Different Approaches in Therapy Aiming to Stabilize an Unstable Atherosclerotic Plaque
Abstract
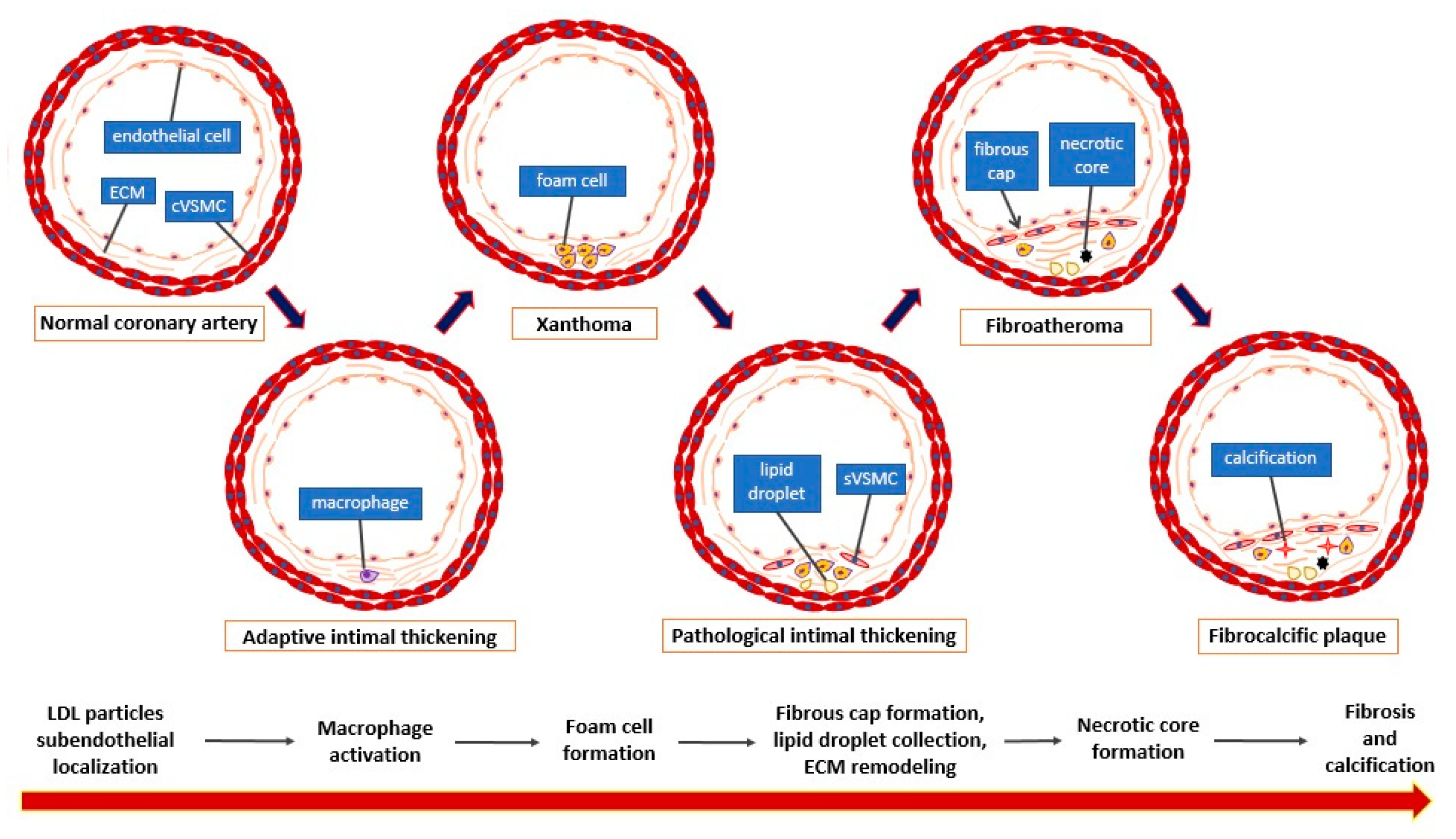
1. Introduction
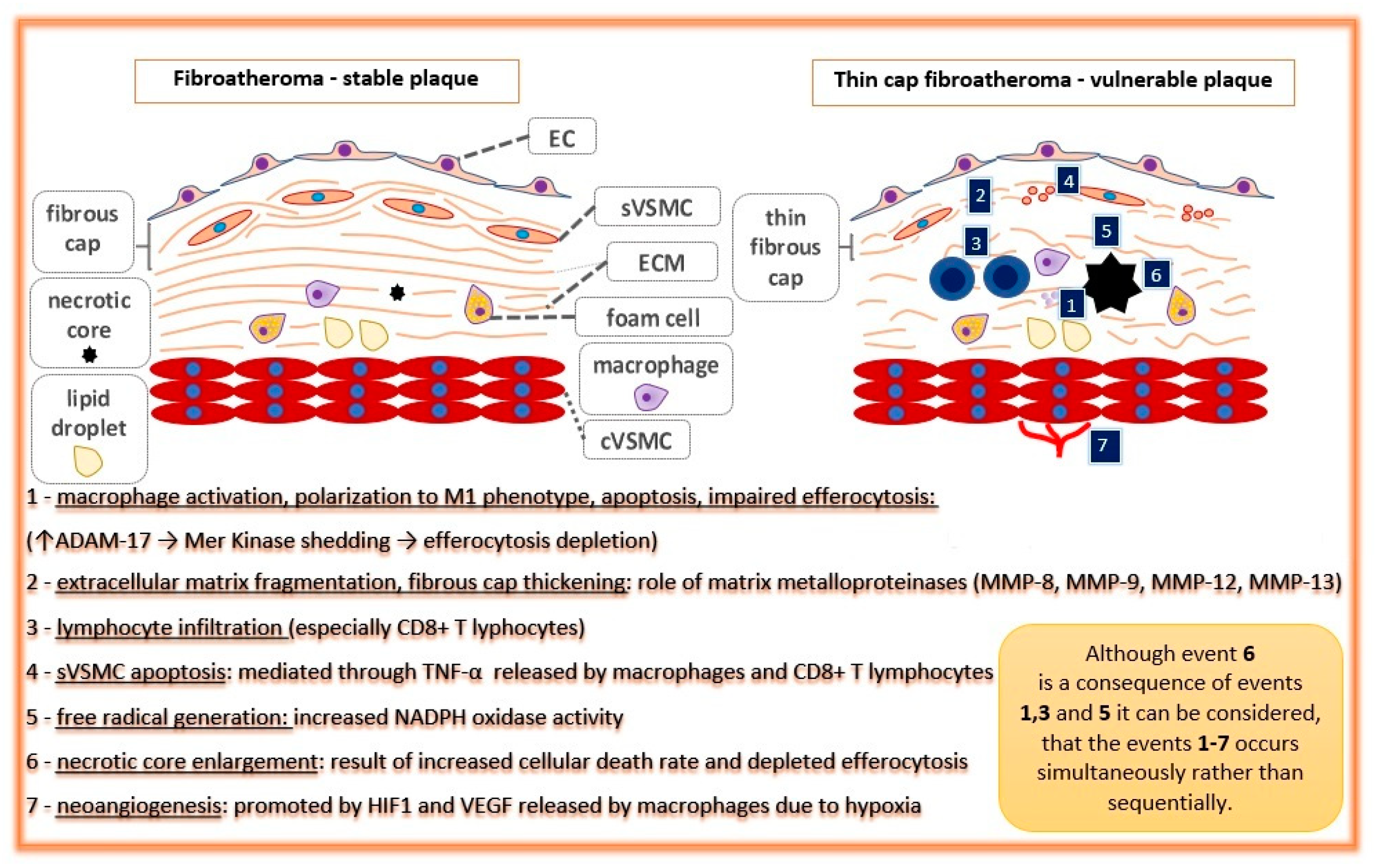
2. General Considerations
3. Approaches Directed at Specific Molecular Pathways
3.1. Approach towards Regulation of Metabolism
3.2. Approach towards Macrophages and Cellular Death Mechanisms
3.3. Approach toward Inflammation and Immune Reactions
3.4. Approach towards Reactive Oxygen Species—Antioxidation Therapy
3.5. Approach towards Extracellular Matrix Remodeling and Neovascularization
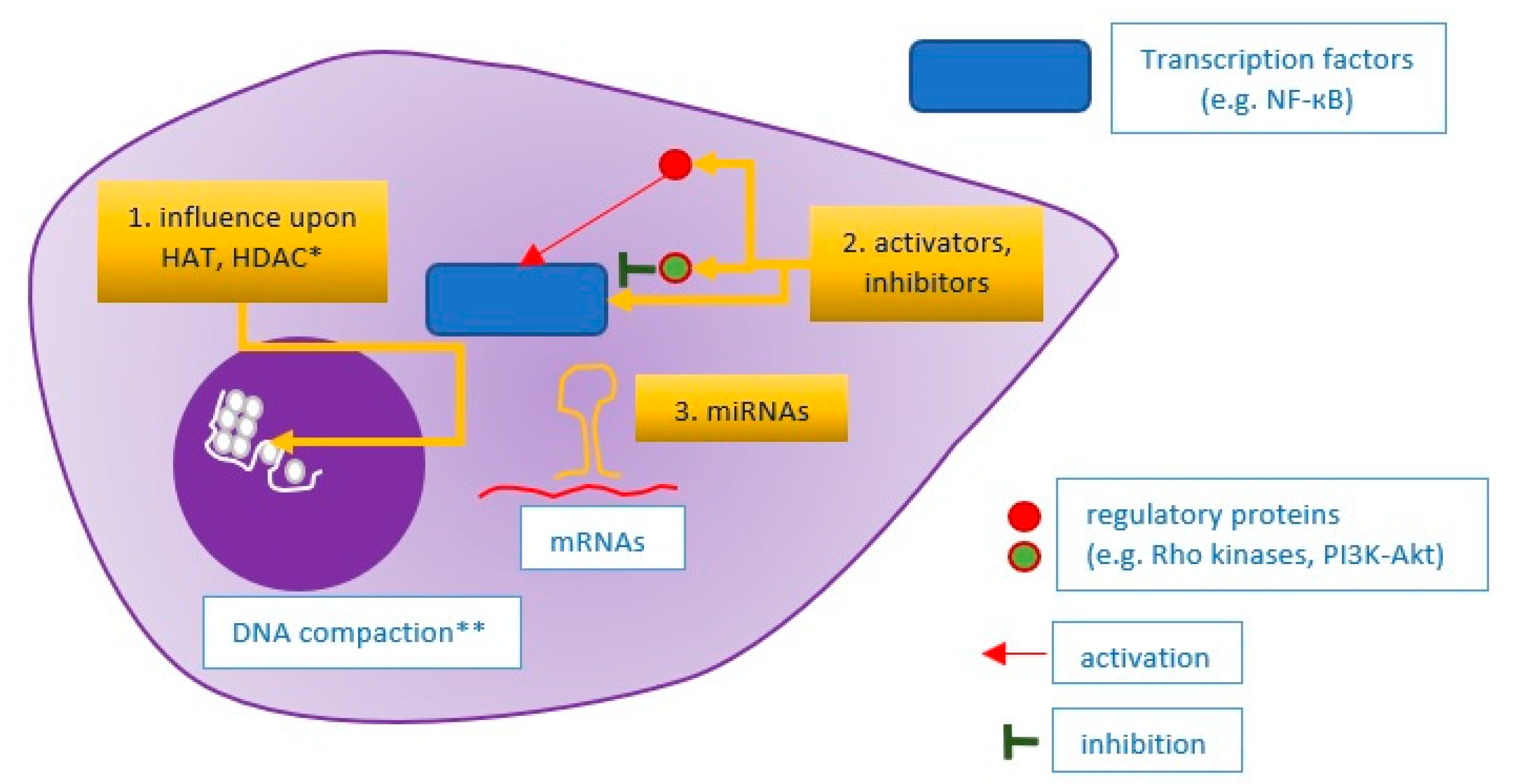
3.6. Specific Approaches—Summary
4. An Integrated Approach
5. Clinical Studies Conforming Plaque Stabilization
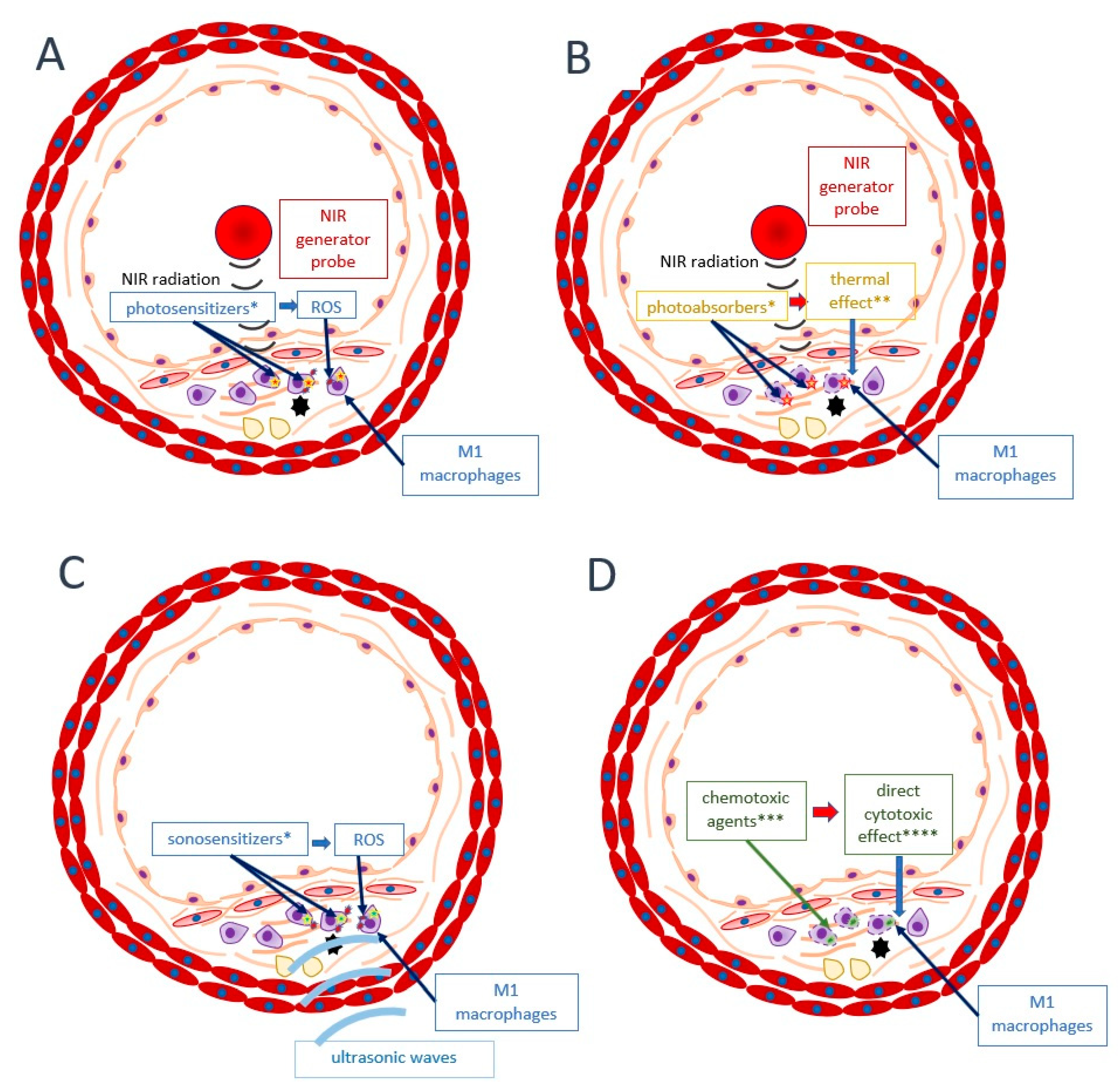
6. Alternative Approaches
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [PubMed]
- Tibaut, M.; Mekis, D.; Petrovic, D. Pathophysiology of Myocardial Infarction and Acute Management Strategies. Cardiovasc. Hematol. Agents Med. Chem. 2017, 14, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Cannon, C.P. Acute coronary syndromes: Diagnosis and management, part I. Mayo Clin. Proc. 2009, 84, 917–938. [Google Scholar] [CrossRef] [PubMed]
- Aufderheide, T.P. Arrhythmias associated with acute myocardial infarction and thrombolysis. Emerg. Med. Clin. N. Am. 1998, 16, 583–600. [Google Scholar] [CrossRef]
- Saleh, M.; Ambrose, J.A. Understanding myocardial infarction. F1000Research 2018, 7, 1378. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Garcia-Cardena, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Geovanini, G.R.; Libby, P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef]
- Khan, M.H.; Rochlani, Y.; Yandrapalli, S.; Aronow, W.S.; Frishman, W.H. Vulnerable Plaque: A Review of Current Concepts in Pathophysiology and Imaging. Cardiol. Rev. 2020, 28, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47 (Suppl. 8), C13–C18. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; de Winther, M.P.; Weber, C.; Daemen, M.J.; Lutgens, E.; Soehnlein, O. Atherosclerotic plaque destabilization: Mechanisms, models, and therapeutic strategies. Circ. Res. 2014, 114, 214–226. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Fuster, V. The myth of the “vulnerable plaque”: Transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J. Am. Coll. Cardiol. 2015, 65, 846–855. [Google Scholar] [CrossRef]
- Zhang, Y.; Koradia, A.; Kamato, D.; Popat, A.; Little, P.J.; Ta, H.T. Treatment of atherosclerotic plaque: Perspectives on theranostics. J. Pharm. Pharmacol. 2019, 71, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Shi, W.L.; Xin, Q.Q.; Chen, K.J.; Cong, W.H. Holistic Regulation of Angiogenesis with Chinese Herbal Medicines as a New Option for Coronary Artery Disease. Evid. Based Complement. Alternat. Med. 2018, 2018, 3725962. [Google Scholar] [CrossRef]
- Song, G.; Tian, H.; Qin, S.; Sun, X.; Yao, S.; Zong, C.; Luo, Y.; Liu, J.; Yu, Y.; Sang, H.; et al. Hydrogen decreases athero-susceptibility in apolipoprotein B-containing lipoproteins and aorta of apolipoprotein E knockout mice. Atherosclerosis 2012, 221, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef]
- Veseli, B.E.; Perrotta, P.; De Meyer, G.R.A.; Roth, L.; Van der Donckt, C.; Martinet, W.; De Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017, 816, 3–13. [Google Scholar] [CrossRef]
- Shiomi, M.; Koike, T.; Ito, T. Contribution of the WHHL rabbit, an animal model of familial hypercholesterolemia, to elucidation of the anti-atherosclerotic effects of statins. Atherosclerosis 2013, 231, 39–47. [Google Scholar] [CrossRef]
- Hartwig, H.; Silvestre-Roig, C.; Hendrikse, J.; Beckers, L.; Paulin, N.; Van der Heiden, K.; Braster, Q.; Drechsler, M.; Daemen, M.J.; Lutgens, E.; et al. Atherosclerotic Plaque Destabilization in Mice: A Comparative Study. PLoS ONE 2015, 10, e0141019. [Google Scholar] [CrossRef]
- Jain, M.; Frobert, A.; Valentin, J.; Cook, S.; Giraud, M.N. The Rabbit Model of Accelerated Atherosclerosis: A Methodological Perspective of the Iliac Artery Balloon Injury. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Gomez-Garre, D.; Munoz-Pacheco, P.; Gonzalez-Rubio, M.L.; Aragoncillo, P.; Granados, R.; Fernandez-Cruz, A. Ezetimibe reduces plaque inflammation in a rabbit model of atherosclerosis and inhibits monocyte migration in addition to its lipid-lowering effect. Br. J. Pharmacol. 2009, 156, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Muller-Wieland, D.; Kotzka, J.; Krone, W. Stabilization of atherosclerotic plaque during lipid lowering. Curr. Opin. Lipidol. 1997, 8, 348–353. [Google Scholar] [CrossRef]
