Development of a Topical Insulin Polymeric Nanoformulation for Skin Burn Regeneration: An Experimental Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Immortalized Human Keratinocytes Cells (HaCat)
2.1.3. Animals
2.2. Methods
2.2.1. Preparation of NPs
2.2.2. Preparation POLX-Based Hydrogel
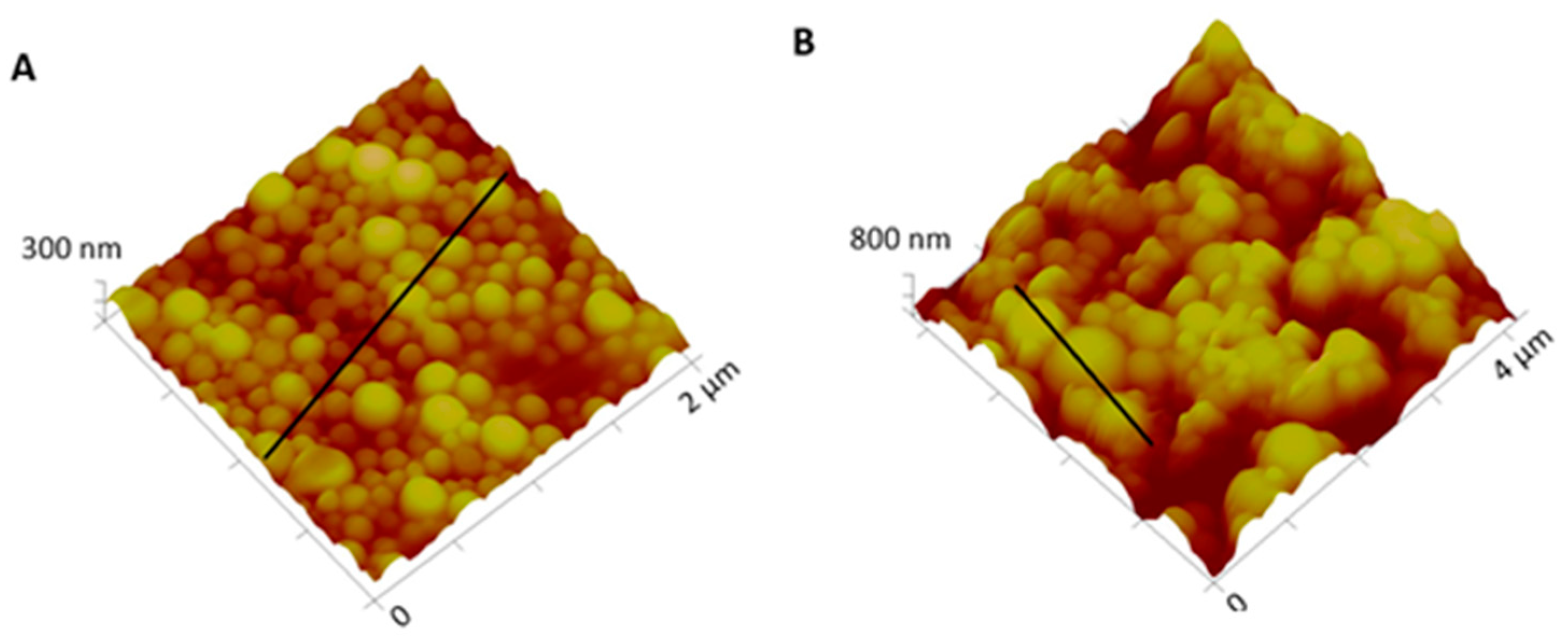
2.2.3. NPs Characterization
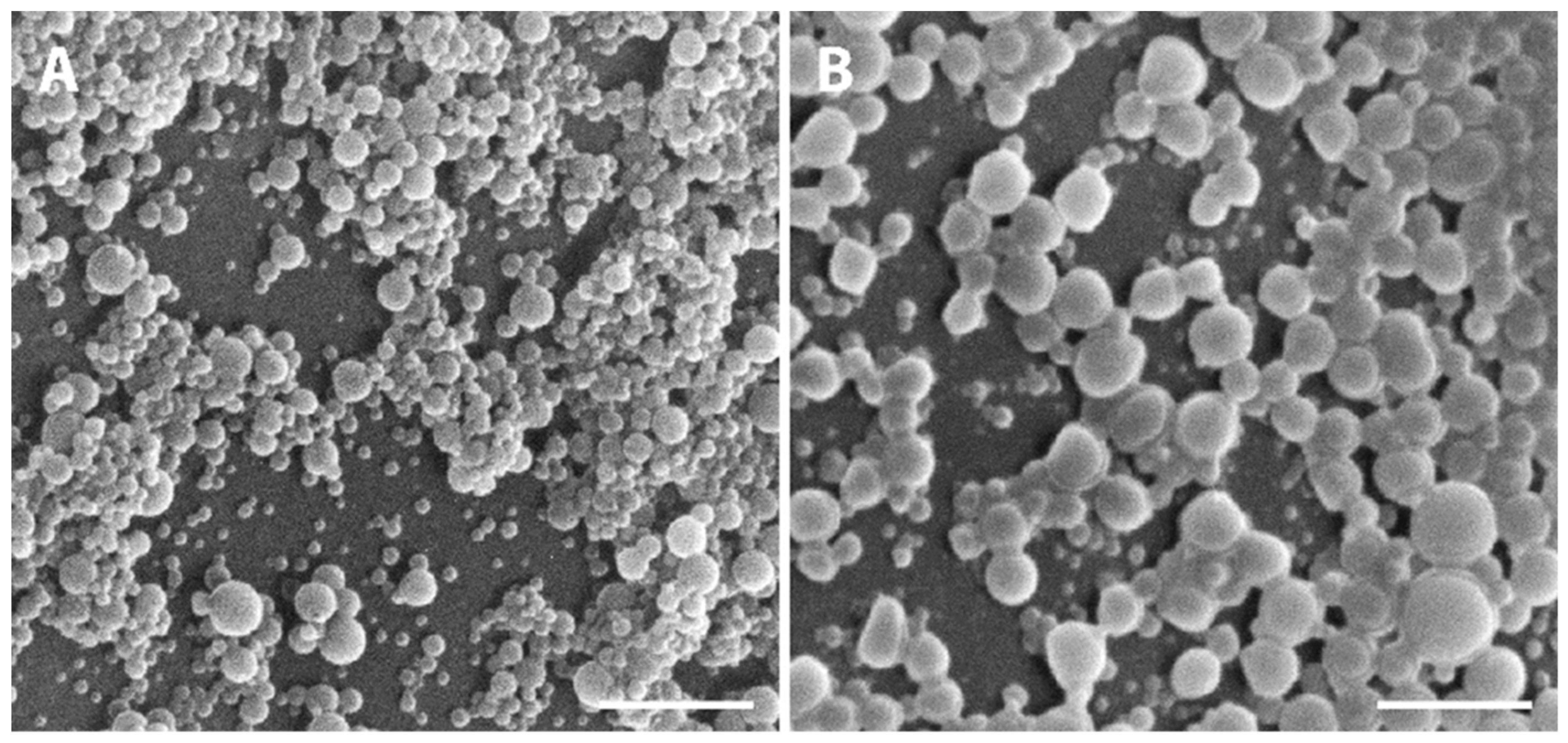
2.2.4. Surface and Morphological Analysis
2.2.5. Determination of Encapsulation Efficiency (EE)
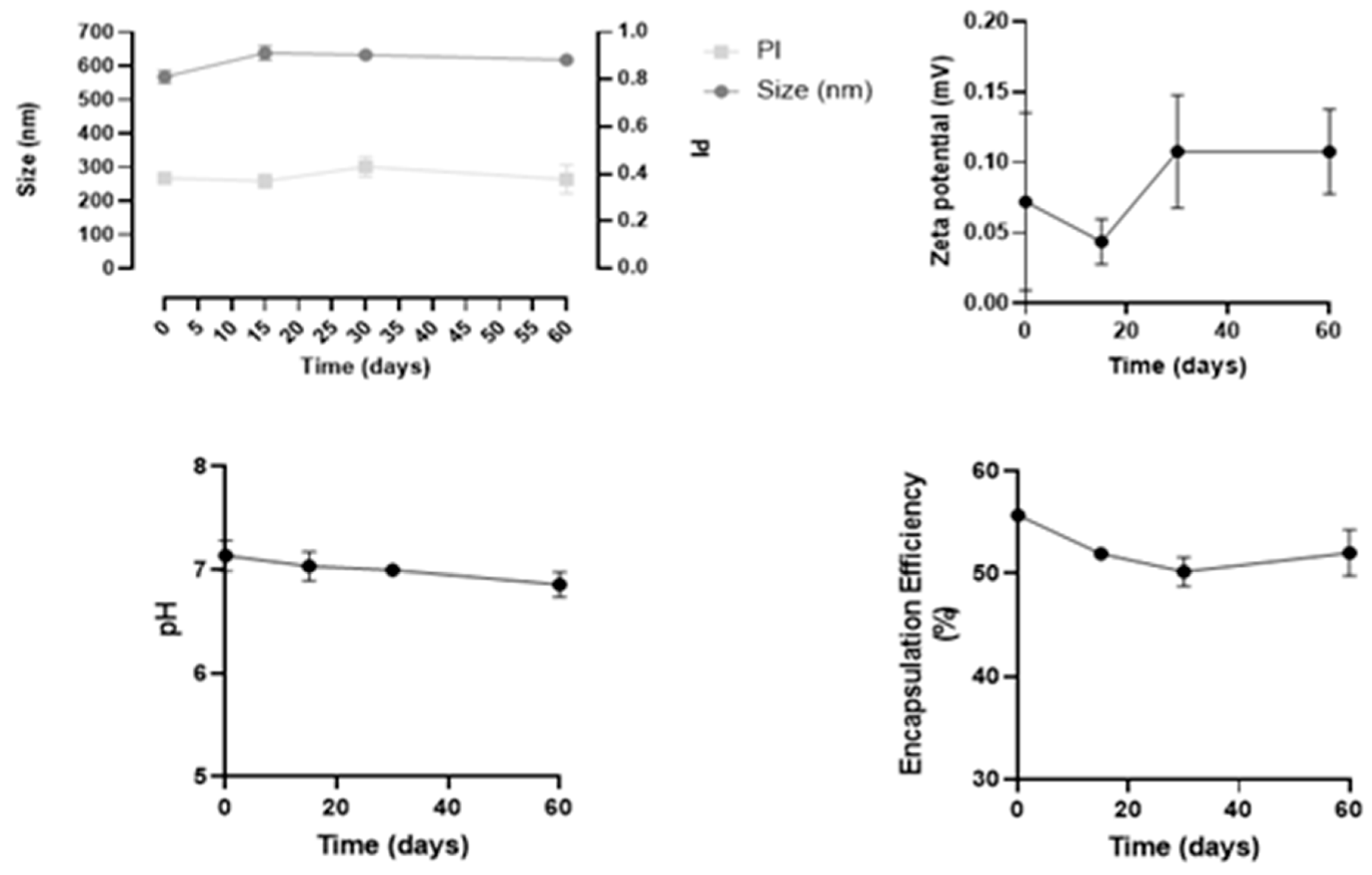
2.2.6. Physical Stability of Insulin Nanoformulation over Time
2.2.7. Protein Structural Stability after Encapsulation
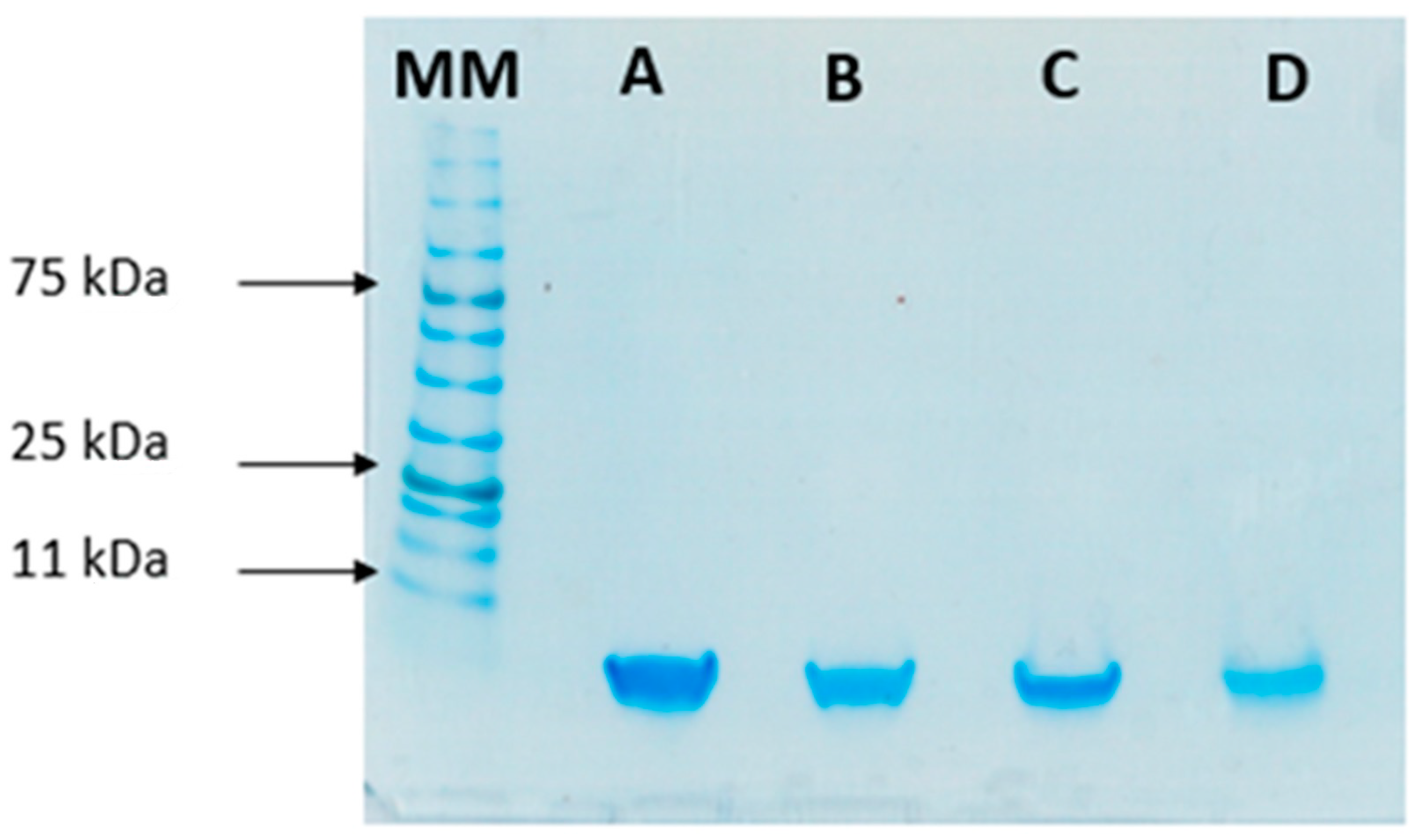
SDS-PAGE
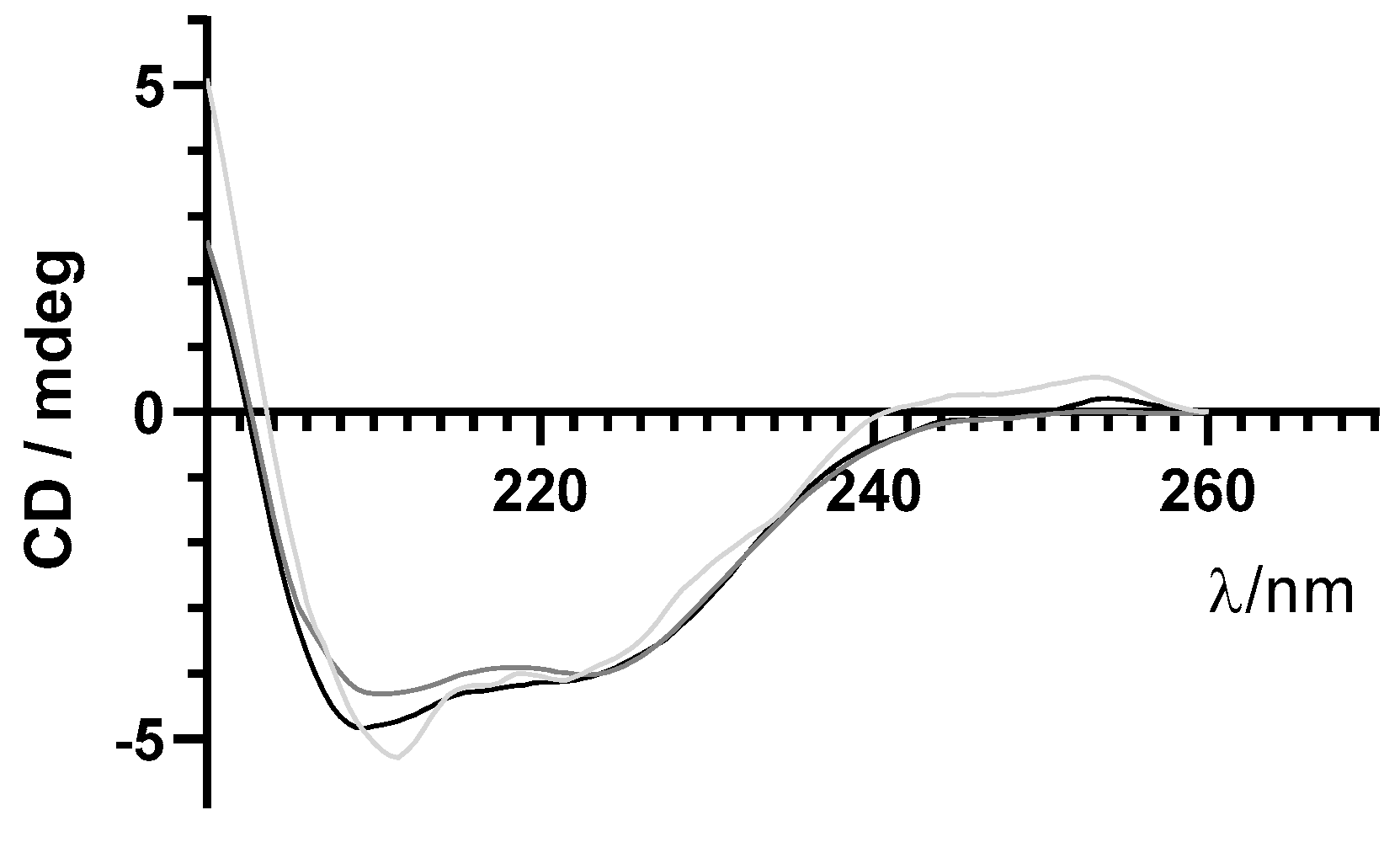
Circular Dichroism
2.2.8. Release Behavior of Insulin Formulations over Time
In Vitro Diffusion Assay Using Franz Cells
2.2.9. Preliminary In Vitro Safety Assessment
In Vitro Assessment
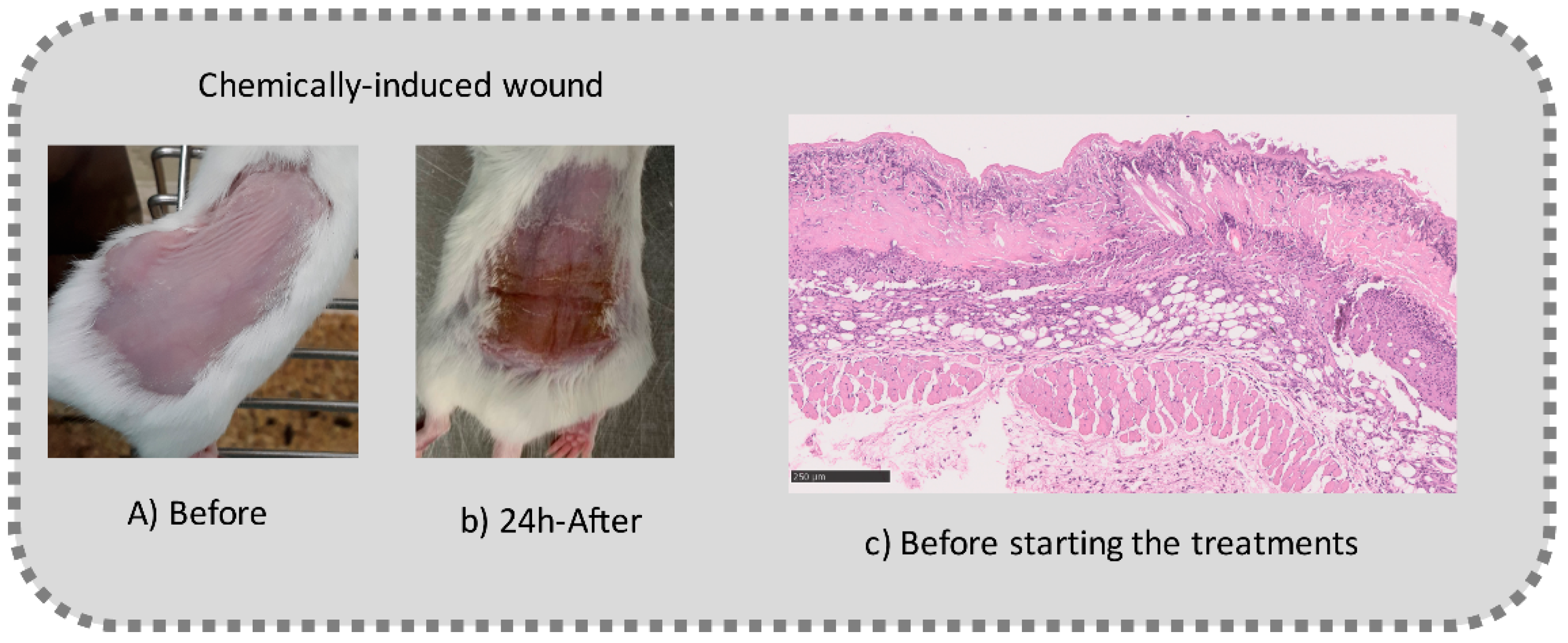
2.2.10. Preliminary In Vivo Efficacy Assessment
2.2.11. Statistical Analysis
3. Results
3.1. NPs Characterization: Size, PI, Zeta Potential, Particle Morphology, and EE
3.2. Physical Stability of Nanoformulation over Time
3.3. Protein Stability after Encapsulation Process
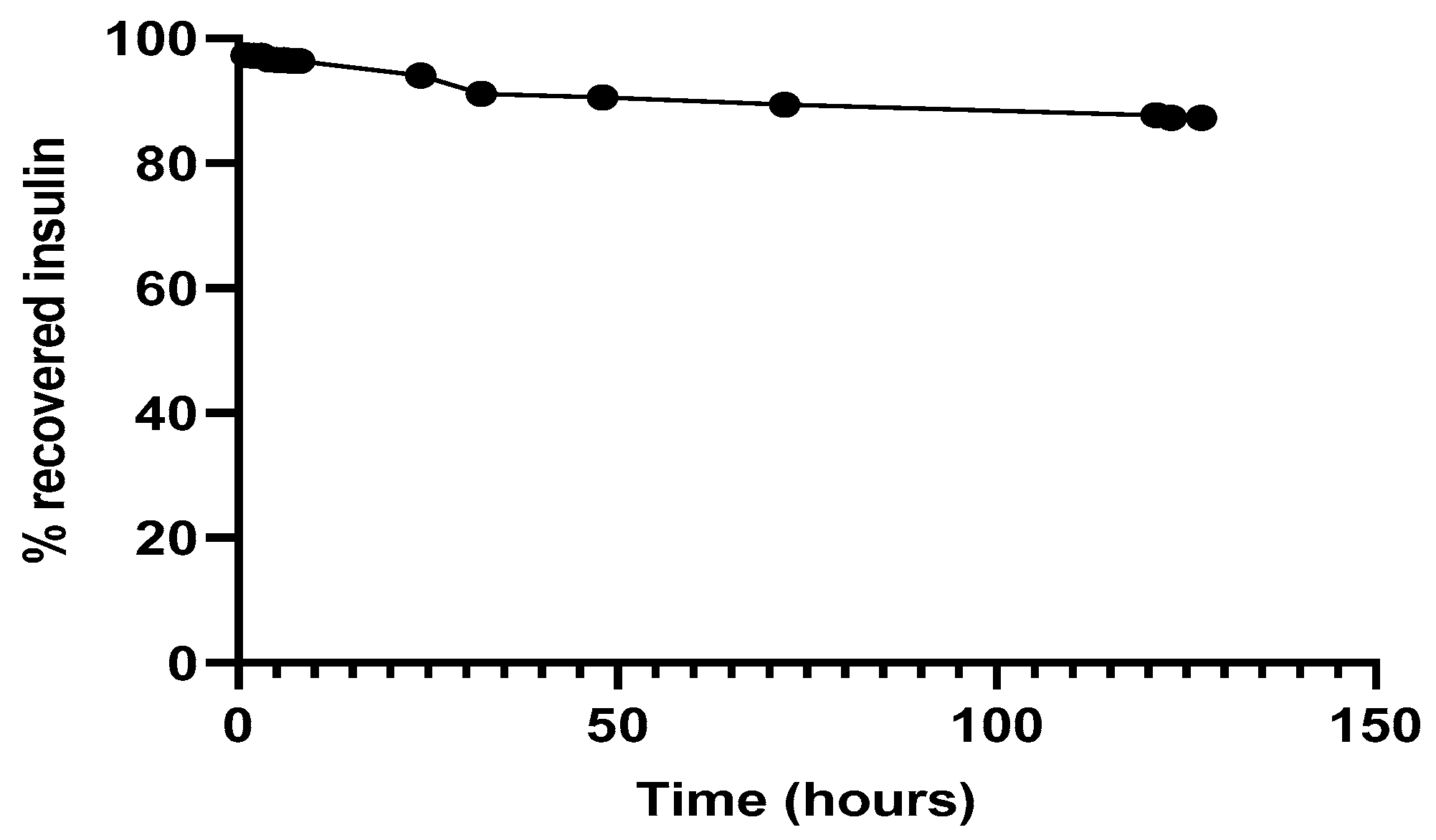
3.4. Protein Recovery after Encapsulation Process
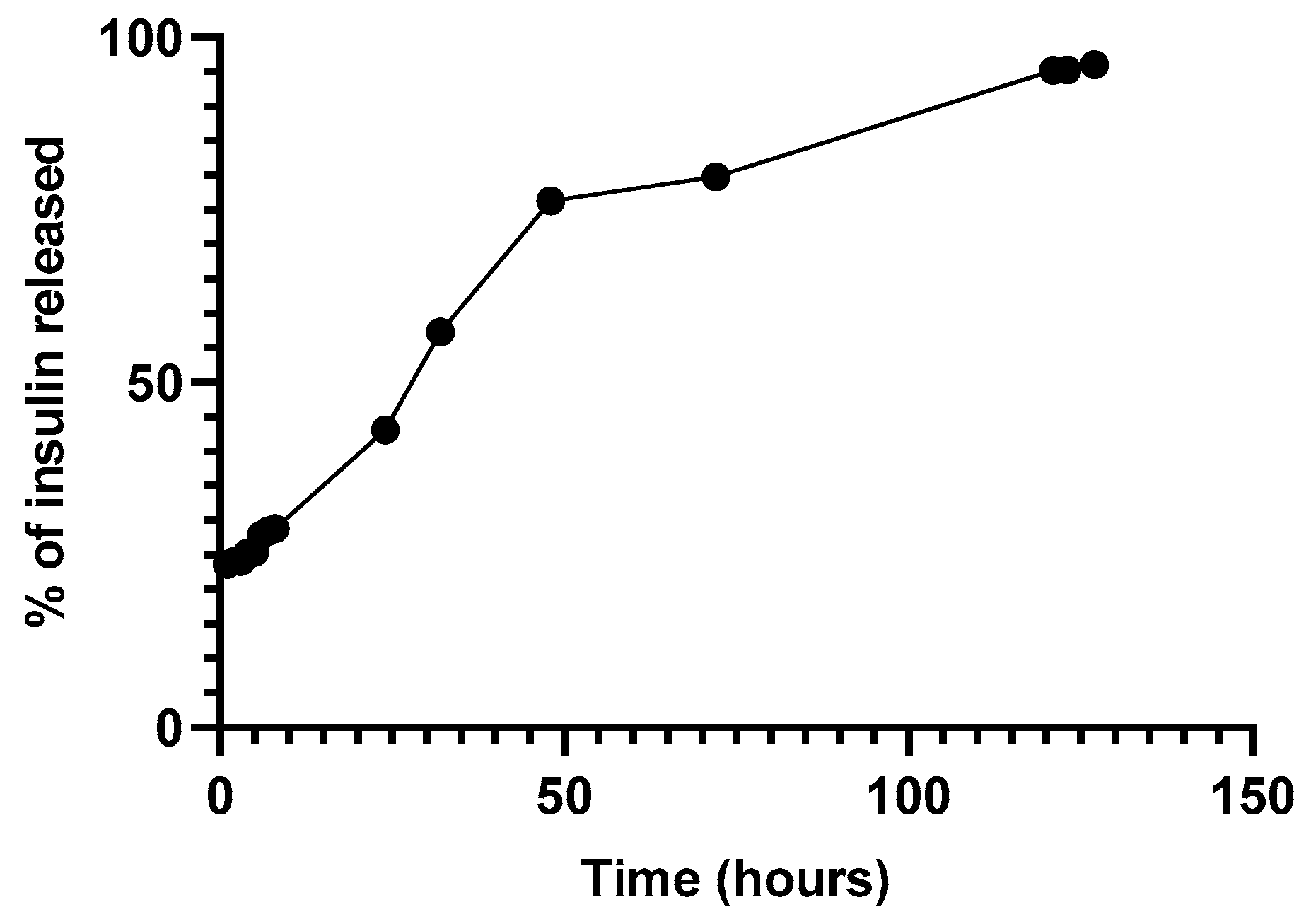
In Vitro Release Assay
3.5. Preliminary Safety and Efficacy Assessments
3.5.1. In Vitro Safety Assessment
3.5.2. In Vivo Preliminary Efficacy Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Olawoye, O.A. Open burn wound dressing: A practical option in resource constrained settings. Ann. Burn. Fire Disasters 2013, 26, 1–4. [Google Scholar]
