4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, a Major Active Metabolite of Bisphenol A, Triggers Pancreatic β-Cell Death via a JNK/AMPKα Activation-Regulated Endoplasmic Reticulum Stress-Mediated Apoptotic Pathway
Abstract
1. Introduction
2. Results
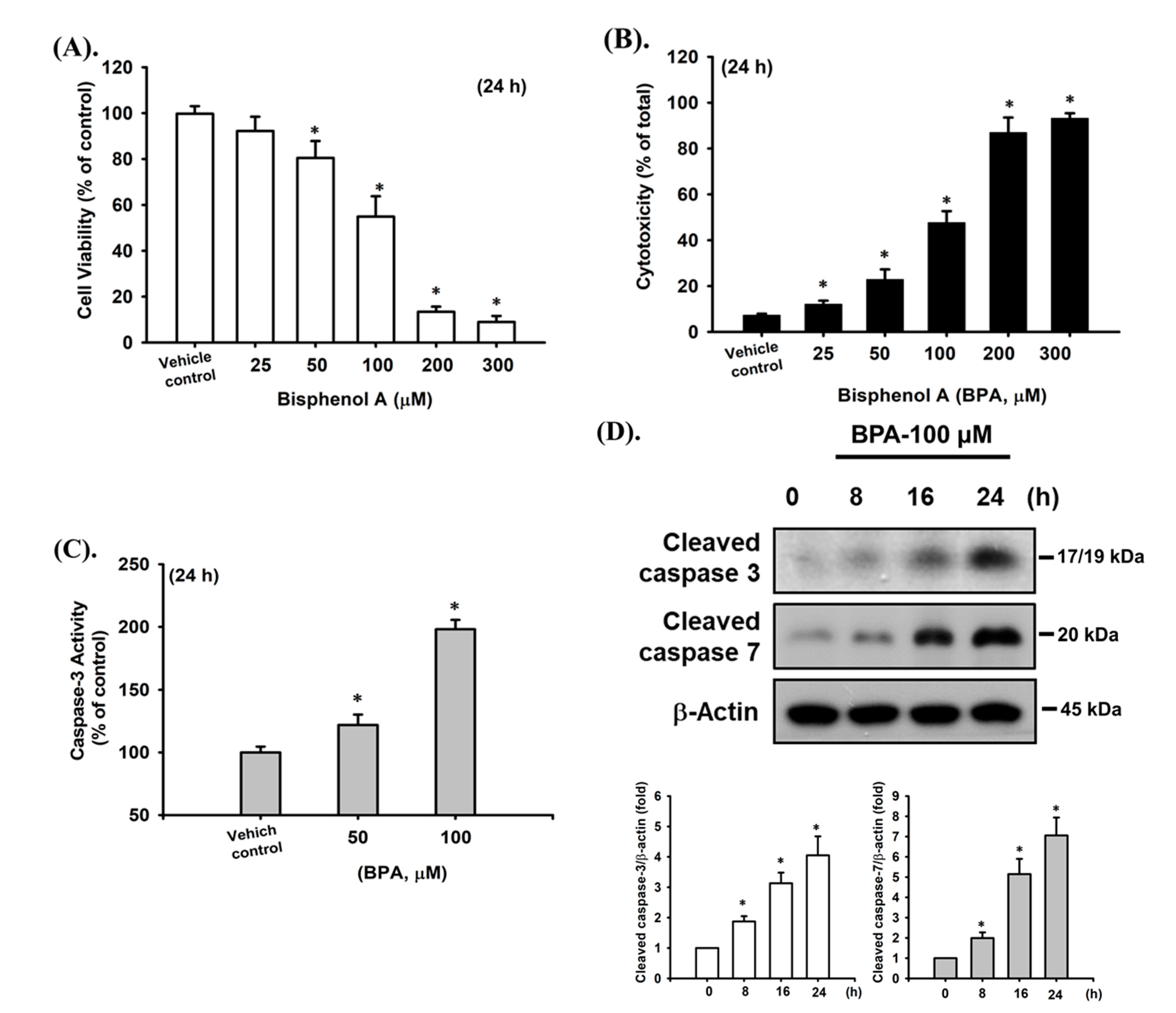
2.1. BPA-Induced Cytotoxicity and Apoptosis in Pancreatic β-Cells
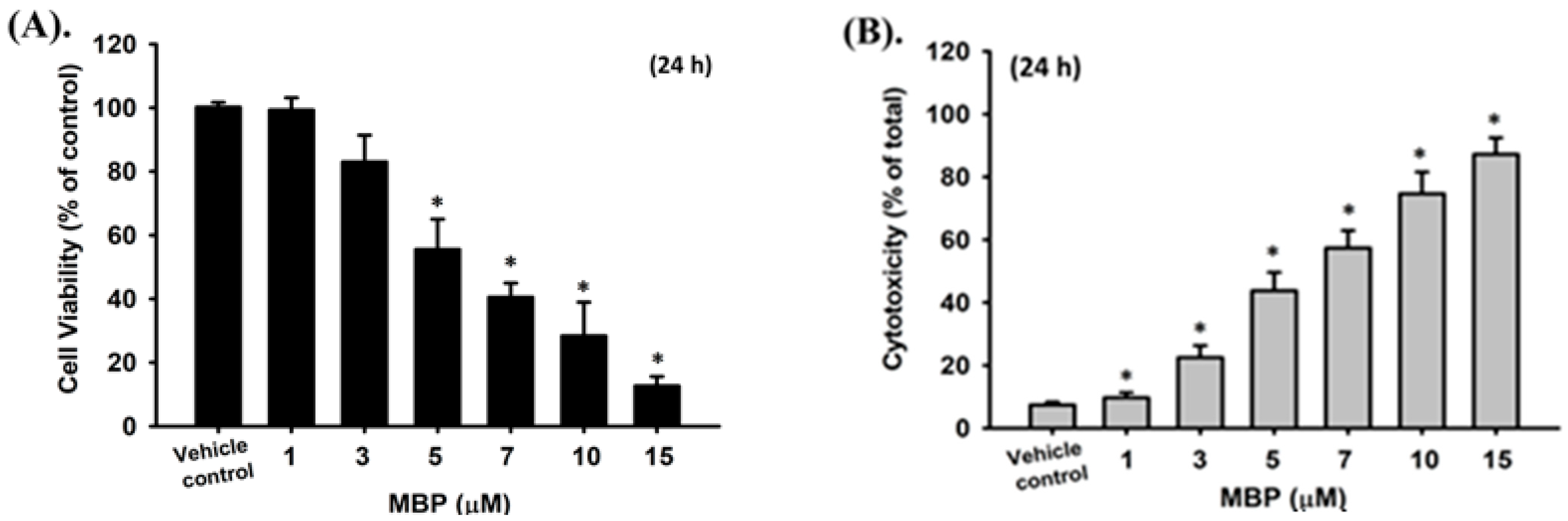
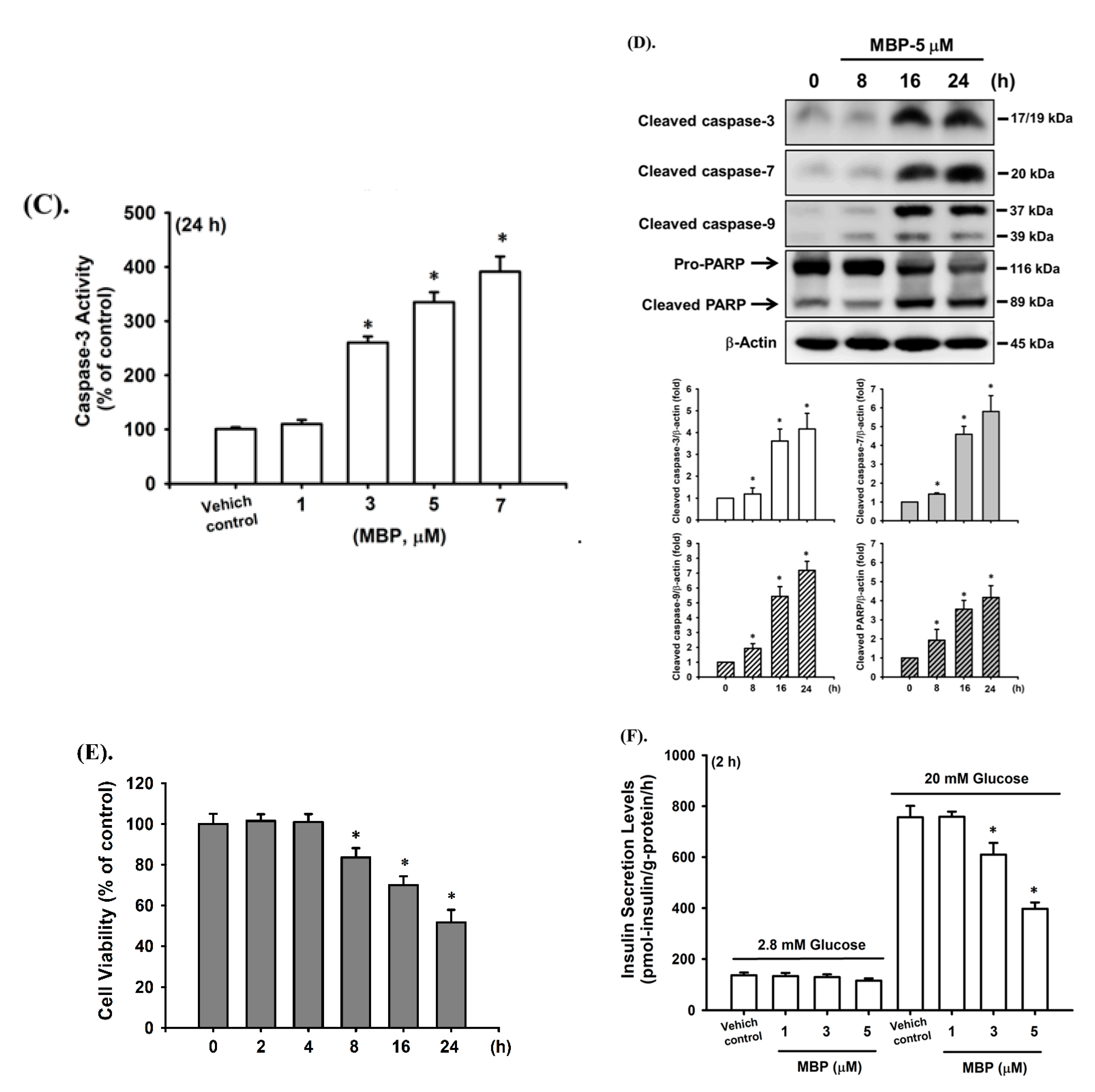
2.2. MBP-Induced Cell Apoptosis in RIN-m5F Cells
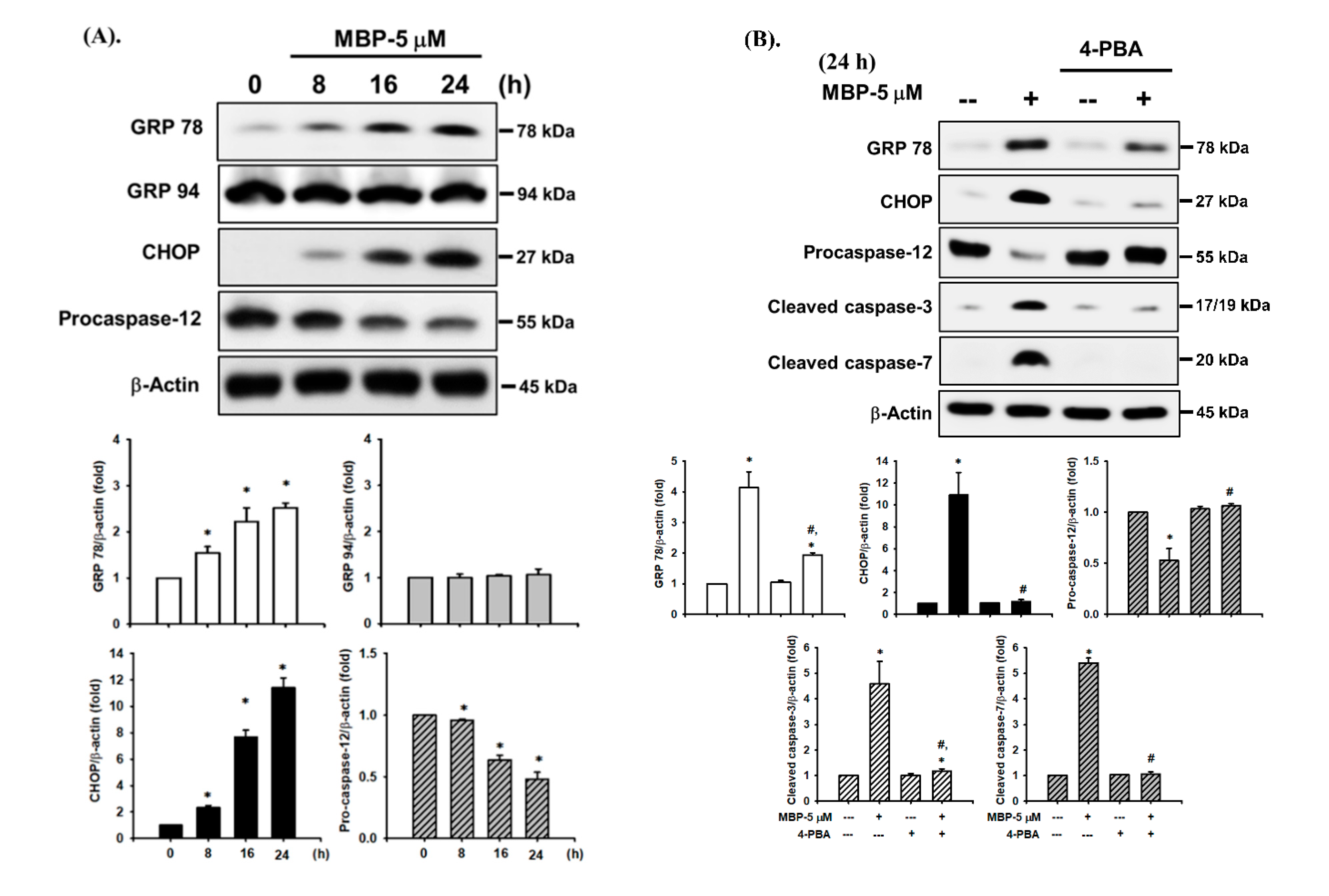
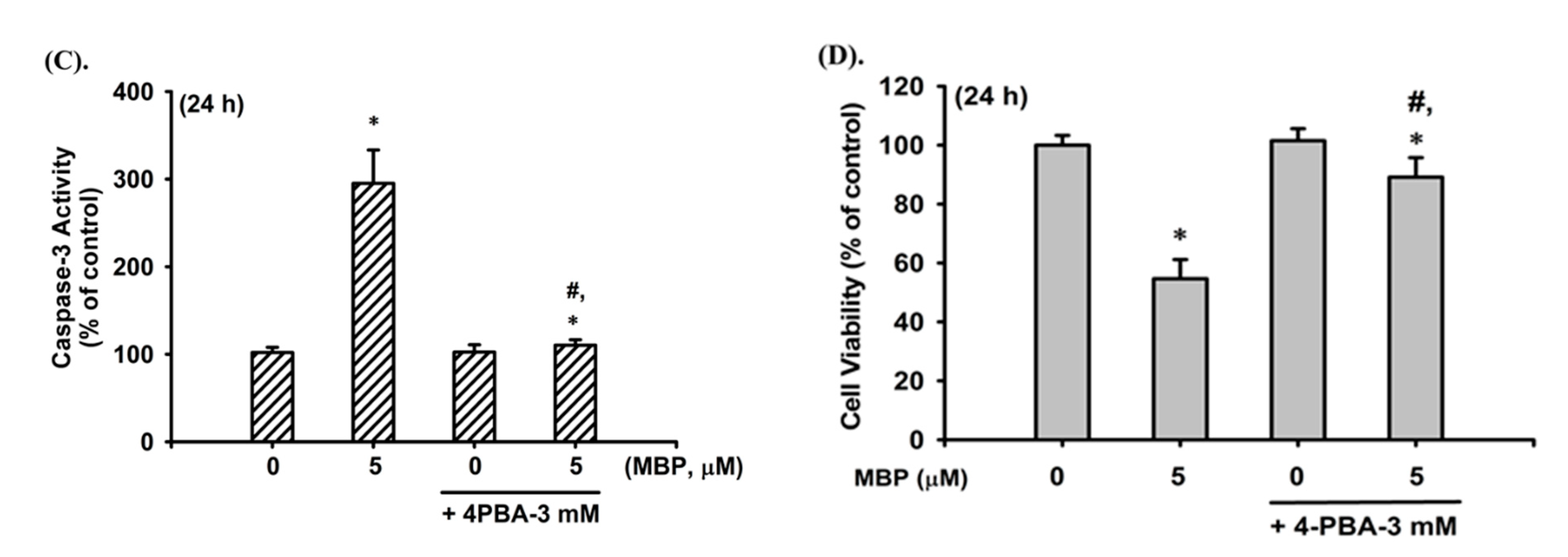
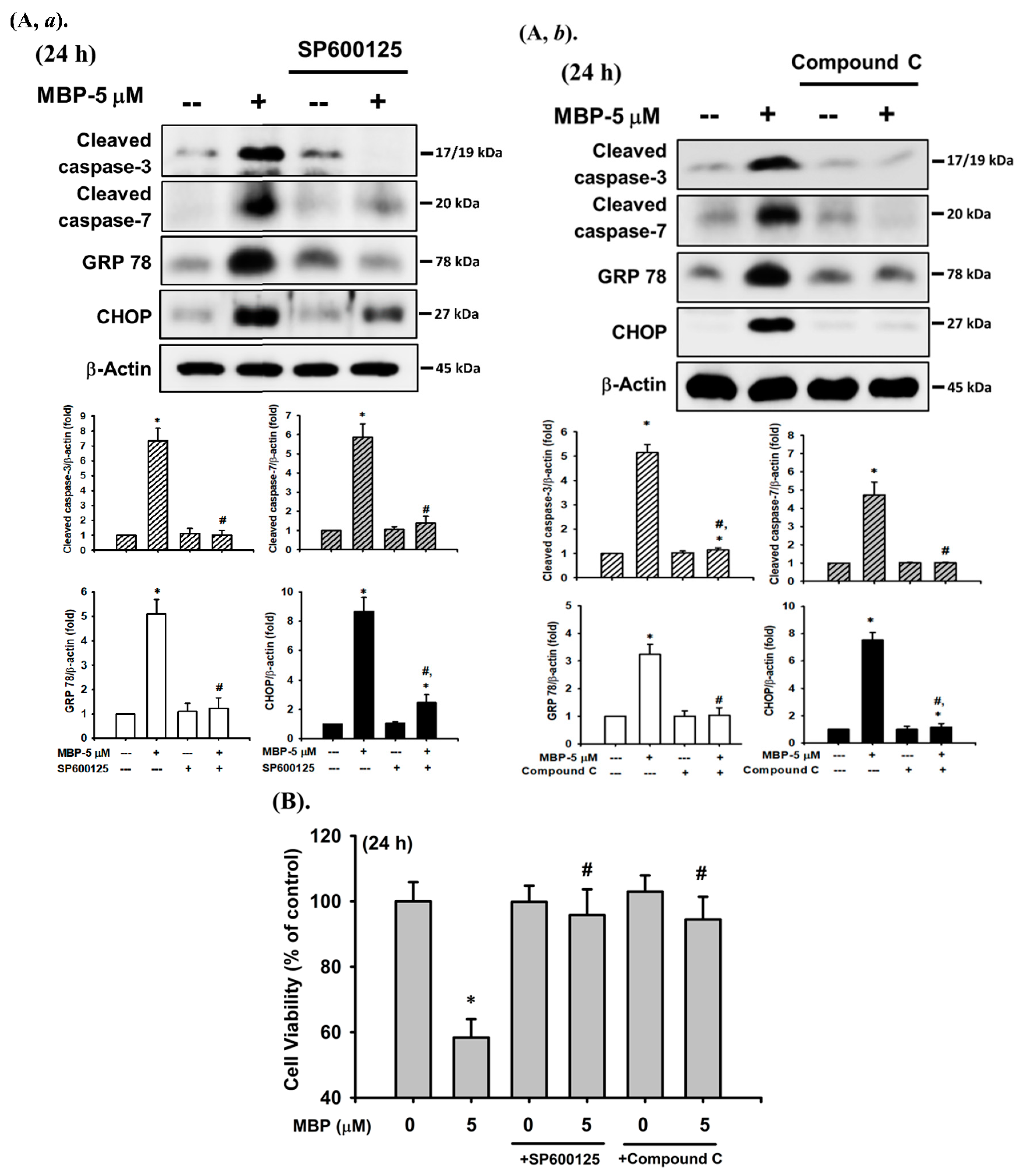
2.3. MBP-Induced ER Stress Response in RIN-m5F Cells
