Thixotropic Hydrogels Composed of Self-Assembled Nanofibers of Double-Hydrophobic Elastin-Like Block Polypeptides
Abstract
1. Introduction
2. Results
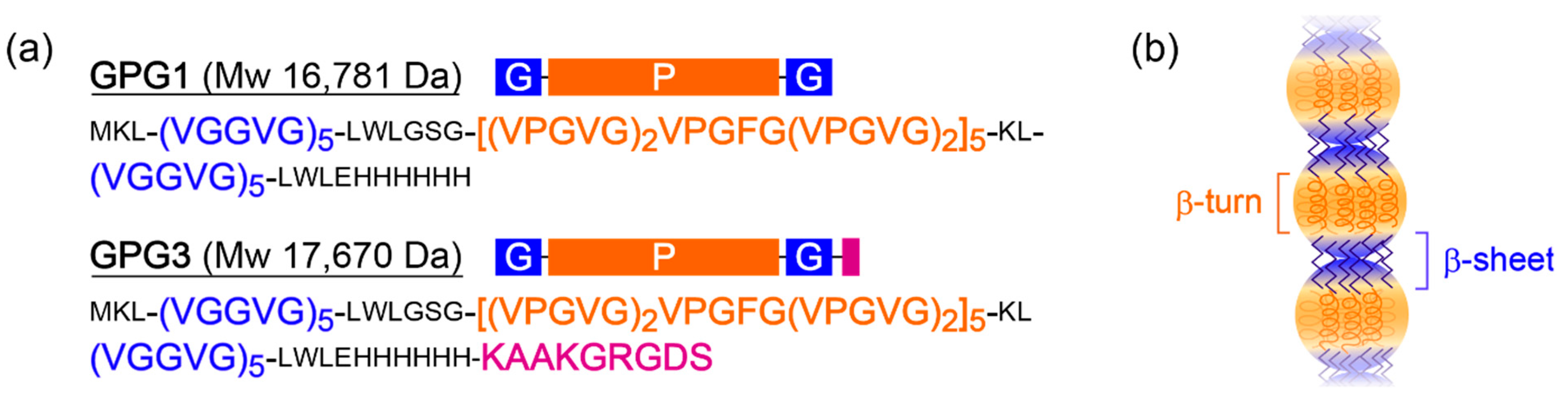
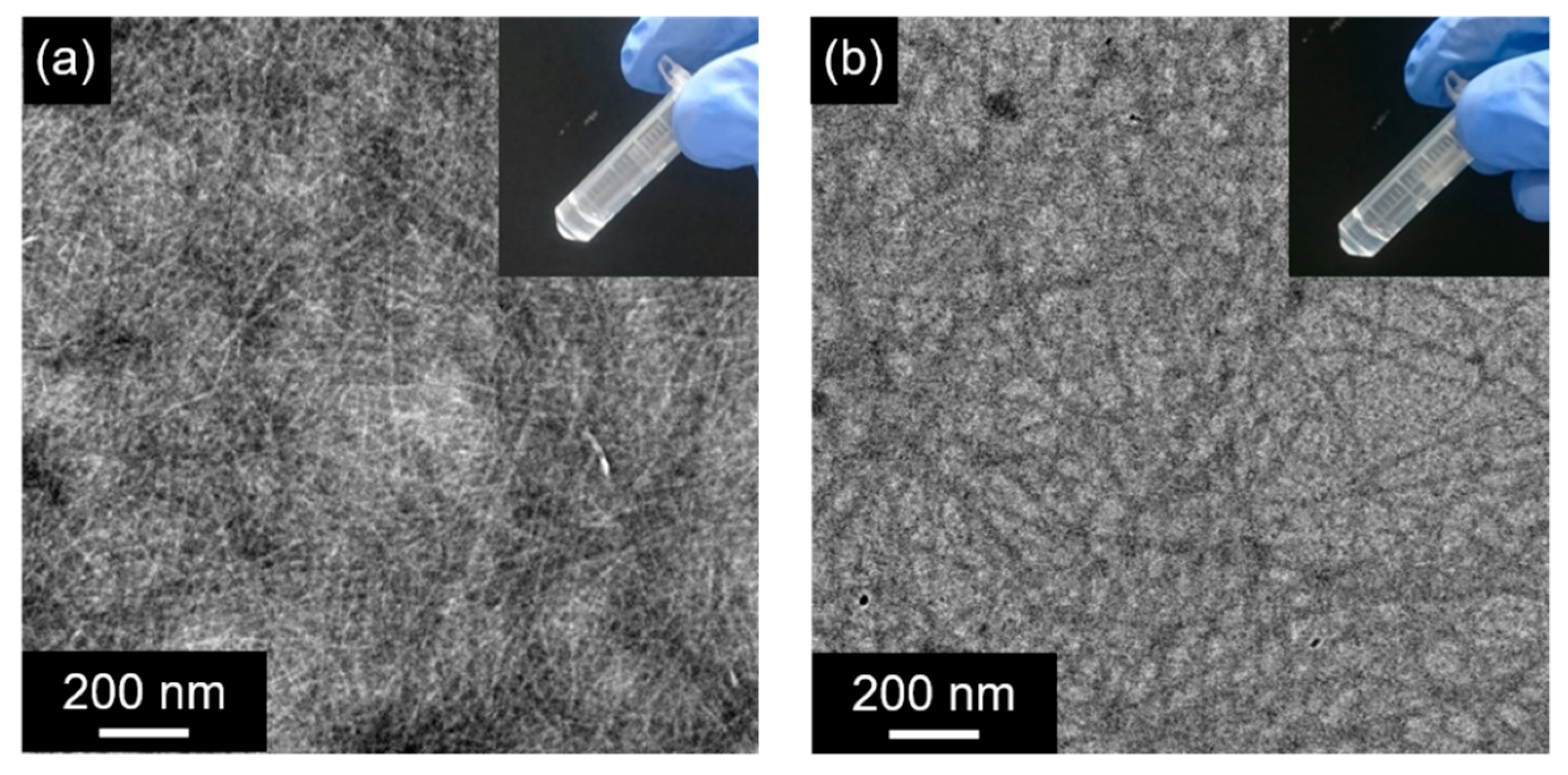
2.1. Formation of Hydrogels
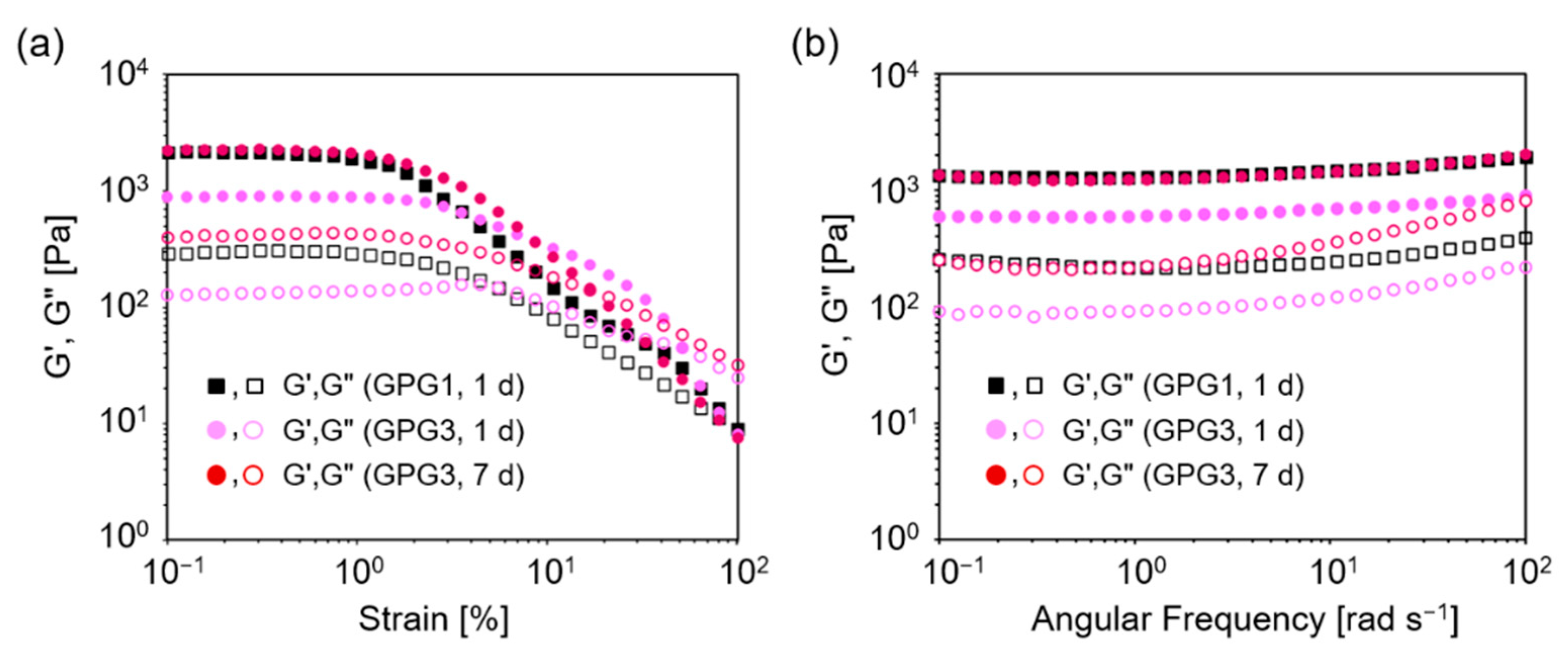
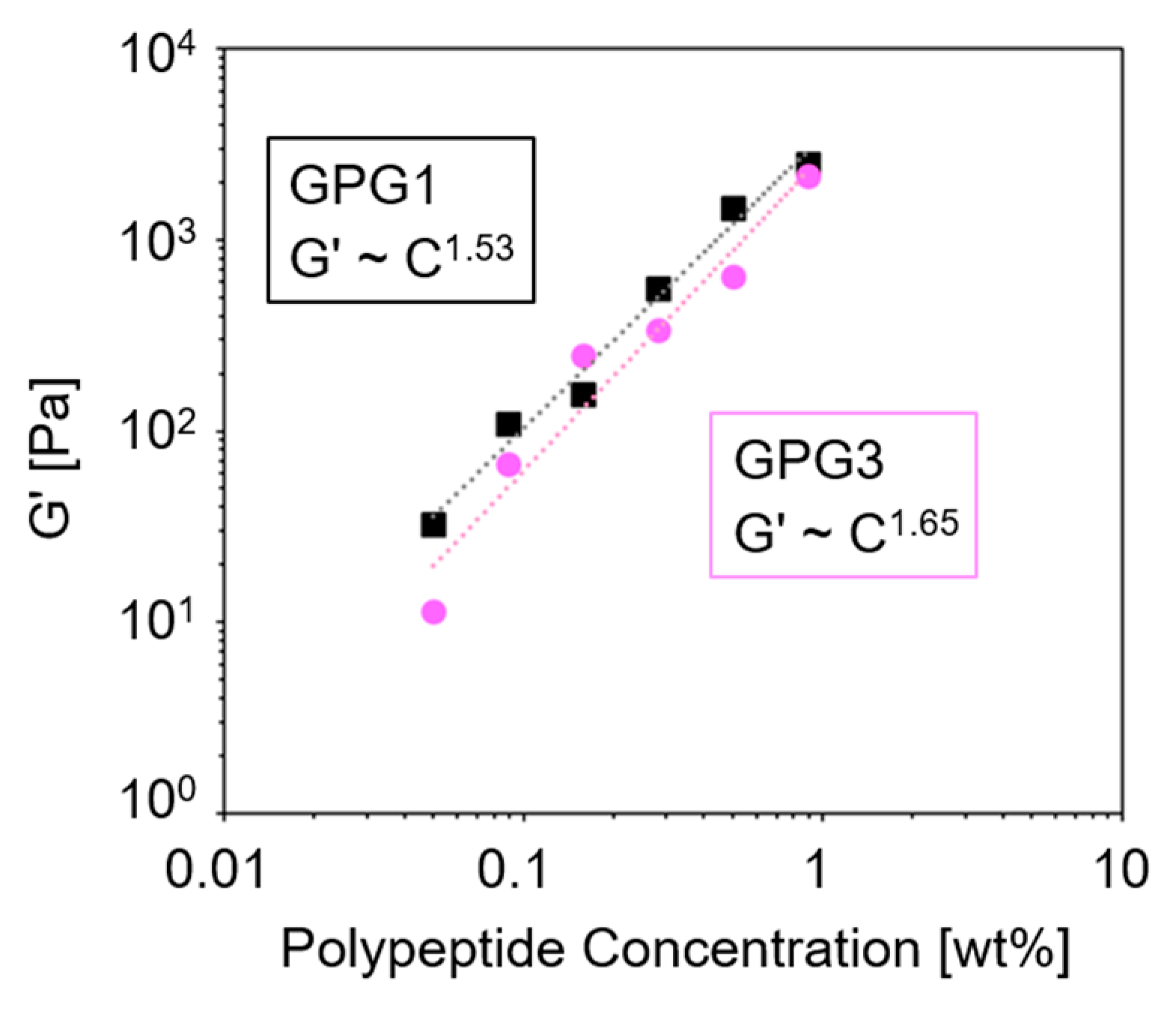
2.2. Dynamic Rheological Characteristics of Hydrogels
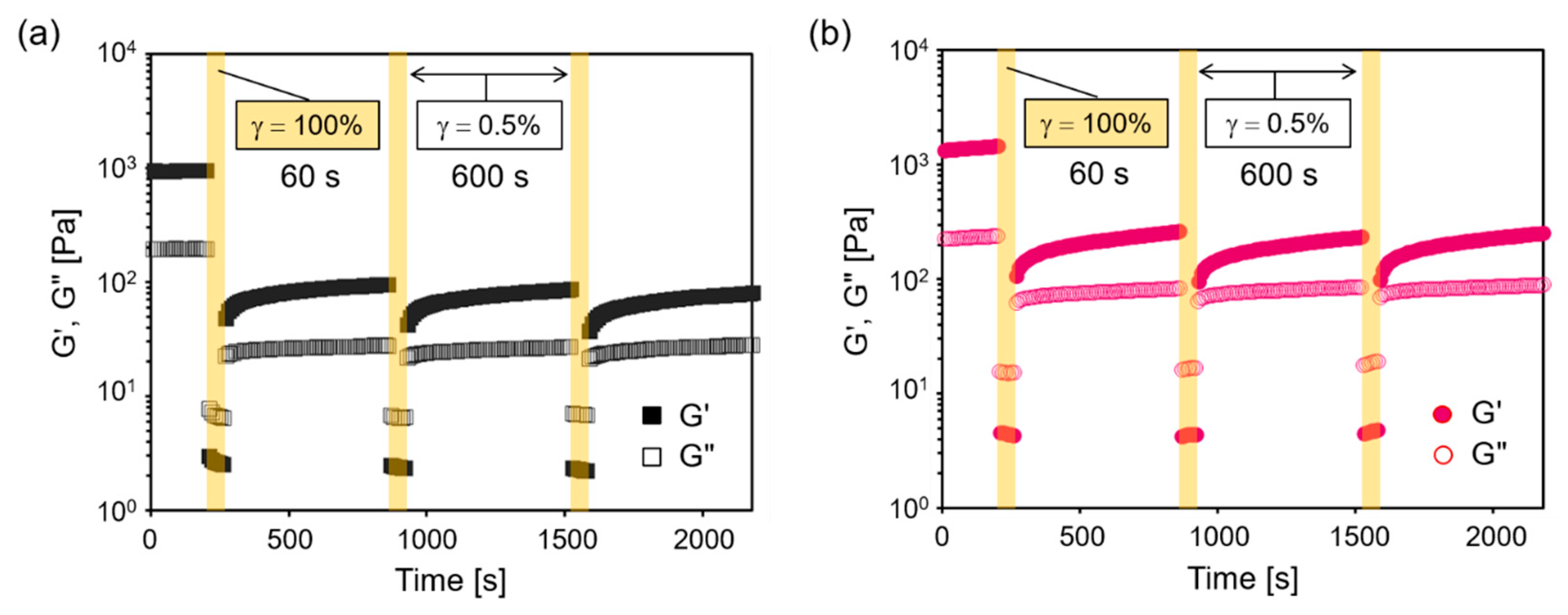
2.3. Thixotropicity of Hydrogels
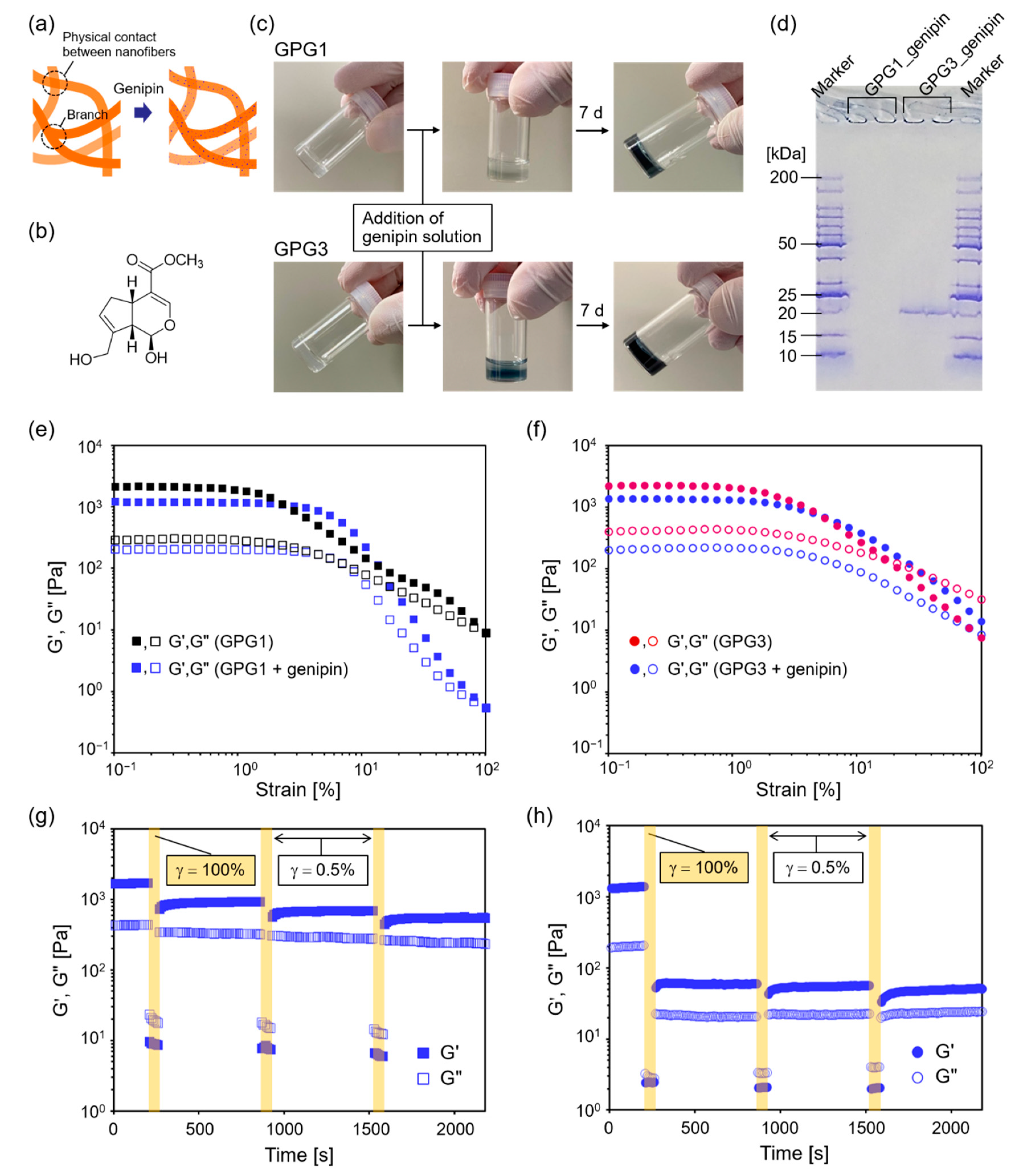
2.4. Effects of Chemical Crosslinks Using Genipin
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Rheological Measurements
4.3. TEM
4.4. Genipin Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Da Silva, K.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Three-dimensional printing of extracellular matrix (ECM)-mimicking scaffolds: A critical review of the current ECM materials. J. Biomed. Mater. Res. Part A 2020, 108, 2324–2350. [Google Scholar] [CrossRef]
- Mithieux, S.M.; Weiss, A.S. Elastin. Adv. Protein Chem. 2005, 70, 437–461. [Google Scholar] [CrossRef]
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta 2013, 1832, 866–875. [Google Scholar] [CrossRef]
- Nettles, D.L.; Chilkoti, A.; Setton, L.A. Applications of elastin-like polypeptides in tissue engineering. Adv. Drug Deliv. Rev. 2010, 62, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Sugawara-Narutaki, A.; Le, D.H.T. Elastin-like polypeptides as building motifs toward designing functional nanobiomaterials. Mol. Syst. Des. Eng. 2019, 4, 545–565. [Google Scholar] [CrossRef]
