A Novel Volume-Stable Collagen Matrix Induces Changes in the Behavior of Primary Human Oral Fibroblasts, Periodontal Ligament, and Endothelial Cells
Abstract
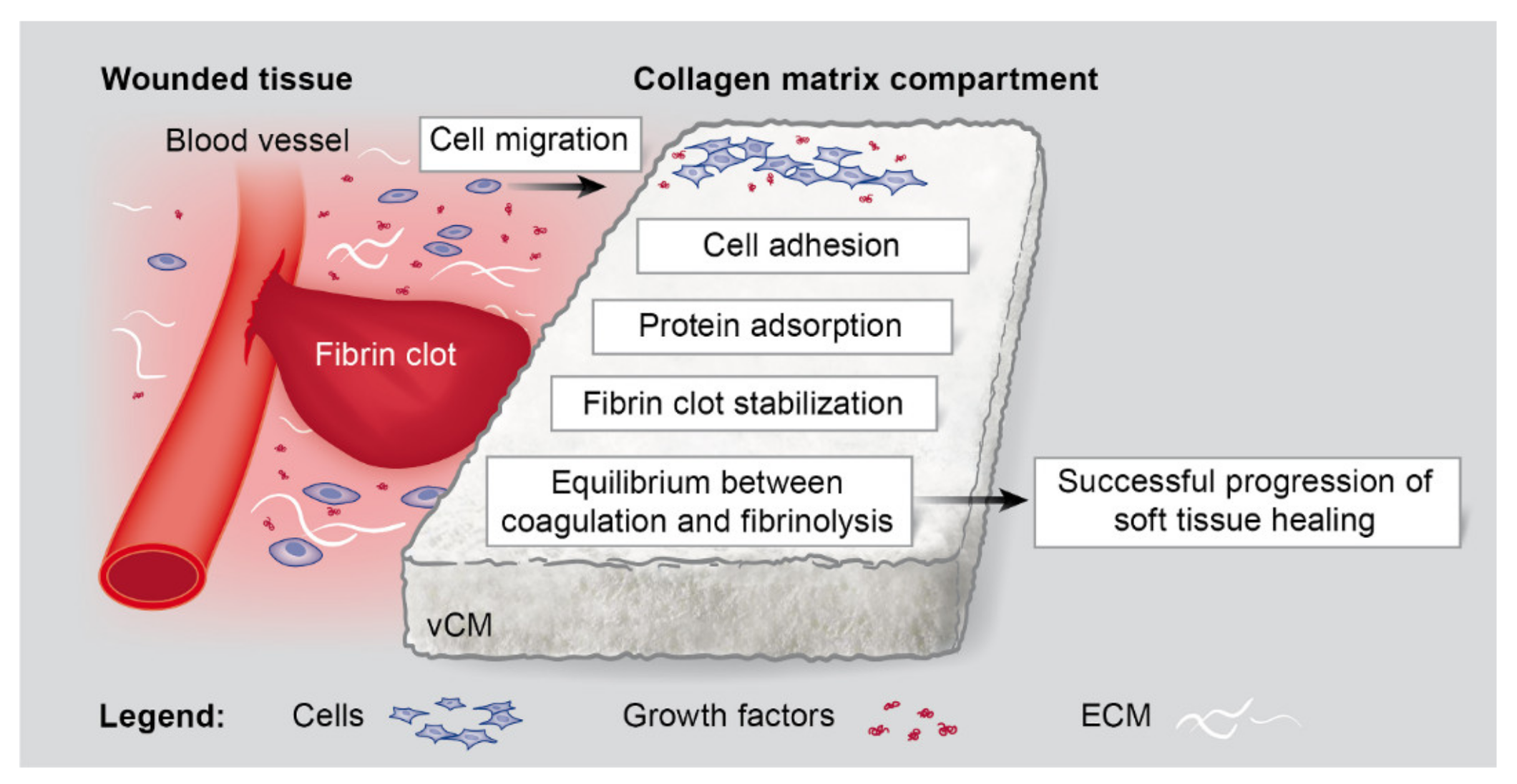
1. Introduction
2. Results
2.1. Strongly Increased Migratory Potential of Primary hPDLCs, hOFs, and HUVECs toward the Volume-Stable Collagen Matrix
2.2. Significantly Increased Adhesive Properties of Primary hPDLCs, hOFs, and HUVECs on the Volume-Stable Collagen Matrix
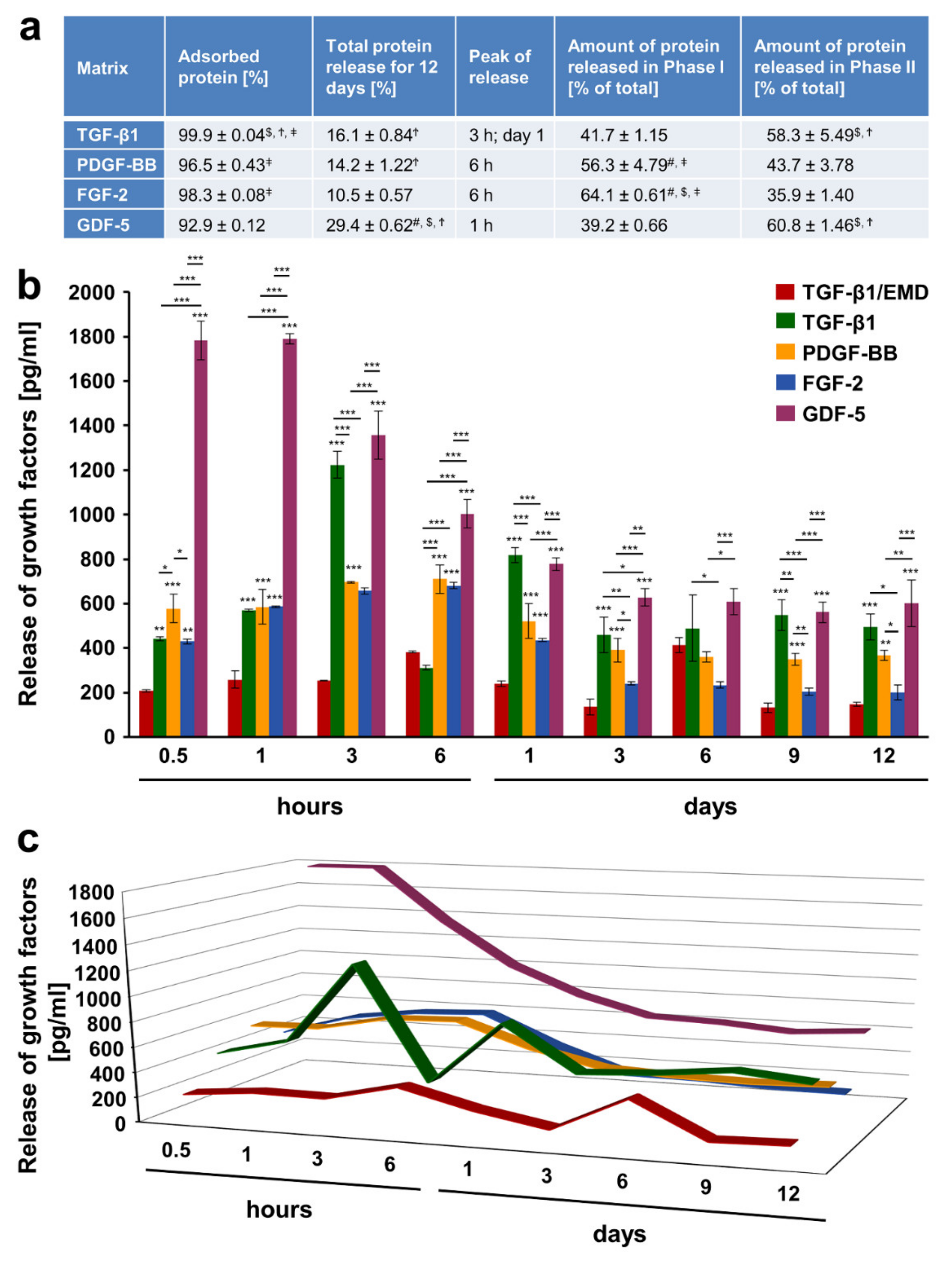
2.3. Adsorption and Release of Recombinant Growth Factors from the Volume-Stable Collagen Matrix over a 12-Day Period
2.4. Release Kinetics of Growth Factors from the Volume-Stable Collagen Matrix
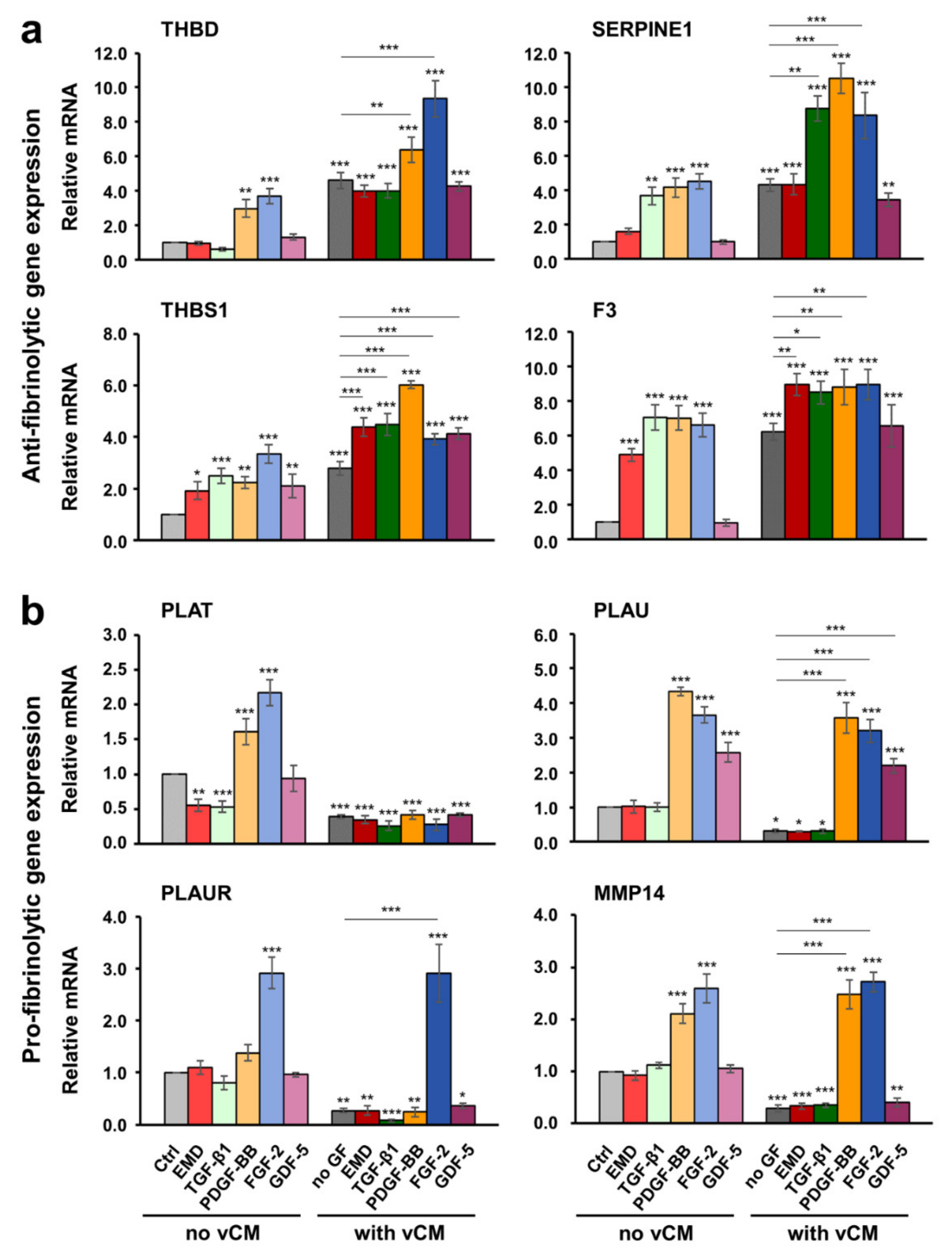
2.5. Increased Antifibrinolytic and Inhibited Profibrinolytic Gene Expression in Primary hPDLCs, hOFs, and HUVECs Grown on the Native/Uncoated Volume-Stable Collagen Matrix
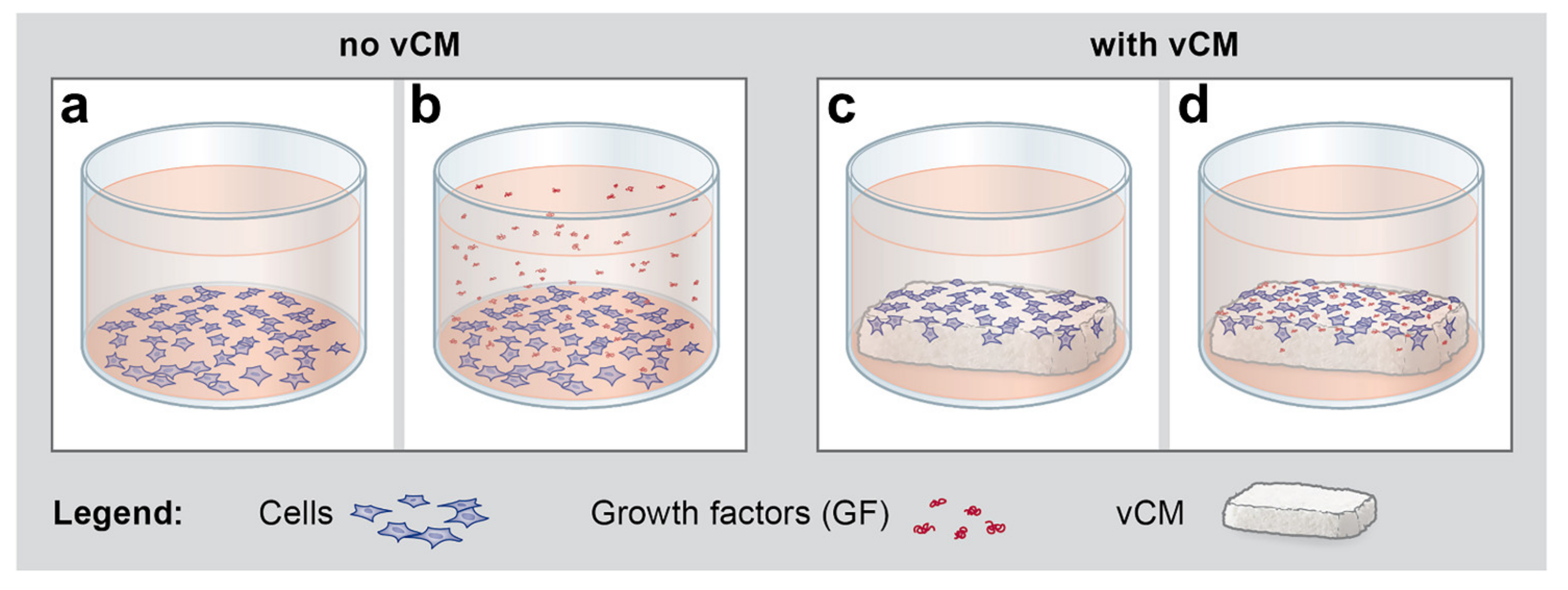
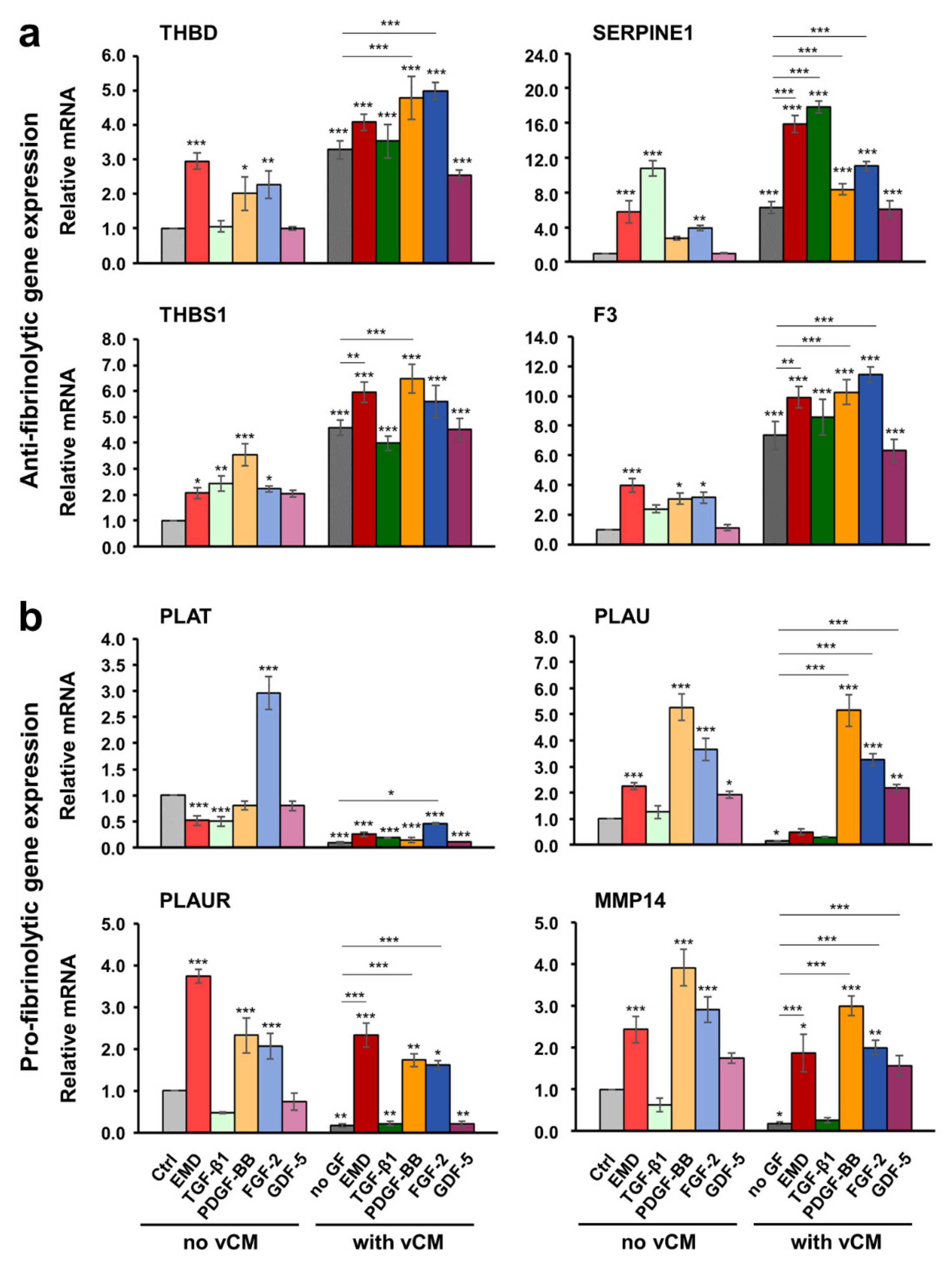
2.6. Growth Factors Applied in Suspension or as a Coating on the Volume-Stable Collagen Matrix Exhibit Cell Type-Dependent Effects on Anti- and Pro-fibrinolytic Gene Expression. Potential Contribution of Growth factors to the Equilibrium between Coagulation and Fibrinolysis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Migration Assay
4.3. Cell Adhesion Assay
4.4. Adsorption and Release of Growth Factors from Volume-Stable Collagen Matrix
4.5. Gene Expression Analyses by qRT-PCR
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vignoletti, F.; Nunez, J.; Sanz, M. Soft tissue wound healing at teeth, dental implants and the edentulous ridge when using barrier membranes, growth and differentiation factors and soft tissue substitutes. J. Clin. Periodontol. 2014, 41 (Suppl. S15), S23–S35. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Cortellini, P.; Pellegrini, G.; Nieri, M.; Bonaccini, D.; Allegri, M.; Bouchard, P.; Cairo, F.; Conforti, G.; Fourmousis, I.; et al. Xenogenic collagen matrix or autologous connective tissue graft as adjunct to coronally advanced flaps for coverage of multiple adjacent gingival recession: Randomized trial assessing non-inferiority in root coverage and superiority in oral health-related quality of life. J. Clin. Periodontol. 2018, 45, 78–88. [Google Scholar] [CrossRef]