- Pikuła, M.; Langa, P.; Kosikowska, P.; Trzonkowski, P. Kom{ó}rki macierzyste i czynniki wzrostu w gojeniu ran * Stem cells and growth factors in wound healing. Adv. Hyg. Exp. Med. 2015, 69, 874–885. [Google Scholar]
- Xiaoqing, D.; Xu, J.; Wang, W.; Luo, H.; Liang, X.; Zhang, L.; Wang, H.; Wang, P.; Changet, J. Repair effect of diabetic ulcers with recombinant human epidermal growth factor loaded by sustained-release microspheres. Sci. China Ser. C Life Sci. 2008, 51, 1039–1044. [Google Scholar]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and biomarkers for wound healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef]
- Hrynyk., M.; Neufeld, R.J. Insulin and wound healing. Burns 2014, 40, 1433–1446. [Google Scholar] [CrossRef]
- AbdelKader, D.H.; Osman, M.A.; Elgizawy, S.A.; Faheem, A.M.; McCarron, P.A. The role of insulin in wound healing process: Mechanism of action and pharmaceutical applications. J. Anal. Pharm. Res. 2018, 2, 1–6. [Google Scholar]
- Apikoglu-Rabus, S.; Izzettin, F.V.; Turan, P.; Ercan, F. Effect of topical insulin on cutaneous wound healing in rats with or without acute diabetes: Original article. Clin. Exp. Dermatol. 2010, 35, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E. Effects of insulin on wound healing: A review of animal and human evidences. Life Sci. 2017, 174, 59–67. [Google Scholar] [CrossRef]
- Yah, C.S.; Simate, G.S. Nanoparticles as potential new generation broad spectrum antimicrobial agents. DARU J. Pharm. Sci. 2015, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1551–1573. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Ghosh, B.; Mukhopadhyay, M. Development of nanotechnology for advancement and application in wound healing: A review. IET Nanobiotechnol. 