wolff, R.A.; Wang, H.; Schnabel, C.A.; et al. Performance and prognostic utility of the 92-gene assay in the molecular subclassification of ampullary adenocarcinoma. BMC Cancer 2016, 16, 668. [Google Scholar] [CrossRef][Green Version]
- Pea, A.; Riva, G.; Bernasconi, R.; Sereni, E.; Lawlor, R.T.; Scarpa, A.; Luchini, C. Ampulla of Vater carcinoma: Molecular landscape and clinical implications. World J. Gastrointest. Oncol. 2018, 10, 370–380. [Google Scholar] [CrossRef]
- Mafficini, A.; Amato, E.; Cataldo, I.; Rusev, B.C.; Bertoncello, L.; Corbo, V.; Simbolo, M.; Luchini, C.; Fassan, M.; Cantù, C.; et al. Ampulla of Vater carcinoma: Sequencing analysis identifies TP53 status as a novel independent prognostic factor and potentially actionable ERBB, PI3K, and WNT pathways gene mutations. Ann. Surg. 2018, 267, 149–156. [Google Scholar] [CrossRef]
- Gingras, M.C.; Covington, K.R.; Chang, D.K.; Donehower, L.A.; Gill, A.J.; Ittmann, M.M.; Creighton, C.J.; Johns, A.L.; Shinbrot, E.; Dewal, N.; et al. Ampullary cancers harbor ELF3 tumor suppressor gene mutations and exhibit frequent WNT dysregulation. Cell Rep. 2016, 14, 907–919. [Google Scholar] [CrossRef]
- Yachida, S.; Wood, L.D.; Suzuki, M.; Takai, E.; Totoki, Y.; Kato, M.; Luchini, C.; Arai, Y.; Nakamura, H.; Hama, N.; et al. Genomic sequencing identifies ELF3 as a driver of ampullary carcinoma. Cancer Cell. 2016, 29, 229–240. [Google Scholar] [CrossRef]
- Alexander, J.R.; Andrews, J.M.; Buchi, K.N.; Lee, R.G.; Becker, J.M.; Burt, R.W. High prevalence of adenomatous polyps of the duodenal papilla in familial adenomatous polyposis. Dig. Dis. Sci. 1989, 34, 167–170. [Google Scholar] [CrossRef]
- Achille, A.; Scupoli, M.T.; Magalini, A.R.; Zamboni, G.; Romanelli, M.G.; Orlandini, S.; Biasi, M.O.; Lemoine, N.R.; Accolla, R.S.; Scarpa, A. APC gene mutations and allelic losses in sporadic ampullary tumours: Evidence of genetic difference from tumours associated with familial adenomatous polyposis. Int. J. Cancer 1996, 68, 305–312. [Google Scholar] [CrossRef]
- Achille, A.; Baron, A.; Zamboni, G.; Di Pace, C.; Orlandini, S.; Scarpa, A. Chromosome 5 allelic losses are early events in tumours of the papilla of Vater and occur at sites similar to those of gastric cancer. Br. J. Cancer 1998, 78, 1653–1660. [Google Scholar] [CrossRef][Green Version]
- Scarpa, A.; Zamboni, G.; Achille, A.; Capelli, P.; Bogina, G.; Iacono, C.; Serio, G.; Accolla, R.S. ras-family gene mutations in neoplasia of the ampulla of Vater. Int. J. Cancer 1994, 59, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Schönleben, F.; Qiu, W.; Allendorf, J.D.; Chabot, J.A.; Remotti, H.E.; Su, G.H. Molecular analysis of PIK3CA, BRAF, and RAS oncogenes in periampullary and ampullary adenomas and carcinomas. J. Gastrointest. Surg. 2009, 13, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.R.; Klimstra, D.S.; Cordon-Cardo, C.; Paty, P.B.; Park, P.Y.; Brennan, M.F. K-ras mutation in adenomas and carcinomas of the ampulla of Vater. Clin. Cancer Res. 1997, 3, 129–133. [Google Scholar] [PubMed]
- Chung, C.H.; Wilentz, R.E.; Polak, M.M.; Ramsoekh, T.B.; Noorduyn, L.A.; Gouma, D.J.; Huibregtse, K.; Offerhaus, G.J.; Slebos, R.J. Clinical significance of K-ras oncogene activation in ampullary neoplasms. J. Clin. Pathol. 1996, 49, 460–464. [Google Scholar] [CrossRef]
- Gallinger, S.; Vivona, A.A.; Odze, R.D.; Mitri, A.; O’Beirne, C.P.; Berk, T.C.; Bapat, B.V. Somatic APC and K-ras codon 12 mutations in periampullary adenomas and carcinomas from familial adenomatous polyposis patients. Oncogene 1995, 10, 1875–1878. [Google Scholar] [PubMed]
- Imai, Y.; Tsurutani, N.; Oda, H.; Inoue, T.; Ishikawa, T. Genetic instability and mutation of the TGF-beta-receptor-II gene in ampullary carcinomas. Int. J. Cancer 1998, 76, 407–411. [Google Scholar] [CrossRef]
- McCarthy, D.M.; Hruban, R.H.; Argani, P.; Howe, J.R.; Conlon, K.C.; Brennan, M.F.; Zahurak, M.; Wilentz, R.E.; Cameron, J.L.; Yeo, C.J.; et al. Role of the DPC4 tumor suppressor gene in adenocarcinoma of the ampulla of Vater: Analysis of 140 cases. Mod. Pathol. 2003, 16, 272–278. [Google Scholar] [CrossRef]
- Sessa, F.; Furlan, D.; Zampatti, C.; Carnevali, I.; Franzi, F.; Capella, C. Prognostic factors for ampullary carcinomas: Tumor stage, tumor histology, tumor location, immunohistochemistry and microsatellite instability. Virchows Arch. 2007, 45, 649–657. [Google Scholar] [CrossRef]