- Cosgarea, R.; Juncar, R.; Arweiler, N.; Lascu, L.; Sculean, A. Clinical evaluation of a porcine acellular dermal matrix for the treatment of multiple adjacent class I, II, and III gingival recessions using the modified coronally advanced tunnel technique. Quintessence Int. 2016, 47, 739–747. [Google Scholar] [CrossRef]
- Shirakata, Y.; Sculean, A.; Shinohara, Y.; Sena, K.; Takeuchi, N.; Bosshardt, D.D.; Noguchi, K. Healing of localized gingival recessions treated with a coronally advanced flap alone or combined with an enamel matrix derivative and a porcine acellular dermal matrix: A preclinical study. Clin. Oral Investig. 2016, 20, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Pietruska, M.; Skurska, A.; Podlewski, Ł.; Milewski, R.; Pietruski, J. Clinical evaluation of Miller class I and II recessions treatment with the use of modified coronally advanced tunnel technique with either collagen matrix or subepithelial connective tissue graft: A randomized clinical study. J. Clin. Periodontol. 2019, 46, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Leong, S.; Liang, Y.-C.; Huang, R.C.C.; Chen, C.S.; Yu, S.M. Immobilization of growth factors on collagen scaffolds mediated by polyanionic collagen mimetic peptides and its effect on endothelial cell morphogenesis. Biomacromolecules 2008, 9, 2929–2936. [Google Scholar] [CrossRef]
- Nica, C.; Lin, Z.; Sculean, A.; Asparuhova, M.B. Adsorption and release of growth factors from four different porcine-derived collagen matrices. Materials 2020, 13, 2635. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Nica, C.; Sculean, A.; Asparuhova, M.B. Enhanced Wound Healing Potential of Primary Human Oral Fibroblasts and Periodontal Ligament Cells Cultured on Four Different Porcine-Derived Collagen Matrices. Materials 2020, 13, 3819. [Google Scholar] [CrossRef]
- Fischer, K.R.; Fickl, S.; Mardas, N.; Bozec, L.; Donos, N. Stage-two surgery using collagen soft tissue grafts: Clinical cases and ultrastructural analysis. Quintessence Int. 2014, 45, 853–860. [Google Scholar] [CrossRef]
- Sculean, A.; Mihatovic, I.; Shirakata, Y.; Bosshardt, D.D.; Schwarz, F.; Iglhaut, G. Healing of localized gingival recessions treated with coronally advanced flap alone or combined with either a resorbable collagen matrix or subepithelial connective tissue graft. A preclinical study. Clin. Oral Investig. 2015, 19, 903–909. [Google Scholar] [CrossRef]