2019, 13, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Semete, B.; Booysen, L.; Lemmer, Y.; Kalombo, L.; Katata, L.; Verschoor, J.; Swai, H.S. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Memvanga, P.B.; Rieux, A. Combined effect of PLGA and curcumin on wound healing activity. J. Control. Release 2013, 171, 208–215. [Google Scholar]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug-polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef]
- Murakami, H.; Kobayashi, M.; Takeuchi, H.; Kawashima, Y. Preparation of poly(DL-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int. J. Pharm. 1999, 187, 143–152. [Google Scholar] [CrossRef]
- Reis, C.P.; Martinho, N.; Rosado, C.; Fernandes, A.S.; Roberto, A. Design of polymeric nanoparticles and its applications as drug delivery systems for acne treatment. Drug Dev. Ind. Pharm. 2014, 40, 409–417. [Google Scholar] [CrossRef]
- Soga, O.; Van Nostrum, C.F.; Fens, M.; Rijcken, C.J.F.; Schiffelers, R.M.; Storm, G.; Hennink, W.E. Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery. J. Control. Release 2005, 103, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.; Dandekar, P.; Jain, R. Nanotoxicology: Evaluating toxicity potential of drug-nanoparticles. In Nanoparticulate Drug Delivery; Woodhead Publishing Ltd.: Cambridge, UK, 2012; pp. 123–155. [Google Scholar]
- Braiman-Wiksman, L.; Solomonik, I.; Spira, R.; Tennenbaum, T. Novel insights into wound healing sequence of events. Toxicol. Pathol. 2007, 35, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Sultana, J.; Molla, M.R.; Kamal, M.; Shahidullah, M.; Begum, F.; Bashar, M.A. Histological differences in wound healing in maxillofacial region in patients with or without risk factors. Bangladesh J. Pathol. 1970, 24, 3–8. [Google Scholar] [CrossRef]
