Myocardial Accumulations of Reg3A, Reg3γ and Oncostatin M Are Associated with the Formation of Granulomata in Patients with Cardiac Sarcoidosis
Abstract
1. Introduction
2. Results
2.1. Patients and General Experimental Design
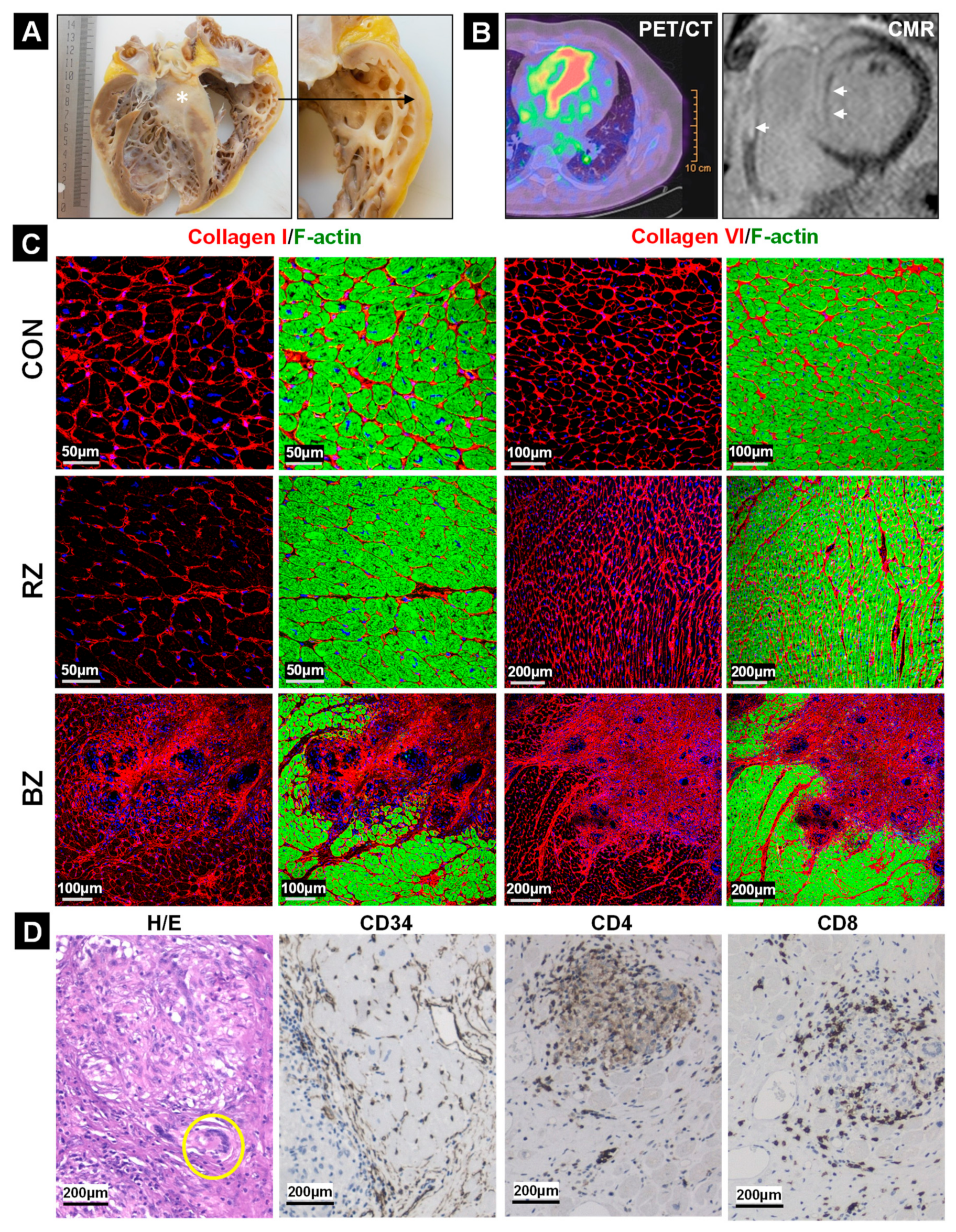
2.2. The Development of Granulomata (GL) Is Associated with Loss of Cardiomyocyte Mass and Scar Formation
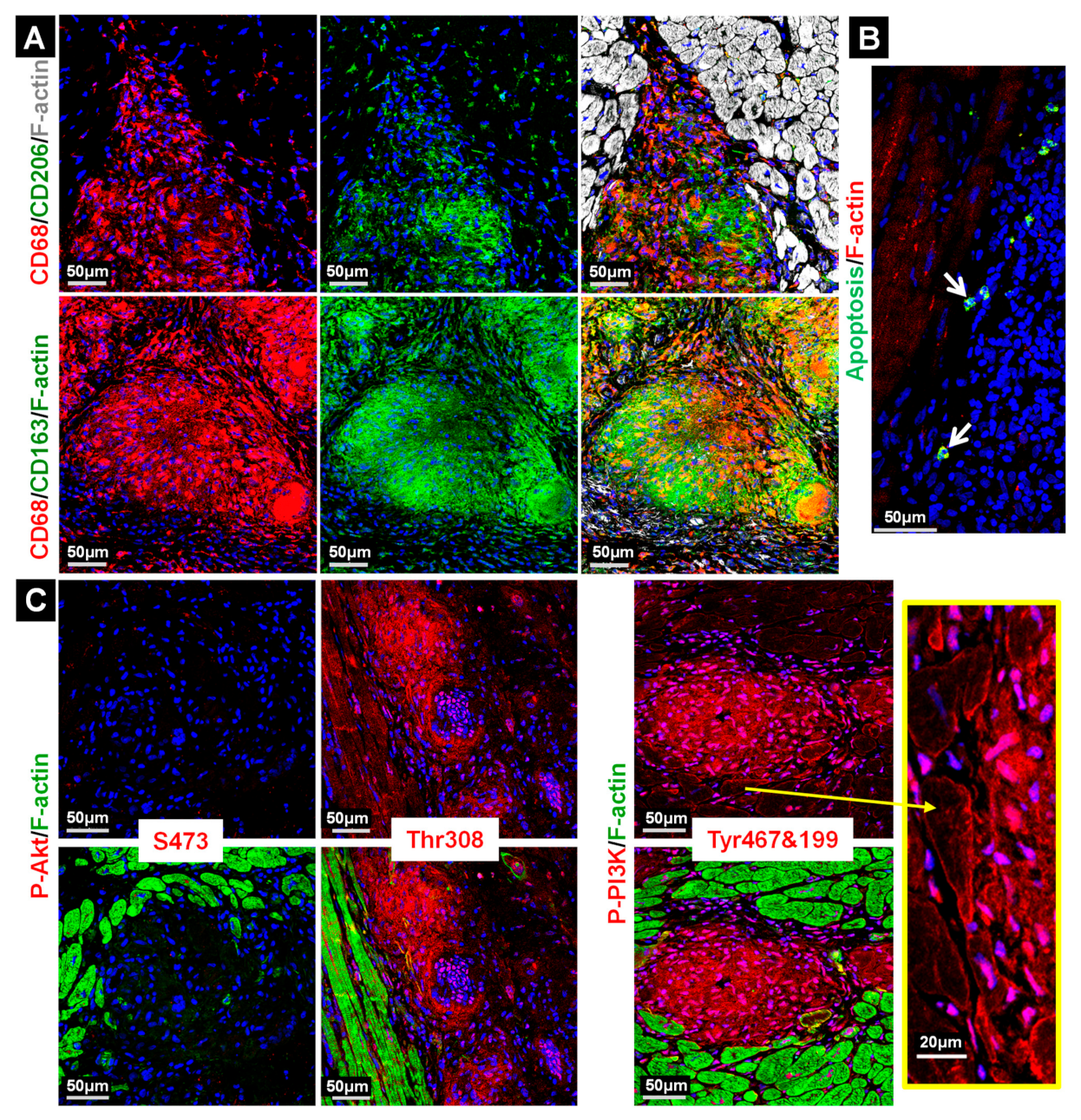
2.3. Macrophages in the Granuloma Show Mild Apoptosis and Are Highly Activated
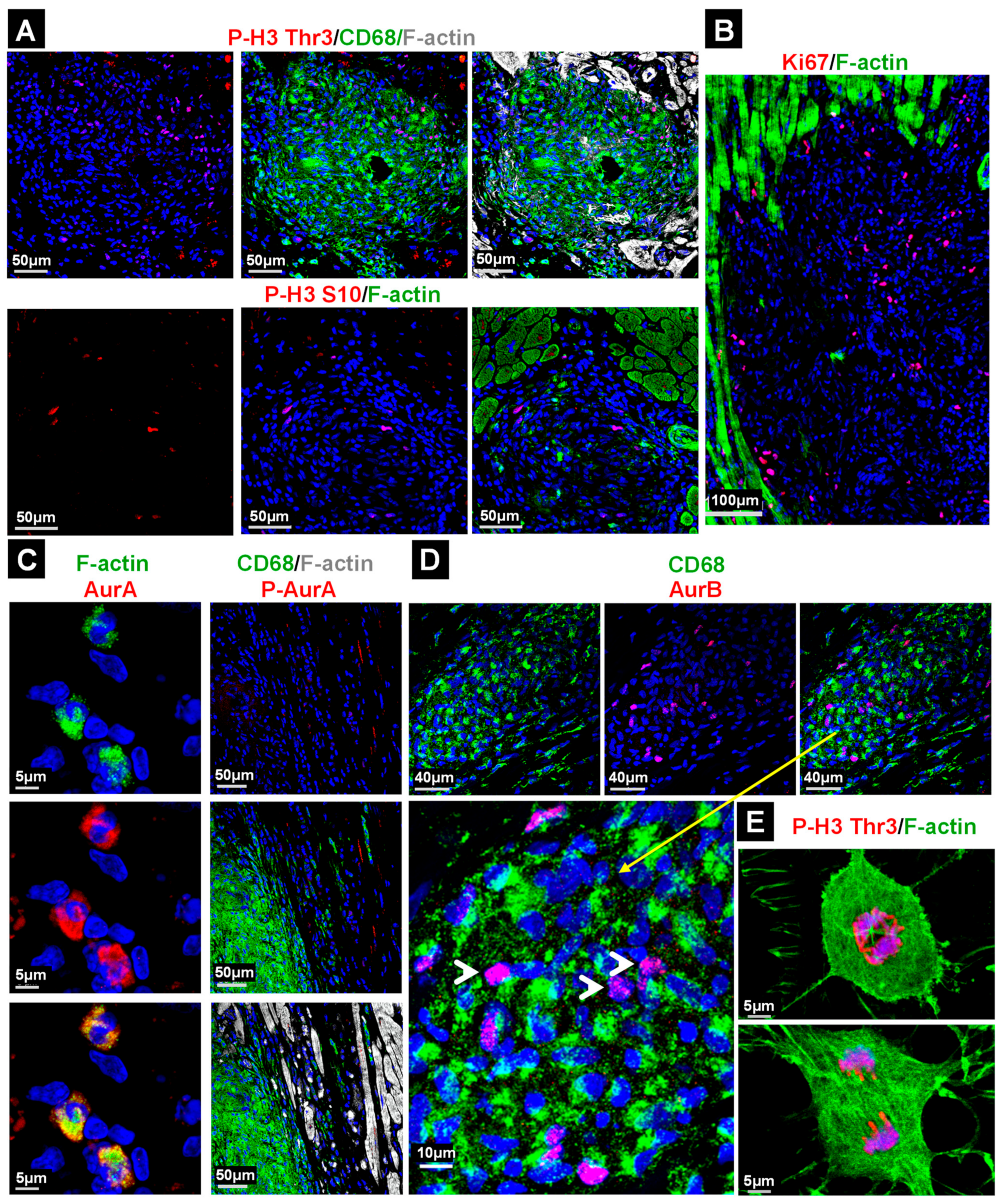
2.4. The Majority of Cell Cycle Marker Positive Cells in the Granuloma Are Non-Macrophages
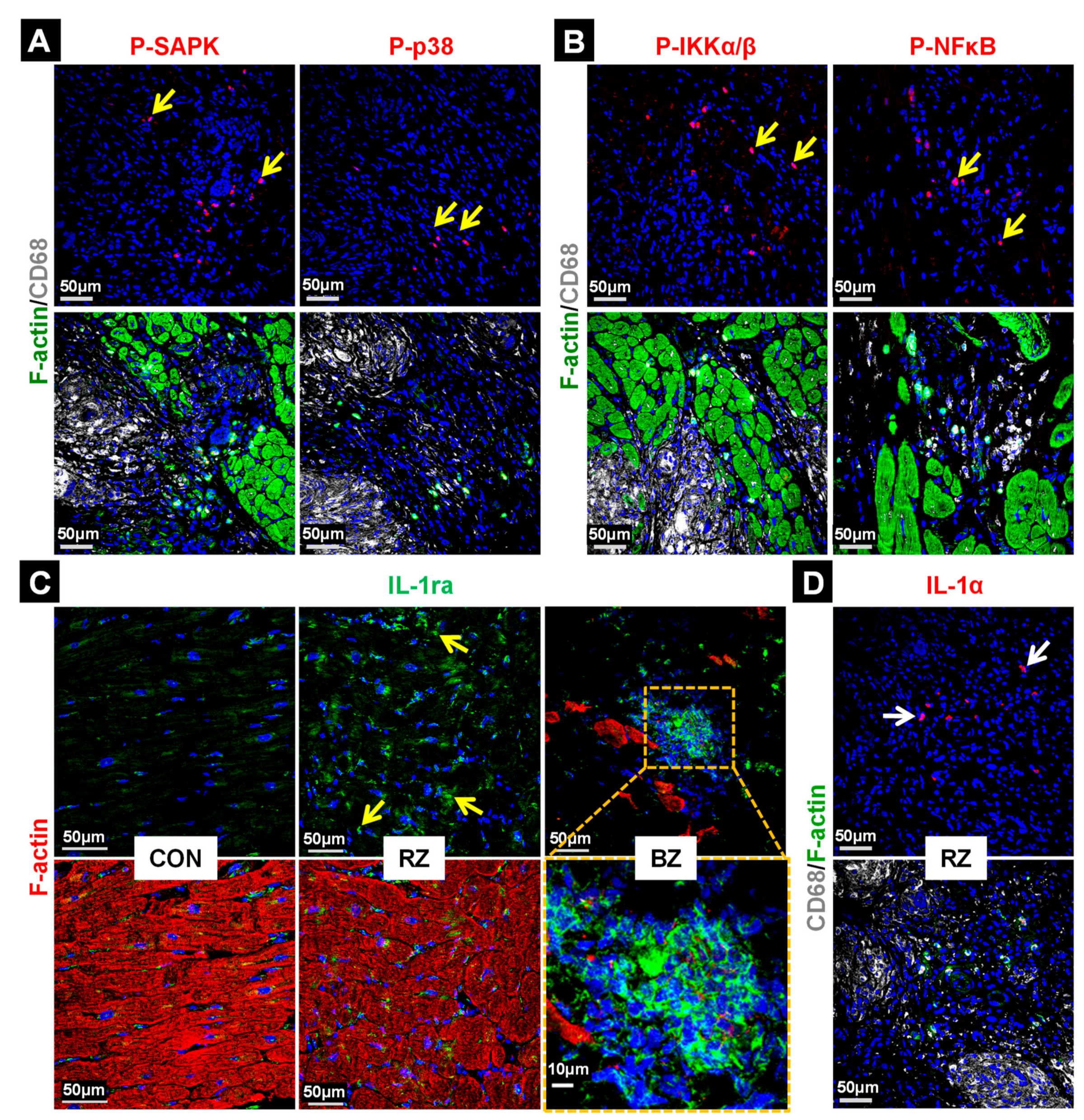
2.5. Stress and Inflammatory Pathways Are Mildly Activated in the Granuloma and the Anti-Inflammatory Status Appears to Be Predominant
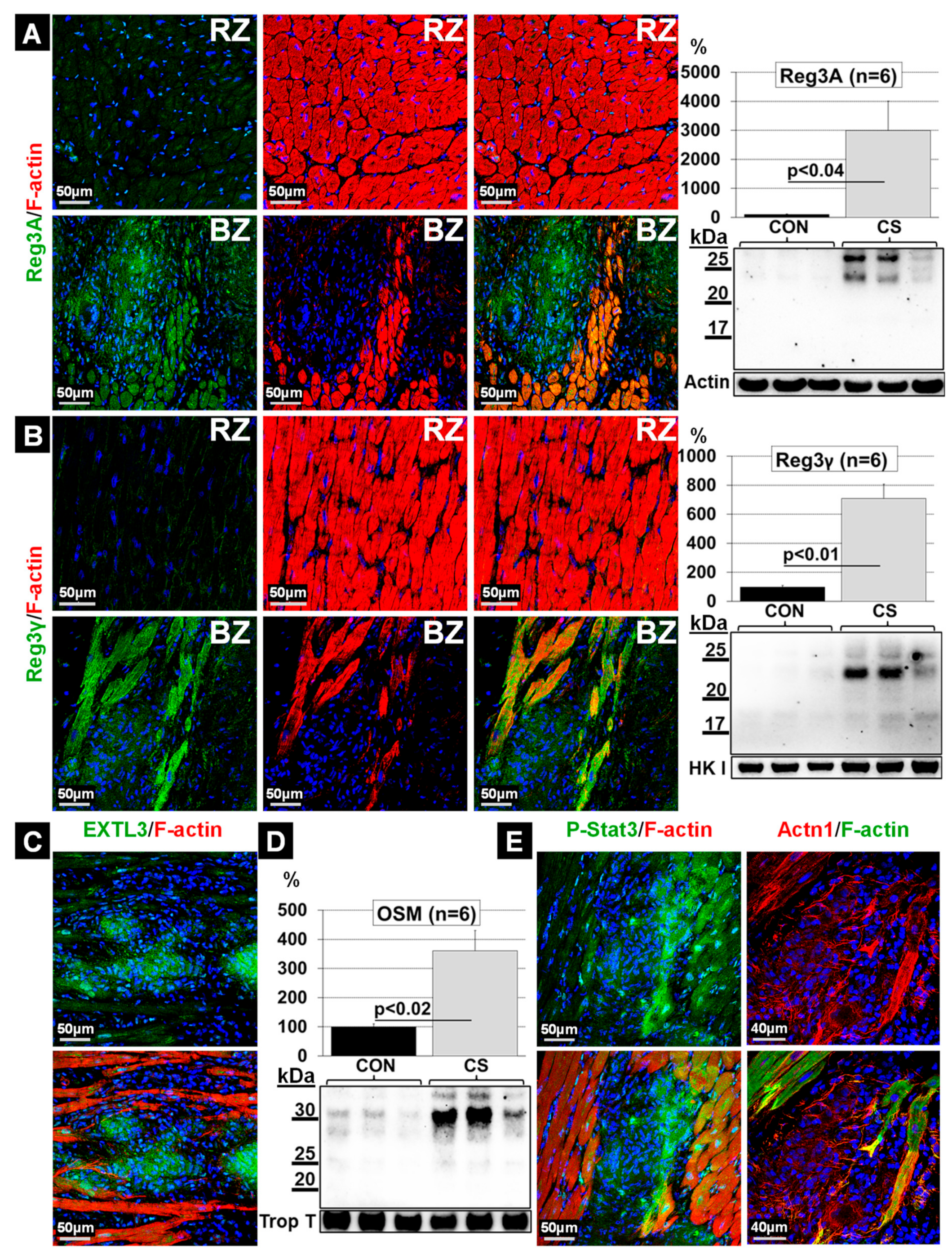
2.6. Elevations of Oncostatin M Correlate with Dedifferentiated Cardiomyocytes and Increased Amounts of Reg3A and Reg3γ in the Border Zone
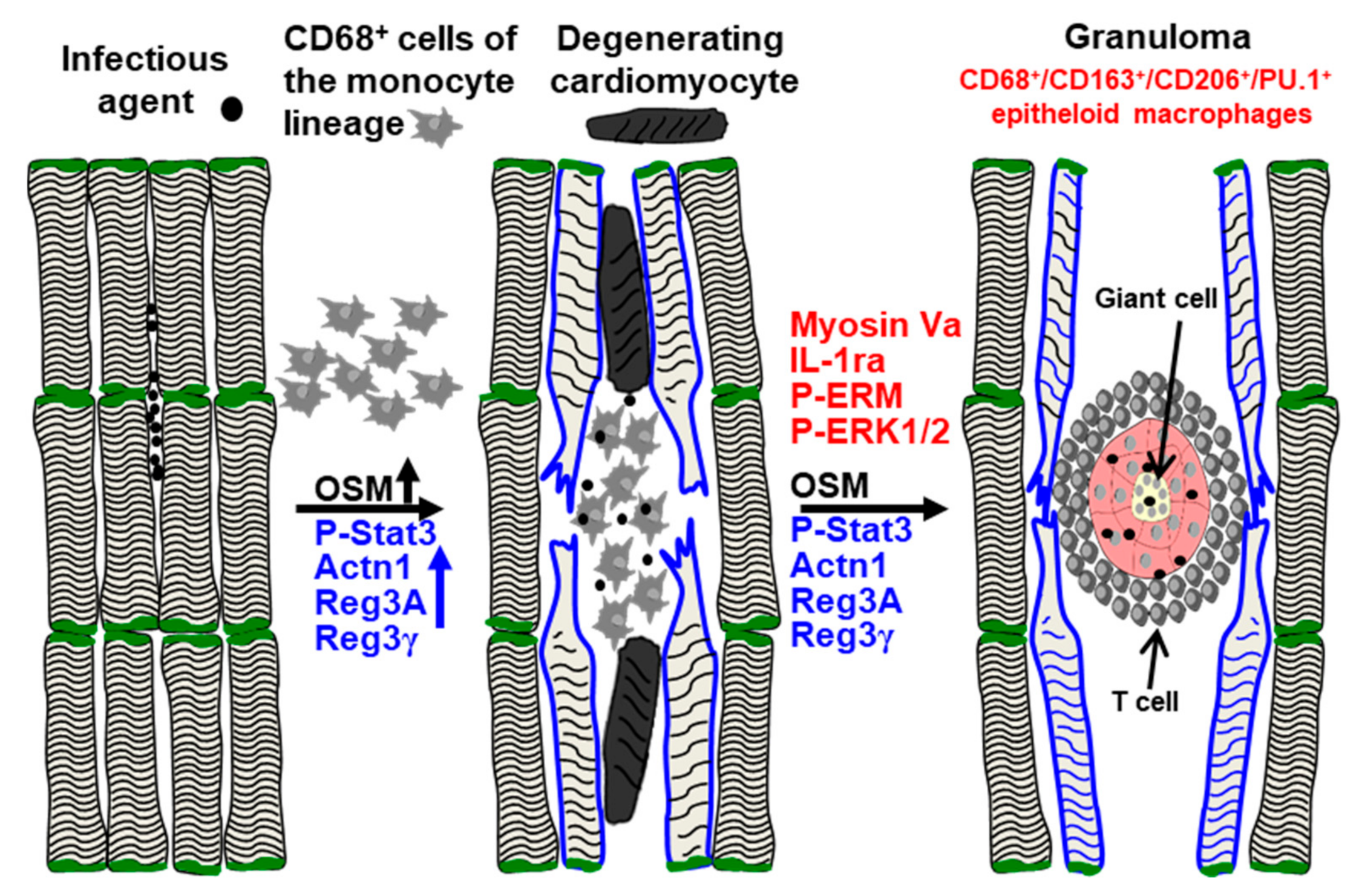
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Fluorescence Microscopy
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birnie, D.H.; Nery, P.B.; Ha, A.C.; Beanlands, R.S.B. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2016, 68, 411–421. [Google Scholar] [CrossRef]
- Rybicki, B.A.; Iannuzzi, M.C. Epidemiology of Sarcoidosis: Recent Advances and Future Prospects. Semin. Respir. Crit. Care Med. 2007, 28, 022–035. [Google Scholar] [CrossRef]
- Birnie, D.H.; Kandolin, R.; Nery, P.B.; Kupari, M. Cardiac manifestations of sarcoidosis: Diagnosis and management. Eur. Heart J. 2017, 38, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Silverman, K.J.; Hutchins, G.M.; Bulkley, B.H. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978, 58, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Lubitz, S.A.; Frankel, Z.; Wisnivesky, J.P.; Einstein, A.J.; Goldman, M.; Machac, J.; Teirstein, A. Cardiac Involvement in Patients with Sarcoidosis: Diagnostic and Prognostic Value of Outpatient Testing. Chest 2008, 133, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Lagana, S.M.; Parwani, A.V.; Nichols, L.C. Cardiac Sarcoidosis: A Pathology-Focused Review. Arch. Pathol. Lab. Med. 2010, 134, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.; Thakral, D.; Emeagwali, N.; Galan, A.; King, B. Tofacitinib Treatment and Molecular Analysis of Cutaneous Sarcoidosis. N. Engl. J. Med. 2018, 379, 2540–2546. [Google Scholar] [CrossRef]
- Ayoub, C.; Pena, E.; Ohira, H.; Dick, A.; Leung, E.; Nery, P.B.; Birnie, D.; Beanlands, R.S.B. Advanced Imaging of Cardiac Sarcoidosis. Curr. Cardiol. Rep. 2015, 17, 17. [Google Scholar] [CrossRef]
- Richter, M.; Lautze, H.J.; Walther, T.; Braun, T.; Kostin, S.; Kubin, T. The failing heart is a major source of circulating FGF23 via oncostatin M receptor activation. J. Heart Lung Transplant. 2015, 34, 1211–1214. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Berge, B.; Sen-Hild, B.; Troidl, K.; Gajawada, P.; Kubin, N.; Valeske, K.; Schranz, D.; Akinturk, H.; Schonburg, M.; et al. Radixin Relocalization and Nonmuscle alpha-Actinin Expression Are Features of Remodeling Cardiomyocytes in Adult Patients with Dilated Cardiomyopathy. Dis. Mark. 2020, 2020, 9356738. [Google Scholar]
- Clucas, J.; Valderrama, F. ERM proteins in cancer progression. J. Cell Sci. 2014, 127, 267–275. [Google Scholar] [CrossRef]
- Desnos, C.; Huet, S.; Darchen, F. ‘Should I stay or should I go?’: Myosin V function in organelle trafficking. Biol. Cell 2007, 99, 411–423. [Google Scholar] [CrossRef]
- Hume, D.A. The Many Alternative Faces of Macrophage Activation. Front. Immunol. 2015, 6, 370. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Sultan, S.; Taylor, S.S.; Higgins, J.M. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 2005, 19, 472–488. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [PubMed]