- Moreira, A.R.O.; Santamaria, M.P.; Silvério, K.G.; Casati, M.Z.; Nociti Junior, F.H.; Sculean, A.; Sallum, E.A. Coronally advanced flap with or without porcine collagen matrix for root coverage: A randomized clinical trial. Clin. Oral Investig. 2016, 20, 2539–2549. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Moest, T.; Lutz, R.; Wehrhan, F.; Neukam, F.W.; Schlegel, K.A. Long-term outcomes after vestibuloplasty with a porcine collagen matrix (Mucograft(®)) versus the free gingival graft: A comparative prospective clinical trial. Clin. Oral Implants Res. 2016, 27, e125–e133. [Google Scholar] [CrossRef] [PubMed]
- Rotundo, R.; Genzano, L.; Patel, D.; D’Aiuto, F.; Nieri, M. Adjunctive benefit of a xenogenic collagen matrix associated with coronally advanced flap for the treatment of multiple gingival recessions: A superiority, assessor-blind, randomized clinical trial. J. Clin. Periodontol. 2019, 46, 1013–1023. [Google Scholar] [CrossRef]
- De Resende, D.R.B.; Greghi, S.L.A.; Siqueira, A.F.; Benfatti, C.A.M.; Damante, C.A.; Ragghianti Zangrando, M.S. Acellular dermal matrix allograft versus free gingival graft: A histological evaluation and split-mouth randomized clinical trial. Clin. Oral Investig. 2019, 23, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Cieślik-Wegemund, M.; Wierucka-Młynarczyk, B.; Tanasiewicz, M.; Gilowski, Ł. Tunnel Technique With Collagen Matrix Compared With Connective Tissue Graft for Treatment of Periodontal Recession: A Randomized Clinical Trial. J. Periodontol. 2016, 87, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Barootchi, S.; Di Gianfilippo, R.; Modarressi, M.; Cairo, F.; Rasperini, G.; Wang, H.L. Acellular dermal matrix and coronally advanced flap or tunnel technique in the treatment of multiple adjacent gingival recessions. A 12-year follow-up from a randomized clinical trial. J. Clin. Periodontol. 2019, 46, 937–948. [Google Scholar] [CrossRef]
- Matoh, U.; Petelin, M.; Gašperšič, R. Split-Mouth Comparison of Coronally Advanced Flap with Connective Tissue Graft or Collagen Matrix for Treatment of Isolated Gingival Recessions. Int. J. Periodontics Restor. Dent. 2019, 39, 439–446. [Google Scholar] [CrossRef]
- Wei, P.C.; Laurell, L.; Lingen, M.W.; Geivelis, M. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 2. A histological comparative study. J. Periodontol. 2002, 73, 257–265. [Google Scholar] [CrossRef]
- Núñez, J.; Caffesse, R.; Vignoletti, F.; Guerra, F.; San Roman, F.; Sanz, M. Clinical and histological evaluation of an acellular dermal matrix allograft in combination with the coronally advanced flap in the treatment of Miller class I recession defects: An experimental study in the mini-pig. J. Clin. Periodontol. 2009, 36, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.; Hüsler, J.; Jung, R.E. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef]