- Malvern Instruments Ltd. Zetasizer Nano Series; Malvern Instruments Ltd.: Malvern, UK, 2014; p. 20. [Google Scholar]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of Nanomaterials: Tools and Challenges; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef]
- Panyam, J.; Dali, M.M.; Sahoo, S.K.; Ma, W. Polymer degradation and in vitro release of a model protein from poly (D, L-lactide-co-glycolid) nano- and microparticles. J. Control. Release 2003, 92, 173–187. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-castro, A.; Ca, M.; Horobin, R.W. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J. Effects of topical insulin on wound healing: A review of animal and human evidences. Diabetes Metab. Syndr. Obes. 2020, 13, 719–727. [Google Scholar] [CrossRef]
- Belfield, W.O.; Golinsky, S.; Compton, M.D. The use of insulin in open-wound healing. Vet. Med. Small Anim. Clin. 1970, 65, 455–460. [Google Scholar]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Potential of insulin nanoparticle formulations for oral delivery and diabetes treatment. J. Control. Release 2017, 264, 247–275. [Google Scholar] [CrossRef]
- Garcia, M.L.; Calpena, A.C. Biopharmaceutical profile of hydrogels containing pranoprofen-loaded PLGA nanoparticles for skin administration: In vitro, ex vivo and in vivo characterization. Int. J. Pharm. 2016, 501, 350–361. [Google Scholar]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinology 2009, 1, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Jatana, S.; Palmer, B.C.; Phelan, S.J.; Delouise, L.A. Immunomodulatory effects of nanoparticles on skin allergy. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Li, B.; Hou, Y.; Yang, J.; Yi, L. Development of a novel morphological paclitaxel-loaded PLGA microspheres for effective cancer therapy: In vitro and in vivo evaluations. Drug Deliv. 2018, 25, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Choi, K.; Jang, S.; Shin, H. Molecules role of disulfide bonds in the structure and activity of human. Mol. Cells 2003, 16, 323–330. [Google Scholar]
- Pocker, Y.; Biswas, S.B. Conformational dynamics of insulin in solution. Circular dichroic studies. Biochemistry 1980, 19, 5043–5049. [Google Scholar] [CrossRef]
- Mittal, G.; Sahana, D.K.; Bhardwaj, V.; Kumar, M.N.V.R. Estradiol loaded PLGA nanoparticles for oral administration: Effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo vol. J. Control. Release 2007, 119, 77–85. [Google Scholar] [CrossRef]
- Holgado, M.A.; Arias, J.L.; Mun, I.; Co, M.J.; Ferna, M. Insulin-Loaded PLGA microparticles: Flow focusing versus double emulsion solvent evaporation. J. Microencapsul. 2011, 28, 430–441. [Google Scholar]
- Corrigan, O.I.; Li, X. Quantifying drug release from PLGA nanoparticulates. Eur. J. Pharm. Sci. 2009, 37, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef]
- Amaral, M.; Martins, A.S.; Catarino, J.; Faísca, P.; Kumar, P. How can biomolecules improve mucoadhesion of oral insulin? A comprehensive insight using ex vivo, in silico and in vivo models. Biomolecules 2020, 10, 675. [Google Scholar] [CrossRef]
- De Chimie, R.R.; Bercea, M.; Darie, R.N.; Morariu, S. Rheological investigation of xanthan/pluronic F127 hydrogels. Rev. Roum. Chim. 2013, 58, 189–196. [Google Scholar]