- Kuhnast, S.; van der Hoorn, J.W.; Pieterman, E.J.; van den Hoek, A.M.; Sasiela, W.J.; Gusarova, V.; Peyman, A.; Schafer, H.L.; Schwahn, U.; Jukema, J.W.; et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J. Lipid Res. 2014, 55, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Ibanez, B.; Giannarelli, C.; Cimmino, G.; Santos-Gallego, C.G.; Alique, M.; Pinero, A.; Vilahur, G.; Fuster, V.; Badimon, L.; Badimon, J.J. Recombinant HDL(Milano) exerts greater anti-inflammatory and plaque stabilizing properties than HDL(wild-type). Atherosclerosis 2012, 220, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Kuhnast, S.; van der Tuin, S.J.; van der Hoorn, J.W.; van Klinken, J.B.; Simic, B.; Pieterman, E.; Havekes, L.M.; Landmesser, U.; Luscher, T.F.; Willems van Dijk, K.; et al. Anacetrapib reduces progression of atherosclerosis, mainly by reducing non-HDL-cholesterol, improves lesion stability and adds to the beneficial effects of atorvastatin. Eur. Heart J. 2015, 36, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Van Craeyveld, E.; Gordts, S.C.; Nefyodova, E.; Jacobs, F.; De Geest, B. Regression and stabilization of advanced murine atherosclerotic lesions: A comparison of LDL lowering and HDL raising gene transfer strategies. J. Mol. Med. 2011, 89, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.J.; Zhou, S.N.; Shan, M.R.; Wang, X.Q.; Zhang, M.; Chen, Y.; Zhang, Y.; Wang, S.X.; Guo, T. AMPKalpha inactivation destabilizes atherosclerotic plaque in streptozotocin-induced diabetic mice through AP-2alpha/miRNA-124 axis. J. Mol. Med. 2018, 96, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Kavurma, M.M.; Rayner, K.J.; Karunakaran, D. The walking dead: Macrophage inflammation and death in atherosclerosis. Curr. Opin. Lipidol. 2017, 28, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Babaev, V.R.; Yeung, M.; Erbay, E.; Ding, L.; Zhang, Y.; May, J.M.; Fazio, S.; Hotamisligil, G.S.; Linton, M.F. Jnk1 Deficiency in Hematopoietic Cells Suppresses Macrophage Apoptosis and Increases Atherosclerosis in Low-Density Lipoprotein Receptor Null Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1122–1131. [Google Scholar] [CrossRef]
- Linton, M.F.; Tao, H.; Linton, E.F.; Yancey, P.G. SR-BI: A Multifunctional Receptor in Cholesterol Homeostasis and Atherosclerosis. Trends Endocrinol. Metab. 2017, 28, 461–472. [Google Scholar] [CrossRef]
- Evans, T.D.; Jeong, S.J.; Zhang, X.; Sergin, I.; Razani, B. TFEB and trehalose drive the macrophage autophagy-lysosome system to protect against atherosclerosis. Autophagy 2018, 14, 724–726. [Google Scholar] [CrossRef]
- Abderrazak, A.; Couchie, D.; Mahmood, D.F.; Elhage, R.; Vindis, C.; Laffargue, M.; Mateo, V.; Buchele, B.; Ayala, M.R.; El Gaafary, M.; et al. Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation 2015, 131, 1061–1070. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhou, D.; Zhang, L.S.; Deng, F.X.; Shu, S.; Wang, L.J.; Wu, Y.; Guo, N.; Zhou, J.; et al. Angiotensin II deteriorates advanced atherosclerosis by promoting MerTK cleavage and impairing efferocytosis through the AT1R/ROS/p38 MAPK/ADAM17 pathway. Am. J. Physiol. Cell Physiol. 2019, 317, C776–C787. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, R.; Geikowski, A.; Weidner, C.; Witzke, A.; Kodelja, V.; Schwarz, T.; Gabriel, M.; Erker, T.; Sauer, S. Foam cell specific LXRalpha ligand. PLoS ONE 2013, 8, e57311. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Goel, A.; Mehta, J.L. LOX-1: Regulation, Signaling and Its Role in Atherosclerosis. Antioxidants 2019, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Ogura, S.; Little, P.J.; Xu, S.W.; Sawamura, T. Targeting LOX-1 in atherosclerosis and vasculopathy: Current knowledge and future perspectives. Ann. N. Y. Acad. Sci. 2019, 1443, 34–53. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Wolf, M.P.; Hunziker, P. Atherosclerosis: Insights into Vascular Pathobiology and Outlook to Novel Treatments. J. Cardiovasc. Transl. Res. 2020, 13, 744–757. [Google Scholar] [CrossRef]
- van Dijk, R.A.; Duinisveld, A.J.; Schaapherder, A.F.; Mulder-Stapel, A.; Hamming, J.F.; Kuiper, J.; de Boer, O.J.; van der Wal, A.C.; Kolodgie, F.D.; V irmani, R.; et al. A change in inflammatory footprint precedes plaque instability: A systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J. Am. Heart Assoc. 2015, 4, e001403. [Google Scholar] [CrossRef] [PubMed]
- Sliva, J.; Charalambous, C.; Bultas, J.; Karetova, D. A new strategy for the treatment of atherothrombosis—Inhibition of inflammation. Physiol. Res. 2019, 68 (Suppl. 1), S17–S30. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.; Wagner, C.S.; Demmer, P.; Callies, S.; Solter, G.; Loghmani-khouzani, H.; Hu, N.; Schuett, H.; Tietge, U.J.; Warnecke, G.; et al. Acute perioperative-stress-induced increase of atherosclerotic plaque volume and vulnerability to rupture in apolipoprotein-E-deficient mice is amenable to statin treatment and IL-6 inhibition. Dis. Model Mech. 2015, 8, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Segers, D.; Lipton, J.A.; Leenen, P.J.; Cheng, C.; Tempel, D.; Pasterkamp, G.; Moll, F.L.; de Crom, R.; Krams, R. Atherosclerotic Plaque Stability Is Affected by the Chemokine CXCL10 in Both Mice and Humans. Int. J. Inflam. 2011, 2011, 936109. [Google Scholar] [CrossRef][Green Version]