- Mathes, S.H.; Wohlwend, L.; Uebersax, L.; von Mentlen, R.; Thoma, D.S.; Jung, R.E.; Görlach, C.; Graf-Hausner, U. A bioreactor test system to mimic the biological and mechanical environment of oral soft tissues and to evaluate substitutes for connective tissue grafts. Biotechnol. Bioeng. 2010, 107, 1029–1039. [Google Scholar] [CrossRef]
- Thoma, D.S.; Jung, R.E.; Schneider, D.; Cochran, D.L.; Ender, A.; Jones, A.A.; Görlach, C.; Uebersax, L.; Graf-Hausner, U.; Hämmerle, C.H. Soft tissue volume augmentation by the use of collagen-based matrices: A volumetric analysis. J. Clin. Periodontol. 2010, 37, 659–666. [Google Scholar] [CrossRef]
- Ferrantino, L.; Bosshardt, D.; Nevins, M.; Santoro, G.; Simion, M.; Kim, D. Tissue Integration of a Volume-Stable Collagen Matrix in an Experimental Soft Tissue Augmentation Model. Int. J. Periodontics Restorative Dent. 2016, 36, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Zeltner, M.; Jung, R.E.; Hämmerle, C.H.; Hüsler, J.; Thoma, D.S. Randomized controlled clinical study comparing a volume-stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: Linear volumetric soft tissue changes up to 3 months. J. Clin. Periodontol. 2017, 44, 446–453. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue Response to a Porous Collagen Matrix Used for Soft Tissue Augmentation. Materials 2019, 12, 3721. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Zhang, S.; Sculean, A.; Staehli, A.; Bosshardt, D.D. Tissue Integration and Degradation of a Porous Collagen-Based Scaffold Used for Soft Tissue Augmentation. Materials 2020, 13, 2420. [Google Scholar] [CrossRef] [PubMed]
- Imber, J.C.; Bosshardt, D.D.; Stähli, A.; Saulacic, N.; Deschner, J.; Sculean, A. Pre-clinical evaluation of the effect of a volume-stable collagen matrix on periodontal regeneration in two-wall intrabony defects. J. Clin. Periodontol. 2021. [Google Scholar] [CrossRef]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41, S6–S22. [Google Scholar] [CrossRef]
- Tezono, K.; Sarker, K.P.; Kikuchi, H.; Nasu, M.; Kitajima, I.; Maruyama, I. Bioactivity of the vascular endothelial growth factor trapped in fibrin clots: Production of IL-6 and IL-8 in monocytes by fibrin clots. Haemostasis 2001, 31, 71–79. [Google Scholar] [CrossRef]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef]