- Lorchner, H.; Poling, J.; Gajawada, P.; Hou, Y.; Polyakova, V.; Kostin, S.; Adrian-Segarra, J.M.; Boettger, T.; Wietelmann, A.; Warnecke, H.; et al. Myocardial healing requires Reg3beta-dependent accumulation of macrophages in the ischemic heart. Nat. Med. 2015, 21, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Busse-Wicher, M.; Wicher, K.B.; Kusche-Gullberg, M. The exostosin family: Proteins with many functions. Matrix Biol. 2014, 35, 25–33. [Google Scholar] [CrossRef]
- Cao, Y.; Tian, Y.; Liu, Y.; Su, Z. Reg3β: A Potential Therapeutic Target for Tissue Injury and Inflammation-Associated Disorders. Int. Rev. Immunol. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Doughan, A.R.; Williams, B.R. Cardiac sarcoidosis. Heart 2006, 92, 282–288. [Google Scholar] [CrossRef]
- Chiu, C.-Z.; Nakatani, S.; Zhang, G.; Tachibana, T.; Ohmori, F.; Yamagishi, M.; Kitakaze, M.; Tomoike, H.; Miyatake, K. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am. J. Cardiol. 2005, 95, 143–146. [Google Scholar] [CrossRef]
- Nagai, T.; Nagano, N.; Sugano, Y.; Asaumi, Y.; Aiba, T.; Kanzaki, H.; Kusano, K.; Noguchi, T.; Yasuda, S.; Ogawa, H.; et al. Effect of Corticosteroid Therapy on Long-Term Clinical Outcome and Left Ventricular Function in Patients With Cardiac Sarcoidosis. Circ. J. 2015, 79, 1593–1600. [Google Scholar] [CrossRef]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) Pathway. Sci. Signal. 2010, 3, cm1. [Google Scholar] [CrossRef]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef]
- Chen, Z.; Downing, S.; Tzanakakis, E.S. Four Decades After the Discovery of Regenerating Islet-Derived (Reg) Proteins: Current Understanding and Challenges. Front. Cell Dev. Biol. 2019, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Kiji, T.; Dohi, Y.; Takasawa, S.; Okamoto, H.; Nonomura, A.; Taniguchi, S. Activation of regenerating gene Reg in rat and human hearts in response to acute stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H277–H284. [Google Scholar] [CrossRef] [PubMed]