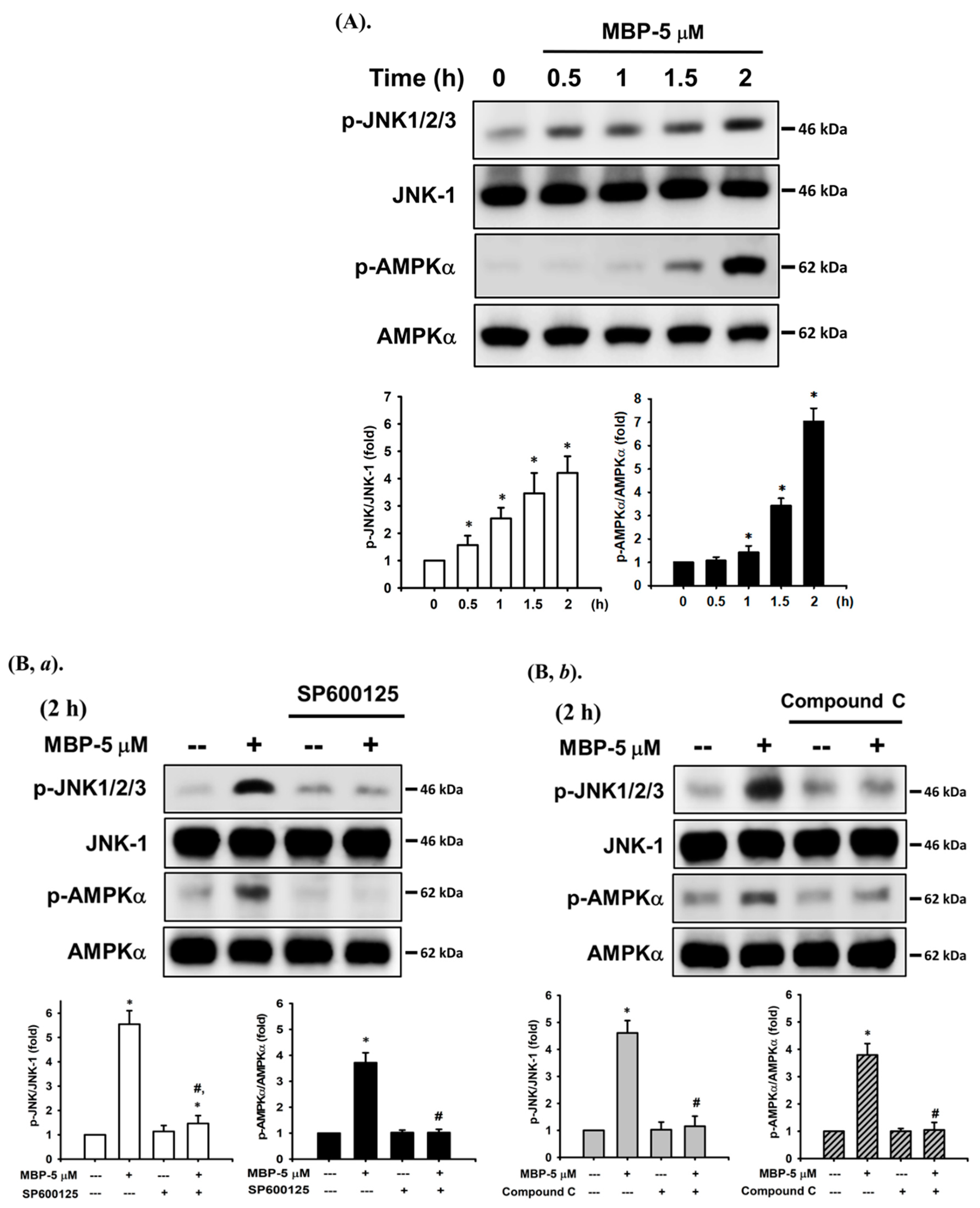
2.4. JNK and AMPK Signaling Played Crucial Roles in MBP-Induced β-Cell Apoptosis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Pancreatic β-Cell-Derived RIN-m5F Cell Culture
4.3. Cell Viability and Cytotoxicity Assay
4.4. Determination of Insulin Secretion
4.5. Measurement of Caspase-3 Activity
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MBP | 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene |
| BPA | bisphenol A |
| PARP | poly (ADP-ribose) polymerase |
| ER | endoplasmic reticulum |
| GRP | glucose-regulated protein |
| CHOP | C/EBP homologous protein |