- Gomez, D.; Baylis, R.A.; Durgin, B.G.; Newman, A.A.C.; Alencar, G.F.; Mahan, S.; St Hilaire, C.; Muller, W.; Waisman, A.; Francis, S.E.; et al. Interleukin-1beta has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat. Med. 2018, 24, 1418–1429. [Google Scholar] [CrossRef]
- Bhaskar, V.; Yin, J.; Mirza, A.M.; Phan, D.; Vanegas, S.; Issafras, H.; Michelson, K.; Hunter, J.J.; Kantak, S.S. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis 2011, 216, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Spartalis, M.; Spartalis, E.; Tzatzaki, E.; Tsilimigras, D.I.; Moris, D.; Kontogiannis, C.; Kaminiotis, V.V.; Paschou, S.A.; Chatzidou, S.; Siasos, G.; et al. The Beneficial Therapy with Colchicine for Atherosclerosis via Anti-inflammation and Decrease in Hypertriglyceridemia. Cardiovasc. Hematol. Agents Med. Chem. 2018, 16, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, A.; Vilchez-Tschischke, J.P.; Mateo, J.; Sanchez-Gonzalez, J.; Espana, S.; Fernandez-Jimenez, R.; Lopez-Melgar, B.; Fernandez Friera, L.; Lopez-Martin, G.J.; Fuster, V.; et al. Effects of Colchicine on Atherosclerotic Plaque Stabilization: A Multimodality Imaging Study in an Animal Model. J. Cardiovasc. Transl. Res. 2020, 14, 150–160. [Google Scholar] [CrossRef]
- Aguilar, E.C.; Leonel, A.J.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.; Capettini, L.S.; Lemos, V.S.; Santos, R.A.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFkappaB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tong, L.; Zhang, L.; Li, H.; Wan, Y.; Zhang, T. Tanshinone II A stabilizes vulnerable plaques by suppressing RAGE signaling and NF-kappaB activation in apolipoprotein-E-deficient mice. Mol. Med. Rep. 2016, 14, 4983–4990. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Sun, Z.; Nie, P.; Yuan, R.; Cai, Z.; Wu, C.; Hu, L.; Jin, S.; Zhou, H.; Zhang, X.; et al. Sulindac-derived retinoid X receptor-alpha modulator attenuates atherosclerotic plaque progression and destabilization in ApoE(-/-) mice. Br. J. Pharmacol. 2019, 176, 2559–2572. [Google Scholar] [CrossRef]
- Liu, J.; Lin, J.; He, S.; Wu, C.; Wang, B.; Liu, J.; Duan, Y.; Liu, T.; Shan, S.; Yang, K.; et al. Transgenic Overexpression of IL-37 Protects Against Atherosclerosis and Strengthens Plaque Stability. Cell. Physiol. Biochem. 2018, 45, 1034–1050. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, T.; Wu, Y.; Yang, L.; Wang, L.; Fan, X.; Zhang, W.; Feng, J.; Yu, H.; Yang, Y.; et al. Pioglitazone stabilizes atherosclerotic plaque by regulating the Th17/Treg balance in AMPK-dependent mechanisms. Cardiovasc. Diabetol. 2017, 16, 140. [Google Scholar] [CrossRef]
- Kobiyama, K.; Saigusa, R.; Ley, K. Vaccination against atherosclerosis. Curr. Opin. Immunol. 2019, 59, 15–24. [Google Scholar] [CrossRef]
- Lassegue, B.; Griendling, K.K. NADPH oxidases: Functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Judkins, C.P.; Diep, H.; Broughton, B.R.; Mast, A.E.; Hooker, E.U.; Miller, A.A.; Selemidis, S.; Dusting, G.J.; Sobey, C.G.; Drummond, G.R. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE-/- mice. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H24–H32. [Google Scholar] [CrossRef]
- Gargiulo, S.; Testa, G.; Gamba, P.; Staurenghi, E.; Poli, G.; Leonarduzzi, G. Oxysterols and 4-hydroxy-2-nonenal contribute to atherosclerotic plaque destabilization. Free Radic. Biol. Med. 2017, 111, 140–150. [Google Scholar] [CrossRef]
- Martinet, W.; Knaapen, M.W.; De Meyer, G.R.; Herman, A.G.; Kockx, M.M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 2002, 106, 927–932. [Google Scholar] [CrossRef]
- Gargiulo, S.; Rossin, D.; Testa, G.; Gamba, P.; Staurenghi, E.; Biasi, F.; Poli, G.; Leonarduzzi, G. Up-regulation of COX-2 and mPGES-1 by 27-hydroxycholesterol and 4-hydroxynonenal: A crucial role in atherosclerotic plaque instability. Free Radic. Biol. Med. 2018, 129, 354–363. [Google Scholar] [CrossRef]
- Prunet, C.; Montange, T.; Vejux, A.; Laubriet, A.; Rohmer, J.F.; Riedinger, J.M.; Athias, A.; Lemaire-Ewing, S.; Neel, D.; Petit, J.M.; et al. Multiplexed flow cytometric analyses of pro- and anti-inflammatory cytokines in the culture media of oxysterol-treated human monocytic cells and in the sera of atherosclerotic patients. Cytometry A 2006, 69, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Lamb, F.S.; Choi, H.; Miller, M.R.; Stark, R.J. TNFalpha and Reactive Oxygen Signaling in Vascular Smooth Muscle Cells in Hypertension and Atherosclerosis. Am. J. Hypertens. 2020, 33, 902–913. [Google Scholar] [PubMed]