- Säkkinen, H.; Aro, J.; Kaikkonen, L.; Ohukainen, P.; Näpänkangas, J.; Tokola, H.; Ruskoaho, H.; Rysä, J. Mitogen-activated protein kinase p38 target regenerating islet-derived 3γ expression is upregulated in cardiac inflammatory response in the rat heart. Physiol. Rep. 2016, 4, e12996. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Feng, X.; Li, Z.; Hou, R.; Han, C.; Zhang, Y. Gene expression profile of cardiomyocytes in hypertrophic heart induced by continuous norepinephrine infusion in the rats. Cell. Mol. Life Sci. 2003, 60, 2200–2209. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, H.; Wang, H.; Lu, H.; Chen, R.; Xu, H.; Su, Z.; Shao, X. Reg3β from cardiomyocytes regulated macrophage migration, proliferation and functional skewing in experimental autoimmune myocarditis. Am. J. Clin. Exp. Immunol. 2018, 7, 8–15. [Google Scholar] [PubMed]
- Kubin, T.; Cetinkaya, A.; Kubin, N.; Bramlage, P.; Sen-Hild, B.; Gajawada, P.; Akintürk, H.; Schönburg, M.; Schaper, W.; Choi, Y.-H.; et al. The MEK/ERK Module Is Reprogrammed in Remodeling Adult Cardiomyocytes. Int. J. Mol. Sci. 2020, 21, 6348. [Google Scholar] [CrossRef] [PubMed]
- Kubin, T.; Poling, J.; Kostin, S.; Gajawada, P.; Hein, S.; Rees, W.; Wietelmann, A.; Tanaka, M.; Lorchner, H.; Schimanski, S.; et al. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 2011, 9, 420–432. [Google Scholar] [CrossRef]
- Poling, J.; Gajawada, P.; Richter, M.; Lorchner, H.; Polyakova, V.; Kostin, S.; Shin, J.; Boettger, T.; Walther, T.; Rees, W.; et al. Therapeutic targeting of the oncostatin M receptor-beta prevents inflammatory heart failure. Basic Res. Cardiol. 2014, 109, 396. [Google Scholar] [CrossRef]
- Hermanns, H.M. Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015, 26, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Kolattukudy, P.E.; Quach, T.; Bergese, S.; Breckenridge, S.; Hensley, J.; Altschuld, R.; Gordillo, G.; Klenotic, S.; Orosz, C.; Parker-Thornburg, J. Myocarditis induced by targeted expression of the MCP-1 gene in murine cardiac muscle. Am. J. Pathol. 1998, 152, 101–111. [Google Scholar] [PubMed]
