Activation of Insulin Signaling by Botanical Products
Abstract
1. Introduction
2. An Overview of the Regulation of Glucose Transport by the Activation of Insulin Signaling
3. Insulin Receptor Activation by Botanical Compounds
4. Inhibition of Phosphatases for the Activation of Insulin Signaling
4.1. PTP1B Inhibition by Botanical Compounds
4.2. Inhibition of Other Phosphatases by Botanical Products
5. GLUT4 Translocation
Botanical Products Affecting GUT4 Expression, Translocation and Functionality
6. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activates kinase |
| APS | Adapter protein with a PH and SH2 domain |
| AS160 | Akt substrate of 160 kDa |
| CA | Corosolic acid |
| ERK | Extracellular-signal-regulated kinase |
| ESR | Estrogen receptor |
| GEF | Guanine nucleotide exchange factor |
| GLP-1 | glucagon-like peptide 1 |
| GLUT4 | Glucose transporter 4 |
| GSPE | Grape-seed procyanidins extract |
| GSV | GLUT4 storage vesicles |
| IR | Insulin receptor |
| IRS-1 | Insulin receptor substrate 1 |
| LBD | Ligand binding domain |
| MEF2 | Myocyte enhancer factor 2 |
| PGG | Penta-galloyl-glucose |
| PI3K | Phosphatidyl insoitol-3-kinase |
| PKB | Protein kinase B |
| PM | Plasma membrane |
| PP2A | Protein phosphatase 2A |
| PPARγ | Peroxisome proliferator-activated receptors γ |
| PTP1B | Protein tyrosine phosphatase 1B |
| HFD | High fat diet |
| RXR | Retinoid X receptor |
| SGLT2 | Sodium-Glucose transporter 2 |
| SHP2 | SH2 containing protein tyrosine phosphatase-2 |
| TCPTP | T-cell protein tyrosine phosphatase |
| T2D | Type 2 diabetes |
| TZD | Thiazolidinediones |
| UA | Ursolic acid |
References
- Wilmot, E.; Idris, I. Early onset type 2 diabetes: Risk factors, clinical impact and management. Ther. Adv. Chronic Dis. 2014, 5, 234–244. [Google Scholar] [CrossRef]
- Marin-Penalver, J.J.; Martin-Timon, I.; Sevillano-Collantes, C.; Del Canizo-Gomez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef]
- Zhang, Y.; Parajuli, K.R.; Fava, G.E.; Gupta, R.; Xu, W.; Nguyen, L.U.; Zakaria, A.F.; Fonseca, V.A.; Wang, H.; Mauvais-Jarvis, F.; et al. GLP-1 Receptor in Pancreatic α-Cells Regulates Glucagon Secretion in a Glucose-Dependent Bidirectional Manner. Diabetes 2019, 68, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Fusco, J.; Xiao, X.; Prasadan, K.; Sheng, Q.; Chen, C.; Ming, Y.-C.; Gittes, G. GLP-1/Exendin-4 induces β-cell proliferation via the epidermal growth factor receptor. Sci. Rep. 2017, 7, 9100. [Google Scholar] [CrossRef]
- Glossmann, H.H.; Lutz, O.M.D. Pharmacology of metformin—An update. Eur. J. Pharmacol. 2019, 865, 172782. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diab. Rep. 2019, 19, 151. [Google Scholar] [CrossRef]
- Batista, T.M.; Haider, N.; Kahn, C.R. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia 2021, 64, 994–1006. [Google Scholar] [CrossRef]
- Bhat, S.; Chowta, M.; Chowta, N.; Shastry, R.; Kamath, P. Proportion of type 2 diabetic patients achieving treatment goals and the survey of patient’s attitude towards insulin initiation in patients with inadequate glycaemic control with oral anti-diabetic drugs. Curr. Diabetes Rev. 2020. [Google Scholar] [CrossRef]
- Lautie, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Nazarian-Samani, Z.; Sewell, R.D.E.; Lorigooini, Z.; Rafieian-Kopaei, M. Medicinal Plants with Multiple Effects on Diabetes Mellitus and Its Complications: A Systematic Review. Curr. Diabetes Rep. 2018, 18, 72. [Google Scholar] [CrossRef]
- Governa, P.; Baini, G.; Borgonetti, V.; Cettolin, G.; Giachetti, D.; Magnano, A.R.; Miraldi, E.; Biagi, M. Phytotherapy in the Management of Diabetes: A Review. Molecules 2018, 23, 105. [Google Scholar] [CrossRef]
- Menting, J.G.; Whittaker, J.; Margetts, M.B.; Whittaker, L.J.; Kong, G.K.; Smith, B.J.; Watson, C.J.; Zakova, L.; Kletvikova, E.; Jiracek, J.; et al. How insulin engages its primary binding site on the insulin receptor. Nature 2013, 493, 241–245. [Google Scholar] [CrossRef]
- Hubbard, S.R. The insulin receptor: Both a prototypical and atypical receptor tyrosine kinase. Cold Spring Harb. Perspect. Biol. 2013, 5, a008946. [Google Scholar] [CrossRef]
- Singh, P.; Alex, J.M.; Bast, F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: Novel treatment strategies for cancer. Med. Oncol. 2014, 31, 805. [Google Scholar] [CrossRef]