- Kohlgruber, S.; Upadhye, A.; Dyballa-Rukes, N.; McNamara, C.A.; Altschmied, J. Regulation of Transcription Factors by Reactive Oxygen Species and Nitric Oxide in Vascular Physiology and Pathology. Antioxid. Redox Signal. 2017, 26, 679–699. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K. Soy Isoflavones Inhibit Endothelial Cell Dysfunction and Prevent Cardiovascular Disease. J. Cardiovasc. Pharmacol. 2019, 74, 201–209. [Google Scholar] [CrossRef]
- Li, W.; Hellsten, A.; Jacobsson, L.S.; Blomqvist, H.M.; Olsson, A.G.; Yuan, X.M. Alpha-tocopherol and astaxanthin decrease macrophage infiltration, apoptosis and vulnerability in atheroma of hyperlipidaemic rabbits. J. Mol. Cell. Cardiol. 2004, 37, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Giordano, P.; Scicchitano, P.; Locorotondo, M.; Mandurino, C.; Ricci, G.; Carbonara, S.; Gesualdo, M.; Zito, A.; Dachille, A.; Caputo, P.; et al. Carotenoids and cardiovascular risk. Curr. Pharm. Des. 2012, 18, 5577–5589. [Google Scholar] [CrossRef]
- Sozen, E.; Demirel, T.; Ozer, N.K. Vitamin E: Regulatory role in the cardiovascular system. IUBMB Life 2019, 71, 507–515. [Google Scholar] [CrossRef]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef]
- Quesada, I.M.; Lucero, A.; Amaya, C.; Meijles, D.N.; Cifuentes, M.E.; Pagano, P.J.; Castro, C. Selective inactivation of NADPH oxidase 2 causes regression of vascularization and the size and stability of atherosclerotic plaques. Atherosclerosis 2015, 242, 469–475. [Google Scholar] [CrossRef]
- Zhang, H.L.; Jia, K.Y.; Sun, D.; Yang, M. Protective effect of HSP27 in atherosclerosis and coronary heart disease by inhibiting reactive oxygen species. J. Cell. Biochem. 2019, 120, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Holm Nielsen, S.; Jonasson, L.; Kalogeropoulos, K.; Karsdal, M.A.; Reese-Petersen, A.L.; Auf dem Keller, U.; Genovese, F.; Nilsson, J.; Goncalves, I. Exploring the role of extracellular matrix proteins to develop biomarkers of plaque vulnerability and outcome. J. Intern. Med. 2020, 287, 493–513. [Google Scholar] [CrossRef] [PubMed]
- Kowara, M.; Cudnoch-Jedrzejewska, A.; Opolski, G.; Wlodarski, P. MicroRNA regulation of extracellular matrix components in the process of atherosclerotic plaque destabilization. Clin. Exp. Pharmacol. Physiol. 2017, 44, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Shami, A.; Goncalves, I.; Hultgardh-Nilsson, A. Collagen and related extracellular matrix proteins in atherosclerotic plaque development. Curr. Opin. Lipidol. 2014, 25, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Barchuk, M.; Miksztowicz, V. Behavior of Metalloproteinases in Adipose Tissue, Liver and Arterial Wall: An Update of Extracellular Matrix Remodeling. Cells 2019, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Newby, A.C. Proteinases and plaque rupture: Unblocking the road to translation. Curr. Opin. Lipidol. 2014, 25, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, X.; Feng, Y.; Dong, G.; Wang, Y.; Yang, J. The Role of Matrix Metalloproteinase-9 in Atherosclerotic Plaque Instability. Mediat. Inflamm. 2020, 2020, 3872367. [Google Scholar] [CrossRef]
- Bultmann, A.; Li, Z.; Wagner, S.; Gawaz, M.; Ungerer, M.; Langer, H.; May, A.E.; Munch, G. Loss of protease activity of ADAM15 abolishes protective effects on plaque progression in atherosclerosis. Int, J, Cardiol. 2011, 152, 382–385. [Google Scholar] [CrossRef]
- Johnson, J.L.; Jenkins, N.P.; Huang, W.C.; Di Gregoli, K.; Sala-Newby, G.B.; Scholtes, V.P.; Moll, F.L.; Pasterkamp, G.; Newby, A.C. Relationship of MMP-14 and TIMP-3 expression with macrophage activation and human atherosclerotic plaque vulnerability. Mediat. Inflamm. 2014, 2014, 276457. [Google Scholar] [CrossRef]
- Sigala, F.; Savvari, P.; Liontos, M.; Sigalas, P.; Pateras, I.S.; Papalampros, A.; Basdra, E.K.; Kolettas, E.; Papavassiliou, A.G.; Gorgoulis, V.G. Increased expression of bFGF is associated with carotid atherosclerotic plaques instability engaging the NF-kappaB pathway. J. Cell. Mol. Med. 2010, 14, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Wang, C.; Zhong, W.; Li, B.; Wang, Z.; Shao, C.; Chen, Y.; Yan, J. Activation of CD137 Signaling Promotes Angiogenesis in Atherosclerosis via Modulating Endothelial Smad1/5-NFATc1 Pathway. J. Am. Heart Assoc. 2017, 6, e004756. [Google Scholar] [CrossRef]
- Guo, M.; Cai, Y.; Yao, X.; Li, Z. Mathematical modeling of atherosclerotic plaque destabilization: Role of neovascularization and intraplaque hemorrhage. J. Theor. Biol. 2018, 450, 53–65. [Google Scholar] [CrossRef]