- Hou, Y.; Adrian-Segarra, J.M.; Richter, M.; Kubin, N.; Shin, J.; Werner, I.; Walther, T.; Schonburg, M.; Poling, J.; Warnecke, H.; et al. Animal Models and “Omics” Technologies for Identification of Novel Biomarkers and Drug Targets to Prevent Heart Failure. BioMed Res. Int. 2015, 2015, 212910. [Google Scholar] [CrossRef]
- Kubin, N.; Richter, M.; Sen-Hild, B.; Akinturk, H.; Schonburg, M.; Kubin, T.; Cetinkaya, A. Macrophages represent the major pool of IL-7Ralpha expressing cells in patients with myocarditis. Cytokine 2020, 130, 155053. [Google Scholar] [CrossRef] [PubMed]

| Demographic and Risk Factors | Mean ± s.e.m. | Sildenafil 20 mg (%) | 17 |
| Number of patients | 6 | ASS (%) | 0 |
| DCM (%) | 100 | Amiodaron (%) | 67 |
| CHD (%) | 0 | L Thyroxin (%) | 17 |
| Etiology of DCM | 2x pulmonary involvement | Statins (%) | 0 |
| 2x acute myocarditis | Laboratory parameters | ||
| Age (years) | 48.2 ± 4.3 | Sodium (mmol/L) | 138 ± 1 |
| Gender male (%) | 17 | Potassium (mmol/L) | 4.1 ± 0.2 |
| NYHA class | 3.5 ± 0.5 | Creatinine (mg/dL) | 1.06 ± 0.2 |
| Body height (cm) | 169 ± 2.9 | Hemoglobin (g/dL) | 12.2 ± 1 |
| Weight (Kg) | 60.3 ± 4.2 | Hematocrit (%) | 36.2 ± 4.1 |
| BMI (kg/m2) | 21 ± 1 | CRP (mg/L) | 0.8 ± 0.4 |
| Prior myocardial infarction (%) | 0 | Leukocytes Ts/µL | 6.5 ± 0.5 |
| Prior PCI or CABG (%) | 0 | Total cholesterol (mg/dL) | 196 ± 26 |
| Prior smoking (%) | 0 | LDH U/L | 265 ± 41 |
| Prior hypertension (%) | 17 | SGOT (IU/L) | 37.2 ± 13 |
| PRIOR COPD (%) | 17 | SGPT (IU/L) | 36.4 ± 16.6 |
| Prior diabetes mellitus (%) | 0 | CK U/L | 47.7 ± 6.6 |
| Thrombocytopenia (%) | 0 | NT-pro BNP pg/mL | 5580 ± 4021 |
| Prior hypercholesterolemia (%) | 0 | Quick (Thromboplastin time) (%) | 74.2 ± 8.3 |
| Pre CABG (%) | 0 | PTT (s) | 29.5 ± 1.9 |