- Sarfstein, R.; Werner, H. Minireview: Nuclear insulin and insulin-like growth factor-1 receptors: A novel paradigm in signal transduction. Endocrinology 2013, 154, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R. Insulin Signaling in the Control of Glucose and Lipid Homeostasis. Handb. Exp. Pharmacol. 2016, 233, 51–71. [Google Scholar] [CrossRef]
- Ye, L.; Maji, S.; Sanghera, N.; Gopalasingam, P.; Gorbunov, E.; Tarasov, S.; Epstein, O.; Klein-Seetharaman, J. Structure and dynamics of the insulin receptor: Implications for receptor activation and drug discovery. Drug Discov Today 2017, 22, 1092–1102. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Tiganis, T. PTP1B and TCPTP--nonredundant phosphatases in insulin signaling and glucose homeostasis. FEBS J 2013, 280, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Haeusler, R.A.; McGraw, T.E.; Accili, D. Biochemical and cellular properties of insulin receptor signalling. Nat. Rev. Mol. Cell Biol. 2018, 19, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Youngren, J.F.; Manchem, V.P.; Kozlowski, M.; Zhang, B.B.; Maddux, B.A.; Goldfine, I.D. Small molecule insulin receptor activators potentiate insulin action in insulin-resistant cells. Diabetes 2001, 50, 2323–2328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.; Miyazaki, M.; Romeo, G.R.; Shoelson, S.E. Insulin receptor activation with transmembrane domain ligands. J. Biol. Chem. 2014, 289, 19769–19777. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.J.; Lei, Z.; Yiannikouris, A.; Yerramreddy, T.R.; Li, X.; Kincaid, H.; Eastridge, K.; Gadberry, H.; Power, C.; Xiao, R.; et al. Non-peptidyl small molecule, adenosine, 5′-Se-methyl-5′-seleno-, 2′,3′-diacetate, activates insulin receptor and attenuates hyperglycemia in type 2 diabetic Lepr(db/db) mice. Cell. Mol. Life Sci. 2020, 77, 1623–1643. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.J.; Lei, Z.; Nation, L.; Li, X.; Yiannikouris, A.; Yerramreddy, T.R.; Kincaid, H.; Eastridge, K.; Xiao, R.; Goettl, R.; et al. Oral administration of NPC43 counters hyperglycemia and activates insulin receptor in streptozotocin-induced type 1 diabetic mice. BMJ Open Diabetes Res Care 2020, 8. [Google Scholar] [CrossRef]
- Pender, C.; Goldfine, I.D.; Manchem, V.P.; Evans, J.L.; Spevak, W.R.; Shi, S.; Rao, S.; Bajjalieh, S.; Maddux, B.A.; Youngren, J.F. Regulation of insulin receptor function by a small molecule insulin receptor activator. J. Biol. Chem. 2002, 277, 43565–43571. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, C.; Wang, D.; Endoh, K.; Fukushi, Y.; Arakawa, K.; Fujikawa, S. Analysis of supercooling activity of tannin-related polyphenols. Cryobiology 2013, 67, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kim, J.; Li, J.; Liu, F.; Liu, X.; Himmeldirk, K.; Ren, Y.; Wagner, T.E.; Chen, X. Natural anti-diabetic compound 1,2,3,4,6-penta-Ogalloyl-d-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway. Biochem. Biophys. Res. Commun. 2005, 336, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Himmeldirk, K.; Chen, X. Synthesis and structure-activity relationship study of antidiabetic penta-Ogalloyl-d-glucopyranose and its analogues. J. Med. Chem. 2006, 49, 2829–2837. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22, 606. [Google Scholar] [CrossRef]
- Navarro, M.; Arnaez, E.; Moreira, I.; Quesada, S.; Azofeifa, G.; Wilhelm, K.; Vargas, F.; Chen, P. Polyphenolic Characterization, Antioxidant, and Cytotoxic Activities of Mangifera indica Cultivars from Costa Rica. Foods 2019, 8, 384. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Mangos and their bioactive components: Adding variety to the fruit plate for health. Food Funct. 2017, 8, 3010–3032. [Google Scholar] [CrossRef]
- Liu, X.; Kim, J.K.; Li, Y.; Li, J.; Liu, F.; Chen, X. Tannic acid stimulates glucose transport and inhibits adipocyte differentiation in 3T3-L1 cells. J. Nutr. 2005, 135, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, M.; Yamagishi, T.; Nonaka, G. Novel hydrolyzable tannins from Nuphar Japonicum DC. Chem. Pharm. Bull. 1982, 30, 1094–1097. [Google Scholar] [CrossRef]
- Liu, F.; Kim, J.; Li, Y.; Liu, X.; Li, J.; Chen, X. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J. Nutr. 2001, 131, 2242–2247. [Google Scholar] [CrossRef]