- Annabi, N.; Mithieux, S.M.; Zorlutuna, P.; Camci-Unal, G.; Weiss, A.S.; Khademhosseini, A. Engineered cell-laden human protein-based elastomer. Biomaterials 2013, 34, 5496–5505. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Sani, E.S.; Spencer, A.R.; Guan, Y.; Weiss, A.S.; Annabi, N. Human-recombinant-elastin-based bioinks for 3D bioprinting of vascularized soft tissues. Adv. Mater. 2020, 32, e2003915. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W. Molecular machines: How motion and other functions of living organisms can result from reversible chemical changes. Angew. Chem. Int. Ed. 1993, 32, 819–841. [Google Scholar] [CrossRef]
- McHale, M.K.; Setton, L.A.; Chilkoti, A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005, 11, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- González de Torre, I.; Santos, M.; Quintanilla, L.; Testera, A.; Alonso, M.; Rodríguez-Cabello, J.C. Elastin-like recombinamer catalyst-free click gels: Characterization of poroelastic and intrinsic viscoelastic properties. Acta Biomater. 2014, 10, 2495–2505. [Google Scholar] [CrossRef]
- Madl, C.M.; Katz, L.M.; Heilshorn, S.C. Bio-orthogonally crosslinked, engineered protein hydrogels with tunable mechanics and biochemistry for cell encapsulation. Adv. Funct. Mater. 2016, 26, 3612–3620. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Fonseca, A.; Santiago Maniega, S.; Gorbenko Del Blanco, D.; Catalán Bernardos, B.; Vega Castrillo, A.; Álvarez Barcia, Á.J.; Alonso, M.; Aguado, H.J.; Rodríguez-Cabello, J.C. Elastin-like recombinamer hydrogels for improved skeletal muscle healing through modulation of macrophage polarization. Front. Bioeng. Biotechnol. 