- Mosher, D.F.; Misenheimer, T.M.; Stenflo, J.; Hogg, P.J. Modulation of fibrinolysis by thrombospondin. Ann. N. Y. Acad. Sci. 1992, 667, 64–69. [Google Scholar] [CrossRef]
- Anonick, P.K.; Yoo, J.K.; Webb, D.J.; Gonias, S.L. Characterization of the antiplasmin activity of human thrombospondin-1 in solution. Biochem. J. 1993, 289 (Pt 3), 903–909. [Google Scholar] [CrossRef]
- Poon, I.K.; Patel, K.K.; Davis, D.S.; Parish, C.R.; Hulett, M.D. Histidine-rich glycoprotein: The Swiss Army knife of mammalian plasma. Blood 2011, 117, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Landmesser, U.; Rauch, U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc. Med. 2016, 26, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Suzuki, Y.; Iwaki, T.; Sano, H.; Honkura, N.; Castellino, F.J. Recognition of Plasminogen Activator Inhibitor Type 1 as the Primary Regulator of Fibrinolysis. Curr. Drug Targets 2019, 20, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Cesarman-Maus, G.; Hajjar, K.A. Molecular mechanisms of fibrinolysis. Br. J. Haematol. 2005, 129, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Pluskota, E.; Soloviev, D.A.; Plow, E.F. Convergence of the adhesive and fibrinolytic systems: Recognition of urokinase by integrin alpha Mbeta 2 as well as by the urokinase receptor regulates cell adhesion and migration. Blood 2003, 101, 1582–1590. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. Primary hyperfibrinolysis: Facts and fancies. Thromb. Res. 2018, 166, 71–75. [Google Scholar] [CrossRef]
- Juhan-Vague, I.; Alessi, M.C.; Morange, P.E. Hypofibrinolysis and increased PAI-1 are linked to atherothrombosis via insulin resistance and obesity. Ann. Med. 2000, 32 (Suppl. S1), 78–84. [Google Scholar]
- Mah, W.; Jiang, G.; Olver, D.; Cheung, G.; Kim, B.; Larjava, H.; Häkkinen, L. Human Gingival Fibroblasts Display a Non-Fibrotic Phenotype Distinct from Skin Fibroblasts in Three-Dimensional Cultures. PLoS ONE 2014, 9, e90715. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, M. Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef]
- Bachetti, T.; Morbidelli, L. Endothelial cells in culture: A model for studying vascular functions. Pharmacol. Res. 2000, 42, 9–19. [Google Scholar] [CrossRef]
- Cochran, D.L.; Oh, T.J.; Mills, M.P.; Clem, D.S.; McClain, P.K.; Schallhorn, R.A.; McGuire, M.K.; Scheyer, E.T.; Giannobile, W.V.; Reddy, M.S.; et al. A randomized clinical trial evaluating rh-FGF-2/β-TCP in periodontal defects. J. Dent. Res. 2016, 95, 523–530. [Google Scholar] [CrossRef]