- Achille, A.; Biasi, M.O.; Zamboni, G.; Bogina, G.; Iacono, C.; Talamini, G.; Capella, G.; Scarpa, A. Cancers of the papilla of Vater: Mutator phenotype is associated with good prognosis. Clin. Cancer. Res. 1997, 3, 1841–1847. [Google Scholar]
- Ruemmele, P.; Dietmaier, W.; Terracciano, L.; Tornillo, L.; Bataille, F.; Kaiser, A.; Wuensch, P.H.; Heinmoeller, E.; Homayounfar, K.; Luettges, J.; et al. Histopathologic features and microsatellite instability of cancers of the papilla of vater and their precursor lesions. Am. J. Surg. Pathol. 2009, 33, 691–704. [Google Scholar] [CrossRef]
- Ohike, N.; Kim, G.E.; Tajiri, T.; Krasinskas, A.; Basturk, O.; Coban, I.; Bandyopadhyay, S.; Morohoshi, T.; Goodman, M.; Kooby, D.A.; et al. Intra-ampullary papillary-tubular neoplasm (IAPN): Characterization of tumoral intraepithelial neoplasia occurring within the ampulla: A clinicopathologic analysis of 82 cases. Am. J. Surg. Pathol. 2010, 34, 1731–1748. [Google Scholar] [CrossRef]
- Han, S.; Jang, K.T.; Choi, D.W.; Choi, S.H.; Heo, J.S.; Han, I.W.; Park, D.; Ryu, Y. Prognostic impact of intra-ampullary papillary-tubular neoplasm versus flat dysplasia as precursor lesions of ampullary adenocarcinoma. Dig. Surg. 2020, 37, 505–514. [Google Scholar] [CrossRef]
- Galiatsatos, P.; Foulkes, W.D. Familial adenomatous polyposis. Am. J. Gastroenterol. 2006, 101, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Al-Tassan, N.H.; Chmiel, J.; Maynard, N.; Fleming, A.L.; Livingston, G.T.; Williams, A.K.; Hodges, D.R.; Davies, S.S.; David, J.R.; Sampson, J.P.; et al. Inherited variants of MYH associated with somatic G:C-->T:A mutations in colorectal tumors. Nat. Genet. 2002, 30, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, F.; Viel, A.; Bignami, M. Role of MUTYH in human cancer. Mutat. Res. 2013, 743, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Palles, C.; Cazier, J.B.; Howarth, K.M.; Domingo, E.; Jones, A.M.; Broderick, P.; Kemp, Z.; Spain, S.L.; Guarino, E.; Salguero, I. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat. Genet. 2013, 45, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Bellido, F.; Pineda, M.; Aiza, G.; Valdés-Mas, R.; Navarro, M.; Puente, D.A.; Pons, T.; González, S.; Iglesias, S.; Darder, E.; et al. POLE and POLD1 mutations in 529 kindred with familial colorectal cancer and/or polyposis: Review of reported cases and recommendations for genetic testing and surveillance. Genet. Med. 2016, 18, 325–332. [Google Scholar] [CrossRef]
- Weren, R.D.; Ligtenberg, M.J.; Kets, C.M.; de Voer, R.M.; Verwiel, E.T.; Spruijt, L.; van Zelst-Stams, W.A.; Jongmans, M.C.; Gilissen, C.; Hehir-Kwa, J.Y.; et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat. Genet. 2015, 47, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.; Nardi, F.; Bechi, P.; Taddei, G.; Gozzo, P.; Romagnoli, P. Extracolonic polyps in familial polyposis coli and Gardner’s syndrome. Dis. Colon Rectum 1985, 28, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Sarre, R.G.; Frost, A.G.; Jagelman, D.G.; Petras, R.E.; Sivak, M.V.; McGannon, E. Gastric and duodenal polyps in familial adenomatous polyposis: A prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut 1987, 28, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Bülow, S.; Christensen, I.J.; Højen, H.; Björk, J.; Elmberg, M.; Järvinen, H.; Lepistö, A.; Nieuwenhuis, M.; Vasen, H. Duodenal surveillance improves the prognosis after duodenal cancer in familial adenomatous polyposis. Colorectal Dis. 2012, 14, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, I.; Kellokumpu, I.; Jarvinen, H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy 1999, 31, 412–416. [Google Scholar] [CrossRef]
- Galle, T.S.; Juel, K.; Bulow, S. Causes of death in familial adenomatous polyposis. Scand. J. Gastroenterol. 1999, 34, 808–812. [Google Scholar]
- Bülow, S.; Alm, T.; Fausa, O.; Hultcrantz, R.; Järvinen, H.; Vasen, H.; DAF Project Group. Duodenal adenomatosis in familial adenomatous polyposis. Int. J. Colorectal Dis. 1995, 10, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Church, J.M.; McGannon, E.; Hull-Boiner, S.; Sivak, M.V.; Van Stolk, R.; Jagelman, D.G.; Fazio, V.W.; Oakley, J.R.; Lavery, I.C.; Milsom, J.W. Gastroduodenal polyps in patients with familial adenomatous polyposis. Dis. Colon Rectum. 1992, 35, 1170–1173. [Google Scholar] [CrossRef]
- Vasen, H.F.; Möslein, G.; Alonso, A.; Aretz, S.; Bernstein, I.; Bertario, L.; Blanco, I.; Bülow, S.; Burn, J.; Capella, G.; et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008, 57, 704–713. [Google Scholar] [CrossRef]