- Klein, G.; Kim, J.; Himmeldirk, K.; Cao, Y.; Chen, X. Antidiabetes and Anti-obesity Activity of Lagerstroemia speciosa. Evid. Based Complement. Alternat. Med. 2007, 4, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, T.; Sakane, I.; Takihara, T.; Ozaki, Y.; Takeuchi, H.; Kuroyanagi, M. Hypoglycemic effect of extracts from Lagerstroemia speciosa L. leaves in genetically diabetic KK-AY mice. Biosci. Biotechnol. Biochem. 1996, 60, 204–208. [Google Scholar] [CrossRef]
- Mohan, C.G.; Viswanatha, G.L.; Savinay, G.; Rajendra, C.E.; Halemani, P.D. 1,2,3,4,6 Penta-Ogalloyl-beta-d-glucose, a bioactivity guided isolated compound from Mangifera indica inhibits 11beta-HSD-1 and ameliorates high fat diet-induced diabetes in C57BL/6 mice. Phytomedicine 2013, 20, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.A.; Li, W.; Peterson, S.K.; Brown, A.; Kuvibidila, S.; Perkins-Veazie, P.; Clarke, S.L.; Smith, B.J. Mango modulates body fat and plasma glucose and lipids in mice fed a high-fat diet. Br. J. Nutr. 2011, 106, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, Y.; Kim, J.; Ren, Y.; Himmeldirk, K.; Liu, Y.; Qian, Y.; Liu, F.; Chen, X. Orally efficacious novel small molecule 6-chloro-6-deoxy-1,2,3,4-tetra-Ogalloyl-alpha-d-glucopyranose selectively and potently stimulates insulin receptor and alleviates diabetes. J. Mol. Endocrinol. 2013, 51, 15–26. [Google Scholar] [CrossRef][Green Version]
- Cao, Y.; Himmeldirk, K.B.; Qian, Y.; Ren, Y.; Malki, A.; Chen, X. Biological and biomedical functions of Penta-Ogalloyl-d-glucose and its derivatives. J. Nat. Med. 2014, 68, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.; Hogger, P. Oligomeric procyanidins of French maritime pine bark extract (Pycnogenol) effectively inhibit alpha-glucosidase. Diabetes Res. Clin. Pract. 2007, 77, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Bowser, S.M.; Moore, W.T.; McMillan, R.P.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Hulver, M.W.; Neilson, A.P. High-molecular-weight cocoa procyanidins possess enhanced insulin-enhancing and insulin mimetic activities in human primary skeletal muscle cells compared to smaller procyanidins. J. Nutr. Biochem. 2017, 39, 48–58. [Google Scholar] [CrossRef]
- Montagut, G.; Onnockx, S.; Vaqué, M.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.; Arola, L.; Pirson, I.; et al. Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J. Nutr. Biochem. 2010, 21, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Wang, L.; Nanba, F.; Ito, C.; Toda, T.; Ashida, H. Procyanidin Promotes Translocation of Glucose Transporter 4 in Muscle of Mice through Activation of Insulin and AMPK Signaling Pathways. PLoS ONE 2016, 11, e0161704. [Google Scholar] [CrossRef]
- Dorenkott, M.R.; Griffin, L.E.; Goodrich, K.M.; Thompson-Witrick, K.A.; Fundaro, G.; Ye, L.; Stevens, J.R.; Ali, M.; O’Keefe, S.F.; Hulver, M.W.; et al. Oligomeric cocoa procyanidins possess enhanced bioactivity compared to monomeric and polymeric cocoa procyanidins for preventing the development of obesity, insulin resistance, and impaired glucose tolerance during high-fat feeding. J. Agric. Food Chem. 2014, 62, 2216–2227. [Google Scholar] [CrossRef]
- Pinent, M.; Blay, M.; Blade, M.C.; Salvado, M.J.; Arola, L.; Ardevol, A. Grape seed-derived procyanidins have an antihyperglycemic effect in streptozotocin-induced diabetic rats and insulinomimetic activity in insulin-sensitive cell lines. Endocrinology 2004, 145, 4985–4990. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Issac, A.; Nm, J.; Ninan, E.; Maliakel, B.; Kuttan, R. Effects of the polyphenol content on the anti-diabetic activity of Cinnamomum zeylanicum extracts. Food Funct. 2014, 5, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liu, G.; Hu, Z.; Shi, W.; Chen, B.; Zou, P.; Li, X. Grape seed proanthocyanidins protect against streptozotocininduced diabetic nephropathy by attenuating endoplasmic reticulum stressinduced apoptosis. Mol. Med. Rep. 2018, 18, 1447–1454. [Google Scholar] [CrossRef]
- Wei, J.; Wu, H.; Zhang, H.; Li, F.; Chen, S.; Hou, B.; Shi, Y.; Zhao, L.; Duan, H. Anthocyanins inhibit high glucose-induced renal tubular cell apoptosis caused by oxidative stress in db/db mice. Int. J. Mol. Med. 2018, 41, 1608–1618. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Garcia-Villanova, B.; Guerra-Hernandez, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef]