- Sangiorgio, J.P.M.; Neves, F.; Rocha Dos Santos, M.; França-Grohmann, I.L.; Casarin, R.C.V.; Casati, M.Z.; Santamaria, M.P.; Sallum, E.A. Xenogenous collagen matrix and/or enamel matrix derivative for treatment of localized gingival recessions: A randomized clinical trial. Part I: Clinical outcomes. J. Periodontol. 2017, 88, 1309–1318. [Google Scholar] [CrossRef]
- Gruber, R.; Karreth, F.; Kandler, B.; Fuerst, G.; Rot, A.; Fischer, M.B.; Watzek, G. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets 2004, 15, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Mozgan, E.-M.; Edelmayer, M.; Janjić, K.; Pensch, M.; Fischer, M.B.; Moritz, A.; Agis, H. Release kinetics and mitogenic capacity of collagen barrier membranes supplemented with secretome of activated platelets—The in vitro response of fibroblasts of the periodontal ligament and the gingiva. BMC Oral Health 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Stähli, A.; Miron, R.J.; Bosshardt, D.D.; Sculean, A.; Gruber, R. Collagen membranes adsorb the transforming growth factor-β receptor I kinase-dependent activity of enamel matrix derivative. J. Periodontol. 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aroca, S.; Molnár, B.; Windisch, P.; Gera, I.; Salvi, G.E.; Nikolidakis, D.; Sculean, A. Treatment of multiple adjacent Miller class I and II gingival recessions with a Modified Coronally Advanced Tunnel (MCAT) technique and a collagen matrix or palatal connective tissue graft: A randomized, controlled clinical trial. J. Clin. Periodontol. 2013, 40, 713–720. [Google Scholar] [CrossRef]
- Enriquez-Ochoa, D.; Robles-Ovalle, P.; Mayolo-Deloisa, K.; Brunck, M.E.G. Immobilization of Growth Factors for Cell Therapy Manufacturing. Front. Bioeng. Biotechnol. 2020, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okada, H.; Takemura, G.; Takada, C.; Tomita, H.; Yano, H.; Muraki, I.; Zaikokuji, R.; Kuroda, A.; Fukuda, H.; et al. Recombinant thrombomodulin protects against LPS-induced acute respiratory distress syndrome via preservation of pulmonary endothelial glycocalyx. Br. J. Pharmacol. 2020, 177, 4021–4033. [Google Scholar] [CrossRef] [PubMed]
