Hypertensive Effect of Downregulation of the Opioid System in Mouse Model of Different Activity of the Endogenous Opioid System
Abstract
1. Introduction
2. Results
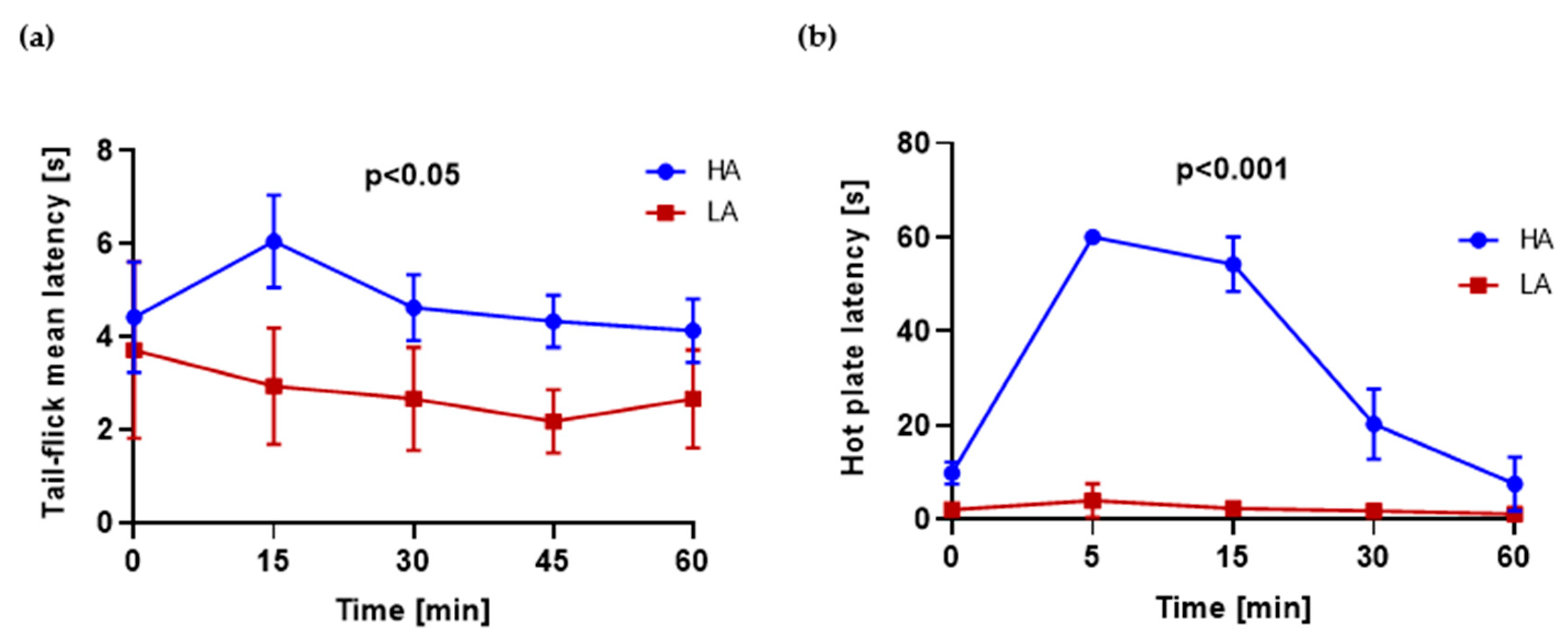
2.1. HA and LA Mice Have Respectively High and Low Analgesia Measured by Tail-Flick Test and Hot Plate Test
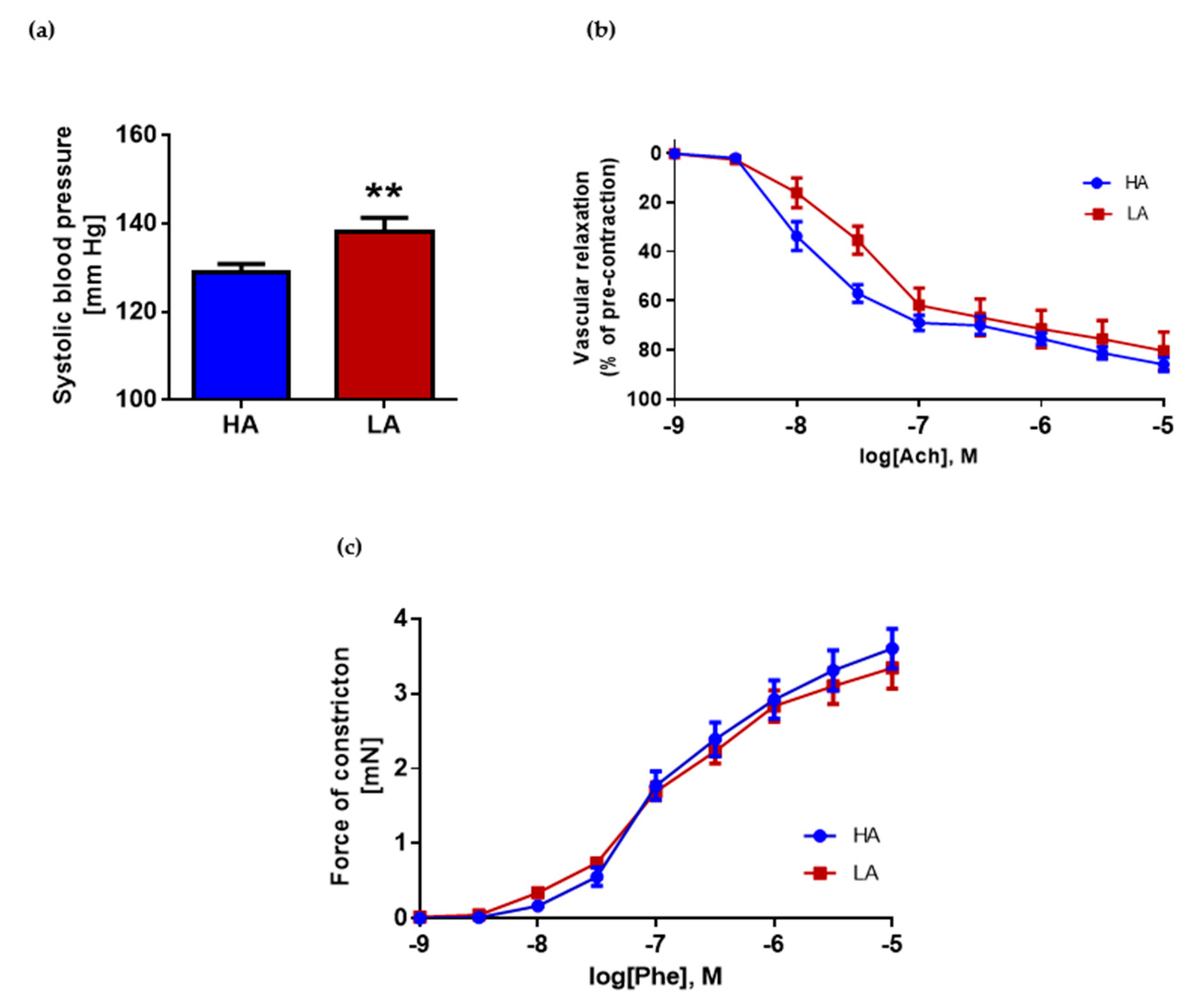
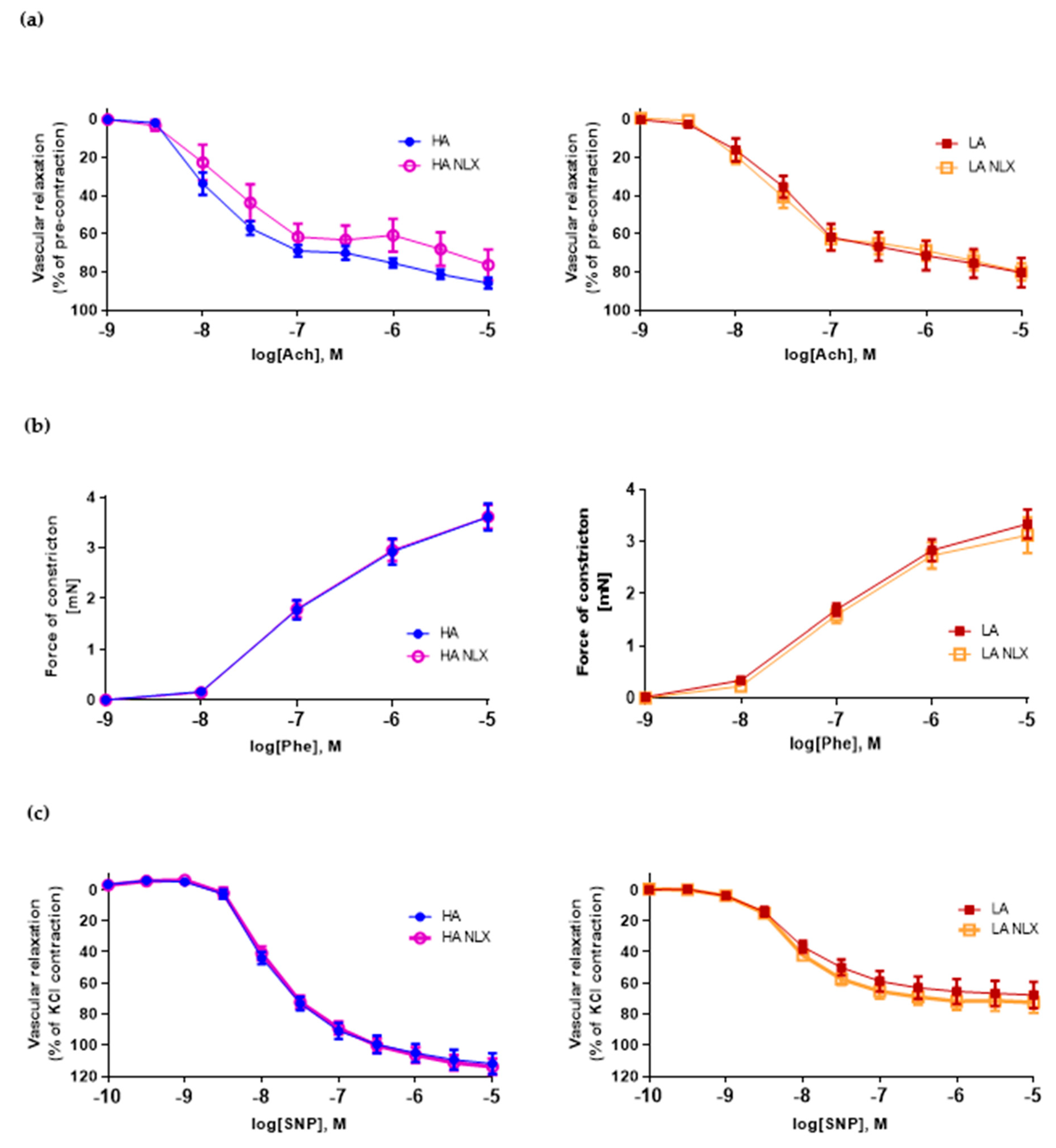
2.2. Low Activity of Opioid System Manifests Elevated Blood Pressure, without Effect on Endothelium Dependent Vascular Relaxation and Constraction in Aorta
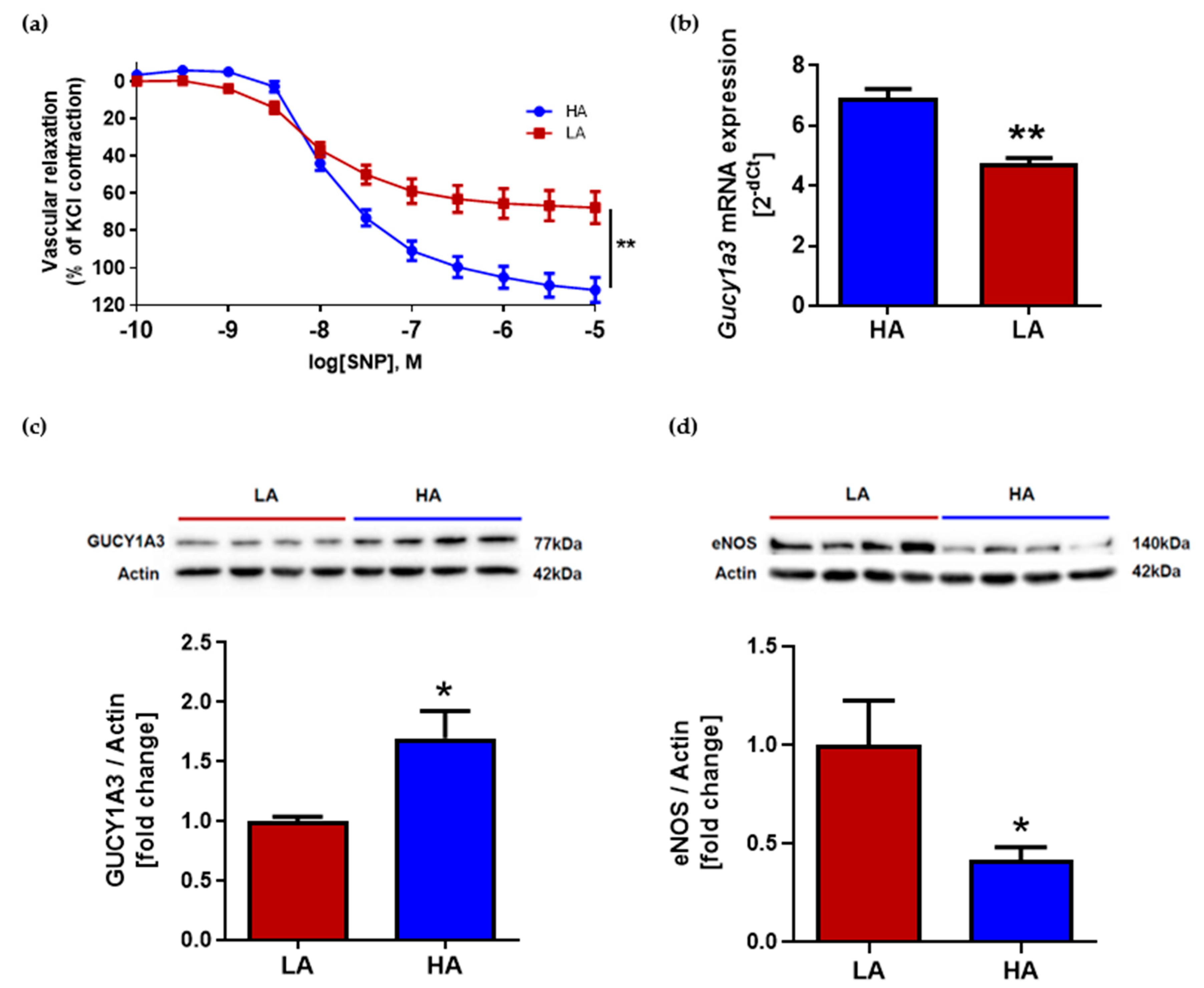
2.3. Low Activity of Opioid System Causes Worse Endothelium-Independent Relaxation Which Correspond with Decreased Level of Guanylyl Cyclase (Gucy1A3) Despite of Elevated eNOS Expression
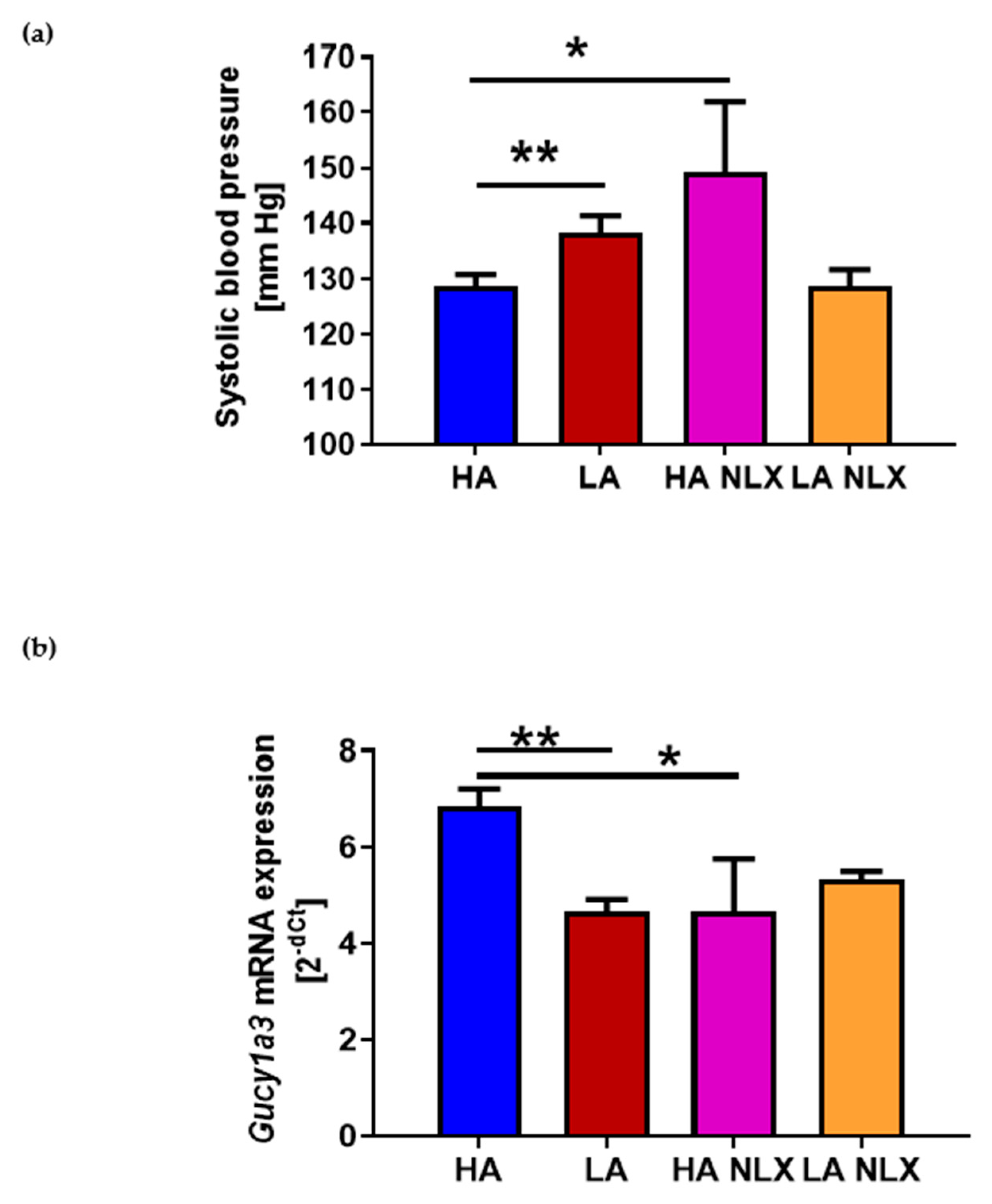
2.4. Naloxone Treatment Elevates Blood Pressure in Mice with High Activity of Opioid System and Decreases Guanylyl Cyclase mRNA Expression in Aorta
2.5. Aortic Segments Treated In Vitro with Naloxone Exert No Changes in Both Endothelium Dependent and Independent Vascular Function in LA and HA Models of Opioid System Activity
3. Discussion
4. Materials and Methods
4.1. Selection Protocol for the HA and LA Lines
4.2. Measurement of Blood Pressure
4.3. Hot-Plate Test
4.4. Tail-Flick Test
4.5. Naloxone Administration
4.6. Determination of Vascular Function
4.7. Measurement of mRNA Expression
4.8. Western Blot
4.9. Data and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, M.L.; Brase, D.A.; Welch, S.P.; Dewey, W.L. The Role of Endogenous Peptides in the Action of Opioid Analgesics. Ann. Emerg. Med. 1986, 15, 1030–1035. [Google Scholar] [CrossRef]