- Sutariya, V.; Miladore, N.; Geldenhuys, W.; Bhatia, D.; Wehrung, D.; Nakamura, H. Thermoreversible gel for delivery of activin receptor-like kinase 5 inhibitor SB-505124 for glaucoma filtration surgery. Pharm. Dev. Technol. 2013, 18, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Madibally, S.V.; Solomon, V.; Mitchell, R.N.; van de Water, L.; Yarmush, M.L.; Toner, M. Influence of insulin therapy on burn wound healing in rats. J. Surg. Res. 2003, 100, 92–100. [Google Scholar] [CrossRef]
- Porporato, P.E.; Payen, V.L.; De Saedeleer, C.J.; Préat, V.; Thissen, J.E.; Feron, O.; Sonveaux, P. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012, 15, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.-C.; Yang, K.-C.; Lin, C.-W.; Chen, Y.-K.; Lu, T.-Y.; Chen, H.-Y.; Cheng, N.-C.; Yu, J. Human adipose-derived stem cell secreted extracellular matrix incorporated into electrospun poly(Lactic-co-glycolic acid) nanofibrous dressing for enhancing wound healing. Polymers 2019, 11, 1609. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, Z. Effect of local insulin injection on wound vascularization in patients with diabetic foot ulcer. Exp. Ther. Med. 2016, 11, 397–402. [Google Scholar]
- Bhittani, M.K.; Rehman, M.; Altaf, H.N.; Altaf, O.S. Effectiveness of topical insulin dressings in management of diabetic foot ulcers. World J. Surg. 2019, 44, 2–7. [Google Scholar] [CrossRef]
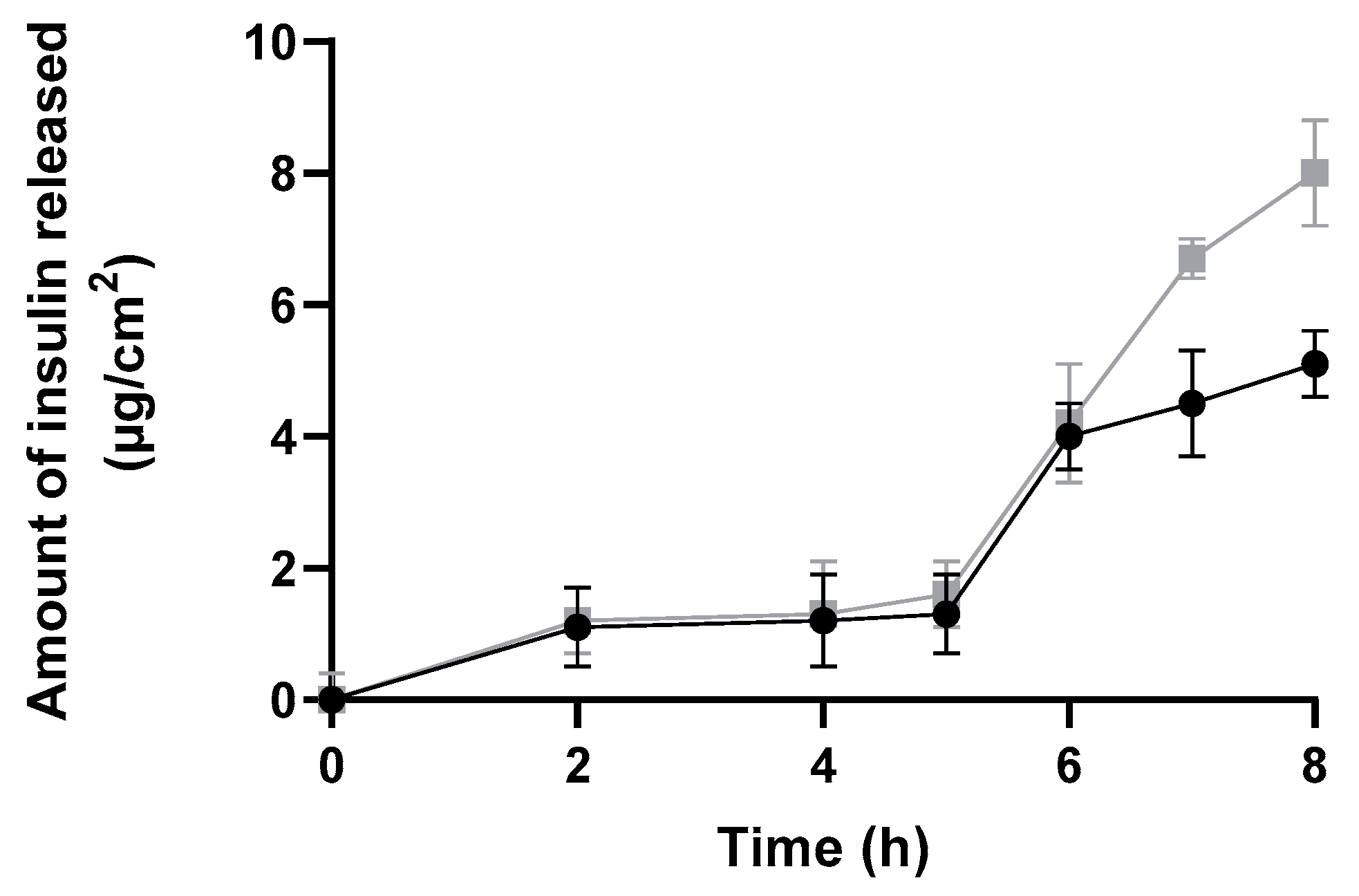
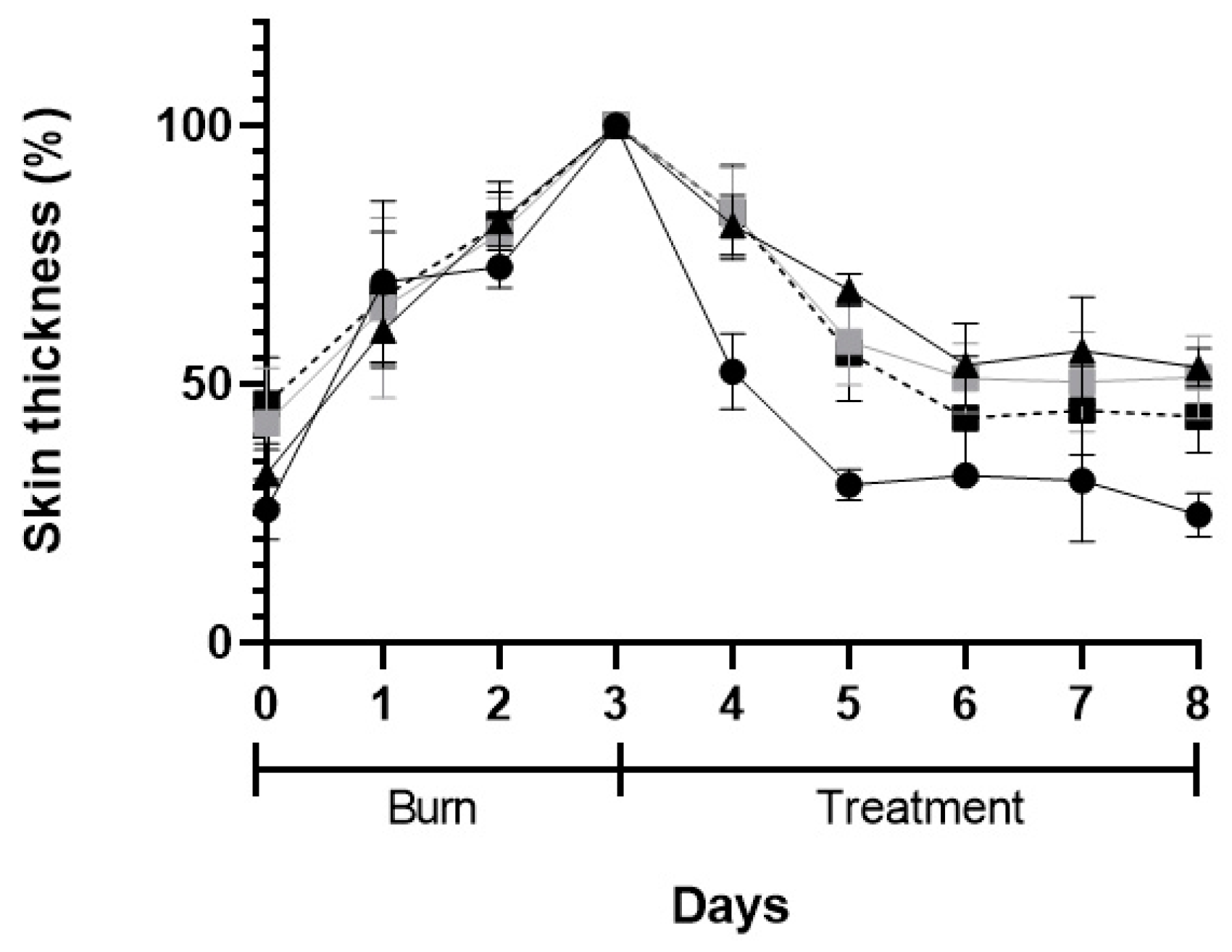
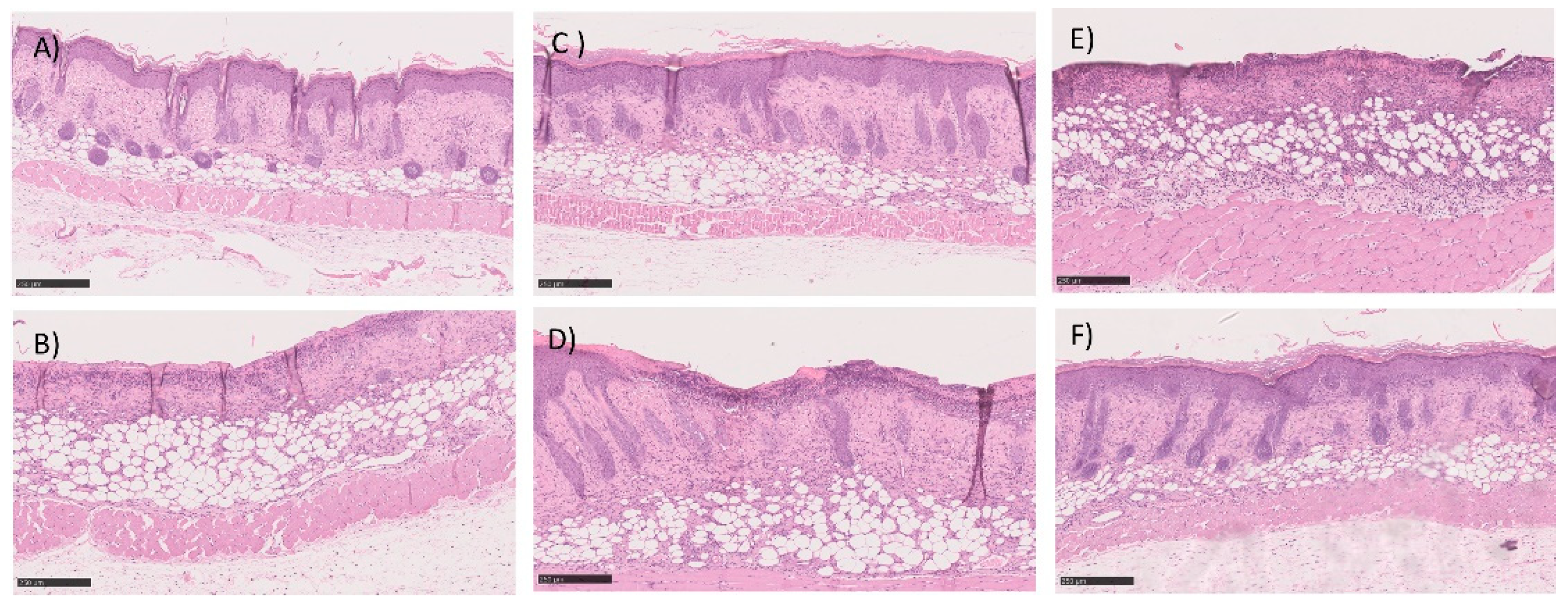
| Formulations and Parameters | Size (nm) | PdI | Zeta Potential (mV) | EE (%) | pH |
|---|---|---|---|---|---|
| Empty NPs | 223 ± 1 | 0.19 ± 0.01 | −1.03 ± 0.26 | - | 5.5 ± 0.1 |
| Insulin-loaded NPs | 557 ± 19 | 0.38 ± 0.02 | 0.07 ± 0.06 | 55.3 ± 0.01 | 7.1 ± 0.2 |