- Yu, H.C.; Wu, J.; Zhang, H.X.; Zhang, H.S.; Qiao, T.T.; Zhang, J.X.; Zhang, G.L.; Sui, J.; Li, L.W.; Zhang, L.R.; et al. Antidepressant-like and anti-oxidative efficacy of Campsis grandiflora flower. J. Pharm. Pharmacol. 2015, 67, 1705–1715. [Google Scholar] [CrossRef]
- Cui, X.Y.; Kim, J.H.; Zhao, X.; Chen, B.Q.; Lee, B.C.; Pyo, H.B.; Yun, Y.P.; Zhang, Y.H. Antioxidative and acute anti-inflammatory effects of Campsis grandiflora flower. J. Ethnopharmacol. 2006, 103, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Jager, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.H.; Ha, Y.J.; Shim, E.K.; Choi, S.Y.; Jin, J.L.; Yun-Choi, H.S.; Lee, J.R. Insulin-mimetic and insulin-sensitizing activities of a pentacyclic triterpenoid insulin receptor activator. Biochem. J. 2007, 403, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.J.G.; Frederico, M.J.S.; Cazarolli, L.H.; Mendes, C.P.; Bretanha, L.C.; Schmidt, É.C.; Bouzon, Z.L.; de Medeiros Pinto, V.A.; da Fonte Ramos, C.; Pizzolatti, M.G.; et al. The mechanism of action of ursolic acid as insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.-y.; Hu, L.-h.; Li, J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Biophys. Acta (BBA) Gen. Subj. 2006, 1760, 1505–1512. [Google Scholar] [CrossRef]
- Xie, L.; Lee, S.Y.; Andersen, J.N.; Waters, S.; Shen, K.; Guo, X.L.; Moller, N.P.; Olefsky, J.M.; Lawrence, D.S.; Zhang, Z.Y. Cellular effects of small molecule PTP1B inhibitors on insulin signaling. Biochemistry 2003, 42, 12792–12804. [Google Scholar] [CrossRef] [PubMed]
- Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A.L.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan, C.C.; et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 1999, 283, 1544–1548. [Google Scholar] [CrossRef]
- Klaman, L.D.; Boss, O.; Peroni, O.D.; Kim, J.K.; Martino, J.L.; Zabolotny, J.M.; Moghal, N.; Lubkin, M.; Kim, Y.B.; Sharpe, A.H.; et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 2000, 20, 5479–5489. [Google Scholar] [CrossRef]
- Lund, I.K.; Hansen, J.A.; Andersen, H.S.; Moller, N.P.; Billestrup, N. Mechanism of protein tyrosine phosphatase 1B-mediated inhibition of leptin signalling. J. Mol. Endocrinol. 2005, 34, 339–351. [Google Scholar] [CrossRef]
- Eleftheriou, P.; Geronikaki, A.; Petrou, A. PTP1b Inhibition, A Promising Approach for the Treatment of Diabetes Type II. Curr. Top. Med. Chem. 2019, 19, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, N.; Karthikeyan, C.; Trivedi, P.; Moorthy, N. Recent Advances in Protein Tyrosine Phosphatase 1B Targeted Drug Discovery for Type II Diabetes and Obesity. Curr. Drug Targets 2018, 19, 551–575. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Yu, H.; Huang, X.-F. Selective binding modes and allosteric inhibitory effects of lupane triterpenes on protein tyrosine phosphatase 1B. Sci. Rep. 2016, 6, 20766. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Maguire-Nguyen, K.K.; Rando, T.A.; Zasloff, M.A.; Strange, K.B.; Yin, V.P. The protein tyrosine phosphatase 1B inhibitor MSI-1436 stimulates regeneration of heart and multiple other tissues. npj Regen. Med. 2017, 2, 4. [Google Scholar] [CrossRef]
- Vintonyak, V.V.; Antonchick, A.P.; Rauh, D.; Waldmann, H. The therapeutic potential of phosphatase inhibitors. Curr. Opin. Chem. Biol. 2009, 13, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Jung, K.-W.; Woo, E.-R.; Kim, Y.-M. Docking Study of Biflavonoids, Allosteric Inhibitors of Protein Tyrosine Phosphatase 1B. Bull. Korean Chem. Soc. 2008, 29. [Google Scholar] [CrossRef]
- Reddy, R.H.; Kim, H.; Cha, S.; Lee, B.; Kim, Y.J. Structure-Based Virtual Screening of Protein Tyrosine Phosphatase Inhibitors: Significance, Challenges, and Solutions. J. Microbiol. Biotechnol. 2017, 27, 878–895. [Google Scholar] [CrossRef]
- Lee, M.S.; Thuong, P.T. Stimulation of glucose uptake by triterpenoids from Weigela subsessilis. Phytother. Res. 2010, 24, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Yang, S.; He, L.; Oh, H.; Kim, B.S.; Oh, W.K.; Kim, B.Y.; Ahn, J.S. Inhibition of protein tyrosine phosphatase 1B by ursane-type triterpenes isolated from Symplocos paniculata. Planta Med. 2006, 72, 261–263. [Google Scholar] [CrossRef]