2020, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Contessotto, P.; Orbanić, D.; Da Costa, M.; Jin, C.; Owens, P.; Chantepie, S.; Chinello, C.; Newell, J.; Magni, F.; Papy-Garcia, D.; et al. Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep. Sci. Transl. Med. 2021, 13, eaaz5380. [Google Scholar] [CrossRef]
- Marsico, G.; Jin, C.; Abbah, S.A.; Brauchle, E.M.; Thomas, D.; Rebelo, A.L.; Orbanić, D.; Chantepie, S.; Contessotto, P.; Papy-Garcia, D.; et al. Elastin-like hydrogel stimulates angiogenesis in a severe model of critical limb ischemia (CLI): An insight into the glyco-host response. Biomaterials 2021, 269, 120641. [Google Scholar] [CrossRef]
- McDaniel, J.R.; Radford, D.C.; Chilkoti, A. A unified model for de novo design of elastin-like polypeptides with tunable inverse transition temperatures. Biomacromolecules 2013, 14, 2866–2872. [Google Scholar] [CrossRef]
- Glassman, M.J.; Olsen, B.D. Arrested phase separation of elastin-like polypeptide solutions yields stiff, thermoresponsive gels. Biomacromolecules 2015, 16, 3762–3773. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Keeley, F.W. Viscoelastic properties and gelation of an elastin-like polypeptide. J. Rheol. 2009, 53, 1215. [Google Scholar] [CrossRef]
- Roberts, S.; Harmon, T.S.; Schaal, J.L.; Miao, V.; Li, K.J.; Hunt, A.; Wen, Y.; Oas, T.G.; Collier, J.H.; Pappu, R.V.; et al. Injectable tissue integrating networks from recombinant polypeptides with tunable order. Nat. Mater. 2018, 17, 1154–1163. [Google Scholar] [CrossRef]
- Misbah, M.H.; Quintanilla, L.; Alonso, M.; Rodríguez-Cabello, J.C. Evolution of amphiphilic elastin-like co-recombinamer morphologies from micelles to a lyotropic hydrogel. Polymer 2015, 81, 37–44. [Google Scholar] [CrossRef]