- Moreno, P.R.; Purushothaman, K.R.; Fuster, V.; Echeverri, D.; Truszczynska, H.; Sharma, S.K.; Badimon, J.J.; O’Connor, W.N. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation 2004, 110, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Taruya, A.; Tanaka, A.; Nishiguchi, T.; Matsuo, Y.; Ozaki, Y.; Kashiwagi, M.; Shiono, Y.; Orii, M.; Yamano, T.; Ino, Y.; et al. Vasa Vasorum Restructuring in Human Atherosclerotic Plaque Vulnerability: A Clinical Optical Coherence Tomography Study. J. Am. Coll. Cardiol. 2015, 65, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, P.; Veseli, B.E.; Van der Veken, B.; Roth, L.; Martinet, W.; De Meyer, G.R.Y. Pharmacological strategies to inhibit intra-plaque angiogenesis in atherosclerosis. Vasc. Pharmacol. 2019, 112, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Van der Veken, B.; De Meyer, G.R.Y.; Martinet, W. Axitinib attenuates intraplaque angiogenesis, haemorrhages and plaque destabilization in mice. Vasc. Pharmacol. 2018, 100, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.L. Macrophage iron, hepcidin, and atherosclerotic plaque stability. Exp. Biol. Med. 2007, 232, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Vinchi, F.; Porto, G.; Simmelbauer, A.; Altamura, S.; Passos, S.T.; Garbowski, M.; Silva, A.M.N.; Spaich, S.; Seide, S.E.; Sparla, R.; et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur. Heart J. 2020, 41, 2681–2695. [Google Scholar] [CrossRef]
- Vujic, N.; Schlager, S.; Eichmann, T.O.; Madreiter-Sokolowski, C.T.; Goeritzer, M.; Rainer, S.; Schauer, S.; Rosenberger, A.; Woelfler, A.; Doddapattar, P.; et al. Monoglyceride lipase deficiency modulates endocannabinoid signaling and improves plaque stability in ApoE-knockout mice. Atherosclerosis 2016, 244, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Erbay, E.; Babaev, V.R.; Mayers, J.R.; Makowski, L.; Charles, K.N.; Snitow, M.E.; Fazio, S.; Wiest, M.M.; Watkins, S.M.; Linton, M.F.; et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 2009, 15, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhuo, X.; Ma, A. STAT6 Upregulation Promotes M2 Macrophage Polarization to Suppress Atherosclerosis. Med. Sci. Monit. Basic Res. 2017, 23, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J.; Bourgeois, M.; Harbison, R.D. Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders. Cardiovasc. Toxicol. 2018, 18, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Laso, V.; Sastre, C.; Mendez-Barbero, N.; Egido, J.; Martin-Ventura, J.L.; Gomez-Guerrero, C.; Blanco-Colio, L.M. TWEAK blockade decreases atherosclerotic lesion size and progression through suppression of STAT1 signaling in diabetic mice. Sci. Rep. 2017, 7, 46679. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Tsai, M.C.; Chern, C.Y.; Tsao, T.P.; Lin, F.Y.; Chen, S.J.; Tsui, P.F.; Liu, Y.W.; Lu, H.J.; Wu, W.L.; et al. A chalcone derivative, 1m-6, exhibits atheroprotective effects by increasing cholesterol efflux and reducing inflammation-induced endothelial dysfunction. Br. J. Pharmacol. 2020, 177, 5375–5392. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, S.; Francisci, D.; Mencarelli, A.; Renga, B.; Schiaroli, E.; D’Amore, C.; Baldelli, F.; Fiorucci, S. Efficacy of the CCR5 antagonist maraviroc in reducing early, ritonavir-induced atherogenesis and advanced plaque progression in mice. Circulation 2013, 127, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Tang, X. Analysis of interleukin-17 and interleukin-18 levels in animal models of atherosclerosis. Exp. Ther. Med. 2019, 18, 517–522. [Google Scholar] [CrossRef]
- Niessner, A.; Shin, M.S.; Pryshchep, O.; Goronzy, J.J.; Chaikof, E.L.; Weyand, C.M. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation 2007, 116, 2043–2052. [Google Scholar] [CrossRef]
- Khambata, R.S.; Ghosh, S.M.; Rathod, K.S.; Thevathasan, T.; Filomena, F.; Xiao, Q.; Ahluwalia, A. Antiinflammatory actions of inorganic nitrate stabilize the atherosclerotic plaque. Proc. Natl. Acad. Sci. USA 2017, 114, E550–E559. [Google Scholar] [CrossRef]
- Kyaw, T.; Winship, A.; Tay, C.; Kanellakis, P.; Hosseini, H.; Cao, A.; Li, P.; Tipping, P.; Bobik, A.; Toh, B.H. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 2013, 127, 1028–1039. [Google Scholar] [CrossRef]
- Ou, H.X.; Guo, B.B.; Liu, Q.; Li, Y.K.; Yang, Z.; Feng, W.J.; Mo, Z.C. Regulatory T cells as a new therapeutic target for atherosclerosis. Acta Pharmacol. Sin. 2018, 39, 1249–1258. [Google Scholar] [CrossRef]
- Fiorelli, S.; Porro, B.; Cosentino, N.; Di Minno, A.; Manega, C.M.; Fabbiocchi, F.; Niccoli, G.; Fracassi, F.; Barbieri, S.; Marenzi, G.; et al. Activation of Nrf2/HO-1 Pathway and Human Atherosclerotic Plaque Vulnerability: An In Vitro and In Vivo Study. Cells 2019, 8, 356. [Google Scholar] [CrossRef]
