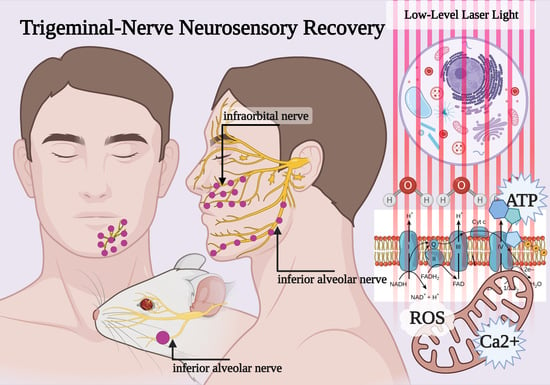
Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review
Abstract
1. Introduction
1.1. Trigeminal Nerve Damage
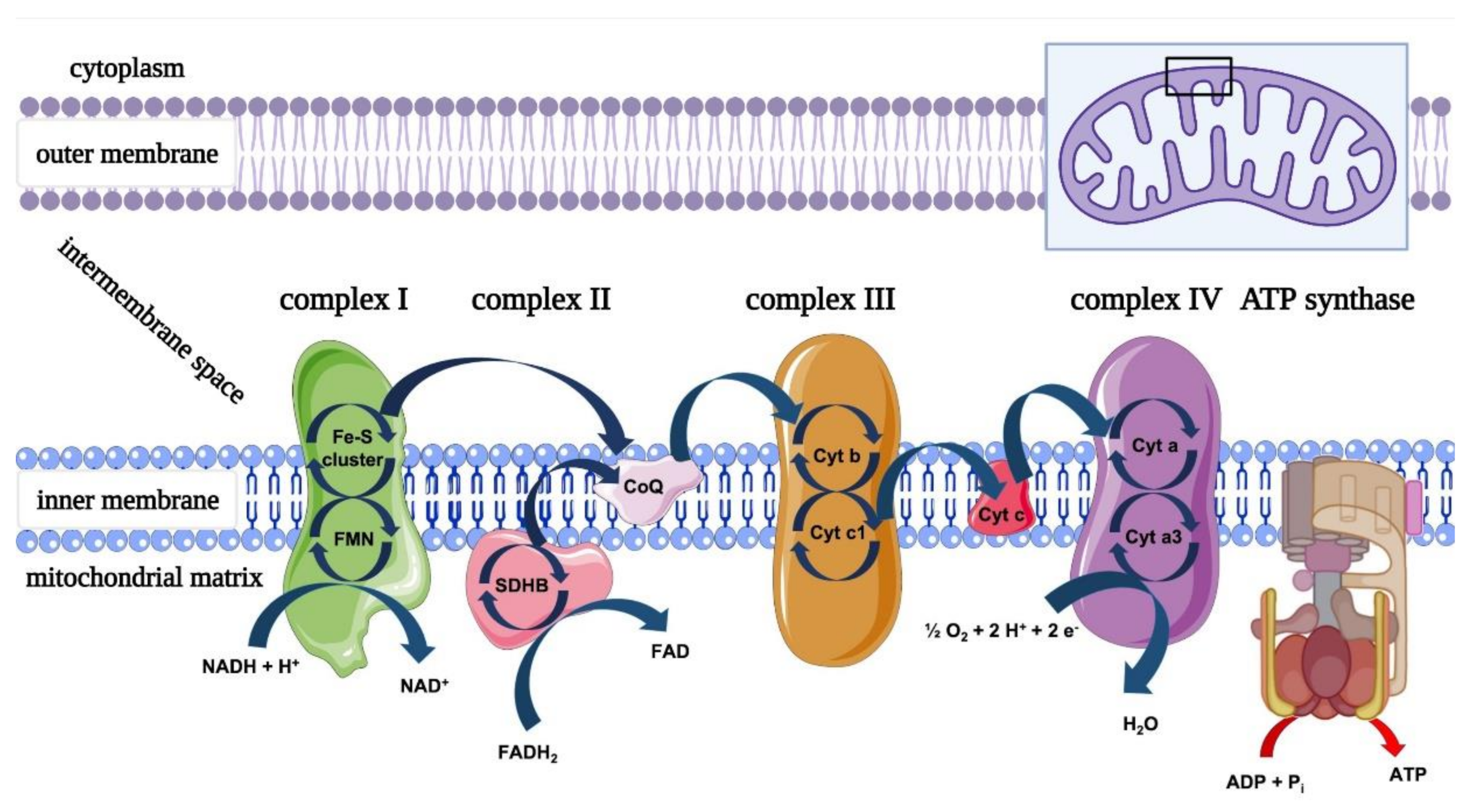
1.2. Mitochondrial Dysfunction in Nerve Damage
1.3. Questions
2. Methods for Articles Selection
3. Result of Articles Screening
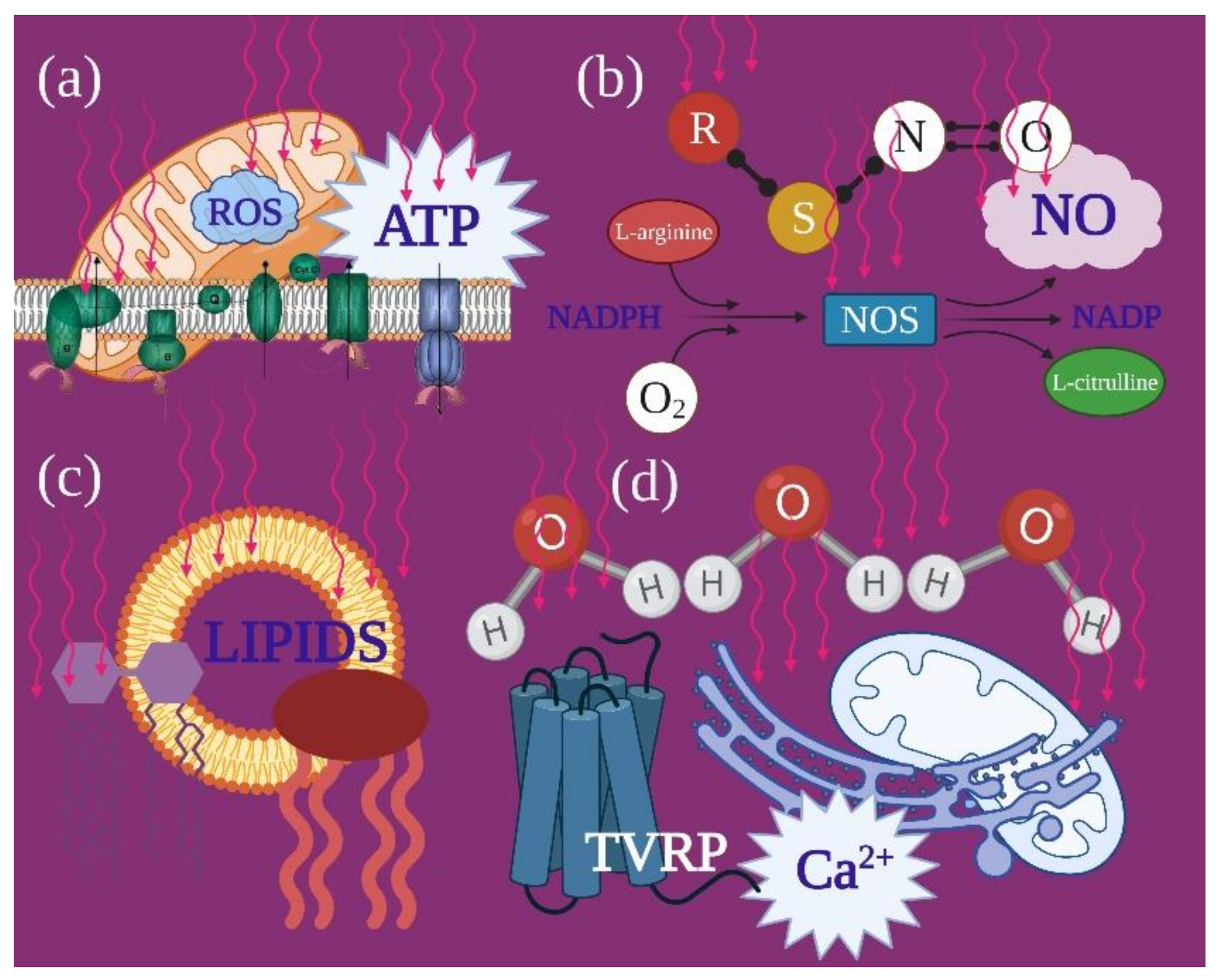
3.1. Mitochondria and PBM
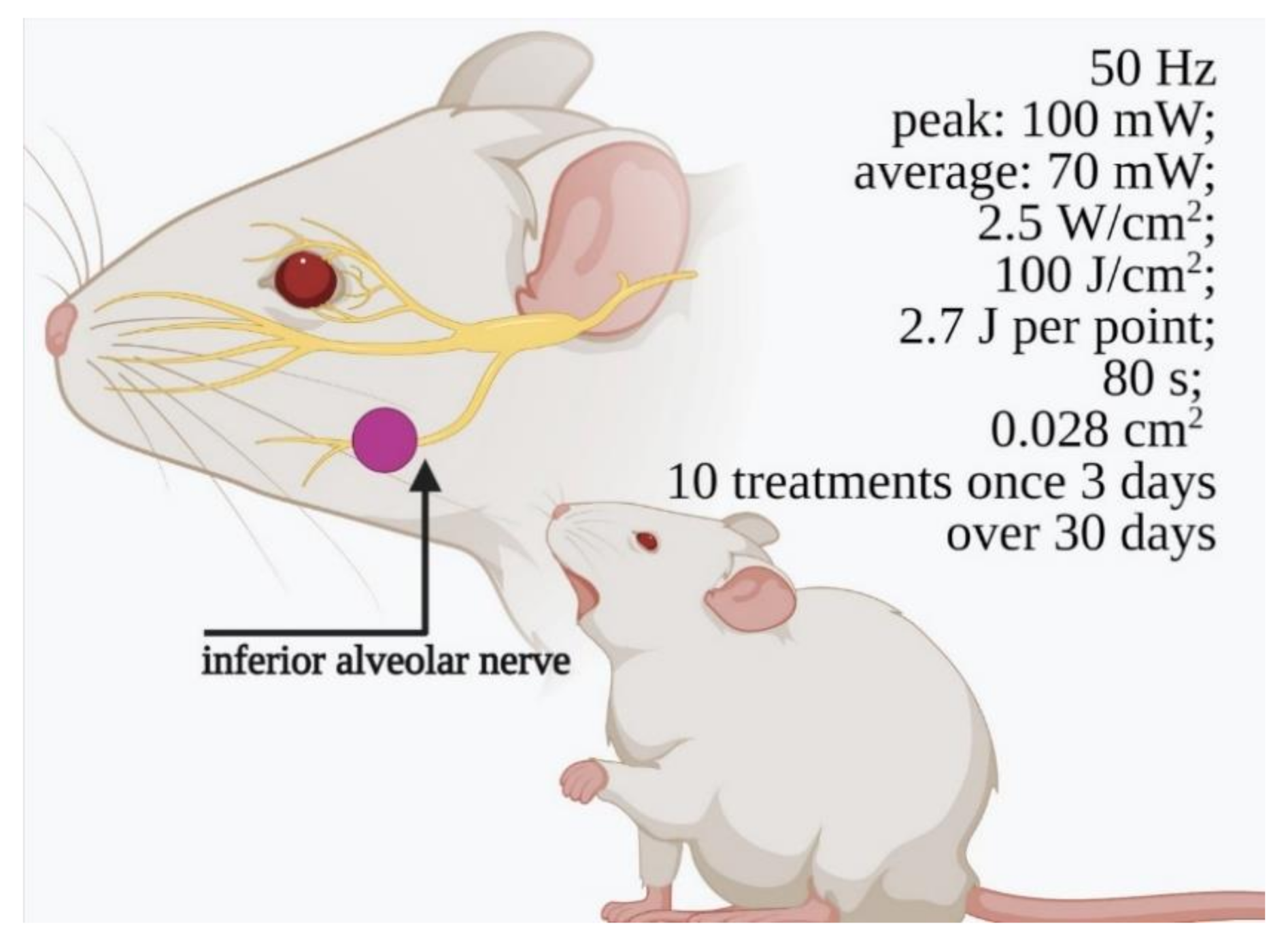
3.2. In Vivo Preclinical Studies (Animal Model, Trigeminal Branches Nerves and PBM)
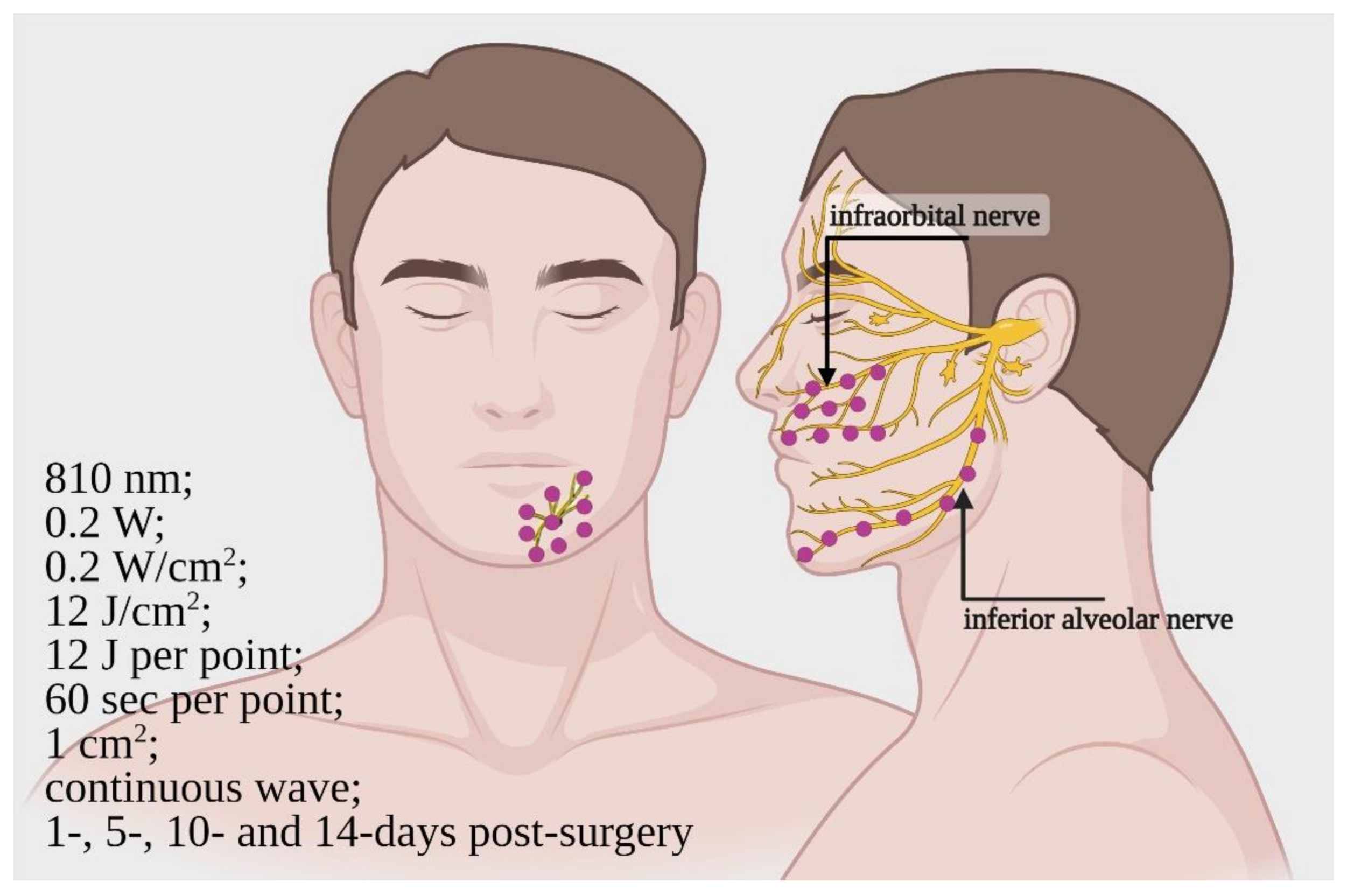
3.3. Clinical Studies (Patient, Trigeminal Branches Nerves and PBM)
4. Discussion
4.1. Photobiomodulation and Mitochondrial Bioenergetics
4.2. In Vivo Preclinical Studies
4.3. Clinical Studies
5. Conclusions
- The reliability of photobiomodulatory event strongly bases on biological and physical–chemical evidence. Its principal player is the mitochondrion, whether its cytochromes are directly involved as photoacceptor or indirectly through a vibrational and energetic variation of bound water and water as a photoacceptor.
- Moving from a microscopic point of view to preclinical and clinical, photobiomodulation seems to confirm its role as effective medical support for trigeminal disease. Irradiations in both pulsed and continuous wave mode affect the IAN nerve regeneration and neurosensory recovery through accurate wavelengths and doses.
- The 808-nm and 100 J/cm2 (0.07 W; 2.5 W/cm2; pulsed 50 Hz; 27 J per point; 80 s) on rats and 800-nm and 0.2 W/cm2 (0.2 W; 12 J/cm2; 12 J per point; 60 s, CW) on humans irradiated with the modalities previously described, resulted as trustworthy therapies, which could be supported by extensive studies.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, P.P. Observation on the recovery of sensation following inferior alveolar nerve injuries. Br. J. Oral Maxillofac. Surg. 1988, 26, 177–189. [Google Scholar] [CrossRef]
- LaBlanc, J.P.; Gregg, J.M. Trigeminal nerve injury: Diagnosis and management. Oral Maxillofac. Surg. Clin. NA 1995, 53, 1330–1333. [Google Scholar]
- Chiapasco, M.; Pedrinazzi, M.; Motta, J.; Crescentini, M.; Ramundo, G. Chirurgia dei terzi molari inferiori e lesioni del nervo linguale [Surgery of lower third molars and lesions of the lingual nerve]. Minerva Stomatol. 1996, 45, 517–522. [Google Scholar] [PubMed]
- Miloro, M. Trigeminal Nerve Injuries, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2013; Volume 1, pp. 27–61. [Google Scholar]
- Simpson, H.E. Injuries to the inferior dental and mental nerves. J. Oral Surg. 1958, 16, 300–305. [Google Scholar]
- Alling, C.C. Dysesthesia of the lingual and inferior alveolar nerves following third molar surgery. J. Oral Maxillofac. Surg. 1958, 44, 454–457. [Google Scholar] [CrossRef]
- Miloro, M. Surgical access for inferior alveolar nerve repair. J. Oral Maxillofac. Surg. 1995, 53, 1224–1225. [Google Scholar] [CrossRef]
- Buchman, A.L. Side effects of corticosteroid therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef]
- Flatters, S.J.L. The contribution of mitochondria to sensory processing and pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 119–146. [Google Scholar]
- Javadov, S.; Kozlov, A.V.; Camara, A.K.S. Mitochondria in Health and Diseases. Cells 2020, 9, 1177. [Google Scholar] [CrossRef]
- Lim, T.K.Y.; Rone, M.B.; Lee, S.; Antel, J.P.; Zhang, J. Mitochondrial and bioenergetic dysfunction in trauma-induced painful peripheral neuropathy. Mol. Pain 2015, 11, 58. [Google Scholar] [CrossRef]
- Flatters, S.J.L.; Bennett, G.J. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain 2006, 122, 245–257. [Google Scholar] [CrossRef]
- Xiao, W.H.; Zheng, H.; Zheng, F.Y.; Nuydens, R.; Meert, T.F.; Bennett, G.J. Mitochondrial abnormality in sensory, but not motor, axons in paclitaxel-evoked painful peripheral neuropathy in the rat. Neuroscience 2011, 199, 461–469. [Google Scholar] [CrossRef]
- Sivitz, W.I.; Yorek, M.A. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 537–577. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Semino-Mora, C.; Leon-Monzon, M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2′3′-dideoxycytidine (ddC). Lab. Investig. 2001, 81, 1537–1544. [Google Scholar] [CrossRef]
- Carter, G.T.; Jensen, M.P.; Galer, B.S.; Kraft, G.H.; Crabtree, L.D.; Beardsley, R.M. Neuropathic pain in Charcot-Marie-Tooth disease. Arch. Phys. Med. Rehabil. 1998, 79, 1560–1564. [Google Scholar] [CrossRef]