| 4-PBA | 4-phenylbutyric acid |
| JNK | c-Jun N-terminal kinase |
| AMPK | AMP-activated protein kinase |
| DM | diabetes mellitus |
References
- Ingelfinger, J.R.; Jarcho, J.A. Increase in the Incidence of Diabetes and Its Implications. N. Engl. J. Med. 2017, 376, 1473–1474. [Google Scholar] [CrossRef]
- Diabetes-Statistics & Facts. 2021. Available online: https://www.statista.com/topics/1723/diabetes/#dossierSummary_chapter1 (accessed on 1 March 2021).
- Song, Y.; Chou, E.L.; Baecker, A.; You, N.C.; Song, Y.; Sun, Q.; Liu, S. Endocrine-disrupting chemicals, risk of type 2 diabetes, and diabetes-related metabolic traits: A systematic review and meta-analysis. J. Diabetes 2016, 8, 516–532. [Google Scholar] [CrossRef]
- Global Industry Analysts. Bisphenol A—Global Market Trajectory & Analytics; Global Industry Analysts Inc.: San Jose, CA, USA, 2021; pp. 1–174. [Google Scholar]
- World Health Organization (WHO). Toxicological and Health Aspects of Bisphenol A; WHO: Geneva, Switzerland; FAO: Rome, Italy, 2011. [Google Scholar]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. environment Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Maahs, D.M.; West, N.A.; Lawrence, J.M.; Mayer-Davis, E.J. Epidemiology of type 1 diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 481–497. [Google Scholar] [CrossRef]
- Stojanoska, M.M.; Milosevic, N.; Milic, N.; Abenavoli, L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders. Endocrine 2017, 55, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Weldingh, N.M.; Jorgensen-Kaur, L.; Becher, R.; Holme, J.A.; Bodin, J.; Nygaard, U.C.; Bolling, A.K. Bisphenol A Is More Potent than Phthalate Metabolites in Reducing Pancreatic beta-Cell Function. Biomed. Res. Int. 2017, 2017, 4614379. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA). Bisphenol a Casrn 80-05-7. Available online: https://cfpub.Epa.Gov/ncea/iris2/chemicallanding.Cfm?Substance_nmbr=356 (accessed on 1 March 2021).