- Judy, W.V.; Hari, S.P.; Stogsdill, W.W.; Judy, J.S.; Naguib, Y.M.A.; Passwater, R. Antidiabetic activity of a standardized extract (Glucosol™) from Lagerstroemia speciosa leaves in Type II diabetics: A dose-dependence study. J. Ethnopharmacol. 2003, 87, 115–117. [Google Scholar] [CrossRef]
- Xu, S.; Wang, G.; Peng, W.; Xu, Y.; Zhang, Y.; Ge, Y.; Jing, Y.; Gong, Z. Corosolic acid isolated from Eriobotrya japonica leaves reduces glucose level in human hepatocellular carcinoma cells, zebrafish and rats. Sci. Rep. 2019, 9, 4388. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, W.; Zhou, Y.-Y.; Zhang, Y.-N.; Li, J.-Y.; Hu, L.-H.; Li, J. Corosolic acid stimulates glucose uptake via enhancing insulin receptor phosphorylation. Eur. J. Pharmacol. 2008, 584, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Kim, B.Y.; Osada, H.; Ahn, J.S. Inhibition of protein tyrosine phosphatase 1B by lupeol and lupenone isolated from Sorbus commixta. J. Enzyme Inhib. Med. Chem. 2009, 24, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Sturm, S.; Gil, R.R.; Chai, H.B.; Ngassapa, O.D.; Santisuk, T.; Reutrakul, V.; Howe, A.; Moss, M.; Besterman, J.M.; Yang, S.L.; et al. Lupane derivatives from Lophopetalum wallichii with farnesyl protein transferase inhibitory activity. J. Nat. Prod. 1996, 59, 658–663. [Google Scholar] [CrossRef]
- Khan, M.F.; Maurya, C.K.; Dev, K.; Arha, D.; Rai, A.K.; Tamrakar, A.K.; Maurya, R. Design and synthesis of lupeol analogues and their glucose uptake stimulatory effect in L6 skeletal muscle cells. Bioorg. Med. Chem. Lett. 2014, 24, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Ali, M.Y.; Jung, H.A.; Oh, S.H.; Choi, R.J.; Kim, E.J. Protein tyrosine phosphatase 1B inhibitory activity of alkaloids from Rhizoma Coptidis and their molecular docking studies. J. Ethnopharmacol. 2015, 171, 28–36. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Huang, C. Berberine inhibits PTP1B activity and mimics insulin action. Biochem. Biophys. Res. Commun. 2010, 397, 543–547. [Google Scholar] [CrossRef]
- Geng, F.H.; Li, G.H.; Zhang, X.; Zhang, P.; Dong, M.Q.; Zhao, Z.J.; Zhang, Y.; Dong, L.; Gao, F. Berberine improves mesenteric artery insulin sensitivity through up-regulating insulin receptor-mediated signalling in diabetic rats. Br. J. Pharmacol. 2016, 173, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef]
- Xu, X.; Yi, H.; Wu, J.; Kuang, T.; Zhang, J.; Li, Q.; Du, H.; Xu, T.; Jiang, G.; Fan, G. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed. Pharmacother. 2021, 133, 110984. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuk, H.J.; Kim, J.Y.; Kim, D.W.; Song, Y.H.; Tan, X.F.; Curtis-Long, M.J.; Park, K.H. Novel chromenedione derivatives displaying inhibition of protein tyrosine phosphatase 1B (PTP1B) from Flemingia philippinensis. Bioorg. Med. Chem. Lett. 2016, 26, 318–321. [Google Scholar] [CrossRef]
- Sasaki, T.; Li, W.; Higai, K.; Quang, T.H.; Kim, Y.H.; Koike, K. Protein tyrosine phosphatase 1B inhibitory activity of lavandulyl flavonoids from roots of Sophora flavescens. Planta Med. 2014, 80, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Zhu, Y.Y.; Feng, J.Y.; Zhang, J.G.; Hu, F.; Prasad, C.; Wei, Z.J. Morin as an imminent functional food ingredient: An update on its enhanced efficacy in the treatment and prevention of metabolic syndromes. Food Funct. 2020, 11, 8424–8443. [Google Scholar] [CrossRef] [PubMed]
- Zargari, F.; Lotfi, M.; Shahraki, O.; Nikfarjam, Z.; Shahraki, J. Flavonoids as potent allosteric inhibitors of protein tyrosine phosphatase 1B: Molecular dynamics simulation and free energy calculation. J. Biomol. Struct. Dyn. 2018, 36, 4126–4142. [Google Scholar] [CrossRef] [PubMed]
- Paoli, P.; Cirri, P.; Caselli, A.; Ranaldi, F.; Bruschi, G.; Santi, A.; Camici, G. The insulin-mimetic effect of Morin: A promising molecule in diabetes treatment. Biochim. Biophys. Acta 2013, 1830, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Kai, K.; Ishii, M.; Ishii, T.; Akagawa, M. Safranal, a novel protein tyrosine phosphatase 1B inhibitor, activates insulin signaling in C2C12 myotubes and improves glucose tolerance in diabetic KK-Ay mice. Mol. Nutr. Food Res. 2014, 58, 1177–1189. [Google Scholar] [CrossRef]
- Obanda, D.N.; Zhao, P.; Richard, A.J.; Ribnicky, D.; Cefalu, W.T.; Stephens, J.M. Stinging Nettle (Urtica dioica L.) Attenuates FFA Induced Ceramide Accumulation in 3T3-L1 Adipocytes in an Adiponectin Dependent Manner. PLoS ONE 2016, 11, e0150252. [Google Scholar] [CrossRef]