- Lenaz, G.; Genova, M.L. Structure and Organization of Mitochondrial Respiratory Complexes: A New Understanding of an Old Subject. Antioxid. Redox Signal. 2010, 12, 961–1008. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Jessu, R.; Aeddula, N.R. Physiology, Sodium Potassium Pump; StatPearls Publishing: Treasoure Island, FL, USA, 2021. [Google Scholar]
- Stevens, C.W. Editorial, New pathways for an old molecule: The role of the Na +-K + ATPase pump in peripheral neuropathy. J. Neurol. Sci. 2014, 340, 3–4. [Google Scholar] [CrossRef]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef]
- Lippe, G.; Coluccino, G.; Zancani, M.; Baratta, W.; Crusiz, P. Mitochondrial F-ATP Synthase and Its Transition into an Energy-Dissipating Molecular Machine. Oxidative Med. Cell. Longev. 2019, 2019, 8743257. [Google Scholar] [CrossRef]
- Gorman, A.M. Neuronal cell death in neurodegenerative diseases: Recurring themes around protein handling. J. Cell. Mol. Med. 2008, 12, 2263–2280. [Google Scholar] [CrossRef]
- Lim, T.K.Y.; Shi, X.Q.; Johnson, J.M.; Rone, M.B.; Antel, J.P.; David, S. Peripheral nerve injury induces persistent vascular dysfunction and endoneurial hypoxia, contributing to the genesis of neuropathic pain. J. Neurosci. 2015, 35, 3346–3359. [Google Scholar] [CrossRef] [PubMed]
- Venditti, P.; Di Stefano, L.; Di Meo, S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 2013, 13, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramón, S.; Liu, L.; Reale, M.; Rivas-Arancibia, S.; Solleiro-Villavicencio, H. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4 + T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 1, 114. [Google Scholar]
- Tiwari, B.S.; Belenghi, B.; Levine, A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002, 128, 1271–1281. [Google Scholar] [CrossRef]
- Steen, K.H.; Steen, A.E.; Kreysel, H.W.; Reeh, P.W. Inflammatory mediators potentiate pain induced by experimental tissue acidosis. Pain 1996, 66, 163–170. [Google Scholar] [CrossRef]
- Lee, S.; Min, K.-T. The Interface between ER and Mitochondria: Molecular Compositions and Functions. Mol. Cells 2018, 41, 1000–1007. [Google Scholar]
- Siau, C.; Bennett, G.J. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth. Analg. 2006, 102, 1485–1490. [Google Scholar] [CrossRef]
- Amaroli, A.; Colombo, E.; Zekiy, A.; Aicardi, S.; Benedicenti, S.; De Angelis, N. Interaction between Laser Light and Osteoblasts: Photobiomodulation as a Trend in the Management of Socket Bone Preservation-A Review. Biology 2020, 9, 409. [Google Scholar] [CrossRef]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and Clinical Applications of Red and Near-Infrared Photobiomodulation on Endothelial Dysfunction: A Review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Benedicenti, S.; Amaroli, A.; Sălăgean, T.; Pop, I.D.; Todea, D.; Bordea, I.R. Photobiomodulation Therapy in Oral Mucositis and Potentially Malignant Oral Lesions: A Therapy Towards the Future. Cancers 2020, 12, 1949. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- De Freitas Rodrigues, A.; De Oliveira Martins, D.; Chacur, M.; Luz, J.G.C. The effectiveness of photobiomodulation in the management of temporomandibular pain sensitivity in rats: Behavioral and neurochemical effects. Lasers Med. Sci. 2020, 35, 447–453. [Google Scholar] [CrossRef]
- Desiderá, A.C.; Nascimento, G.C.; Gerlach, R.F.; Leite-Panissi, C.R. Laser therapy reduces gelatinolytic activity in the rat trigeminal ganglion during temporomandibular joint inflammation. Oral Dis. 2015, 21, 652–658. [Google Scholar] [CrossRef]
- Diker, N.; Aytac, D.; Helvacioglu, F.; Oguz, Y. Comparative effects of photobiomodulation therapy at wavelengths of 660 and 808 nm on regeneration of inferior alveolar nerve in rats following crush injury. Lasers Med. Sci. 2020, 35, 413–420. [Google Scholar] [CrossRef]
- Hakimiha, N.; Dehghan, M.M.; Manaheji, H.; Zaringhalam, J.; Farzad-Mohajeri, S.; Fekrazad, R.; Moslemi, N. Recovery of inferior alveolar nerve by photobiomodulation therapy using two laser wavelengths: A behavioral and immunological study in rat. J. Photochem. Photobiol. B 2020, 204, 111785. [Google Scholar] [CrossRef]
- Martins, D.O.; Dos Santos, F.M.; Ciena, A.P.; Watanabe, I.S.; De Britto, L.R.G.; Lemos, J.B.D.; Chacur, M. Neuropeptide expression and morphometric differences in crushed alveolar inferior nerve of rats: Effects of photobiomodulation. Lasers Med. Sci. 2017, 32, 833–840. [Google Scholar] [CrossRef]