- Yoshihara, S.; Mizutare, T.; Makishima, M.; Suzuki, N.; Fujimoto, N.; Igarashi, K.; Ohta, S. Potent estrogenic metabolites of bisphenol A and bisphenol B formed by rat liver S9 fraction: Their structures and estrogenic potency. Toxicol. Sci. 2004, 78, 50–59. [Google Scholar] [CrossRef]
- Ishibashi, H.; Watanabe, N.; Matsumura, N.; Hirano, M.; Nagao, Y.; Shiratsuchi, H.; Kohra, S.; Yoshihara, S.; Arizono, K. Toxicity to early life stages and an estrogenic effect of a bisphenol A metabolite, 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene on the medaka (Oryzias latipes). Life Sci. 2005, 77, 2643–2655. [Google Scholar] [CrossRef]
- Okuda, K.; Takiguchi, M.; Yoshihara, S. In vivo estrogenic potential of 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, an active metabolite of bisphenol A, in uterus of ovariectomized rat. Toxicol. Lett. 2001, 197, 7–11. [Google Scholar] [CrossRef]
- Moreman, J.; Takesono, A.; Trznadel, M.; Winter, M.J.; Perry, A.; Wood, M.E.; Rogers, N.J.; Kudoh, T.; Tyler, C.R. Estrogenic Mechanisms and Cardiac Responses Following Early Life Exposure to Bisphenol A (BPA) and Its Metabolite 4-Methyl-2,4-bis(p-hydroxyphenyl)pent-1-ene (MBP) in Zebrafish. Environ. Sci. Technol. 2018, 52, 6656–6665. [Google Scholar] [CrossRef]
- Mentor, A.; Bornehag, C.G.; Jonsson, M.; Mattsson, A. A suggested bisphenol A metabolite (MBP) interfered with reproductive organ development in the chicken embryo while a human-relevant mixture of phthalate monoesters had no such effects. J. Toxicol. Environ. Health A 2020, 83, 66–81. [Google Scholar] [CrossRef]
- Hirao-Suzuki, M.; Takeda, S.; Okuda, K.; Takiguchi, M.; Yoshihara, S. Repeated Exposure to 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene (MBP), an Active Metabolite of Bisphenol A, Aggressively Stimulates Breast Cancer Cell Growth in an Estrogen Receptor beta (ERbeta)-Dependent Manner. Mol. Pharm. 2019, 95, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Su, C.C.; Lee, K.I.; Chen, Y.W. Effects of Bisphenol A Metabolite 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene on Lung Function and Type 2 Pulmonary Alveolar Epithelial Cell Growth. Sci. Rep. 2016, 6, 39254. [Google Scholar] [CrossRef]
- Garcia-Arevalo, M.; Alonso-Magdalena, P.; Servitja, J.M.; Boronat-Belda, T.; Merino, B.; Villar-Pazos, S.; Medina-Gomez, G.; Novials, A.; Quesada, I.; Nadal, A. Maternal Exposure to Bisphenol-A During Pregnancy Increases Pancreatic beta-Cell Growth During Early Life in Male Mice Offspring. Endocrinology 2016, 157, 4158–4171. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, X.; Qiu, L.; Wei, J.; Huang, Q.; Fang, C.; Ye, T.; Kang, M.; Shen, H.; Dong, S. Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma (INS-1) cells. Cell Death Dis. 2013, 4, e460. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Yen, C.C.; Lee, K.I.; Su, C.C.; Yang, C.Y.; Wu, C.C.; Hsieh, S.S.; Ueng, K.C.; Huang, C.F. Molybdenum induces pancreatic beta-cell dysfunction and apoptosis via interdependent of JNK and AMPK activation-regulated mitochondria-dependent and ER stress-triggered pathways. Toxicol. Appl. Pharm. 2016, 294, 54–64. [Google Scholar] [CrossRef]
- Huang, C.C.; Kuo, C.Y.; Yang, C.Y.; Liu, J.M.; Hsu, R.J.; Lee, K.I.; Su, C.C.; Wu, C.C.; Lin, C.T.; Liu, S.H.; et al. Cadmium exposure induces pancreatic beta-cell death via a Ca2+-triggered JNK/CHOP-related apoptotic signaling pathway. Toxicology 2019, 425, 152252. [Google Scholar] [CrossRef]
- Remedi, M.S.; Emfinger, C. Pancreatic beta-cell identity in diabetes. Diabetes Obes. Metab. 2016, 18, 110–116. [Google Scholar] [CrossRef]
- Anuradha, R.; Saraswati, M.; Kumar, K.G.; Rani, S.H. Apoptosis of beta cells in diabetes mellitus. DNA Cell Biol. 2014, 33, 743–748. [Google Scholar] [CrossRef]
- Ling, C.; Groop, L. Epigenetics: A molecular link between environmental factors and type 2 diabetes. Diabetes 2009, 58, 2718–2725. [Google Scholar] [CrossRef]
- Zimmet, P.Z.; Magliano, D.J.; Herman, W.H.; Shaw, J.E. Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol. 2014, 2, 56–64. [Google Scholar] [CrossRef]
- Chang, K.C.; Hsu, C.C.; Liu, S.H.; Su, C.C.; Yen, C.C.; Lee, M.J.; Chen, K.L.; Ho, T.J.; Hung, D.Z.; Wu, C.C.; et al. Cadmium induces apoptosis in pancreatic beta-cells through a mitochondria-dependent pathway: The role of oxidative stress-mediated c-Jun N-terminal kinase activation. PLoS ONE 2013, 8, e54374. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lan, K.C.; Tsai, J.R.; Weng, T.I.; Yang, C.Y.; Liu, S.H. Tributyltin exposure at noncytotoxic doses dysregulates pancreatic beta-cell function in vitro and in vivo. Arch. Toxicol. 2017, 91, 3135–3144. [Google Scholar] [CrossRef]