- Nagapudi, K.; Brinkman, W.T.; Thomas, B.S.; Park, J.O.; Srinivasarao, M.; Wright, E.; Conticello, V.P.; Chaikof, E.L. Viscoelastic and mechanical behavior of recombinant protein elastomers. Biomaterials 2005, 26, 4695–4706. [Google Scholar] [CrossRef]
- Haghpanah, J.S.; Yuvienco, C.; Roth, E.W.; Liang, A.; Tu, R.S.; Montclare, J.K. Supramolecular assembly and small molecule recognition by genetically engineered protein block polymers composed of two SADs. Mol. BioSyst. 2010, 6, 1662–1667. [Google Scholar] [CrossRef]
- Mizuguchi, Y.; Mashimo, Y.; Mie, M.; Kobatake, E. Temperature-responsive multifunctional protein hydrogels with elastin-like polypeptides for 3-D angiogenesis. Biomacromolecules 2020, 21, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Dandu, R.; Von Cresce, A.; Briber, R.; Dowell, P.; Cappello, J.; Ghandehari, H. Silk–elastinlike protein polymer hydrogels: Influence of monomer sequence on physicochemical properties. Polymer 2009, 50, 366–374. [Google Scholar] [CrossRef]
- Xia, X.-X.; Xu, Q.; Hu, X.; Qin, G.; Kaplan, D.L. Tunable self-assembly of genetically engineered silk-elastin-like protein polymers. Biomacromolecules 2011, 12, 3844–3850. [Google Scholar] [CrossRef]
- Fernández-Colino, A.; Arias, F.J.; Alonso, M.; Rodríguez-Cabello, J.C. Self-organized ECM-mimetic model based on an amphiphilic multiblock silk-elastin-like corecombinamer with a concomitant dual physical gelation process. Biomacromolecules 2014, 15, 3781–3793. [Google Scholar] [CrossRef]