- Linard, C.; Tissedre, F.; Busson, E.; Holler, V.; Leclerc, T.; Strup-Perrot, C.; Couty, L.; L’Homme, B.; Benderitter, M.; Lafont, A.; et al. Therapeutic potential of gingival fibroblasts for cutaneous radiation syndrome: Comparison to bone marrow-mesenchymal stem cell grafts. Stem Cells Dev. 2015, 24, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qiu, X.; Zhang, Y.; Fu, K.; Zhao, X.; Wu, J.; Hu, Y.; Zhu, W.; Guo, H. Basic Fibroblast Growth Factor Ameliorates Endothelial Dysfunction in Radiation-Induced Bladder Injury. BioMed Res. Int. 2015, 2015, 967680. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.C.; Lo, I.C.; Huang, Y.W.; Tsai, I.C.; Cheng, H.P.; Shi, G.Y.; Wu, H.L.; Jiang, M.J. The chondroitin sulfate moiety mediates thrombomodulin-enhanced adhesion and migration of vascular smooth muscle cells. J. Biomed. Sci. 2018, 25, 14. [Google Scholar] [CrossRef] [PubMed]
- Lo, I.C.; Lin, T.M.; Chou, L.H.; Liu, S.L.; Wu, L.W.; Shi, G.Y.; Wu, H.L.; Jiang, M.J. Ets-1 mediates platelet-derived growth factor-BB-induced thrombomodulin expression in human vascular smooth muscle cells. Cardiovasc. Res. 2009, 81, 771–779. [Google Scholar] [CrossRef]
- Kaneko, T.; Fujii, S.; Matsumoto, A.; Goto, D.; Ishimori, N.; Watano, K.; Furumoto, T.; Sugawara, T.; Sobel, B.E.; Kitabatake, A. Induction of plasminogen activator inhibitor-1 in endothelial cells by basic fibroblast growth factor and its modulation by fibric acid. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Choung, H.W.; Lim, K.T.; Jin, B.; Jin, C.; Chung, J.H.; Choung, P.H. Recombinant Human Plasminogen Activator Inhibitor-1 Promotes Cementogenic Differentiation of Human Periodontal Ligament Stem Cells. Tissue Eng. Part A 2015, 21, 2817–2828. [Google Scholar] [CrossRef]
- Aburima, A.; Berger, M.; Spurgeon, B.E.J.; Webb, B.A.; Wraith, K.S.; Febbraio, M.; Poole, A.W.; Naseem, K.M. Thrombospondin-1 promotes hemostasis through modulation of cAMP signaling in blood platelets. Blood 2021, 137, 678–689. [Google Scholar] [CrossRef]
- Tan, K.; Lawler, J. The interaction of Thrombospondins with extracellular matrix proteins. J. Cell Commun. Signal. 2009, 3, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Jin, L.; Ruebel, K.H.; Scheithauer, B.W.; Lloyd, R.V. Regulation of VEGF-A, VEGFR-I, thrombospondin-1, -2, and -3 expression in a human pituitary cell line (HP75) by TGFbeta1, bFGF, and EGF. Endocrine 2004, 24, 141–146. [Google Scholar] [CrossRef]
- Breitkopf, K.; Sawitza, I.; Westhoff, J.H.; Wickert, L.; Dooley, S.; Gressner, A.M. Thrombospondin 1 acts as a strong promoter of transforming growth factor beta effects via two distinct mechanisms in hepatic stellate cells. Gut 2005, 54, 673–681. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Poczatek, M. Activation of latent TGF-beta by thrombospondin-1: Mechanisms and physiology. Cytokine Growth Factor Rev. 2000, 11, 59–69. [Google Scholar] [CrossRef]