- Miloro, M.; Halkias, L.E.; Mallery, S.; Travers, S.; Rashid, R.G. Low-level laser effect on neural regeneration in Gore-Tex tubes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 93, 27–34. [Google Scholar] [CrossRef]
- Noma, D.; Fujita, S.; Zama, M.; Mayahara, K.; Motoyoshi, M.; Kobayashi, M. Application of oxytocin with low-level laser irradiation suppresses the facilitation of cortical excitability by partial ligation of the infraorbital nerve in rats: An optical imaging study. Brain Res. 2020, 1728, 146588. [Google Scholar] [CrossRef]
- Sasaki, R.T.; Grossi, N.G.; Zeni, R.T.; Saez, D.M.; Gonçalves, I.D.; Da Silva, M.C.P. Effect of Laser Photobiomodulation with Gradual or Constant Doses in the Regeneration of Rats’ Mental Nerve After Lesion by Compression. Photomed. Laser Surg. 2017, 35, 408–414. [Google Scholar] [CrossRef]
- Yucesoy, T.; Kutuk, N.; Canpolat, D.G.; Alkan, A. Comparison of Ozone and Photo-Biomodulation Therapies on Mental Nerve Injury in Rats. J. Oral Maxillofac. Surg. 2017, 75, 2323–2332. [Google Scholar] [CrossRef]
- Buchaim, D.V.; Rodrigues Ade, C.; Buchaim, R.L.; Barraviera, B.; Junior, R.S.; Junior, G.M.; Bueno, C.R.; Roque, D.D.; Dias, D.V.; Dare, L.R.; et al. The new heterologous fibrin sealant in combination with low-level laser therapy (LLLT) in the repair of the buccal branch of the facial nerve. Lasers Med. Sci. 2016, 31, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, D.V.; Andreo, J.C.; Ferreira Junior, R.S.; Barraviera, B.; Rodrigues, A.C.; Macedo, M.C.; Rosa Junior, G.M.; Shinohara, A.L.; Santos German, I.J.; Pomini, K.T.; et al. Efficacy of Laser Photobiomodulation on Morphological and Functional Repair of the Facial Nerve. Photomed. Laser Surg. 2017, 35, 442–449. [Google Scholar] [CrossRef]
- De Oliveira Martins, D.; Martinez dos Santos, F.; Evany de Oliveira, M.; de Britto, L.R.; Benedito Dias Lemos, J.; Chacur, M. Laser therapy and pain-related behavior after injury of the inferior alveolar nerve: Possible involvement of neurotrophins. J. Neurotrauma 2013, 30, 480–486. [Google Scholar] [CrossRef]
- Yuca, Y.; Yucesoy, T.; Tok, O.E.; Alkan, A. The efficiency of ozone therapy and low-level laser therapy in rat facial nerve injury. J. Craniomaxillofac. Surg. 2020, 48, 308–314. [Google Scholar] [CrossRef]
- Haghighat, A.; Khosrawi, S.; Tamizifar, A.; Haghighat, M. Does Low-Level Laser Photobiomodulation Improve Neurosensory Recovery After Orthognathic Surgery? A Clinical Trial with Blink Reflex. J. Oral Maxillofac. Surg. 2021, 79, 685–693. [Google Scholar] [CrossRef]
- Mohammad, E.; Motamedi, M.H.K. Effect of Low-Level Laser on the Healing of Neurosensory Disturbance Following Sagittal Split Ramus Osteotomy: A Double-Blind, Randomized Clinical Trial. Iran. Red Crescent Med. J. 2018. [Google Scholar] [CrossRef]
- Führer-Valdivia, A.; Noguera-Pantoja, A.; Ramírez-Lobos, V.; Solé-Ventura, P. Low-level laser effect in patients with neurosensory impairment of mandibular nerve after sagittal split ramus osteotomy. Randomized clinical trial, controlled by placebo. Med. Oral Patol. Oral Cir. Bucal 2014, 19, 327–334. [Google Scholar] [CrossRef]
- Miloro, M.; Criddle, T.R. Does Low-Level Laser Therapy Affect Recovery of Lingual and Inferior Alveolar Nerve Injuries? J. Oral Maxillofac. Surg. 2018, 76, 2669–2675. [Google Scholar] [CrossRef]
- Santos, F.T.; Sciescia, R.; Santos, P.L.; Weckwerth, V.; Dela Coleta Pizzol, K.E.; Queiroz, T.P. Is Low-Level Laser Therapy Effective on Sensorineural Recovery after Bilateral Sagittal Split Osteotomy? Randomized Trial. J. Oral Maxillofac. Surg. 2019, 77, 164–173. [Google Scholar] [CrossRef]
- Sharifi, R.; Fekrazad, R.; Taheri, M.M.; Kasaeian, A.; Babaei, A. Effect of photobiomodulation on recovery from neurosensory disturbances after sagittal split ramus osteotomy: A triple-blind randomised controlled trial. Br. J. Oral Maxillofac. Surg. 2020, 58, 535–541. [Google Scholar] [CrossRef]
- De Oliveira, R.F.; Da Silva, A.C.; Simões, A.; Youssef, M.N.; De Freitas, P.M. Laser Therapy in the Treatment of Paresthesia: A Retrospective Study of 125 Clinical Cases. Photomed. Laser Surg. 2015, 33, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Girão Evangelista, Í.; Pontes Tabosa, F.B.; Bezerra, A.V.; De Araújo Neto, E.V., Jr. Low-Level Laser Therapy in the Treatment of Inferior Alveolar Nerve Paresthesia after Surgical Exeresis of a Complex Odontoma. J. Lasers Med. Sci. 2019, 10, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Esteves Pinto Faria, P.; Temprano, A.; Piva, F.; Sant’ana, E.; Pimenta, D. Low-level laser therapy for neurosensory recovery after sagittal ramus osteotomy. Minerva Stomatol. 2020, 69, 141–147. [Google Scholar] [CrossRef]