| Insulin-loaded NPs after solvent evaporation | 520 ± 8 | 0.40 ± 0.02 | 0.25 ± 0.12 | 56.4 ± 0.03 | 8.7 ± 0.2 |
| Sample | Concentration (µg/mL) | % of Viable HaCat MTT 12 h | % of Viable HaCat MTT 24 h |
|---|---|---|---|
| Commercial insulin solution in POLX gel | 2.5 | 100.0 ± 4.9 | 79.2 ± 7.2 |
| 20 | 90.1 ± 5.4 | 65.3 ± 2.9 | |
| 80 | 71.5 ± 5.6 | 52.7 ± 7.1 | |
| Empty NPs in POLX gel | 2.5 | 96.8 ± 9.8 | 56.8 ± 8.7 |
| 20 80 | 87.2 ± 7.1 60.4 ± 3.6 | 55.2 ± 6.5 47.7 ± 4.8 | |
| Insulin-loaded NPs in POLX gel | 2.5 | 93.3 ± 6.4 | 46.0 ± 6.1 |
| 20 80 | 69.7 ± 4.6 56.2 ± 9.1 | 39.4 ± 5.6 36.0 ± 1.7 |
| Sample | Day 2 | Day 5 | Day 8 |
|---|---|---|---|
| Negative control (POLX gel) |  | 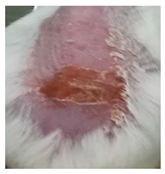 | 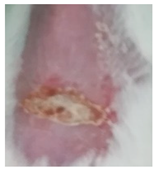 |
| Positive control (Commercial cream of silver sulfadiazine) | 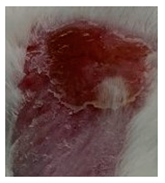 | 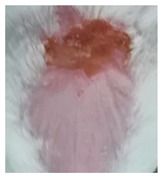 |  |
| Commercial insulin solution in POLX gel | 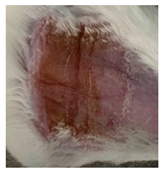 |  | 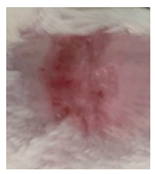 |
| Insulin-loaded NPs in POLX gel |  | 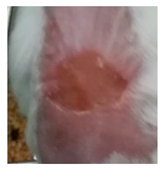 | 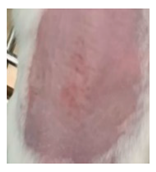 |
| Samples | Epidermal Closure | Epidermal Differentiation | Amount of Granulation | Inflammatory Infiltration | Collagen Fiber Orientation | Pattern of Collagen | Total Score |
|---|---|---|---|---|---|---|---|
| Negative control (POLX gel) | 0.5 ± 0.4 | 1.0 ± 0.7 | 3.0 ± 0.7 | 2.5 ± 0.4 | 2.5 ± 0.4 | 2.5 ± 0.4 | 12.0 ± 2.8 |
| Positive control (Commercial cream of silver sulfadiazine) | 0.7 ± 0.3 | 1.3 ± 0.5 | 3.3 ± 0.5 | 2.7 ± 0.3 | 2.3 ± 0.5 | 2.3 ± 0.5 | 12.7 ± 2.7 |