- Jackson, D.E. A Note on the Pharmacological Action of Opium Alkaloids. J. Pharmacol. Exp. Ther. 1914, 6, 57–72. [Google Scholar]
- Feuerstein, G.; Sirén, A.L. The Opioid System in Cardiac and Vascular Regulation of Normal and Hypertensive States. Circulation 1987, 75, I125–I129. [Google Scholar] [PubMed]
- Schultz, J.E.J.; Gross, G.J. Opioids and Cardioprotection. Pharmacol. Ther. 2001, 89, 123–137. [Google Scholar] [CrossRef]
- Najafipour, H.; Beik, A. The Impact of Opium Consumption on Blood Glucose, Serum Lipids and Blood Pressure, and Related Mechanisms. Front. Physiol. 2016, 7, 436. [Google Scholar] [CrossRef] [PubMed]
- Masoomi, M.; Ramezani, M.A.; Karimzadeh, H. The Relationship of Opium Addiction with Coronary Artery Disease. Int. J. Prev. Med. 2010, 1, 182–186. [Google Scholar]
- Lewkowski, M.D.; Young, S.N.; Ghosh, S.; Ditto, B. Effects of Opioid Blockade on the Modulation of Pain and Mood by Sweet Taste and Blood Pressure in Young Adults. Pain 2008, 135, 75–81. [Google Scholar] [CrossRef]
- McCubbin, J.A.; Helfer, S.G.; Switzer, F.S.; Galloway, C.; Griffith, W.V. Opioid Analgesia in Persons at Risk for Hypertension. Psychosom. Med. 2006, 68, 116–120. [Google Scholar] [CrossRef]
- Feuerstein, G.; Molineaux, C.J.; Rosenberger, J.G.; Faden, A.I.; Cox, B.M. Dynorphins and Leu-Enkephalin in Brain Nuclei and Pituitary of WKY and SHR Rats. Peptides 1983, 4, 225–229. [Google Scholar] [CrossRef]