- Guarini, D.; Gracia, B.; Ramírez-Lobos, V.; Noguera-Pantoja, A.; Solé-Ventura, P. Laser Biophotomodulation in Patients with Neurosensory Disturbance of the Inferior Alveolar Nerve after Sagittal Split Ramus Osteotomy: A 2-Year Follow-Up Study. Photomed. Laser Surg. 2018, 36, 3–9. [Google Scholar] [CrossRef]
- Khullar, S.M.; Emami, B.; Westermark, A.; Haanaes, H.R. Effect of low-level laser treatment on neurosensory deficits subsequent to sagittal split ramus osteotomy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1996, 82, 132–138. [Google Scholar] [CrossRef]
- Miloro, M.; Repasky, M. Low-level laser effect on neurosensory recovery after sagittal ramus osteotomy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 89, 12–18. [Google Scholar] [CrossRef]
- Ozen, T.; Orhan, K.; Gorur, I.; Ozturk, A. Efficacy of low level laser therapy on neurosensory recovery after injury to the inferior alveolar nerve. Head Face Med. 2006, 15, 2–3. [Google Scholar] [CrossRef]
- Pol, R.; Gallesio, G.; Riso, M.; Ruggiero, T.; Scarano, A.; Mortellaro, C.; Mozzati, M. Effects of Superpulsed, Low-Level Laser Therapy on Neurosensory Recovery of the Inferior Alveolar Nerve. J. Craniofac. Surg. 2016, 27, 1215–1219. [Google Scholar] [CrossRef]
- Qi, W.; Wang, Y.; Huang, Y.Y. Photobiomodulation therapy for management of inferior alveolar nerve injury post-extraction of impacted lower third molars. Laser Dent. Sci. 2020, 4, 25–32. [Google Scholar] [CrossRef]
- Abdelkarim Elafifi, H.; Acevedo Carrero, M.; Parada Avendaño, I.; España-Tost, A.; Arnabat-Domínguez, J. Effect of Photobiomodulation (Diode 810 nm) on Long-Standing Neurosensory Alterations of the Inferior Alveolar Nerve: A Case Series Study. Photobiomodul. Photomed. Laser Surg. 2021, 39, 4–9. [Google Scholar] [CrossRef]
- Eshghpour, M.; Shaban, B.; Ahrari, F.; Erfanian, M.; Shadkam, E. Is Low-Level Laser Therapy Effective for Treatment of Neurosensory Deficits Arising from Sagittal Split Ramus Osteotomy? J. Oral Maxillofac. Surg. 2017, 75, 2085–2090. [Google Scholar] [CrossRef]
- Gasperini, G.; de Siqueira, I.C.; Costa, L.R. Lower-level laser therapy improves neurosensory disorders resulting from bilateral mandibular sagittal split osteotomy: A randomized crossover clinical trial. J. Craniomaxillofac. Surg. 2014, 42, e130–e133. [Google Scholar] [CrossRef]
- Piotrowska, A.; Wierzbicka, J.; Żmijewski, M.A. Vitamin D in the skin physiology and pathology. Acta Biochim. Pol. 2016, 63, 17–29. [Google Scholar] [CrossRef]
- Werner, J.S.; Peterzell, D.H.; Scheetz, A.J. Light, vision, and aging. Optom. Sci. 1990, 67, 214–229. [Google Scholar] [CrossRef]
- Rojas, J.C.; Gonzalez-Lima, F. Low-level light therapy of the eye and brain. Eye Brain 2011, 14, 49–67. [Google Scholar]
- Mester, E.; Ludany, G.; Selyei, M.; Szende, B.; Total, G.J. The stimulating effect of low power laser rays on biological systems. Laser Rev. 1968, 1, 3. [Google Scholar]
- Arany, P.R.; Nayak, R.S.; Hallikerimath, S.; Limaye, A.M.; Kale, A.D.; Kondaiah, P. Activation of latent TGF-beta1 by low-power laser in vitro correlates with increased TGF-beta1 levels in laser-enhanced oral wound healing. Wound Repair Regen. 2007, 15, 866–874. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of all Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Karu, T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J. Photochem. Photobiol. B 2014, 140, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Pastore, D.; Greco, M.; Passarella, S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int. J. Radiat. Biol. 2000, 76, 863–870. [Google Scholar] [CrossRef]