- Spigelman, A.D.; Williams, C.B.; Talbot, I.C.; Domizio, P.; Phillips, R.K. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 1989, 2, 783–785. [Google Scholar] [CrossRef]
- Ruo, L.; Coit, D.G.; Brennan, M.F.; Guillem, J.G. Long-term follow up of patients with familial adenomatous polyposis undergoing pancreaticoduodenal surgery. J. Gastrointest. Surg. 2002, 6, 671–675. [Google Scholar] [CrossRef]
- Syngal, S.; Brand, R.E.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am. J. Gastroenterol. 2015, 110, 223–262. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Hamilton, S.R.; Krush, A.J.; Piantadosi, S.; Hylind, L.M.; Celano, P.; Booker, S.V.; Robinson, C.R.; Offerhaus, G.J. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N. Engl. J. Med. 1993, 328, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Winde, G.; Schmid, K.W.; Schlegel, W.; Fischer, R.; Osswald, H.; Bünte, H. Complete reversion and prevention of rectal adenomas in colectomized patients with familial adenomatous polyposis by rectal low-dose sulindac maintenance treatment: Advantages of a low-dose nonsteroidal anti-inflammatory drug regimen in reversing adenomas exceeding 33 months. Dis. Colon. Rectum. 1995, 38, 813–830. [Google Scholar] [PubMed]
- Cruz-Correa, M.; Hylind, L.M.; Romans, K.E.; Booker, S.V.; Giardiello, F.M. Long-term treatment with sulindac in familial adenomatous polyposis: A prospective cohort study. Gastroenterology 2002, 22, 641–645. [Google Scholar] [CrossRef]
- Conio, M.; Gostout, C.J. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Gastrointest. Endosc. 2001, 53, 265–266. [Google Scholar] [CrossRef]
- Debinski, H.S.; Trojan, J.; Nugent, K.P.; Spigelman, A.D.; Phillips, R.K. Effect of sulindac on small polyps in familial adenomatous polyposis. Lancet 1995, 345, 855–856. [Google Scholar] [CrossRef]
- Nugent, K.P.; Farmer, K.C.; Spigelman, A.D.; Williams, C.B.; Phillips, R.K. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br. J. Surg. 1993, 80, 1618–1619. [Google Scholar] [CrossRef] [PubMed]
- Samadder, N.J.; Neklason, D.W.; Boucher, K.M.; Byrne, K.R.; Kanth, P.; Samowitz, W.; Jones, D.; Tavtigian, S.V.; Done, M.W.; Berry, T.; et al. Effect of sulindac and erlotinib vs placebo on duodenal neoplasia in familial adenomatous polyposis: A randomized clinical trial. JAMA 2016, 315, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.; Jones, N.; Christian, D.; Engel, C.; Nielsen, M.; Kaufmann, A.; Steinke, V.; Vasen, H.F.; Propping, P.; Sampson, J.R.; et al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology 2009, 137, 1976–1985. [Google Scholar] [CrossRef]
- Walton, S.J.; Kallenberg, F.G.; Clark, S.K.; Dekker, E.; Latchford, A. Frequency and Features of Duodenal Adenomas in Patients With MUTYH-associated Polyposis. Clin. Gastroenterol. Hepatol. 2016, 14, 986–992. [Google Scholar] [CrossRef]
- Van Leerdam, M.E.; Roos, V.H.; van Hooft, J.E.; Dekker, E.; Jover, R.; Kaminski, M.F.; Latchford, A.; Neumann, H.; Pellisé, M.; Saurin, J.C.; et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2019, 51, 877–895. [Google Scholar] [CrossRef]
- Monahan, K.J.; Bradshaw, N.; Dolwani, S.; Desouza, B.; Dunlop, M.G.; East, J.E.; Ilyas, M.; Kaur, A.; Lalloo, F.; Latchford, A.; et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/ United Kingdom Cancer Genetics Group (UKCGG). Gut 2020, 69, 411–444. [Google Scholar] [CrossRef]
- Nielsen, M.; Poley, J.W.; Verhoef, S.; van Puijenbroek, M.; Weiss, M.M.; Burger, G.T.; Dommering, C.J.; Vasen, H.F.; Kuipers, E.J.; Wagner, A.; et al. Duodenal carcinoma in MUTYH-associated polyposis. J. Clin. Pathol. 2006, 59, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.; Franken, P.F.; Reinards, T.H.; Weiss, M.M.; Wagner, A.; van der Klift, H.; Kloosterman, S.; Houwing-Duistermaat, J.J.; Aalfs, C.M.; Ausems, M.G.; et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP). J. Med. Genet. 2005, 42, e54. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.E.; Hurley, J.J.; Meuser, E.; Jose, S.; Ashelford, K.E.; Mort, M.; Idziaszczyk, S.; Maynard, J.; Brito, H.L.; Harry, M.; et al. Burden and profile of somatic mutation in duodenal adenomas from patients with familial adenomatous- and MUTYH-associated polyposis. Clin. Cancer Res. 2017, 23, 6721–6732. [Google Scholar] [CrossRef] [PubMed]