- Li, S.-J.; Wonga, S.C.; Hong, J.-S.; Ingenito, A.J. Age-Related Changes in Opioid Peptide Concentrations in Brain and Pituitary of Spontaneously Hypertensive Rats. Pharmacology 1992, 44, 245–256. [Google Scholar] [CrossRef]
- FARSANG, C.; KUNOS, G. Naloxone Reverses the Antihypertensive Effect of Clonidine. Br. J. Pharmacol. 1979, 67, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Sahin, N.; Ozdem, S.; Sadan, G. The Effect of Nitric Oxide Synthase Blockade on Responses to Morphine in Rat Aortic Rings. Auton. Autacoid Pharmacol. 2002, 22, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.-F.; Liu, Y.-C.; Tseng, F.-L.; Sung, Y.-H.; Huang, C.-C.; Jiang, M.-J.; Tsai, Y.-C. High-dose morphine impairs vascular endothelial function by increased production of superoxide anions. J. Am. Soc. Anesthesiol. 2007, 106, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Bądzyńska, B.; Lipkowski, A.W.; Sadowski, J. An Antihypertensive Opioid: Biphalin, a Synthetic Non-Addictive Enkephalin Analog Decreases Blood Pressure in Spontaneously Hypertensive Rats. Pharmacol. Rep. 2016, 68. [Google Scholar] [CrossRef]
- Bądzyńska, B.; Lipkowski, A.W.; Olszyński, K.H.; Sadowski, J. Different Blood Pressure Responses to Opioids in 3 Rat Hypertension Models: Role of the Baseline Status of Sympathetic and Renin–Angiotensin Systems. Can. J. Physiol. Pharmacol. 2016, 94. [Google Scholar] [CrossRef]
- Poznański, P.; Lesniak, A.; Bujalska-Zadrozny, M.; Strzemecka, J.; Sacharczuk, M. Bidirectional Selection for High and Low Stress-Induced Analgesia Affects G-Protein Activity. Neuropharmacology 2019, 144. [Google Scholar] [CrossRef]