- Yu, W.; Naim, J.O.; McGowan, M.; Ippolito, K.; Lanzafame, R.J. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem. Photobiol. 1997, 66, 866–871. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. An 808-nm Diode Laser with a Flat-Top Handpiece Positively Photobiomodulates Mitochondria Activities. Photomed. Laser Surg. 2016, 34, 564–571. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. 808-nm laser therapy with a flat-top handpiece photobiomodulates mitochondria activities of Paramecium primaurelia (Protozoa). Lasers Med. Sci. 2016, 31, 741–747. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. The protozoan, Paramecium primaurelia, as a non-sentient model to test laser light irradiation: The effects of an 808nm infrared laser diode on cellular respiration. Altern. Lab. Anim. 2015, 43, 155–162. [Google Scholar] [CrossRef]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Signore, A.; Ravera, S. Photobiomodulation and Oxidative Stress: 980 nm Diode Laser Light Regulates Mitochondrial Activity and Reactive Oxygen Species Production. Oxidative Med. Cell. Longev. 2021, 2021, 6626286. [Google Scholar] [CrossRef]
- Ravera, S.; Ferrando, S.; Agas, D.; De Angelis, N.; Raffetto, M.; Sabbieti, M.G.; Signore, A.; Benedicenti, S.; Amaroli, A. 1064 nm Nd:YAG laser light affects transmembrane mitochondria respiratory chain complexes. J. Biophotonics 2019, 12, 201900101. [Google Scholar] [CrossRef]
- Frey, T.G.; Mannella, C.A. The internal structure of mitochondria. Trends Biochem. Sci. 2000, 25, 319–324. [Google Scholar] [CrossRef]
- Koch, H.G.; Schneider, D. Folding, assembly, and stability of transmembrane cytochromes. Curr. Chem. Biol. 2007, 1, 59–74. [Google Scholar]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci. Rep. 2017, 7, 7781. [Google Scholar] [CrossRef]
- Figueroa, X.A.; Pollack, G.H. Exclusion-Zone Formation from Discontinuous Nafion Surfaces. Int. J. Des. Nat. Ecodyn. 2011, 6, 286–296. [Google Scholar] [CrossRef]
- Funk, R.H.W. Biophysical mechanisms complementing “classical” cell biology. Front. Biosci. 2018, 23, 921–939. [Google Scholar] [CrossRef]
- Mahamid, J.; Pfeffer, S.; Schaffer, M.; Villa, E.; Danev, R.; Cuellar, L.K.; Forster, F.; Hyman, A.A.; Plitzko, J.M.; Baumeister, W. Visualizing the molecular sociology at the HeLa cell nuclear periphery. Science 2016, 351, 969–972. [Google Scholar] [CrossRef]
- Pollack, G.H. Cell electrical properties: Reconsidering the origin of the electrical potential. Cell Biol. Int. 2015, 39, 237–242. [Google Scholar] [CrossRef]
- Pollack, G.H. The Fourth Phase of Water: A role in fascia? J. Bodyw. Mov. Ther. 2013, 17, 510–511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sommer, A.P. Mitochondrial cytochrome c oxidase is not the primary acceptor for near infrared light-it is mitochondrial bound water: The principles of low-level light therapy. Ann. Transl. Med. 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Walski, T.; Dyrda, A.; Dzik, M. Near infrared light induces post-translational modifications of human red blood cell proteins. Photochem. Photobiol. Sci. 2015, 14, 2035–2045. [Google Scholar] [CrossRef]
- Morelli, A.M.; Ravera, S.; Calzia, D.; Panfoli, I. Hypothesis of Lipid-Phase-Continuity Proton Transfer for Aerobic ATP Synthesis. J. Cereb. Blood Flow Metab. 2013, 33, 1838–1842. [Google Scholar] [CrossRef]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef]
- Jansen, K.; Wu, M.; Van der Steen, A.F. Photoacoustic imaging of human coronary atherosclerosis in two spectral bands. Photoacoustics 2013, 5, 12–20. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: Role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016, 6, 33719. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Benedicenti, A.; Ferrando, S.; Parker, S.; Selting, W.; Gallus, L.; Benedicenti, S. Photobiomodulation by Infrared Diode Laser: Effects on Intracellular Calcium Concentration and Nitric Oxide Production of Paramecium. Photochem. Photobiol. 2016, 92, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, S.; Agas, D.; Mirata, S.; Signore, A.; De Angelis, N.; Ravera, S.; Utyuzh, A.S.; Parker, S.; Sabbieti, M.G.; Benedicenti, S.; et al. The 808 nm and 980 nm infrared laser irradiation affects spore germination and stored calcium homeostasis: A comparative study using delivery hand-pieces with standard (Gaussian) or flat-top profile. J. Photochem. Photobiol. B 2019, 199, 111627. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, J.H. Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neurosci. Res. 2007, 58, 245–249. [Google Scholar] [CrossRef]
- Ferguson, T.A.; Muir, D. MMP-2 and MMP-9 increase the neurite-promoting potential of schwann cell basal laminae and are upregulated in degenerated nerve. Mol. Cell. Neurosci. 2000, 16, 157–167. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Souza-Barros, L.; Dhaidan, G.; Maunula, M. Skin color and tissue thickness effects on transmittance, reflectance, and skin temperature when using 635 and 808 nm lasers in low intensity therapeutics. Lasers Surg. Med. 2018, 50, 291–301. [Google Scholar] [CrossRef]