- Xu, Z.R.; Li, J.Y.; Dong, X.W.; Tan, Z.J.; Wu, W.Z.; Xie, Q.M.; Yang, Y.M. Apple Polyphenols Decrease Atherosclerosis and Hepatic Steatosis in ApoE-/- Mice through the ROS/MAPK/NF-kappaB Pathway. Nutrients 2015, 7, 7085–7105. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Ke, D.; Li, G. Ghrelin inhibits atherosclerotic plaque angiogenesis and promotes plaque stability in a rabbit atherosclerotic model. Peptides 2017, 90, 17–26. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Jiang, X.; Liu, S.; Liu, Y.; Chen, W.; Yang, J.; Zhang, C.; Zhang, W. Melatonin enhances atherosclerotic plaque stability by inducing prolyl-4-hydroxylase alpha1 expression. J. Hypertens. 2019, 37, 964–971. [Google Scholar] [CrossRef]
- Budatha, M.; Zhang, J.; Zhuang, Z.W.; Yun, S.; Dahlman, J.E.; Anderson, D.G.; Schwartz, M.A. Inhibiting Integrin alpha5 Cytoplasmic Domain Signaling Reduces Atherosclerosis and Promotes Arteriogenesis. J. Am. Heart Assoc. 2018, 7, e007501. [Google Scholar] [CrossRef]
- Parma, L.; Peters, H.A.B.; Sluiter, T.J.; Simons, K.H.; Lazzari, P.; de Vries, M.R.; Quax, P.H.A. bFGF blockade reduces intraplaque angiogenesis and macrophage infiltration in atherosclerotic vein graft lesions in ApoE3*Leiden mice. Sci. Rep. 2020, 10, 15968. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wang, L.; Meng, S.; Wang, Y.; Chen, T.; Wang, C. Berberine reduces both MMP-9 and EMMPRIN expression through prevention of p38 pathway activation in PMA-induced macrophages. Int. J. Cardiol. 2011, 146, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, C.; Wang, J.; Li, X.; Yang, Z.; Sun, X.; Fang, L.; Liu, N. Berberine activates peroxisome proliferator-activated receptor gamma to increase atherosclerotic plaque stability in Apoe(-/-) mice with hyperhomocysteinemia. J. Diabetes Investig. 2016, 7, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ma, Y.; Li, Y.; Zheng, X.; Lv, P.; Zhang, Y.; Li, J.; Ma, M.; Zhang, L.; Li, C. Insulin inhibits inflammation and promotes atherosclerotic plaque stability via PI3K-Akt pathway activation. Immunol. Lett. 2016, 170, 7–14. [Google Scholar] [CrossRef]
- Kagami, S.; Owada, T.; Kanari, H.; Saito, Y.; Suto, A.; Ikeda, K.; Hirose, K.; Watanabe, N.; Iwamoto, I.; Nakajima, H. Protein geranylgeranylation regulates the balance between Th17 cells and Foxp3+ regulatory T cells. Int. Immunol. 2009, 21, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Bot, M.; de Jager, S.C.; MacAleese, L.; Lagraauw, H.M.; van Berkel, T.J.; Quax, P.H.; Kuiper, J.; Heeren, R.M.; Biessen, E.A.; Bot, I. Lysophosphatidic acid triggers mast cell-driven atherosclerotic plaque destabilization by increasing vascular inflammation. J. Lipid Res. 2013, 54, 1265–1274. [Google Scholar] [CrossRef]
- Gu, C.; Wang, F.; Zhao, Z.; Wang, H.; Cong, X.; Chen, X. Lysophosphatidic Acid Is Associated with Atherosclerotic Plaque Instability by Regulating NF-kappaB Dependent Matrix Metalloproteinase-9 Expression via LPA2 in Macrophages. Front. Physiol. 2017, 8, 266. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.Y.; Sun, T.W.; Shen, D.L.; He, F.; Dang, Y.H.; Li, L. Amelioration of atherosclerosis in apolipoprotein E-deficient mice by inhibition of lipoprotein-associated phospholipase A2. Clin. Investig. Med. 2013, 36, E32–E41. [Google Scholar] [CrossRef]
- Solly, E.L.; Dimasi, C.G.; Bursill, C.A.; Psaltis, P.J.; Tan, J.T.M. MicroRNAs as Therapeutic Targets and Clinical Biomarkers in Atherosclerosis. J. Clin. Med. 2019, 8, 2199. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Hu, X.; Gao, F.; Li, M.; Cui, Y.; Wei, X.; Qin, Y.; Zhang, C.; Zhao, Y.; et al. MicroRNA-520c-3p targeting of RelA/p65 suppresses atherosclerotic plaque formation. Int. J. Biochem. Cell Biol. 2021, 131, 105873. [Google Scholar] [CrossRef] [PubMed]
- An, T.H.; He, Q.W.; Xia, Y.P.; Chen, S.C.; Baral, S.; Mao, L.; Jin, H.J.; Li, Y.N.; Wang, M.D.; Chen, J.G.; et al. MiR-181b Antagonizes Atherosclerotic Plaque Vulnerability Through Modulating Macrophage Polarization by Directly Targeting Notch1. Mol. Neurobiol. 2017, 54, 6329–6341. [Google Scholar] [CrossRef]
- Chen, W.; Yu, F.; Di, M.; Li, M.; Chen, Y.; Zhang, Y.; Liu, X.; Huang, X.; Zhang, M. MicroRNA-124-3p inhibits collagen synthesis in atherosclerotic plaques by targeting prolyl 4-hydroxylase subunit alpha-1 (P4HA1) in vascular smooth muscle cells. Atherosclerosis 2018, 277, 98–107. [Google Scholar] [CrossRef]
- Loyer, X.; Mallat, Z.; Boulanger, C.M.; Tedgui, A. MicroRNAs as therapeutic targets in atherosclerosis. Expert Opin. Ther. Targets 2015, 19, 489–496. [Google Scholar] [CrossRef]
- Yiew, K.H.; Chatterjee, T.K.; Hui, D.Y.; Weintraub, N.L. Histone Deacetylases and Cardiometabolic Diseases. Arterioscler Thromb Vasc Biol. 2015, 35, 1914–1919. [Google Scholar] [CrossRef]