| Commercial insulin solution in POLX gel | 0.8 ± 0.2 | 1.5 ± 0.4 | 3.5 ± 0.4 | 2.3 ± 0.2 | 2.8 ± 0.2 | 2.8 ± 0.2 | 13.5 ± 1.6 |
| Insulin-loaded NPs in POLX gel | 0.8 ± 0.2 | 1.5 ± 0.4 | 3.5 ± 0.4 | 2.5 ± 0.3 | 2.8 ± 0.2 | 2.8 ± 0.2 | 13.8 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quitério, M.; Simões, S.; Ascenso, A.; Carvalheiro, M.; Leandro, A.P.; Correia, I.; Viana, A.S.; Faísca, P.; Ascensão, L.; Molpeceres, J.; et al. Development of a Topical Insulin Polymeric Nanoformulation for Skin Burn Regeneration: An Experimental Approach. Int. J. Mol. Sci. 2021, 22, 4087. https://doi.org/10.3390/ijms22084087
Quitério M, Simões S, Ascenso A, Carvalheiro M, Leandro AP, Correia I, Viana AS, Faísca P, Ascensão L, Molpeceres J, et al. Development of a Topical Insulin Polymeric Nanoformulation for Skin Burn Regeneration: An Experimental Approach. International Journal of Molecular Sciences. 2021; 22(8):4087. https://doi.org/10.3390/ijms22084087
Chicago/Turabian StyleQuitério, Maria, Sandra Simões, Andreia Ascenso, Manuela Carvalheiro, Ana Paula Leandro, Isabel Correia, Ana Silveira Viana, Pedro Faísca, Lia Ascensão, Jesús Molpeceres, and et al. 2021. "Development of a Topical Insulin Polymeric Nanoformulation for Skin Burn Regeneration: An Experimental Approach" International Journal of Molecular Sciences 22, no. 8: 4087. https://doi.org/10.3390/ijms22084087
APA StyleQuitério, M., Simões, S., Ascenso, A., Carvalheiro, M., Leandro, A. P., Correia, I., Viana, A. S., Faísca, P., Ascensão, L., Molpeceres, J., Gaspar, M. M., & Reis, C. P. (2021). Development of a Topical Insulin Polymeric Nanoformulation for Skin Burn Regeneration: An Experimental Approach. International Journal of Molecular Sciences, 22(8), 4087. https://doi.org/10.3390/ijms22084087