- Bhatheja, K.; Field, J. Schwann cells: Origins and role in axonal maintenance and regeneration. Int. J. Biochem. Cell. Biol. 2006, 38, 1995–1999. [Google Scholar] [CrossRef]
- Bouçanova, F.; Chrast, R. Metabolic Interaction between Schwann Cells and Axons under Physiological and Disease Conditions. Front. Cell. Neurosci. 2020, 14, 148. [Google Scholar] [CrossRef]
- Ino, D.; Iino, M. Schwann cell mitochondria as key regulators in the development and maintenance of peripheral nerve axons. Cell. Mol. Life Sci. 2017, 74, 827–835. [Google Scholar] [CrossRef]
- Chang, S.Y.; Lee, M.Y.; Chung, P.S. Enhanced mitochondrial membrane potential and ATP synthesis by photobiomodulation increases viability of the auditory cell line after gentamicin-induced intrinsic apoptosis. Sci. Rep. 2019, 9, 19248. [Google Scholar] [CrossRef]
- Wang, R.; Dong, Y.; Lu, Y.; Zhang, W.; Brann, D.W.; Zhang, Q. Photobiomodulation for Global Cerebral Ischemia: Targeting Mitochondrial Dynamics and Functions. Mol. Neurobiol. 2019, 56, 1852–1869. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Abrahamse, H. Photobiomodulation-Induced Differentiation of Immortalized Adipose Stem Cells to Neuronal Cells. Lasers Surg. Med. 2020, 52, 1032–1040. [Google Scholar] [CrossRef]
- Silveira, P.C.L.; Ferreira, G.K.; Zaccaron, R.P.; Glaser, V.; Remor, A.P.; Mendes, C.; Pinho, R.A.; Latini, A. Effects of photobiomodulation on mitochondria of brain, muscle, and C6 astroglioma cells. Med. Eng. Phys. 2019, 71, 108–113. [Google Scholar] [CrossRef]
- Rocha, I.R.C.; Perez-Reyes, E.; Chacur, M. Effect of photobiomodulation on mitochondrial dynamics in peripheral nervous system in streptozotocin-induced type 1 diabetes in rats. Photochem. Photobiol. Sci. 2021, 20, 293–301. [Google Scholar] [CrossRef]
- Wong-Riley, M.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. JBC 2005, 280, 4761–4771. [Google Scholar] [CrossRef]
- Zupin, L.; Barbi, E.; Sagredini, R.; Ottaviani, G.; Crovella, S.; Celsi, F. In vitro effects of photobiomodulation therapy on 50B11 sensory neurons: Evaluation of cell metabolism, oxidative stress, mitochondrial membrane potential (MMP), and capsaicin-induced calcium flow. J. Biophotonics 2021, 14, e202000347. [Google Scholar] [CrossRef]
- Pigatto, G.R.; Silva, C.S.; Parizotto, N.A. Photobiomodulation therapy reduces acute pain and inflammation in mice. J. Photochem. Photobiol. B 2019, 196, 111513. [Google Scholar] [CrossRef]
- Amaroli, A.; Marcoli, M.; Venturini, A.; Passalacqua, M.; Agnati, L.F.; Signore, A.; Raffetto, M.; Maura, G.; Benedicenti, S.; Cervetto, C. Near-infrared laser photons induce glutamate release from cerebrocortical nerve terminals. J. Biophotonics 2018, 11, 201800102. [Google Scholar] [CrossRef]
- Hackett, J.T.; Ueda, T. Glutamate Release. Neurochem. Res. 2015, 40, 2443–2460. [Google Scholar] [CrossRef]
- Chen, T.J.; Kukley, M. Glutamate receptors and glutamatergic signalling in the peripheral nerves. Neural Regen. Res. 2020, 15, 438–447. [Google Scholar]
| Study | Animal/Number | Parameters | Therapy | Area/Damage | Method | Results |
|---|---|---|---|---|---|---|
| [36] * | Rat/36 (3 groups) | 660–808 nm; 0.07 W; 100 J/cm2; 2.5 W/cm2; pulsed 50 Hz; 27 J per point; time = 80 s; distance = n.s.; spot area = 0.028 cm2 | Start = immediately End = 30 days after 1 point every 3 days | Mandibular canal/inferior alveolar nerve crush injury (surgery) | Nerve regeneration by histomorphological analysis | 808 nm: increment of axons and myelinization 660 nm: no effect |
| [37] | Rat/72 (4 groups) | 810–980 nm; 0.2 W; 6 J/cm2; 0.4 W/cm2; continuous wave; 3 J per point; time = 15 s; distance; contact; sport area = 0.5 cm2 | Start = a day after surgery End = 30 days after 3 points daily | Mandibular canal/inferior alveolar nerve crush injury (surgery) | Neurosensory recovery: von Frey test Immunoblot: BDNF; NF-kB; TNF-a; IL-1 b | Neurosensory recovery is improved by both wavelengths. NF-kB; TNF-a; IL-1b decreased by both wavelengths. 810 nm better than 980 nm |
| [41] | Rat/48 (4 groups) | 808 nm; 0.1 W; 120 J/cm2; 3.57 W/cm2; continuous wave; time = 22–33 s; spot area = 0.028 cm2; 3.5 J per point | Start = a day after surgery End = 20 days after 1 point daily | Mental nerve/nerve crush with ultrafine forceps | Nerve regeneration: TEM | Increase of regeneration after 14 days up to 20 days of irradiations |