| pre HTX LVAD (%) | 17 | INR | 1.2 ± 0.15 |
| pre HTX AK-OP (%) | 17 | Hepatitis B & C; HIV | negative |
| pre HTX MK-OP (%) | 0 | Echocardiographic characteristics | |
| pre HTX biv ICD (%) | 67 | LVEF (%) | 23.8 ± 4.1 |
| Cardiac arrhythmia (%) | 50 | LVESD (mm) | 56.6 ± 6 |
| Comedication | LVEDD (mm) | 68 ± 4.8 | |
| Beta blocker (%) | 50 | Hemodynamic parameters | |
| ACE inhibitor and/or AT1 antagonist (%) | 83 | Cardiac index (L/min*m) | 23.8 ± 4.1 |
| Diuretic (%) | 100 | Pulmonary vascular resistance (dynes*s/cm5) | 206 ± 53 |
| Digitalis (%) | 17 | Pulmonary capillary wedge pressure (mmHg) | 26.4 ± 2.2 |
| Aldosterone antagonist (%) | 100 | O2 saturation (%) | 63.6 ± 3 |
| Cat ID | Target | Phospho-Sites | Description/Function | Antibody | Company |
|---|---|---|---|---|---|
| C2456 | Collagen I | - | Extracellular matrix product | Mouse | Sigma |
| 600-401-108 | Collagen VI | - | Extracellular matrix product | Rabbit | Rockland |
| F0766 | CD4 (Clone MT310) | - | T lymphocyte marker | Mouse | Dako |
| MA5-12259 | CD4 (Clone4B12) | - | T lymphocyte marker | Mouse | Thermo-Scientific |
| IS623 | CD8 (Clone C8/144B) | - | T lymphocyte marker | Mouse | Dako |
| 108M-94 | CD8 (Clone C8/144B) | - | T lymphocyte marker | Mouse | Cell Marque |
| 134M-14 | CD34 (Clone QBEnd/10) | - | Endothel/hematogenic progenitor cells | Mouse | Cell Marque |
| M0718 | CD68 (Clone EBM11) | - | Macrophage marker | Mouse | Dako |
| ABIN741570 | CD163 | - | Macrophage marker | Rabbit | Bioss |
| 123003 | CD206 (Clone MR5D3) | - | Macrophage marker | Rat | BIOZOL |
| 3149 | P-Ezrin/Radixin/Moesin | see below | Cytoskeletal/membrane linker | Rabbit | Cell Signaling |
| 3145 | Ezrin | Thr567 | Cytoskeletal/membrane linker | Rabbit | Cell Signaling |
| 2636 | Radixin | Thr564 | Cytoskeletal/membrane linker | Rabbit | Cell Signaling |
| 3150 | Moesin | Thr558 | Cytoskeletal/membrane linker | Rabbit | Cell Signaling |