- Schiano, C.; Benincasa, G.; Franzese, M.; Della Mura, N.; Pane, K.; Salvatore, M.; Napoli, C. Epigenetic-sensitive pathways in personalized therapy of major cardiovascular diseases. Pharmacol. Ther. 2020, 210, 107514. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, Y.; Xiang, C. SIRT2 decreases atherosclerotic plaque formation in low-density lipoprotein receptor-deficient mice by modulating macrophage polarization. Biomed. Pharmacother. 2018, 97, 1238–1242. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Zheng, Y.; Zhou, J.; Yuan, J.; Yu, Y.; Wang, J. Resveratrol Exerts Antioxidant Effects by Activating SIRT2 To Deacetylate Prx1. Biochemistry 2017, 56, 6325–6328. [Google Scholar] [CrossRef]
- Pasterkamp, G.; van der Laan, S.W.; Haitjema, S.; Foroughi Asl, H.; Siemelink, M.A.; Bezemer, T.; van Setten, J.; Dichgans, M.; Malik, R.; Worrall, B.B.; et al. Human Validation of Genes Associated With a Murine Atherosclerotic Phenotype. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1240–1246. [Google Scholar] [CrossRef]
- Willemsen, L.; de Winther, M.P. Macrophage subsets in atherosclerosis as defined by single-cell technologies. J. Pathol. 2020, 250, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Allahverdian, S.; Pannu, P.S.; Francis, G.A. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovasc. Res. 2012, 95, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- White, H.D.; Held, C.; Stewart, R.; Tarka, E.; Brown, R.; Davies, R.Y.; Budaj, A.; Harrington, R.A.; Steg, P.G.; The Stability Investigators; et al. Darapladib for preventing ischemic events in stable coronary heart disease. N. Engl. J. Med. 2014, 370, 1702–1711. [Google Scholar]
- Bowman, L.; Hopewell, J.C.; Chen, F.; Wallendszus, K.; Stevens, W.; Collins, R.; Wiviott, S.D.; Cannon, C.P.; Braunwald, E. HPS3/TIMI55-REVEAL Collaborative Group; et al. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N. Engl. J. Med. 2017, 377, 1217–1227. [Google Scholar] [PubMed]
- Daida, H.; Dohi, T.; Fukushima, Y.; Ohmura, H.; Miyauchi, K. The Goal of Achieving Atherosclerotic Plaque Regression with Lipid-Lowering Therapy: Insights from IVUS Trials. J. Atheroscler. Thromb. 2019, 26, 592–600. [Google Scholar] [CrossRef]
- Schartl, M.; Bocksch, W.; Koschyk, D.H.; Voelker, W.; Karsch, K.R.; Kreuzer, J.; Hausmann, D.; Beckmann, S.; Gross, M. Use of intravascular ultrasound to compare effects of different strategies of lipid-lowering therapy on plaque volume and composition in patients with coronary artery disease. Circulation 2001, 104, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Brown, B.G.; Ganz, P.; Vogel, R.A.; Crowe, T.; Howard, G.; Cooper, C.J.; Brodie, B.; et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. JAMA 2004, 291, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Christoph, M.; Herold, J.; Berg-Holldack, A.; Rauwolf, T.; Ziemssen, T.; Schmeisser, A.; Weinert, S.; Ebner, B.; Said, S.; Strasser, R.H.; et al. Effects of the PPARgamma agonist pioglitazone on coronary atherosclerotic plaque composition and plaque progression in non-diabetic patients: A double-center, randomized controlled VH-IVUS pilot-trial. Heart Vessel. 2015, 30, 286–295. [Google Scholar] [CrossRef]
- Thondapu, V.; Kurihara, O.; Yonetsu, T.; Russo, M.; Kim, H.O.; Lee, H.; Soeda, T.; Minami, Y.; Jang, I.K. Comparison of Rosuvastatin Versus Atorvastatin for Coronary Plaque Stabilization. Am. J. Cardiol. 2019, 123, 1565–1571. [Google Scholar] [CrossRef]
- Geng, C.; Zhang, Y.; Hidru, T.H.; Zhi, L.; Tao, M.; Zou, L.; Chen, C.; Li, H.; Liu, Y. Sonodynamic therapy: A potential treatment for atherosclerosis. Life Sci. 2018, 207, 304–313. [Google Scholar] [CrossRef]
- Yoo, S.W.; Oh, G.; Ahn, J.C.; Chung, E. Non-Oncologic Applications of Nanomedicine-Based Phototherapy. Biomedicines 2021, 9, 113. [Google Scholar] [CrossRef]
- Kharlamov, A.N.; Tyurnina, A.E.; Veselova, V.S.; Kovtun, O.P.; Shur, V.Y.; Gabinsky, J.L. Silica-gold nanoparticles for atheroprotective management of plaques: Results of the NANOM-FIM trial. Nanoscale 2015, 7, 8003–8015. [Google Scholar] [CrossRef] [PubMed]
- Scoville, D.K.; Nolin, J.D.; Ogden, H.L.; An, D.; Afsharinejad, Z.; Johnson, B.W.; Bammler, T.K.; Gao, X.; Frevert, C.W.; Altemeier, W.A.; et al. Quantum dots and mouse strain influence house dust mite-induced allergic airway disease. Toxicol. Appl. Pharmacol. 2019, 368, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Dai, J.; Hou, J.; Xing, L.; Ma, L.; Liu, H.; Xu, M.; Yao, Y.; Hu, S.; Yamamoto, E.; et al. Effective anti-thrombotic therapy without stenting: Intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur. Heart J. 2017, 38, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Antithrombotic Trialists’ Collaboration; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [PubMed]