- Viel, A.; Bruselles, A.; Meccia, E.; Fornasarig, M.; Quaia, M.; Canzonieri, V.; Policicchio, E.; Urso, E.D.; Agostini, M.; Genuardi, M.; et al. Specific mutational signature associated with DNA 8-oxoguaninepersistence in MUTYH-defective colorectal cancer. EBioMedicine 2017, 20, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, V.; Pin, E.; Russo, M.T.; Barone, F.; Degan, P.; Sanchez, M.; Quaia, M.; Minoprio, A.; Turco, E.; Mazzei, F.; et al. Loss of MUTYH function in human cells leads to accumulation of oxidative damage and genetic instability. Oncogene 2013, 32, 4500–4508. [Google Scholar] [CrossRef]
- Thomas, L.E.; Hurley, J.J.; Sanchez, A.A.; Aznárez, M.R.; Backman, A.S.; Bjork, J.; Capella, G.; Clark, S.K.; Colas, C.; Collaborative Group on Duodenal Polyposis in MAP; et al. Duodenal adenomas and cancer in MUTYH-associated polyposis: An international cohort study. Gastroenterology 2020, 160, 952–954.e4. [Google Scholar] [CrossRef]
- Nielsen, M.; Joerink-van de Beld, M.C.; Jones, N.; Vogt, S.; Tops, C.M.; Vasen, H.F.; Sampson, J.R.; Aretz, S.; Hes, F.J. Analysis of MUTYH genotypes and colorectal phenotypes in patients with MUTYH-associated polyposis. Gastroenterology 2009, 136, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Spier, I.; Zhao, B.; Kloth, M.; Marquez, J.; Hinrichsen, I.; Kirfel, J.; Tafazzoli, A.; Horpaopan, S.; Uhlhaas, S.; et al. Exome sequencing identifies biallelic MSH3 germline mutations as a recessive subtype of colorectal adenomatous polyposis. Am. J. Hum. Genet. 2016, 99, 337–351. [Google Scholar] [CrossRef]
- Vasen, H.F.; Watson, P.; Mecklin, J.P.; Lynch, H.T. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology 1999, 116, 1453–1456. [Google Scholar] [CrossRef]
- Koornstra, J.J. Small bowel endoscopy in familial adenomatous polyposis and Lynch syndrome. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bigas, M.A.; Vasen, H.F.; Lynch, H.T.; Watson, P.; Myrhøj, T.; Järvinen, H.J.; Mecklin, J.P.; Macrae, F.; St John, D.J.; Bertario, L.; et al. Characteristics of small bowel carcinoma in hereditary nonpolyposis colorectal carcinoma. International Collaborative Group on HNPCC. Cancer 1998, 83, 240–244. [Google Scholar] [PubMed]
- Park, J.G.; Kim, D.W.; Hong, C.W.; Nam, B.H.; Shin, Y.K.; Hong, S.H.; Kim, I.J.; Lim, S.B.; Aronson, M.; Bisgaard, M.L.; et al. Germ line mutations of mismatch repair genes in hereditary nonpolyposis colorectal cancer patients with small bowel cancer: International Society for Gastrointestinal Hereditary Tumours Collaborative Study. Clin. Cancer Res. 2006, 12, 3389–3393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saurin, J.C.; Pilleul, F.; Soussan, E.B.; Manière, T.; D’Halluin, P.N.; Gaudric, M.; Cellier, C.; Heresbach, D.; Gaudin, J.L.; Capsule Commission of the French Society of Digestive Endoscopy (SFED). Small bowel capsule endoscopy diagnoses early and advanced neoplasm in asymptomatic patients with Lynch syndrome. Endoscopy 2010, 42, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, P.; Vanoli, A.; Arpa, G.; Bonometti, A.; Luinetti, O.; Solcia, E.; Corazza, G.R.; Paulli, M.; Di Sabatino, A. Small bowel carcinomas associated with immune-mediated intestinal disorders: The current knowledge. Cancers 2018, 11, 31. [Google Scholar] [CrossRef]
- Bruno, C.J.; Batts, K.P.; Ahlquist, D.A. Evidence against flat dysplasia as a regional field defect in small bowel adenocarcinoma associated with celiac sprue. Mayo Clin. Proc. 1997, 72, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Rampertab, S.D.; Forde, K.A.; Green, P.H. Small bowel neoplasia in coeliac disease. Gut 2003, 52, 1211–1214. [Google Scholar] [CrossRef]
- Vanoli, A.; Di Sabatino, A.; Martino, M.; Klersy, C.; Grillo, F.; Mescoli, C.; Nesi, G.; Volta, U.; Fornino, D.; Luinetti, O.; et al. Small bowel carcinomas in celiac or Crohn’s disease: Distinctive histophenotypic, molecular and histogenetic patterns. Mod. Pathol. 2017, 30, 1453–1466. [Google Scholar] [CrossRef]
- Potter, D.D.; Murray, J.A.; Donohue, J.H.; Burgart, L.J.; Nagorney, D.M.; van Heerden, J.A.; Plevak, M.F.; Zinsmeister, A.R.; Thibodeau, S.N. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004, 64, 7073–7077. [Google Scholar] [CrossRef]
- Diosdado, B.; Buffart, T.E.; Watkins, R.; Carvalho, B.; Ylstra, B.; Tijssen, M.; Bolijn, A.S.; Lewis, F.; Maude, K.; Verbeke, C.; et al. High-resolution array comparative genomic hybridization in sporadic and celiac disease-related small bowel adenocarcinomas. Clin. Cancer Res. 2010, 16, 1391–1401. [Google Scholar] [CrossRef]