- Obanda, D.N.; Ribnicky, D.; Yu, Y.; Stephens, J.; Cefalu, W.T. An extract of Urtica dioica L. mitigates obesity induced insulin resistance in mice skeletal muscle via protein phosphatase 2A (PP2A). Sci. Rep. 2016, 6, 22222. [Google Scholar] [CrossRef]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.U.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef]
- Fan, S.; Raychaudhuri, S.; Kraus, O.; Shahinozzaman, M.; Lofti, L.; Obanda, D.N. Urtica dioica Whole Vegetable as a Functional Food Targeting Fat Accumulation and Insulin Resistance-a Preliminary Study in a Mouse Pre-Diabetic Model. Nutrients 2020, 12, 1059. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Kane, S.; Sano, E.; Mîinea, C.P.; Asara, J.M.; Lane, W.S.; Garner, C.W.; Lienhard, G.E. Insulin-stimulated Phosphorylation of a Rab GTPase-activating Protein Regulates GLUT4 Translocation*. J. Biol. Chem. 2003, 278, 14599–14602. [Google Scholar] [CrossRef]
- Tunduguru, R.; Thurmond, D.C. Promoting Glucose Transporter-4 Vesicle Trafficking along Cytoskeletal Tracks: PAK-Ing Them Out. Front. Endocrinol. 2017, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Morino, T.; Ishii, T.; Kanzaki, M. Cooperative actions of Tbc1d1 and AS160/Tbc1d4 in GLUT4-trafficking activities. J. Biol. Chem. 2019, 294, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R. Insulin Signaling in the Control of Glucose and Lipid Homeostasis. In Metabolic Control; Herzig, S., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 51–71. [Google Scholar]
- Lee, J.O.; Lee, S.K.; Kim, J.H.; Kim, N.; You, G.Y.; Moon, J.W.; Kim, S.J.; Park, S.H.; Kim, H.S. Metformin Regulates Glucose Transporter 4 (GLUT4) Translocation through AMP-activated Protein Kinase (AMPK)-mediated Cbl/CAP Signaling in 3T3-L1 Preadipocyte Cells*. J. Biol. Chem. 2012, 287, 44121–44129. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Treebak, J.T.; Fentz, J.; Lantier, L.; Viollet, B.; Birk, J.B.; Schjerling, P.; Björnholm, M.; Zierath, J.R.; Wojtaszewski, J.F. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes 2015, 64, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Treebak, J.T.; Taylor, E.B.; Witczak, C.A.; An, D.; Toyoda, T.; Koh, H.J.; Xie, J.; Feener, E.P.; Wojtaszewski, J.F.; Hirshman, M.F.; et al. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am. J. Physiol. Cell Physiol. 2010, 298, C377–C385. [Google Scholar] [CrossRef]
- Gaster, M.; Staehr, P.; Beck-Nielsen, H.; Schrøder, H.D.; Handberg, A. GLUT4 Is Reduced in Slow Muscle Fibers of Type 2 Diabetic Patients. Is Insulin Resistance in Type 2 Diabetes a Slow, Type 1 Fiber Disease? Diabetes 2001, 50, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U.; Christensen, B.; Nielsen, T.S.; Pedersen, S.B.; Ørskov, L.; Lund, S.; Møller, N.; Jessen, N. GLUT4 and UBC9 protein expression is reduced in muscle from type 2 diabetic patients with severe insulin resistance. PLoS ONE 2011, 6, e27854. [Google Scholar] [CrossRef]
- Moraes, P.A.; Yonamine, C.Y.; Pinto Junior, D.C.; Esteves, J.V.D.; Machado, U.F.; Mori, R.C. Insulin acutely triggers transcription of Slc2a4 gene: Participation of the AT-rich, E-box and NFKB-binding sites. Life Sci. 2014, 114, 36–44. [Google Scholar] [CrossRef]
- Im, S.S.; Kwon, S.K.; Kim, T.H.; Kim, H.I.; Ahn, Y.H. Regulation of glucose transporter type 4 isoform gene expression in muscle and adipocytes. IUBMB Life 2007, 59, 134–145. [Google Scholar] [CrossRef]
- Dev, K.; Ramakrishna, E.; Maurya, R. Chapter 4—Glucose Transporter 4 Translocation Activators From Nature. In Discovery and Development of Antidiabetic Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 113–145. [Google Scholar]
- Anandharajan, R.; Pathmanathan, K.; Shankernarayanan, N.P.; Vishwakarma, R.A.; Balakrishnan, A. Upregulation of Glut-4 and PPAR gamma by an isoflavone from Pterocarpus marsupium on L6 myotubes: A possible mechanism of action. J. Ethnopharmacol. 2005, 97, 253–260. [Google Scholar] [CrossRef]