- Huang, C.F.; Yang, C.Y.; Tsai, J.R.; Wu, C.T.; Liu, S.H.; Lan, K.C. Low-dose tributyltin exposure induces an oxidative stress-triggered JNK-related pancreatic beta-cell apoptosis and a reversible hypoinsulinemic hyperglycemia in mice. Sci. Rep. 2018, 8, 5734. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef]
- Hwang, S.; Lim, J.E.; Choi, Y.; Jee, S.H. Bisphenol A exposure and type 2 diabetes mellitus risk: A meta-analysis. BMC Endocr. Disord. 2018, 18, 81. [Google Scholar] [CrossRef]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Olea, N.; Pulgar, R.; Perez, P.; Olea-Serrano, F.; Rivas, A.; Novillo-Fertrell, A.; Pedraza, V.; Soto, A.M.; Sonnenschein, C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 1996, 104, 298–305. [Google Scholar] [CrossRef]
- Bodin, J.; Bolling, A.K.; Becher, R.; Kuper, F.; Lovik, M.; Nygaard, U.C. Transmaternal bisphenol A exposure accelerates diabetes type 1 development in NOD mice. Toxicol. Sci. 2014, 137, 311–323. [Google Scholar] [CrossRef]
- Veissi, M.; Jafarirad, S.; Ahangarpour, A.; Mohaghegh, S.M.; Malehi, A.S. Co-exposure to endocrine disruptors: Effect of bisphenol A and soy extract on glucose homeostasis and related metabolic disorders in male mice. Endocr. Regul. 2018, 52, 76–84. [Google Scholar] [CrossRef]
- Ring, J.A.; Ghabrial, H.; Ching, M.S.; Smallwood, R.A.; Morgan, D.J. Fetal hepatic drug elimination. Pharmacol. Ther. 1999, 84, 429–445. [Google Scholar] [CrossRef]
- Brown, A.R.; Green, J.M.; Moreman, J.; Gunnarsson, L.M.; Mourabit, S.; Ball, J.; Winter, M.J.; Trznadel, M.; Correia, A.; Hacker, C.; et al. Cardiovascular Effects and Molecular Mechanisms of Bisphenol A and Its Metabolite MBP in Zebrafish. Environ. Sci. Technol. 2019, 53, 463–474. [Google Scholar] [CrossRef]
- Huang, C.F.; Liu, S.H.; Su, C.C.; Fang, K.M.; Yen, C.C.; Yang, C.Y.; Tang, F.C.; Liu, J.M.; Wu, C.C.; Lee, K.I.; et al. Roles of ERK/Akt signals in mitochondria-dependent and endoplasmic reticulum stress-triggered neuronal cell apoptosis induced by 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, a major active metabolite of bisphenol A. Toxicology 2021, 455, 152764. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Cardozo, A.K.; Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 2008, 29, 42–61. [Google Scholar] [CrossRef]
- Berridge, M.J. The endoplasmic reticulum: A multifunctional signaling organelle. Cell Calcium 2002, 32, 235–249. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. Protein folding in the endoplasmic reticulum and the unfolded protein response. Handb. Exp. Pharm. 2006, 172, 69–91. [Google Scholar] [CrossRef]
- Faitova, J.; Krekac, D.; Hrstka, R.; Vojtesek, B. Endoplasmic reticulum stress and apoptosis. Cell. Mol. Biol. Lett. 2006, 11, 488–505. [Google Scholar] [CrossRef]
- Rasheva, V.I.; Domingos, P.M. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 2009, 14, 996–1007. [Google Scholar] [CrossRef]
- Lee, K.I.; Lin, J.W.; Su, C.C.; Fang, K.M.; Yang, C.Y.; Kuo, C.Y.; Wu, C.C.; Wu, C.T.; Chen, Y.W. Silica nanoparticles induce caspase-dependent apoptosis through reactive oxygen species-activated endoplasmic reticulum stress pathway in neuronal cells. Toxicol. Vitr. 2020, 63, 104739. [Google Scholar] [CrossRef]
- Lu, T.H.; Tseng, T.J.; Su, C.C.; Tang, F.C.; Yen, C.C.; Liu, Y.Y.; Yang, C.Y.; Wu, C.C.; Chen, K.L.; Hung, D.Z.; et al. Arsenic induces reactive oxygen species-caused neuronal cell apoptosis through JNK/ERK-mediated mitochondria-dependent and GRP 78/CHOP-regulated pathways. Toxicol. Lett. 2014, 224, 130–140. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Biophys. Sin 2014, 46, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Zinszner, H.; Kuroda, M.; Wang, X.; Batchvarova, N.; Lightfoot, R.T.; Remotti, H.; Stevens, J.L.; Ron, D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998, 12, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Umek, R.M.; Friedman, A.D.; McKnight, S.L. CCAAT-enhancer binding protein: A component of a differentiation switch. Science 1991, 251, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef]
- Zhao, L.; Ackerman, S.L. Endoplasmic reticulum stress in health and disease. Curr. Opin. Cell Biol. 2006, 18, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Brozzi, F.; Nardelli, T.R.; Lopes, M.; Millard, I.; Barthson, J.; Igoillo-Esteve, M.; Grieco, F.A.; Villate, O.; Oliveira, J.M.; Casimir, M.; et al. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 2015, 58, 2307–2316. [Google Scholar] [CrossRef]