- Shioi, A.; Ikari, Y. Plaque Calcification During Atherosclerosis Progression and Regression. J. Atheroscler. Thromb. 2018, 25, 294–303. [Google Scholar] [CrossRef]

| Approaches | Examples and Mechanisms | Investigated Drugs * |
|---|---|---|
| Metabolic approach | LDL lowering therapy: |
|
| Recombinant HDL particles [28] | 75 mg/kg of apoA-I(Milano) | |
| Iron depletion → increased iron mobilization from macrophages [88,89] | Iron chelation therapy (deferasirox) | |
| MGL depletion → 2‑AG ↑, CB2R activation [90] | N/A (genetic knock-out organisms used in this experiment) | |
| Cell survival promotion approach |
| |
| ||
| STAT6 upregulation → M2 macrophage polarization [92] | N/A (overexpression by recombinant pcDNA) | |
| Prevention from excessive PARP1 activation by severe DNA damage → prevention from ATP depletion [93] | For example, 3-Aminobenzamide (3-AB), doxycycline, thieno(2,3-c)isoquinolin-5-one (TIQ-A) | |
| Anti-inflammatory approach |
| |
|
| |
| Cytotoxic CD8 + T lymphocyte (Tc) depletion [100] | CD8α or CD8β targeted monoclonal antibody | |
| Regulatory T lymphocyte (Treg) promotion [101]. | For example, IL-2, mycophenolate mofetil, vitamin D, rapamycin, G-CSF | |
| Reactive oxygen species approaches | Downregulation of ROS generators (e.g., NADPH oxidases NOX2) [71] | Nox2 inhibitor peptide (a chimeric 18-amino acid peptide) |
| Attenuation of ROS derivative (e.g., 7β‑OH) activity [60]. | N/A (indirect methods like conjugation by glutathione) | |
| Promotion of ROS scavengers (such as HO-1 induced by Nrf transcriptional factor) [102] | N/A | |
| Direct ROS abruption (e.g., polyphenols) [103] | Different polyphenols (in this study–apple polyphenols) | |
| ECM remodeling and neovascularization approach | Inhibition of matrix metalloproteinase (MMPs) synthesis and activity (especially MMP9) [104] | Ghrelin |
| Promotion of collagen synthesis (e.g., by melatonin through Akt phosphorylation and subsequent P4Hα1 upregulation) [105] | Dietary nitrate treatment (KNO3 or KNO2) | |
| Influence on fibronectin (e.g., blockade of fibronectin-integrin α5 pathway) [106] | In vivo knockdown of phosphodiesterase 4D5 (siRNA) | |
| Inhibition of neovascularization (e.g., through bFGF blockade) [107] | K5 (a small molecule bFGF-inhibitor) |
| STUDY NAME | Treatment | No. of Investigated Patients (Period) | Clinical Outcome (MACE, Mortality) | Plaque Stabilization Effect (IVUS or OCT) |
|---|---|---|---|---|
| GAIN [133] 1 | Atorvastatin (20–80 mg) vs. placebo | 65 and 66 (12 months) | Any ischemic event: 21.5% vs. 31.8% (p = 0.184) | IVUS: Larger hyperechogenicity index 42.2% vs. 10.1%, p = 0.021 |
| REVERSAL [134] 2 | Atorvastatin 80 mg (intensive lipid-lowering) vs. Pravastatin 40 mg(moderate lipid-lowering) | 253 and 249 (18 months) | Death: 0.3% vs. 0.3%—NS Myocardial infarction: 1.2% vs. 2.1%—NS | IVUS: Lower percent atheroma volume change 0.2% vs. 1.6%, p < 0.001 |
| PRECISE-IVUS [37] 3 | Atorvastatin * + ezetimibe (10 mg) vs. Atorvastatin * alone | 102 and 100 (9–12 months) | Cardiovascular events ** 11% vs. 14%—NS | IVUS: Change in normalized TAV −6.6% vs. −1.4%, p < 0.001 |
| GLAGOV [135] 4 | Statin *** + PCSK9i (evolocumab 420 mg monthly) vs. Statin *** alone | 423 and 423 (19 months) | Death: 0.6% vs. 0.8%—NS Non-fatal myocardial infarction: 2.1% vs 2.9%—NS | IVUS: Change in TAV −5.8% vs. –0.9%, p < 0.001 |
| Christoph et al. [136] | Pioglitazone (30 mg) vs. Placebo | 27 and 27 (9 months) | Insignificant differences, no MACE registered | VH-IVUS: Decrease in the necrotic core −1.3% vs. + 2.6%, p = 0.008 |
| Tondapu et al. [137] | Rosuvastatin (10 mg) vs. Atorvastatin (20 mg) | 24 and 19 (12 months) | Not applicable | OCT: Increased FCT **** 171.5 vs. 127.0 μm, p = 0.03; Decreased macrophages |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowara, M.; Cudnoch-Jedrzejewska, A. Different Approaches in Therapy Aiming to Stabilize an Unstable Atherosclerotic Plaque. Int. J. Mol. Sci. 2021, 22, 4354. https://doi.org/10.3390/ijms22094354
Kowara M, Cudnoch-Jedrzejewska A. Different Approaches in Therapy Aiming to Stabilize an Unstable Atherosclerotic Plaque. International Journal of Molecular Sciences. 2021; 22(9):4354. https://doi.org/10.3390/ijms22094354
Chicago/Turabian StyleKowara, Michal, and Agnieszka Cudnoch-Jedrzejewska. 2021. "Different Approaches in Therapy Aiming to Stabilize an Unstable Atherosclerotic Plaque" International Journal of Molecular Sciences 22, no. 9: 4354. https://doi.org/10.3390/ijms22094354
APA StyleKowara, M., & Cudnoch-Jedrzejewska, A. (2021). Different Approaches in Therapy Aiming to Stabilize an Unstable Atherosclerotic Plaque. International Journal of Molecular Sciences, 22(9), 4354. https://doi.org/10.3390/ijms22094354