| [39] | Rabbit/5 (split mouths) | 830 nm; 6.0 J/cm2; 0.06 W/cm2; continuous wave; time = 90 s | Start = immediately End = 10 days after 4 points daily | Mandibular canal/inferior alveolar nerve crush injury (surgery and implant into Gore-Tex tube) | Nerve regeneration by histomorphological analysis | Nerve regeneration in samples with both only tube and tube + PBM. PBM improves the effect |
| Study | Study/Patients/Age | Parameters | Therapy | Damaged Area/Cause | Method | Results |
|---|---|---|---|---|---|---|
| [47] * | RCT, double-blinded/12/average age 23.4 | 810 nm; 0.2 W; 0.2 W/cm2; 12 J/cm2; 12 J; time = 60 s per point; 1 cm2; continuous wave | Start = immediately End = 14 days after 16 points on maxilla and 10 points on mandible side. Irradiation 1, 5, 10 and 14 days after surgery | Inferior alveolar nerve and infraorbital nerve/Bilateral sagittal split osteotomy, Le Fort 1 osteotomy | 2-point discrimination test Blink-test (3-months follow-up) | Improve of neurosensory recovery |
| [48] | RCT, double-blind/40/average age 26 | 810 nm; 0.07 W; 0.14 W/cm2; 8.4 J/cm2; 4.2 J per point; 60 s; 0.5 cm2; continuous wave | Start = immediately End = 2 weeks 1 intraoral point and 3 extraoral points 1, 2 and 3 days after surgery and every other day for the next two weeks (10 section in total) | Inferior alveolar nerve/sagittal split osteotomy | 2-point discrimination test Thermal test Contact direction test Pinprick test (12-months follow-up) | Rapid progression of the nerve healing process |
| [49] | RCT, double-blinded/33/average age 22 | 810 nm; 0.1 W; 0.356 W/cm2; 32 J/cm2; 9 J per point; 90 s; 0.28 cm2; continuous wave | Start = a day after surgery End = after 28 days 3 intraorally points at session. Irradiation at day 2, 3, 5, 10, 14, 21 and 28 after surgery | Inferior alveolar nerve/Bilateral sagittal split osteotomy | VAS 2-point discrimination test Thermal test Pain discrimination (6-months follow-up) | Recovery of neurosensory Impairment of mandibular nerve |
| [50] | RCT/35/average age 39.97 | 830 nm; 0.4 W; 2.67 W/cm2; 20 J/cm2, 40 J/cm2; 6 J intraorally, 3 J extraorally; 7.5 s, 15 s; 0.15 cm2; continuous wave | Start = immediately End = 20 sessions after From 1 to 8 points | inferior alveolar nerve and lingual nerve/local anesthetic, third molar odontectomy, and dental implant placement | visual analogue scale clinical neurosensory testing (3-months follow-up) | No effect |
| [51] | RCT, doubled-blind, split osteotomy/20/average age 35 | 780 nm; 0.07 W; 1.75 W/cm2; 157.5 J/cm2; 6.3 J per point; 90 s; 0.04 cm2, continuous wave | Start = variable End = after 5 sessions with intervals of three to four weeks between the sessions | Inferior alveolar nerve/Bilateral sagittal split osteotomy | Semmes-Weinstein monofilament test (12-months follow-up) | improve neurosensory recovery |
| [52] | RCT, Triple-blind, split osteotomy/20/average age 23 | 980 nm; 0.1 W; 0.2 W/cm2; 12 J/cm2; 6 J per point; 60 s; 0.5 cm2; continuous wave | Start = a day before osteotomy End = 28 days after 12 points per session 1, 3, 7, 14, 21 and 28 day postoperatively | Inferior alveolar nerve/Bilateral sagittal split osteotomy | 2-point discrimination test Thermal test Contact direction test (1-month follow-up) | improve neurosensory recovery |
| Study | Study/Patients | Parameters | Therapy | Damaged Area/Cause | Method | Results |
|---|---|---|---|---|---|---|
| [53] | Clinical-cases/ 125 patients | 808 nm; 0.1 W; 3.57 W/cm2; 100 J/cm2; 2.8 J per point; 28 s; 0.028 cm2; continuous wave | Start = variable End = at the average number 13 laser sections. 1–2 section per week | Inferior alveolar, mental, lingual and maxillary nerves/orthognathic surgery, Inferior alveolar nerve lateralization, third molar extraction, dental implant placement, facial trauma | visual analogue scale (VAS) (at the end of sessions) | Better recovery of sensitivity in younger (14–25 y/o) than older (>61 y/o) patients. PBM acts better in orthognathic surgery and facial trauma than in other cases. |
| [54] | Case report/1 | 660 nm and 808 nm; 0.1 W; 3.57 W/cm2; 140 J/cm2; 4 J per point; 40 s; 0.028 cm2; continuous wave | Start = a day after surgery End = after 10 sessions First irradiation with 660 nm followed by 808 nm. 52 points irradiated per session | Inferior alveolar nerve/removal odontoma | VAS (2-year follow-up) | Improvement of neurosensory |