- Svrcek, M.; Piton, G.; Cosnes, J.; Beaugerie, L.; Vermeire, S.; Geboes, K.; Lemoine, A.; Cervera, P.; El-Murr, N.; Dumont, S.; et al. Small bowel adenocarcinomas complicating Crohn’s disease are associated with dysplasia: A pathological and molecular study. Inflamm. Bowel Dis. 2014, 20, 1584–1592. [Google Scholar] [CrossRef]
- Gui, X.; Köbel, M.; Ferraz, J.; Iacucci, M.; Ghosh, S.; Demetrick, D.J. Newly recognized non-adenomatous lesions associated with enteric carcinomas in inflammatory bowel disease—Report of six rare and unique cases. Ann. Diagn. Pathol. 2020, 44, 151455. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Köbel, M.; Ferraz, J.G.; Iacucci, M.; Ghosh, S.; Liu, S.; Ou, Y.; Perizzolo, M.; Winkfein, R.J.; Rambau, P.; et al. Histological and molecular diversity and heterogeneity of precancerous lesions associated with inflammatory bowel diseases. J. Clin. Pathol. 2020, 73, 391–402. [Google Scholar] [CrossRef]
- Choi, W.T.; Yozu, M.; Miller, G.C.; Shih, A.R.; Kumarasinghe, P.; Misdraji, J.; Harpaz, N.; Lauwers, G.Y. Nonconventional dysplasia in patients with inflammatory bowel disease and colorectal carcinoma: A multicenter clinicopathologic study. Mod. Pathol. 2020, 33, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, G.; McBride, R.; Houldsworth, J.; Harpaz, N.; Polydorides, A.D. Clinicopathological and molecular characterisation of Crohn’s disease-associated small bowel adenocarcinomas. J. Crohns Colitis 2020, 14, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Roche, H.J.; Carr, N.J.; Laing, H.; Bateman, A.C. Hyperplastic polyps of the duodenum: An unusual histological finding. J. Clin. Pathol. 2006, 59, 1305–1306. [Google Scholar] [CrossRef]
- Rosty, C.; Buchanan, D.D.; Walters, R.J.; Carr, N.J.; Bothman, J.W.; Young, J.P.; Brown, I.S. Hyperplastic polyp of the duodenum: A report of 9 cases with immunohistochemical and molecular findings. Hum. Pathol. 2011, 42, 1953–1959. [Google Scholar] [CrossRef]
- Sarbia, M.; Juttner, S.; Bettstetter, M.; Berndt, R. Serrated polyps of the duodenum: Three cases with immunohistological and molecular pathological findings. Pathologe 2013, 34, 347–351. [Google Scholar] [CrossRef]
- Terada, T. Pathologic observations of the duodenum in 615 consecutive duodenal specimens: I. benign lesions. Int. J. Clin. Exp. Pathol. 2012, 5, 46–51. [Google Scholar]
- Liu, X.; Chen, D.; Dugum, M.; Horvath, B.; Yuan, L.; Xiao, S.Y. Syndromic and sporadic inflammatory/hyperplastic small-bowel polyps: A comparative study. Gastroenterol. Rep. 2015, 3, 222–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rubio, C.A. Serrated adenoma of the duodenum. J. Clin. Pathol. 2004, 57, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Rosty, C.; Campbell, C.; Clendenning, M.; Bettington, M.; Buchanan, D.D.; Brown, I.S. Do serrated neoplasms of the small intestine represent a distinct entity? Pathological findings and molecular alterations in a series of 13 cases. Histopathology 2015, 66, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.A. Traditional serrated adenomas of the upper digestive tract. J. Clin. Pathol. 2016, 69, 1–5. [Google Scholar] [CrossRef]
- Park, Y.K.; Jeong, W.J.; Cheon, G.J. Slow-growing early adenocarcinoma arising from traditional serrated adenoma in the duodenum. Case Rep. Gastroenterol. 2016, 10, 257–263. [Google Scholar] [CrossRef]
- Srivastava, A.; Rege, T.A.; Kim, K.M.; Lefferts, J.A.; Park, C.K.; Tsongalis, G.J.; Odze, R.D. Duodenal serrated adenomas: Evidence for serrated carcinogenesis in the proximal small intestine. Mod. Pathol. 2011, 24, 168a–169a. [Google Scholar]
- Taggart, M.; Rashid, A.; Estrella, J.; Abraham, S.C. Serrated polyps of the extracolonic gastrointestinal tract. Histologic findings and genetic alterations. Mod. Pathol. 2012, 25, 182a. [Google Scholar]
- Kiremitci, S.; Cansız Ersoz, C.; Savaş, B.; Ensari, A. Gastric and small intestinal traditional serrated adenomas: A detailed morphologic and immunohistochemical analysis. Turk. J. Gastroenterol. 2020, 31, 441–450. [Google Scholar] [CrossRef]