- Panocka, I.; Marek, P.; Sadowski, B. Differentiation of Neurochemical Basis of Stress-Induced Analgesia in Mice by Selective Breeding. Brain Res. 1986, 397, 156–160. [Google Scholar] [CrossRef]
- Kest, B.; Jenab, S.; Brodsky, M.; Sadowski, B.; Belknap, J.K.; Mogil, J.S.; Inturrisi, C.E. Mu and Delta Opioid Receptor Analgesia, Binding Density, and MRNA Levels in Mice Selectively Bred for High and Low Analgesia. Brain Res. 1999, 816, 381–389. [Google Scholar] [CrossRef]
- Gaciong, Z. Resistant: Hypertension, Patient, or Physician? Pol. Arch. Med. Wewn. 2015, 125, 227–231. [Google Scholar] [CrossRef]
- Corbett, K.; Dugan, A.; Vitale, C.; Gravel, T. Long-Term Effects of Opioids on the Cardiovascular System. Nursing 2019, 49, 47–49. [Google Scholar] [CrossRef]
- Chen, A.; Ashburn, M.A. Cardiac Effects of Opioid Therapy. Pain Med. 2015, 16, S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, M.; Joukar, S.; Beik, A. Opioids and Cardiac Arrhythmia: A Literature Review. Med. Princ. Pract. 2018, 27, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, P.; Lesniak, A.; Korostynski, M.; Szklarczyk, K.; Lazarczyk, M.; Religa, P.; Bujalska-Zadrozny, M.; Sadowski, B.; Sacharczuk, M. Delta-opioid receptor antagonism leads to excessive ethanol consumption in mice with enhanced activity of the endogenous opioid system. Neuropharmacology 2017, 118, 90–101. [Google Scholar] [CrossRef]