- Shi, W.H.; Pathiranage, T.M.S.K.; Marciel, A.B. Rheological properties of engineered protein polymer networks. MRS Bull. 2020, 45, 1048–1054. [Google Scholar] [CrossRef]
- Le, D.H.T.; Hanamura, R.; Pham, D.-H.; Kato, M.; Tirrell, D.A.; Okubo, T.; Sugawara-Narutaki, A. Self-assembly of elastin-mimetic double hydrophobic polypeptides. Biomacromolecules 2013, 14, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Sugawara-Narutaki, A.; Yasunaga, S.; Sugioka, Y.; Le, D.H.T.; Kitamura, I.; Nakamura, J.; Ohtsuki, C. Rheology of dispersions of high-aspect-ratio nanofibers assembled from elastin-like double-hydrophobic polypeptides. Int. J. Mol. Sci. 2019, 20, 6262. [Google Scholar] [CrossRef] [PubMed]
- Le, D.H.T.; Tsutsui, Y.; Sugawara-Narutaki, A.; Yukawa, H.; Baba, Y.; Ohtsuki, C. Double-hydrophobic elastin-like polypeptides with added functional motifs: Self-assembly and cytocompatibility. J. Biomed. Mater. Res. Part A 2017, 105, 2475–2484. [Google Scholar] [CrossRef]
- Tamburro, A.M.; Bochicchio, B.; Pepe, A. Dissection of human tropoelastin: Exon-by-exon chemical synthesis and related conformational studies. Biochemistry 2003, 42, 13347–13362. [Google Scholar] [CrossRef]
- Le, D.H.T.; Okubo, T.; Sugawara-Narutaki, A. Beaded nanofibers assembled from double-hydrophobic elastin-like block polypeptides: Effects of trifluoroethanol. Biopolymers 2015, 103, 175–185. [Google Scholar] [CrossRef]
- Hyun, K.; Kim, S.H.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear as a way to classify the complex fluids. J. Non-Newtonian Fluid Mech. 2002, 107, 51–65. [Google Scholar] [CrossRef]
- Daniel, C.; Hamley, I.H.; Wilhelm, M.; Mingvanish, W. Non-linear rheology of a face-centred cubic phase in a diblock copolymer gel. Rheol. Acta 2001, 40, 39–48. [Google Scholar] [CrossRef]