- Gilad, O.; Rosner, G.; Fliss-Isakov, N.; Aharon-Kaspi, S.; Strul, H.; Gluck, N.; Kariv, R. Clinical and histologic overlap and distinction among various hamartomatous polyposis syndromes. Clin. Transl. Gastroenterol. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Shaco-Levy, R.; Jasperson, K.W.; Martin, K.; Samadder, N.J.; Burt, R.W.; Ying, J.; Bronner, M.P. Morphologic characterization of hamartomatous gastrointestinal polyps in Cowden syndrome, Peutz-Jeghers syndrome, and juvenile polyposis syndrome. Hum. Pathol. 2016, 49, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Pourmand, K.; Itzkowitz, S.H. Small Bowel Neoplasms and Polyps. Curr. Gastroenterol. Rep. 2016, 18, 23. [Google Scholar] [CrossRef]
- Kidambi, T.D.; Kohli, D.R.; Samadder, N.J.; Singh, A. Hereditary Polyposis Syndromes. Curr. Treat. Options Gastro. 2019, 17, 650–665. [Google Scholar] [CrossRef]
- Anderson, B.; Smyrk, T.; Sweester, S. Fibroblastic Polyps: A Novel Polyp Subtype in Cowden Syndrome. ACG Case Rep. J. 2017, 4, e113. [Google Scholar] [CrossRef] [PubMed]
- Nugent, K.P.; Talbot, I.C.; Hodgson, S.V.; Phillips, R.K. Solitary juvenile polyps: Not a marker for subsequent malignancy. Gastroenterology 1993, 105, 698–700. [Google Scholar] [CrossRef]
- Kapetanakis, A.M.; Vini, D.; Plitsis, G. Solitary juvenile polyps in children and colon cancer. Hepato-gastroenterology 1996, 43, 1530–1531. [Google Scholar] [PubMed]
- Kinzler, K.W.; Vogelstein, B. Landscaping the cancer terrain. Science 1998, 280, 1036–1037. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T. The hamartoma-adenoma-carcinoma sequence. J. Pathol. 1999, 188, 1–2. [Google Scholar] [CrossRef]
- Woodford-Richens, K.; Bevan, S.; Churchman, M.; Dowling, B.; Jones, D.; Norbury, C.G.; Hodgson, S.V.; Desai, D.; Neale, K.; Phillips, R.K.; et al. Analysis of genetic and phenotypic heterogeneity in juvenile polyposis. Gut 2000, 46, 656–660. [Google Scholar] [CrossRef]
- Blatter, R.H.; Plasilova, M.; Wenzel, F.; Gokaslan, S.T.; Terracciano, L.; Ashfaq, R.; Heinimann, K. Somatic alterations in juvenile polyps from BMPR1A and SMAD4 mutation carriers. Genes Chromosomes Cancer 2015, 54, 575–582. [Google Scholar] [CrossRef]
- Entius, M.M.; Keller, J.J.; Westerman, A.M.; van Rees, B.P.; van Velthuysen, M.L.; de Goeij, A.F.; Wilson, J.H.; Giardiello, F.M.; Offerhaus, G.J. Molecular genetic alterations in hamartomatous polyps and carcinomas of patients with Peutz-Jeghers syndrome. J. Clin. Pathol. 2001, 54, 6–31. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ellis, I.; Zauber, P.; Iwama, T.; Marchese, C.; Talbot, I.; Xue, W.H.; Yan, Z.Y.; Tomlinson, I. Allelic imbalance at the LKB1 (STK11) locus in tumours from patients with Peutz-Jeghers’ syndrome provides evidence for a hamartoma-(adenoma)-carcinoma sequence. J. Pathol. 1999, 188, 9–13. [Google Scholar] [CrossRef]
- Aretz, S.; Stienen, D.; Uhlhaas, S.; Loff, S.; Back, W.; Pagenstecher, C.; McLeod, D.R.; Graham, G.E.; Mangold, E.; Santer, R.; et al. High proportion of large genomic STK11 deletions in Peutz-Jeghers syndrome. Human Mutat. 2005, 26, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Ma, C.; Zhang, H. The molecular mechanisms that underlie the tumor suppressor function of LKB1. Acta Biochim. Biophys. Sin. Shanghai 2009, 41, 97–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borun, P.; Bartkowiak, A.; Banasiewicz, T.; Nedoszytko, B.; Nowakowska, D.; Teisseyre, M.; Limon, J.; Lubinski, J.; Kubaszewski, L.; Walkowiak, J.; et al. High Resolution Melting analysis as a rapid and efficient method of screening for small mutations in the STK11 gene in patients with Peutz-Jeghers syndrome. BMC Med. Genet. 2013, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Duan, F.X.; Gu, G.L.; Yu, P.F. Mutation analysis of related genes in hamartoma polyp tissue of Peutz-Jeghers syndrome. World J. Gastroenterol. 2020, 6, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Hearle, N.; Schumacher, V.; Menko, F.H.; Olschwang, S.; Boardman, L.A.; Gille, J.J.; Keller, J.J.; Westerman, A.M.; Scott, R.J.; Lim, W. Frequency and spectrum of cancers in the Peutz–Jeghers syndrome. Clin. Cancer. Res. 2006, 12, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lin, W.; Zhao, Y.; Zhu, J.; Sun, T.; Ren, L. Distinct promoter methylation patterns of LKB1 in the hamartomatous polyps of Peutz-Jeghers syndrome and its potential in gastrointestinal malignancy prediction. Orphanet J. Rare Dis. 2020, 15, 208. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Fang, J.Y. Genetics of the hamartomatous polyposis syndromes: A molecular review. Int. J. Colorectal Dis. 2009, 24, 865–874. [Google Scholar] [CrossRef]
- Ishida, H.; Ishibashi, K.; Iwama, T. Malignant tumors associated with juvenile polyposis syndrome in Japan. Surg. Today 2018, 48, 253–263. [Google Scholar] [CrossRef]