- Verdecchia, P.; Angeli, F. Natural History of Hypertension Subtypes. Circulation 2005, 111, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-C.; Shieh, J.-P.; Chung, H.-H.; Hung, C.-H.; Lin, H.J.; Cheng, J.-T. Activation of Peripheral Opioid Μ-Receptors in Blood Vessel May Lower Blood Pressure in Spontaneously Hypertensive Rats. Pharmacology 2011, 87, 257–264. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, J.A.; Surwit, R.S.; Williams, R.B. Endogenous Opiate Peptides, Stress Reactivity, and Risk for Hypertension. Hypertension 1985, 7, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Ginty, A.T.; Carroll, D.; Roseboom, T.J.; Phillips, A.C.; de Rooij, S.R. Depression and Anxiety Are Associated with a Diagnosis of Hypertension 5 Years Later in a Cohort of Late Middle-Aged Men and Women. J. Hum. Hypertens. 2013, 27, 187–190. [Google Scholar] [CrossRef]
- Aronow, W.S. Hypertension and Cognitive Impairment. Ann. Transl. Med. 2017, 5, 259. [Google Scholar] [CrossRef]
- Lesniak, A.; Leszczynski, P.; Bujalska-Zadrozny, M.; Pick, C.G.; Sacharczuk, M. Naloxone Exacerbates Memory Impairments and Depressive-like Behavior after Mild Traumatic Brain Injury (MTBI) in Mice with Upregulated Opioid System Activity. Behav. Brain Res. 2017, 326, 209–216. [Google Scholar] [CrossRef]
- Boeuf, B.; Poirier, V.; Gauvin, F.; Guerguerian, A.-M.; Roy, C.; Farrell, C.; Lacroix, J. Naloxone for Shock. Cochrane Database Syst. Rev. 2003. [Google Scholar] [CrossRef]
- Levin, E.R.; Sharp, B.; Drayer, J.I.; Weber, M.A. Severe Hypertension Induced by Naloxone. Am. J. Med. Sci. 1985, 290, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Kario, K.; Kanegae, H.; Oikawa, T.; Suzuki, K. Hypertension Is Predicted by Both Large and Small Artery Disease. Hypertension 2019, 73, 75–83. [Google Scholar] [CrossRef]