- Greenfield, M.A.; Hoffman, J.R.; de la Cruz, M.O.; Stupp, S.I. Tunable mechanics and peptide nanofiber gels. Langmuir 2010, 26, 3641–3647. [Google Scholar] [CrossRef]
- Raeburn, J.; Mendoza-Cuenca, C.; Cattoz, B.N.; Little, M.A.; Terry, A.E.; Cardoso, A.Z.; Griffiths, P.C.; Adams, D.J. The effect of solvent choice on the gelation and final hydrogel properties of Fmoc–diphenylalanine. Soft Matter 2015, 11, 927–935. [Google Scholar] [CrossRef]
- MacKintosh, F.C.; Käs, J.; Janmey, P.A. Elasticity of semiflexible biopolymer networks. Phys. Rev. Lett. 1995, 75, 4425–4428. [Google Scholar] [CrossRef]
- Morse, D.C. Viscoelasticity of concentrated isotropic solutions of semiflexible polymers. 1. model and stress tensor. Macromolecules 1998, 31, 7030–7043. [Google Scholar] [CrossRef]
- Palmer, A.; Mason, T.G.; Xu, J.; Kuo, S.C.; Wirtz, D. Diffusing wave spectroscopy microrheology of actin filament networks. Biophys. J. 1999, 76, 1063–1071. [Google Scholar] [CrossRef]
- Greth, C.; Roberts, W.W.; Ferry, J.D. Rheology of fibrin clots. II. Linear viscoelastic behavior in shear creep. Biophys. Chem. 1974, 2, 208–217. [Google Scholar] [CrossRef]
- Schmidt, F.G.; Hinner, B.; Sackmann, E.; Tang, J.X. Viscoelastic properties of semiflexible filamentous bacteriophage fd. Phys. Rev. E 2000, 62, 5509–5517. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; García-Parra, E.; Vela-Gutiérrez, G.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Genipin as an emergent tool in the design of biocatalysts: Mechanism of reaction and applications. Catalysts 2019, 9, 1035. [Google Scholar] [CrossRef]