- Anhe, G.F.; Okamoto, M.M.; Kinote, A.; Sollon, C.; Lellis-Santos, C.; Anhe, F.F.; Lima, G.A.; Hirabara, S.M.; Velloso, L.A.; Bordin, S.; et al. Quercetin decreases inflammatory response and increases insulin action in skeletal muscle of ob/ob mice and in L6 myotubes. Eur. J. Pharmacol. 2012, 689, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Yonamine, C.Y.; Alves-Wagner, A.B.; Esteves, J.V.; Okamoto, M.M.; Correa-Giannella, M.L.; Giannella-Neto, D.; Machado, U.F. Diabetes induces tri-methylation at lysine 9 of histone 3at Slc2a4 gene in skeletal muscle: A new target to improve glycemic control. Mol. Cell. Endocrinol. 2019, 481, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhou, L.J.; Mu, P.W.; Liu, S.P.; Chen, S.J.; Fu, X.D.; Wang, T.H. Caveolin-3 is involved in the protection of resveratrol against high-fat-diet-induced insulin resistance by promoting GLUT4 translocation to the plasma membrane in skeletal muscle of ovariectomized rats. J. Nutr. Biochem. 2012, 23, 1716–1724. [Google Scholar] [CrossRef]
- Dresseno, L.P.; Lehnen, A.M.; Teló, G.; Silveira, A.; Markoski, M.M.; Machado, U.F.; Schaan, B.D. Impact of flaxseed and soy nuts as dietary supplements on lipid profile, insulin sensitivity, and GLUT4 expression in ovariectomized rats. Appl. Physiol. Nutr. Metab. 2018, 43, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, K.C.R.; Laurindo, C.P.; Machado, U.F. Estrogen and Glycemic Homeostasis: The Fundamental Role of Nuclear Estrogen Receptors ESR1/ESR2 in Glucose Transporter GLUT4 Regulation. Cells 2021, 10, 99. [Google Scholar] [CrossRef]
- Barros, R.P.; Gabbi, C.; Morani, A.; Warner, M.; Gustafsson, J.A. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E124–E133. [Google Scholar] [CrossRef]
- Campello, R.S.; Alves-Wagner, A.B.; Lucas, T.F.; Mori, R.C.; Furuya, D.T.; Porto, C.S.; Machado, U.F. Estrogen receptor 1 agonist PPT stimulates Slc2a4 gene expression and improves insulin-induced glucose uptake in adipocytes. Curr. Top. Med. Chem. 2012, 12, 2059–2069. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Y.; Morrison, R.F.; Bucher, N.L.; Farmer, S.R. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J. Clin. Investig. 1998, 101, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Vigueira, P.A.; Chambers, K.T.; Hall, A.M.; Mitra, M.S.; Qi, N.; McDonald, W.G.; Colca, J.R.; Kletzien, R.F.; Finck, B.N. Insulin resistance and metabolic derangements in obese mice are ameliorated by a novel peroxisome proliferator-activated receptor γ-sparing thiazolidinedione. J. Biol. Chem. 2012, 287, 23537–23548. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARgamma): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Han, K.L.; Choi, J.S.; Lee, J.Y.; Song, J.; Joe, M.K.; Jung, M.H.; Hwang, J.K. Therapeutic potential of peroxisome proliferators--activated receptor-alpha/gamma dual agonist with alleviation of endoplasmic reticulum stress for the treatment of diabetes. Diabetes 2008, 57, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Kotani, H.; Tanabe, H.; Mizukami, H.; Makishima, M.; Inoue, M. Identification of a naturally occurring rexinoid, honokiol, that activates the retinoid X receptor. J. Nat. Prod. 2010, 73, 1332–1336. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Wang, J.N.; Gu, S.P.; Bu, J.; Kramer, M.P.; Baumgartner, L.; Fakhrudin, N.; Ladurner, A.; Malainer, C.; Vuorinen, A.; et al. Honokiol: A non-adipogenic PPARgamma agonist from nature. Biochim. Biophys. Acta 2013, 1830, 4813–4819. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.E.; Rekman, J.F.; Gunnink, L.K.; Busscher, B.M.; Scott, J.L.; Tidball, A.M.; Stehouwer, N.R.; Johnecheck, G.N.; Looyenga, B.D.; Louters, L.L. Quercetin inhibits glucose transport by binding to an exofacial site on GLUT1. Biochimie 2018, 151, 107–114. [Google Scholar] [CrossRef]
- Strobel, P.; Allard, C.; Perez-Acle, T.; Calderon, R.; Aldunate, R.; Leighton, F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem. J. 2005, 386, 471–478. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, B.; Sui, G.; Shi, J. Phytochemicals Block Glucose Utilization and Lipid Synthesis to Counteract Metabolic Reprogramming in Cancer Cells. Appl. Sci. 2021, 11, 1259. [Google Scholar] [CrossRef]
- Granchi, C.; Fortunato, S.; Minutolo, F. Anticancer agents interacting with membrane glucose transporters. MedChemComm 2016, 7, 1716–1729. [Google Scholar] [CrossRef]
- Bazuine, M.; van den Broek, P.J.A.; Maassen, J.A. Genistein directly inhibits GLUT4-mediated glucose uptake in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2005, 326, 511–514. [Google Scholar] [CrossRef]