- Pei, J.-M.; Chen, M.; Wang, Y.-M.; Wen, J.; Zhu, Y.-L. Kappa-Opioid Receptor Stimulation Contributes to Aortic Artery Dilation through Activation of K(ATP) Channel in the Rats. Acta Physiol. Sin. 2003, 55, 91–95. [Google Scholar]
- Stefano, G.B.; Salzet, M.; Hughes, T.K.; Bilfinger, T. v Delta2 Opioid Receptor Subtype on Human Vascular Endothelium Uncouples Morphine Stimulated Nitric Oxide Release. Int. J. Cardiol. 1998, 64, 43–51. [Google Scholar] [CrossRef]
- Noris, M.; Morigi, M.; Donadelli, R.; Aiello, S.; Foppolo, M.; Todeschini, M.; Orisio, S.; Remuzzi, G.; Remuzzi, A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ. Res. 1995, 76, 536–543. [Google Scholar] [CrossRef]
- Davis, M.E.; Cai, H.; Drummond, G.R.; Harrison, D.G. Shear stress regulates endothelial nitric oxide synthase expression through c-Src by divergent signaling pathways. Circ. Res. 2001, 89, 1073–1080. [Google Scholar] [CrossRef]
- Hiyoshi, H.; Yayama, K.; Takano, M.; Okamoto, H. Angiotensin Type 2 Receptor-Mediated Phosphorylation of eNOS in the Aortas of Mice With 2-Kidney, 1-Clip Hypertension. Hypertension 2005, 45, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, T.P.; Nosalski, R.; Skiba, D.S.; Koziol, J.; Mazur, M.; Justo-Junior, A.S.; Kowalczyk, P.; Kusmierczyk, Z.; Schramm-Luc, A.; Luc, K.; et al. 1,2,3,4,6-Penta-O-Galloyl-β-d-Glucose Modulates Perivascular Inflammation and Prevents Vascular Dysfunction in Angiotensin II-Induced Hypertension. Br. J. Pharmacol. 2019, 176, 1951–1965. [Google Scholar] [CrossRef]