| ab76543 | PU.1/Spi1 | - | Transcription factor | Rabbit | Abcam |
| ab207254 | Runx 1,2,3 | - | Transcription factor | Rabbit | Abcam |
| 3402 | Myosin Va | - | Molecular motor protein | Rabbit | Cell Signaling |
| 9145 | P-Stat3 | Tyr705 | Signaling cascade | Rabbit | Cell Signaling |
| 4370 | P-p44/42 MAPK | Thr202/Tyr204 | Signaling cascade | Rabbit | Cell Signaling |
| 4511 | p-p38 MAPK | Thr180/Tyr182 | Stress signaling cascade | Rabbit | Cell Signaling |
| 4668 | P-SAPK/JNK MAPK | Thr183/Tyr185 | Stress signaling cascade | Rabbit | Cell Signaling |
| 2697 | P-IKKα/β | Ser176/180 | Inflammatory signaling cascade | Rabbit | Cell Signaling |
| 3033 | P-NF-κB p65 | Ser536 | Inflammatory signaling cascade | Rabbit | Cell Signaling |
| ab207254 | P-PI3 Kinase (p85α & γ) | Tyr467&199 | Survival signaling cascade | Rabbit | Abcam |
| 4060 | P-Akt | Ser473 | Survival signaling cascade | Rabbit | Cell Signaling |
| 13038 | P-Akt | Thr308 | Survival signaling cascade | Rabbit | Cell Signaling |
| MAB200 | Interleukin-1α | - | Inflammatory cytokine | Mouse | R&D Systems |
| ab124962 | Interleukin-1 receptor antagonist | - | Blocks Il-1 inflammatory signaling | Rabbit | Abcam |
| ab175392 | Interleukin-1 receptor antagonist | - | Blocks Il-1 inflammatory signaling | Rabbit | Abcam |
| AF-295 | human Oncostatin M (OSM) | - | Cytokine of the IL-6 family | Goat | R&D Systems |
| MAB5965 | human Reg3A | - | Chemokine (macrophage) | Mouse | R&D Systems |
| AP5606c | human Reg3γ | - | Chemokine (macrophage) | Rabbit | Abcepta |
| 9701 | P-Histone 3 | Ser10 | Cell Cycle marker | Rabbit | Cell Signaling |
| 9714 | P-Histone 3 | Thr3 | Cell Cycle marker | Rabbit | Cell Signaling |
| 14475 | Aurora A | - | Cell Cycle marker | Rabbit | Cell Signaling |
| 3079 | P-Aurora A/B/C | Thr288/Thr232/Thr198 | Cell Cycle marker | Rabbit | Cell Signaling |
| 3094 | Aurora B | - | Cell Cycle marker | Rabbit | Cell Signaling |
| ab16667 | Ki67 | - | Cell Cycle marker | Rabbit | Abcam |