| [55] | Case series/20 | 808 nm; 0.1 W; 3.57 W/cm2; 100 J/cm2; 2.8 J per point; 28 s; 0.028 cm2; continuous wave | Start = two day after surgery End = after 10 sessions 25 points at session. Irradiation every 72 h | Inferior alveolar nerve/Bilateral sagittal split osteotomy | VAS (at the end of sessions) | Improvement of neurosensory |
| [56] | Case series/42 | 810 nm; 0.1 W; 0.353 W/cm2; 31.8 J/cm2; 9 J per point; 90 s; 0.283 cm2, continuous wave | Start = immediately End = 28 days after surgery Three intraoral applications on days 1, 2, 3, 5, 10, 14, 21, and 28 after surgery | Inferior alveolar nerve/Bilateral sagittal split osteotomy | VAS 2-point discrimination test Thermal test (2-years follow-up) | Improve neurosensory recovery |
| [57] | Case series/13 | 820 nm; 0.07 W; 0.55 W/cm2; 46 J/cm2; 6 J per point; 86 s; 0.13 cm2; continuous wave | Start = immediately End = from 20 to 63 days after (mean, 31 days). 4 points per treatment along with the distribution of the inferior alveolar nerve, for a total of 20 treatment | Inferior alveolar nerve/Bilateral sagittal split osteotomy | Semmes Weinstein monofilaments test, Thermotester, VAS (at the end of sessions) | Improve neurosensory recovery |
| [58] | Prospective study/6 | 820–830 nm; 0.55 W; 4.2 W/cm2; 46 J/cm2; 6 J per point; 86 s; 0.13 cm2 | Start = immediately End = 7 days 4 points per treatment along with the distribution of the inferior alveolar nerve, irradiated at the day 2, 3, 4 and 7 after surgery | Inferior alveolar nerve/Bilateral sagittal split osteotomy | VAS 2-point discrimination test Thermal test Contact direction test Pinprick test (2-years follow-up) | Improve neurosensory recovery |
| [59] | Case report/4 | 820–830 nm; 0.05 W; 0.25 W/cm2; 22.5 J/cm2; 4.5 J per point; 90 s; 0.2 cm2 | Start = immediately End = 5 weeks after 5 point for session 3 times per week | inferior alveolar nerve or the lingual nerve/third molar odontectomy | brush stroke directional discrimination test, 2-point discrimination test, VAS (9-months follow-up) | Recovery of neurosensory Impairment of nerves |
| [60] | Case series/57 | 904–910 nm; frequency of 1 to 80 kHz; pulsed and superpulsed emissions (200 ns of pulse duration); peak power 40 W; 0.008 to 0.5 W of average power | Start = variable End = after 10 sessions, carried out weekly | inferior alveolar nerve/oral surgical injury | 2-point discrimination test Thermal test Contact direction test Pinprick test (10-weeks follow-up) | neurosensory recovery in 83% of patients |
| [61] | Case series/20 | 808 nm; 0.05 W; 0.016 W/cm2; 3 J/cm2; 9.42 J per point; 188 s; 3.14 cm2; continuous wave | Start = variable End = after 7 sessions once every two days 1 point | inferior alveolar nerve/third molar odontectomy | VAS Clinical neurosensory test (at the end of sessions) | improve neurosensory recovery |
| [62] | Case series/11 | 808 nm; 0.017 W; 0.2 W/cm2; 4 J/cm2; 44 J per session; 20 s; = 0.088 cm2; continuous wave | Start = variable End = after 15 session 15 points intra and extraorally twice per week | inferior alveolar nerve/third molar odontectomy | Zuniga–Essick score, British Medical Research Council scale, VAS (at the end of sessions) | improve neurosensory recovery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravera, S.; Colombo, E.; Pasquale, C.; Benedicenti, S.; Solimei, L.; Signore, A.; Amaroli, A. Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review. Int. J. Mol. Sci. 2021, 22, 4347. https://doi.org/10.3390/ijms22094347
Ravera S, Colombo E, Pasquale C, Benedicenti S, Solimei L, Signore A, Amaroli A. Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review. International Journal of Molecular Sciences. 2021; 22(9):4347. https://doi.org/10.3390/ijms22094347
Chicago/Turabian StyleRavera, Silvia, Esteban Colombo, Claudio Pasquale, Stefano Benedicenti, Luca Solimei, Antonio Signore, and Andrea Amaroli. 2021. "Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review" International Journal of Molecular Sciences 22, no. 9: 4347. https://doi.org/10.3390/ijms22094347
APA StyleRavera, S., Colombo, E., Pasquale, C., Benedicenti, S., Solimei, L., Signore, A., & Amaroli, A. (2021). Mitochondrial Bioenergetic, Photobiomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review. International Journal of Molecular Sciences, 22(9), 4347. https://doi.org/10.3390/ijms22094347