- Riedel, W.; Neeck, G. Nociception, Pain, and Antinociception: Current Concepts. Zeitschrift für Rheumatol. 2001, 60, 404–415. [Google Scholar] [CrossRef]
- Hannig, G.; Tchernychev, B.; Kurtz, C.B.; Bryant, A.P.; Currie, M.G.; Silos-Santiago, I. Guanylate Cyclase-C/CGMP: An Emerging Pathway in the Regulation of Visceral Pain. Front. Mol. Neurosci. 2014, 7, 31. [Google Scholar] [CrossRef]
- Amarante, L.H.; Duarte, I.D.G. The κ-Opioid Agonist (±)-Bremazocine Elicits Peripheral Antinociception by Activation of the l-Arginine/Nitric Oxide/Cyclic GMP Pathway. Eur. J. Pharmacol. 2002, 454, 19–23. [Google Scholar] [CrossRef]
- Granados-Soto, V.; Rufino, M.O.; Gomes Lopes, L.D.; Ferreira, S.H. Evidence for the Involvement of the Nitric Oxide-CGMP Pathway in the Antinociception of Morphine in the Formalin Test. Eur. J. Pharmacol. 1997, 340, 177–180. [Google Scholar] [CrossRef]
- Pacheco, D.F.; Reis, G.M.L.; Francischi, J.N.; Castro, M.S.A.; Perez, A.C.; Duarte, I.D.G. δ-Opioid Receptor Agonist SNC80 Elicits Peripheral Antinociception via Δ1 and Δ2 Receptors and Activation of the L-Arginine/Nitric Oxide/Cyclic GMP Pathway. Life Sci. 2005, 78, 54–60. [Google Scholar] [CrossRef]
- Mixcoatl-Zecuatl, T.; Aguirre-Bañuelos, P.; Granados-Soto, V. Sildenafil Produces Antinociception and Increases Morphine Antinociception in the Formalin Test. Eur. J. Pharmacol. 2000, 400, 81–87. [Google Scholar] [CrossRef]