- Dimida, S.; Demitri, C.; De Benedictis, V.M.; Scalera, F.; Gervaso, F.; Sannino, A. Genipin-cross-linked chitosan-based hydrogels: Reaction kinetics and structure-related characteristics. J. Appl. Polym. Sci. 2015, 132, 42256. [Google Scholar] [CrossRef]
- Baker, A.E.G.; Tam, R.Y.; Shoichet, M.S. Independently tuning the biochemical and mechanical properties of 3D hyaluronan-based hydrogels with oxime and diels–alder chemistry to culture breast cancer spheroids. Biomacromolecules 2017, 18, 4373–4384. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Smith, A.M.; Ligorio, C.; Mykhaylyk, O.O.; Miller, A.F.; Saiani, A. Role of sheet-edge interactions in β-sheet self-assembling peptide hydrogels. Biomacromolecules 2020, 21, 2285–2297. [Google Scholar] [CrossRef]
- Yin, Z.; Wu, F.; Xing, T.; Yadavalli, V.K.; Kundu, S.C.; Lu, S. A silk fibroin hydrogel with reversible sol–gel transition. RSC Adv. 2017, 7, 24085–24096. [Google Scholar] [CrossRef]
- Jacob, R.S.; Ghosh, D.; Singh, P.K.; Basu, S.K.; Jha, N.N.; Das, S.; Sukul, P.K.; Patil, S.; Sathaye, S.; Kumar, A.; et al. Self healing hydrogels composed of amyloid nano fibrils for cell culture and stem cell differentiation. Biomaterials 2015, 54, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zanna, N.; Focaroli, S.; Merlettini, A.; Gentilucci, L.; Teti, G.; Falconi, M.; Tomasini, C. Thixotropic peptide-based physical hydrogels applied to three-dimensional cell culture. ACS Omega 2017, 2, 2374–2381. [Google Scholar] [CrossRef]