- Samadder, N.J.; Baffy, N.; Giridhar, K.V.; Couch, F.J.; Riegert-Johnson, D. Hereditary cancer syndromes-a primer on diagnosis and management, part 2: Gastrointestinal cancer syndromes. Mayo Clin. Proc. 2019, 94, 1099–1116. [Google Scholar] [CrossRef]
- Calva-Cerqueira, D.; Chinnathambi, S.; Pechman, B.; Bair, J.; Larsen-Haidle, J.; Howe, J.R. The rate of germline mutations and large deletions of SMAD4 and BMPR1A in juvenile polyposis. Clin. Genet. 2009, 75, 79–85. [Google Scholar] [CrossRef]
- Sweet, K.; Willis, J.; Zhou, X.P.; Gallione, C.; Sawada, T.; Alhopuro, P.; Khoo, S.K.; Patocs, A.; Martin, C.; Bridgeman, S.; et al. Molecular classification of patients with unexplained hamartomatous and hyperplastic polyposis. JAMA 2005, 294, 2465–2473. [Google Scholar] [CrossRef]
- Gammon, A.; Jasperson, K.; Kohlmann, W.; Burt, R.W. Hamartomatous polyposis syndromes. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 219–231. [Google Scholar] [CrossRef]
- Friedl, W.; Uhlhaas, S.; Schulmann, K.; Stolte, M.; Loff, S.; Back, W.; Mangold, E.; Stern, M.; Knaebel, H.P.; Sutter, C.; et al. Juvenile polyposis: Massive gastric polyposis is more common in MADH4 mutation carriers than in BMPR1A mutation carriers. Hum. Genet. 2002, 111, 108–111. [Google Scholar] [CrossRef]
- Aretz, S.; Stienen, D.; Uhlhaas, S.; Stolte, M.; Entius, M.M.; Loff, S.; Back, W.; Kaufmann, A.; Keller, K.M.; Blaas, S.H. High proportion of large genomic deletions and a genotype phenotype update in 80 unrelated families with juvenile polyposis syndrome. J. Med. Genet. 2007, 44, 702–709. [Google Scholar] [CrossRef]
- Hobert, J.A.; Eng, C. PTEN hamartoma tumor syndrome: An overview. Genet. Med. 2009, 11, 687–694. [Google Scholar] [CrossRef]
- Jelsig, A.M. Hamartomatous polyps—A clinical and molecular genetic study. Dan. Med. J. 2016, 63, 5280. [Google Scholar]
- Tan, M.H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin. Cancer Res. 2012, 18, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Ponz de Leona, M.; Di Gregorio, C.; Giunti, L.; Roncucci, L.; Pedroni, M.; Tinca, A.C.; Crucianelli, F.; Tricarico, R.; Genuardi, M. Duodenal carcinoma in a 37-year-old man with Cowden/Bannayan syndrome. Dig. Liv. Dis. 2013, 45, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Hirooka, Y.; Yamamura, T.; Yamada, K.; Nagura, A.; Yoshimura, T.; Ohmiya, N.; Uehara, K.; Yoshioka, Y.; Nagino, M.; et al. Cowden syndrome complicated by a gastrointestinal stromal tumor. Dig. Endosc. 2014, 23, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Vanoli, A.; La Rosa, S.; Klersy, C.; Grillo, F.; Albarello, L.; Inzani, F.; Maragliano, R.; Manca, R.; Luinetti, O.; Milione, M.; et al. Four neuroendocrine tumor types and neuroendocrine carcinoma of the duodenum: Analysis of 203 cases. Neuroendocrinology 2017, 104, 112–125. [Google Scholar] [CrossRef]
- Vanoli, A.; Albarello, L.; Uncini, S.; Fassan, M.; Grillo, F.; Di Sabatino, A.; Martino, M.; Pasquali, C.; Milanetto, A.C.; Falconi, M.; et al. Neuroendocrine tumors (NETs) of the minor papilla/ampulla: Analysis of 16 cases underlines homology with major ampulla NETs and differences from extra-ampullary duodenal NETs. Am. J. Surg. Pathol. 2019, 43, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, D.S.; Kloppell, G.; La Rosa, S.; Rindi, G. Classification of neuroendocrine neoplasms of the digestive system. In WHO Classification of Tumours: Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 16–22. [Google Scholar]
- Chandrasekharappa, S.C.; Guru, S.C.; Manickam, P.; Olufemi, S.E.; Collins, F.S.; Emmert-Buck, M.R.; Debelenko, L.V.; Zhuang, Z.; Lubensky, I.A.; Liotta, L.A.; et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 1997, 276, 404–407. [Google Scholar] [CrossRef]
- Anlauf, M.; Perren, A.; Henopp, T.; Rudolf, T.; Garbrecht, N.; Schmitt, A.; Raffel, A.; Gimm, O.; Weihe, E.; Knoefel, W.T.; et al. Allelic deletion of the MEN1 gene in duodenal gastrin and somatostatin cell neoplasms and their precursor lesions. Gut 2007, 56, 637–644. [Google Scholar] [CrossRef]
- Anlauf, M.; Perren, A.; Meyer, C.L.; Schmid, S.; Saremaslani, P.; Kruse, M.L.; Weihe, E.; Komminoth, P.; Heitz, P.U.; Klöppel, G. Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology 2005, 128, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Anlauf, M.; Perren, A.; Klöppel, G. Endocrine precursor lesions and microadenomas of the duodenum and pancreas with and without MEN1: Criteria, molecular concepts and clinical significance. Pathobiology 2007, 74, 279–284. [Google Scholar] [CrossRef] [PubMed]