- Lu, T.H.; Su, C.C.; Chen, Y.W.; Yang, C.Y.; Wu, C.C.; Hung, D.Z.; Chen, C.H.; Cheng, P.W.; Liu, S.H.; Huang, C.F. Arsenic induces pancreatic beta-cell apoptosis via the oxidative stress-regulated mitochondria-dependent and endoplasmic reticulum stress-triggered signaling pathways. Toxicol. Lett. 2011, 201, 15–26. [Google Scholar] [CrossRef]
- Yen, Y.P.; Tsai, K.S.; Chen, Y.W.; Huang, C.F.; Yang, R.S.; Liu, S.H. Arsenic induces apoptosis in myoblasts through a reactive oxygen species-induced endoplasmic reticulum stress and mitochondrial dysfunction pathway. Arch. Toxicol. 2012, 86, 923–933. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Kaneto, H.; Matsuoka, T.A.; Katakami, N.; Kawamori, D.; Miyatsuka, T.; Yoshiuchi, K.; Yasuda, T.; Sakamoto, K.; Yamasaki, Y.; Matsuhisa, M. Oxidative stress and the JNK pathway are involved in the development of type 1 and type 2 diabetes. Curr. Mol. Med. 2007, 7, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Yarza, R.; Vela, S.; Solas, M.; Ramirez, M.J. c-Jun N-terminal Kinase (JNK) Signaling as a Therapeutic Target for Alzheimer’s Disease. Front Pharm. 2015, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Carling, D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends. Biochem. Sci. 2004, 29, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. AMP-activated protein kinase phosphorylates retinoblastoma protein to control mammalian brain development. Dev. Cell. 2009, 16, 256–270. [Google Scholar] [CrossRef]
- Terai, K.; Hiramoto, Y.; Masaki, M.; Sugiyama, S.; Kuroda, T.; Hori, M.; Kawase, I.; Hirota, H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol. Cell. Biol. 2005, 25, 9554–9575. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.Y.; Gu, J.; Li, W.; Zhang, M.; Ji, Y.; Li, J.; Chen, L.; Hatch, G.M. Compound K protects pancreatic islet cells against apoptosis through inhibition of the AMPK/JNK pathway in type 2 diabetic mice and in MIN6 beta-cells. Life Sci. 2014, 107, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kefas, B.A.; Heimberg, H.; Vaulont, S.; Meisse, D.; Hue, L.; Pipeleers, D.; Van de Casteele, M. AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia 2003, 46, 250–254. [Google Scholar] [CrossRef][Green Version]
- Kefas, B.A.; Cai, Y.; Ling, Z.; Heimberg, H.; Hue, L.; Pipeleers, D.; Van de Casteele, M. AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J. Mol. Endocrinol. 2003, 30, 151–161. [Google Scholar] [CrossRef]
- Kefas, B.A.; Cai, Y.; Kerckhofs, K.; Ling, Z.; Martens, G.; Heimberg, H.; Pipeleers, D.; Van de Casteele, M. Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochem. Pharm. 2004, 68, 409–416. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-C.; Yang, C.-Y.; Su, C.-C.; Fang, K.-M.; Yen, C.-C.; Lin, C.-T.; Liu, J.-M.; Lee, K.-I.; Chen, Y.-W.; Liu, S.-H.; et al. 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, a Major Active Metabolite of Bisphenol A, Triggers Pancreatic β-Cell Death via a JNK/AMPKα Activation-Regulated Endoplasmic Reticulum Stress-Mediated Apoptotic Pathway. Int. J. Mol. Sci. 2021, 22, 4379. https://doi.org/10.3390/ijms22094379
Huang C-C, Yang C-Y, Su C-C, Fang K-M, Yen C-C, Lin C-T, Liu J-M, Lee K-I, Chen Y-W, Liu S-H, et al. 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, a Major Active Metabolite of Bisphenol A, Triggers Pancreatic β-Cell Death via a JNK/AMPKα Activation-Regulated Endoplasmic Reticulum Stress-Mediated Apoptotic Pathway. International Journal of Molecular Sciences. 2021; 22(9):4379. https://doi.org/10.3390/ijms22094379
Chicago/Turabian StyleHuang, Cheng-Chin, Ching-Yao Yang, Chin-Chuan Su, Kai-Min Fang, Cheng-Chieh Yen, Ching-Ting Lin, Jui-Min Liu, Kuan-I Lee, Ya-Wen Chen, Shing-Hwa Liu, and et al. 2021. "4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, a Major Active Metabolite of Bisphenol A, Triggers Pancreatic β-Cell Death via a JNK/AMPKα Activation-Regulated Endoplasmic Reticulum Stress-Mediated Apoptotic Pathway" International Journal of Molecular Sciences 22, no. 9: 4379. https://doi.org/10.3390/ijms22094379
APA StyleHuang, C.-C., Yang, C.-Y., Su, C.-C., Fang, K.-M., Yen, C.-C., Lin, C.-T., Liu, J.-M., Lee, K.-I., Chen, Y.-W., Liu, S.-H., & Huang, C.-F. (2021). 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, a Major Active Metabolite of Bisphenol A, Triggers Pancreatic β-Cell Death via a JNK/AMPKα Activation-Regulated Endoplasmic Reticulum Stress-Mediated Apoptotic Pathway. International Journal of Molecular Sciences, 22(9), 4379. https://doi.org/10.3390/ijms22094379