- Gavel, P.K.; Kumar, N.; Parmar, H.S.; Das, A.K. Evaluation of a peptide-based coassembled nanofibrous and thixotropic hydrogel for dermal wound healing. ACS Appl. Biomater. 2020, 3, 3326–3336. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chuang, W.-T.; Hsu, S. Gelation mechanism and structural dynamics of chitosan self-healing hydrogels by in situ SAXS and coherent X-ray scattering. ACS Macro Lett. 2019, 8, 1449–1455. [Google Scholar] [CrossRef]
- Inaba, H.; Matsuura, K. Peptide nanomaterials designed from natural supramolecular systems. Chem. Rec. 2019, 19, 843–858. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugioka, Y.; Nakamura, J.; Ohtsuki, C.; Sugawara-Narutaki, A. Thixotropic Hydrogels Composed of Self-Assembled Nanofibers of Double-Hydrophobic Elastin-Like Block Polypeptides. Int. J. Mol. Sci. 2021, 22, 4104. https://doi.org/10.3390/ijms22084104
Sugioka Y, Nakamura J, Ohtsuki C, Sugawara-Narutaki A. Thixotropic Hydrogels Composed of Self-Assembled Nanofibers of Double-Hydrophobic Elastin-Like Block Polypeptides. International Journal of Molecular Sciences. 2021; 22(8):4104. https://doi.org/10.3390/ijms22084104
Chicago/Turabian StyleSugioka, Yusuke, Jin Nakamura, Chikara Ohtsuki, and Ayae Sugawara-Narutaki. 2021. "Thixotropic Hydrogels Composed of Self-Assembled Nanofibers of Double-Hydrophobic Elastin-Like Block Polypeptides" International Journal of Molecular Sciences 22, no. 8: 4104. https://doi.org/10.3390/ijms22084104
APA StyleSugioka, Y., Nakamura, J., Ohtsuki, C., & Sugawara-Narutaki, A. (2021). Thixotropic Hydrogels Composed of Self-Assembled Nanofibers of Double-Hydrophobic Elastin-Like Block Polypeptides. International Journal of Molecular Sciences, 22(8), 4104. https://doi.org/10.3390/ijms22084104