| Subtype | Gene (Encoded Protein) | Prevalence of Mutations | Functional Effect | |
|---|---|---|---|---|
| Sporadic, non-ampullary, intestinal-type adenomas | APC (Adenomatous polyposis coli protein) | 50–55% [15,16] | Regulation of Wnt signaling pathway, cell migration and adhesion, apoptosis | |
| KRAS (KRas) | 5–18% [15,16] | GTPase intracellular signal transducer, regulating proliferation and differentiation | ||
| BRAF (BRaf) | 0–4% [16] | Activation of the MAP kinase transduction pathway | ||
| ERBB2/HER2 (erbB2) | <5% [16] | Protein tyrosine kinase involved in stabilization of peripheral microtubules and transcriptional regulation | ||
| TP53 (p53) | <5% [15,16] | Regulation of cell cycle arrest, apoptosis, senescence and DNA repair | ||
| Pyloric gland adenomas | GNAS (G-alpha subunits of G proteins) | 40% [27] | GPCR-mediated signaling constitutively active; PKA activation | |
| Foveolar adenomas | GNAS (G-alpha subunits of G proteins) | 100% [27] | GPCR-mediated signaling constitutively active; PKA activation | |
| Sporadic, ampullary, intestinal-type adenomas | APC (Adenomatous polyposis coli protein) | 17–44% [15,52] | Regulation of Wnt signaling pathway, cell migration and adhesion, apoptosis | |
| KRAS (KRas) | 30–44% [15,55,56] | GTPase, intracellular signal transducer, regulating proliferation and differentiation | ||
| Syndromic intestinal-type adenomas | FAP | APC (Adenomatous polyposis coli protein) | 17–66% [15,52,96] | Regulation of Wnt signaling pathway, cell migration and adhesion, apoptosis |
| KRAS (KRas) | 10% [96] | GTPase, intracellular signal transducer, regulating proliferation and differentiation | ||
| MAP | MUTYH (Adenine DNA glycosilase) | 100% | Oxidative DNA damage repair (base excision repair) | |
| APC (Adenomatous polyposis coli protein) | 77% [96] | Regulation of Wnt signaling pathway, cell migration and adhesion, apoptosis | ||
| KRAS (KRas) | 33% [96] | GTPase, intracellular signal transducer, regulating proliferation and differentiation | ||
| Crohn’s disease-associated dysplasia | KRAS (KRas) | 15–40% [113,115] | GTPase, intracellular signal transducer, regulating proliferation and differentiation | |
| PIK3CA (Phosphatidylinositol 4,5-biphosphate 3-kinase catalytic subunit alpha isoform) | 0–60% [113,115] | Activation of cell signaling regulating cellular growth, proliferation and morphology | ||
| Peutz-Jeghers polyps | STK11 (STK11) | >90% [143] | Tumor suppressor serine/threonine-protein kinase, controlling AMPK family members | |
| Juvenile polyps | SMAD4 (Smad4/Dpc4) | 20% [151] | Tumor suppressor, mediator of signal transduction by TGF β | |
| BMPR1A (Bone morphogenetic protein receptor type-1A) | 30% [151] | Transmembrane serine/threonine kinases, activation of SMAD transcriptional regulators | ||
| Cowden syndrome polyps | PTEN (Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase) | 80% [154] | Tumor suppressor related to the mTOR pathway through downregulation of the PI3K signaling pathway | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanoli, A.; Grillo, F.; Furlan, D.; Arpa, G.; Grami, O.; Guerini, C.; Riboni, R.; Mastracci, L.; Di Sabatino, A. Small Bowel Epithelial Precursor Lesions: A Focus on Molecular Alterations. Int. J. Mol. Sci. 2021, 22, 4388. https://doi.org/10.3390/ijms22094388
Vanoli A, Grillo F, Furlan D, Arpa G, Grami O, Guerini C, Riboni R, Mastracci L, Di Sabatino A. Small Bowel Epithelial Precursor Lesions: A Focus on Molecular Alterations. International Journal of Molecular Sciences. 2021; 22(9):4388. https://doi.org/10.3390/ijms22094388
Chicago/Turabian StyleVanoli, Alessandro, Federica Grillo, Daniela Furlan, Giovanni Arpa, Oneda Grami, Camilla Guerini, Roberta Riboni, Luca Mastracci, and Antonio Di Sabatino. 2021. "Small Bowel Epithelial Precursor Lesions: A Focus on Molecular Alterations" International Journal of Molecular Sciences 22, no. 9: 4388. https://doi.org/10.3390/ijms22094388
APA StyleVanoli, A., Grillo, F., Furlan, D., Arpa, G., Grami, O., Guerini, C., Riboni, R., Mastracci, L., & Di Sabatino, A. (2021). Small Bowel Epithelial Precursor Lesions: A Focus on Molecular Alterations. International Journal of Molecular Sciences, 22(9), 4388. https://doi.org/10.3390/ijms22094388