- Shijun, H.; Liping, Z.; Yongqiang, Q.; Zhen, L.; Yonghe, Z.; Lihua, L. Morphine-Induced Changes of Adenylate and Guanylate Cyclase in Locus Ceruleus, Periaqueductal Gray, and Substantia Nigra in Rats. Am. J. Drug Alcohol Abus. 2009, 35, 133–137. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skiba, D.S.; Szczepaniak, P.; Siedliński, M.; Poznański, P.; Łazarczyk, M.; Jaskuła, K.; Religa, P.; Sacharczuk, M.; Gaciong, Z. Hypertensive Effect of Downregulation of the Opioid System in Mouse Model of Different Activity of the Endogenous Opioid System. Int. J. Mol. Sci. 2021, 22, 4179. https://doi.org/10.3390/ijms22084179
Skiba DS, Szczepaniak P, Siedliński M, Poznański P, Łazarczyk M, Jaskuła K, Religa P, Sacharczuk M, Gaciong Z. Hypertensive Effect of Downregulation of the Opioid System in Mouse Model of Different Activity of the Endogenous Opioid System. International Journal of Molecular Sciences. 2021; 22(8):4179. https://doi.org/10.3390/ijms22084179
Chicago/Turabian StyleSkiba, Dominik S., Piotr Szczepaniak, Mateusz Siedliński, Piotr Poznański, Marzena Łazarczyk, Kinga Jaskuła, Piotr Religa, Mariusz Sacharczuk, and Zbigniew Gaciong. 2021. "Hypertensive Effect of Downregulation of the Opioid System in Mouse Model of Different Activity of the Endogenous Opioid System" International Journal of Molecular Sciences 22, no. 8: 4179. https://doi.org/10.3390/ijms22084179
APA StyleSkiba, D. S., Szczepaniak, P., Siedliński, M., Poznański, P., Łazarczyk, M., Jaskuła, K., Religa, P., Sacharczuk, M., & Gaciong, Z. (2021). Hypertensive Effect of Downregulation of the Opioid System in Mouse Model of Different Activity of the Endogenous Opioid System. International Journal of Molecular Sciences, 22(8), 4179. https://doi.org/10.3390/ijms22084179