- Nomura, M.; Takahashi, T.; Nagata, N.; Tsutsumi, K.; Kobayashi, S.; Akiba, T.; Yokogawa, K.; Moritani, S.; Miyamoto, K. Inhibitory mechanisms of flavonoids on insulin-stimulated glucose uptake in MC3T3-G2/PA6 adipose cells. Biol. Pharm. Bull. 2008, 31, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, X.J.; Zhao, W.W.; Zhao, W.J.; Jiang, C.H.; Huang, F.; Kou, J.P.; Liu, B.L.; Liu, K. Opposite effects of genistein on the regulation of insulin-mediated glucose homeostasis in adipose tissue. Br. J. Pharmacol. 2013, 170, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Digel, M.; Küch, E.M.; Stremmel, W.; Füllekrug, J. Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. J. Cell. Biochem. 2011, 112, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Tauber, A.L.; Schweiker, S.S.; Levonis, S.M. From tea to treatment; epigallocatechin gallate and its potential involvement in minimizing the metabolic changes in cancer. Nutr. Res. 2020, 74, 23–36. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]

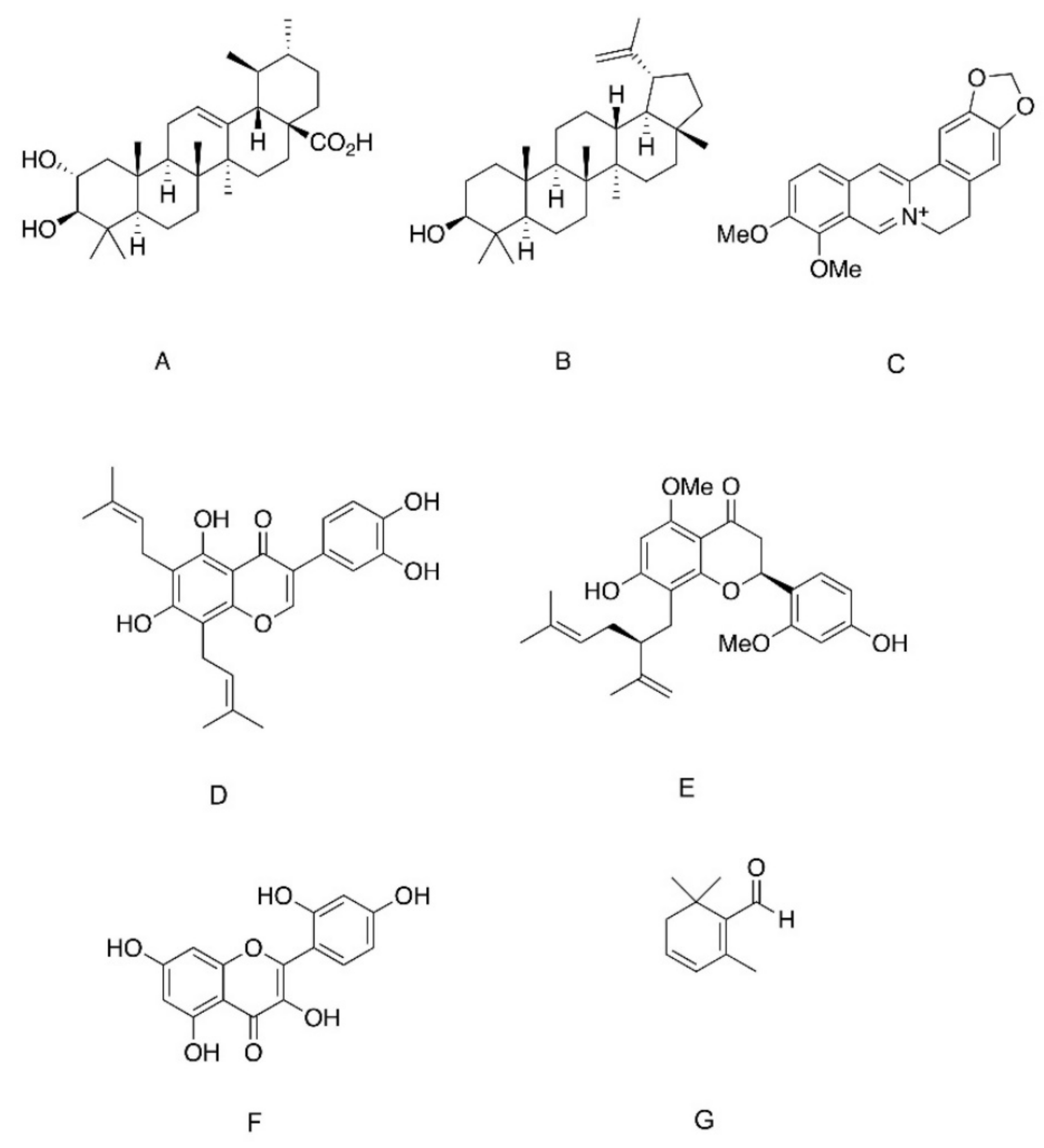
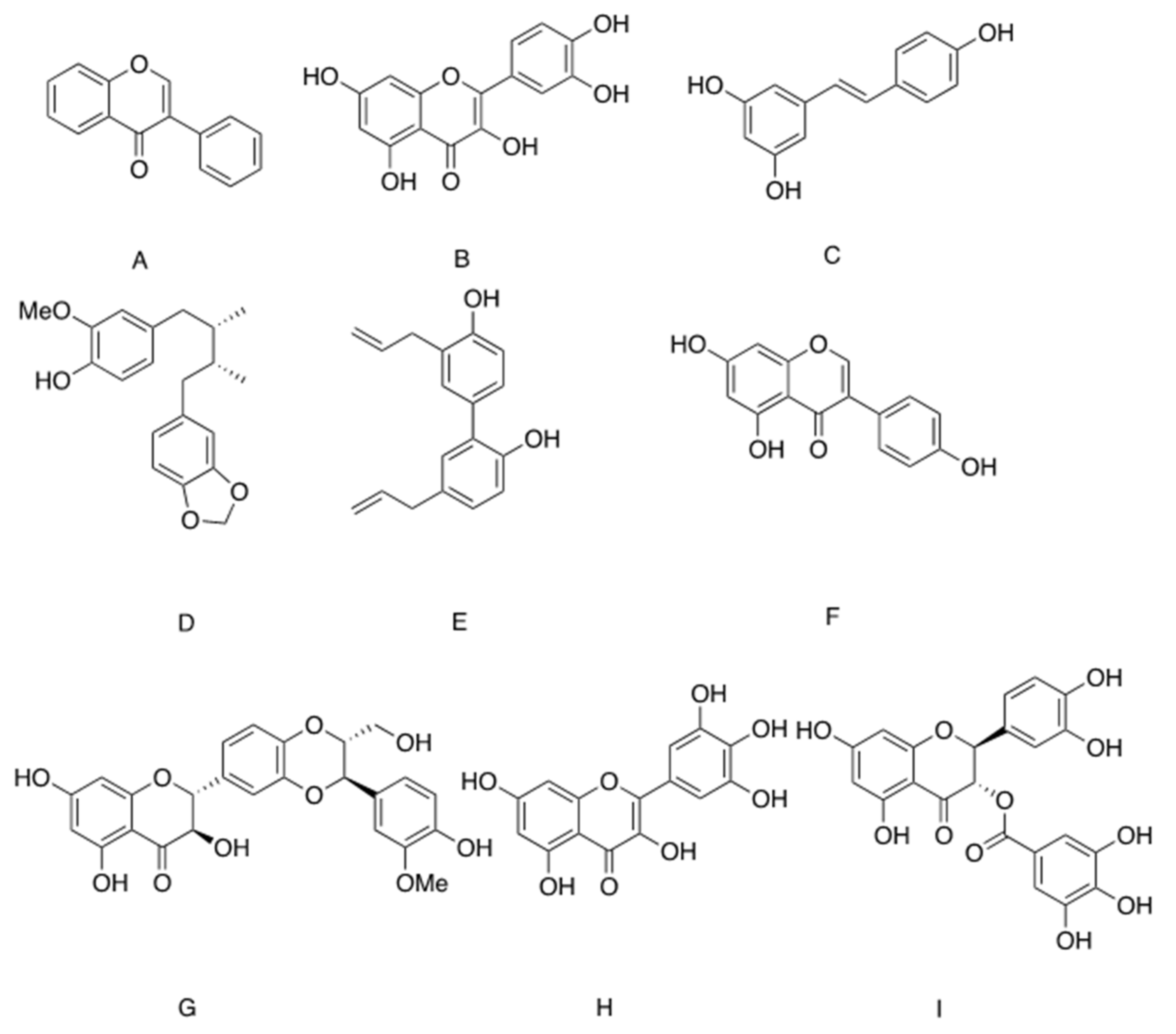
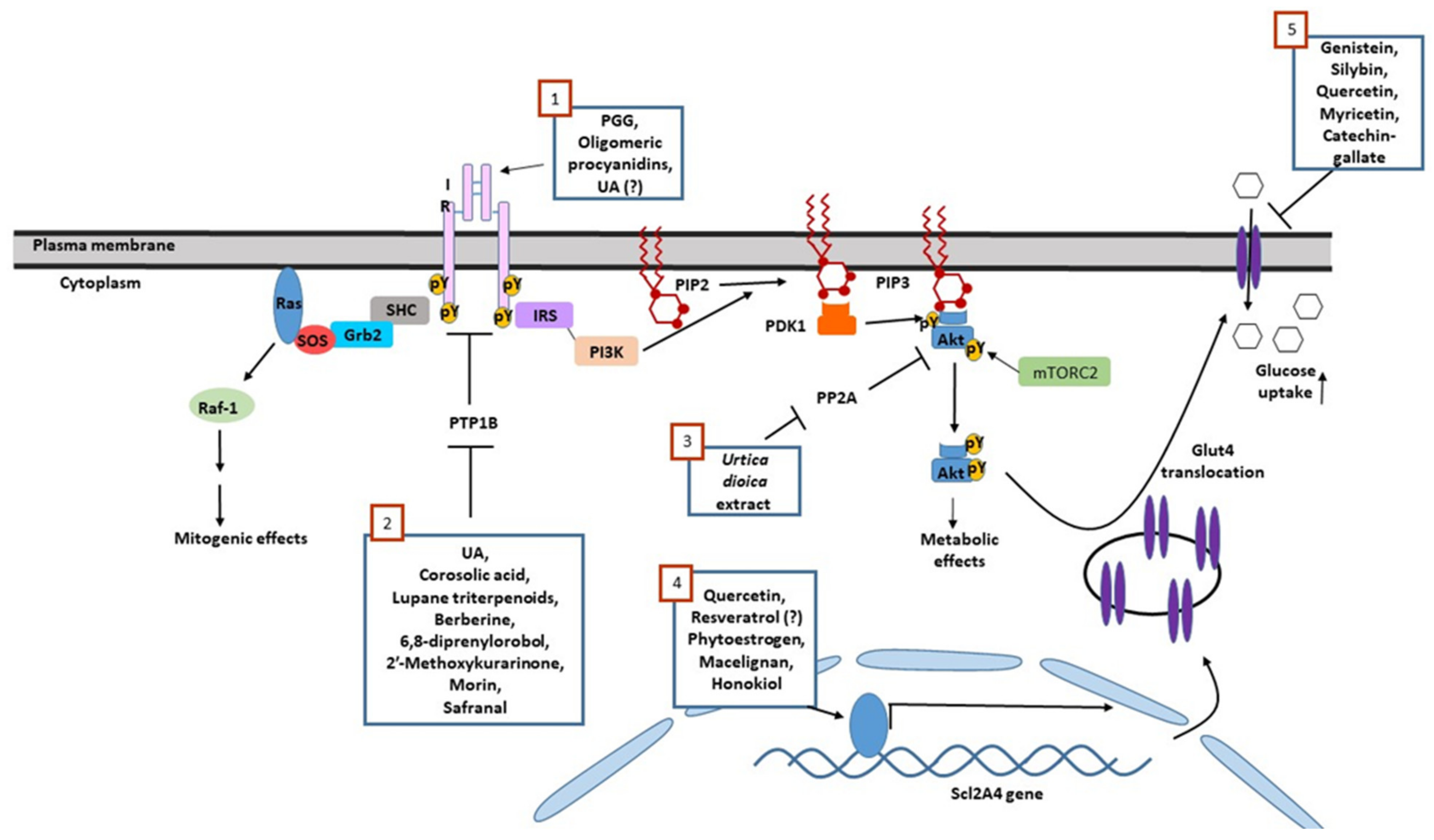
| Compound (Chemical Family) | Natural Origin | Mechanism of Action |
|---|---|---|
| Insulin Receptor Activators | ||
| 1,2,3,4,6-penta-galloyl-α-d-glucopyranose (hydrolysable tannins) | Punica granatum, Mangifera indica, Lagerstroemia speciosa | Binds to the α-subunit of the IR and induces its autophosphorylation |
| Oligomeric procyanidins (condensed tannins) | Cocoa, pine bark, grape seeds | Induce IR autophosphorylation |
| Ursolic acid (triterpenoids) | Campsis grandiflora, Malus domestica, Origanum vulgare, Rosmarinus officinalis, Salvia officinalis, Thymus vulgaris, Lavandula angustifolia | Insulinomimetic only when given at high doses (50 µg/mL). Intensified insulin action via phosphatase inhibition when given at lower doses |
| Phosphatase Inhibitors | ||
| Ursolic acid (triterpenoids) | As depicted above | Competitive inhibitors of PTP1B, TCPTP and SHP2 Enhance insulin-induced IR phosphorylation |
| Corosolic acid (triterpenoids) | Lagerstroemia speciose, Symplocos paniculate and Eriobotrya japonica | Allosteric inhibitors. Bind to WPD loop of PTP1B, leading to a non-competitive PTP inhibition |
| Lupane (triterpenoids) | Lophopetalum wallichii, Bombax ceiba, Sorbus commixta | |
| Berberine (alkaloids) | Berberis vulgaris, Hydrastis canadensis, Cortidis rhizome | |
| 6,8-diprenylorobol (flavonoids) | Flemingia philippinensis | |
| 2′-Methoxykurarinone (flavonoids) | Sophora flavescens | |
| Morin (flavonoids) | Moraceae | |
| Safranal (β carotene) | Crocus sativus | PTP1B inhibitor. Targets the cysteine residue of the catalytic site |
| Inducers of GLUT4 Expression | ||
| Isoflavin (isoflavones) | Pterocarpus marsupium | Stimulates the transcription of Scl2A4/Glut4 |
| Quercetin (flavonoids) | ||
| Resveratrol (flavonoids) | Vitis vinifera Vaccinium macrocarpon | |
| Macelignan (lignans) | Myristica fragrans Houtt | PPAR agonist |
| Honokiol (lignans) | Magnolia | Partial PPAR agonist |
| Inhibitors of GLUT4-Mediated Transport | ||
| Genistein (isoflavones) | soybean, lupinus | Competitive inhibitors of GLUT4, inhibit insulin-dependent glucose transport. However, metabolic benefits might be achieved through anti-inflammatory effects. |
| Silybin (flavonoids) | Silybum marianum | |
| Quercetin (flavonoids) | Widely distributed in plants | |
| Myricetin (flavonoids) |
Comptonia peregrina
Morella cerifera Polygonum bellardii | |
| Catechin-gallate (flavonoids) | Camellia sinensis | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenzweig, T.; Sampson, S.R. Activation of Insulin Signaling by Botanical Products. Int. J. Mol. Sci. 2021, 22, 4193. https://doi.org/10.3390/ijms22084193
Rosenzweig T, Sampson SR. Activation of Insulin Signaling by Botanical Products. International Journal of Molecular Sciences. 2021; 22(8):4193. https://doi.org/10.3390/ijms22084193
Chicago/Turabian StyleRosenzweig, Tovit, and Sanford R. Sampson. 2021. "Activation of Insulin Signaling by Botanical Products" International Journal of Molecular Sciences 22, no. 8: 4193. https://doi.org/10.3390/ijms22084193
APA StyleRosenzweig, T., & Sampson, S. R. (2021). Activation of Insulin Signaling by Botanical Products. International Journal of Molecular Sciences, 22(8), 4193. https://doi.org/10.3390/ijms22084193