| ab68194 | α-actinin-1 | - | non-muscle α-actinin-1 | Rabbit | Abcam |
| A7811 | α-actinin-2 (EA-53) | - | sarcomeric α-actinin-2 | Mouse | Sigma |
| 2024 | Hexokinase 1 | - | Serves as loading control | Rabbit | Cell Signaling |
| ab8295 | Troponin T | - | Serves as loading control | Mouse | Abcam |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajawada, P.; Cetinkaya, A.; von Gerlach, S.; Kubin, N.; Burger, H.; Näbauer, M.; Grinninger, C.; Rolf, A.; Schönburg, M.; Choi, Y.-H.; et al. Myocardial Accumulations of Reg3A, Reg3γ and Oncostatin M Are Associated with the Formation of Granulomata in Patients with Cardiac Sarcoidosis. Int. J. Mol. Sci. 2021, 22, 4148. https://doi.org/10.3390/ijms22084148
Gajawada P, Cetinkaya A, von Gerlach S, Kubin N, Burger H, Näbauer M, Grinninger C, Rolf A, Schönburg M, Choi Y-H, et al. Myocardial Accumulations of Reg3A, Reg3γ and Oncostatin M Are Associated with the Formation of Granulomata in Patients with Cardiac Sarcoidosis. International Journal of Molecular Sciences. 2021; 22(8):4148. https://doi.org/10.3390/ijms22084148
Chicago/Turabian StyleGajawada, Praveen, Ayse Cetinkaya, Susanne von Gerlach, Natalia Kubin, Heiko Burger, Michael Näbauer, Carola Grinninger, Andreas Rolf, Markus Schönburg, Yeong-Hoon Choi, and et al. 2021. "Myocardial Accumulations of Reg3A, Reg3γ and Oncostatin M Are Associated with the Formation of Granulomata in Patients with Cardiac Sarcoidosis" International Journal of Molecular Sciences 22, no. 8: 4148. https://doi.org/10.3390/ijms22084148
APA StyleGajawada, P., Cetinkaya, A., von Gerlach, S., Kubin, N., Burger, H., Näbauer, M., Grinninger, C., Rolf, A., Schönburg, M., Choi, Y.-H., Kubin, T., & Richter, M. (2021). Myocardial Accumulations of Reg3A, Reg3γ and Oncostatin M Are Associated with the Formation of Granulomata in Patients with Cardiac Sarcoidosis. International Journal of Molecular Sciences, 22(8), 4148. https://doi.org/10.3390/ijms22084148