- Belotti, D.; Capelli, C.; Resovi, A.; Introna, M.; Taraboletti, G. Thrombospondin-1 promotes mesenchymal stromal cell functions via TGFβ and in cooperation with PDGF. Matrix Biol. 2016, 55, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.M.; Helkin, A.; Maier, K.G.; Gahtan, V. Thrombospondins Differentially Regulate Proteins Involved in Arterial Remodeling. Physiol. Res. 2019, 68, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Agis, H.; Bauer, M.; Knebl, G.; Watzek, G.; Gruber, R. Effects of platelet-derived growth factor isoforms on plasminogen activation by periodontal ligament and gingival fibroblasts. J. Periodontal. Res. 2008, 43, 334–342. [Google Scholar] [CrossRef]
- Patil, V.A.; Masters, K.S. Engineered Collagen Matrices. Bioengineering 2020, 7, 163. [Google Scholar] [CrossRef]
- Gurbuz, I.; Ferralli, J.; Roloff, T.; Chiquet-Ehrismann, R.; Asparuhova, M.B. SAP domain-dependent Mkl1 signaling stimulates proliferation and cell migration by induction of a distinct gene set indicative of poor prognosis in breast cancer patients. Mol. Cancer 2014, 13, 22. [Google Scholar] [CrossRef]
- Parisi, L.; Buser, D.; Chappuis, V.; Asparuhova, M.B. Cellular responses to deproteinized bovine bone mineral biofunctionalized with bone-conditioned medium. Clin. Oral Investig. 2020, 1–15. [Google Scholar] [CrossRef]


| Gene Symbol | Gene Bank Accession Number | Primer Pair (fwd/rev) | Amplicon Size (bp) |
|---|---|---|---|
| THBD | NM_000361 | 5′- TCCTCTGCGAGTTCCACTTC-3’ 5′- GCCGTAGGTGATCGAGACG-3’ | 89 |
| SERPINE1 | NM_001165413 | 5′- AGTGGACTTTTCAGAGGTGGA-3’ 5′- GCCGTTGAAGTAGAGGGCATT-3’ | 151 |
| THBS1 | NM_003246.3 | 5′- ATCCGCAAAGTGACTGAAGAG-3’ 5′- GCAGATGGTAACTGAGTTCTGAC-3’ | 152 |
| F3 | NM_001993 | 5′- TAAGCACTAAGTCAGGAGATTGG-3’ 5′- TGTCCGAGGTTTGTCTCCAG-3’ | 216 |
| PLAT | NM_000930 | 5′- AAACCCAGATCGAGACTCAAAGC-3’ 5′- GGTAGGCTGACCCATTCCC-3’ | 131 |
| PLAU | NM_001145031 | 5′- GGGAATGGTCACTTTTACCGAG-3’ 5′- GGGCATGGTACGTTTGCTG-3’ | 103 |
| PLAUR | NM_001005377 | 5′- GAGCTATCGGACTGGCTTGAA-3’ 5′- CGGCTTCGGGAATAGGTGAC-3’ | 108 |
| MMP14 | NM_004995.3 | 5′- TCAAAGGAGACAAGCATTGGG-3’ 5′- CGGTAGTACTTGTTTCCACGG-3’ | 165 |
| GAPDH | NM_001256799.2 | 5′- ATCAAGAAGGTGGTGAAGCAG-3’ 5′- TCGTTGTCATACCAGGAAATGAG-3’ | 178 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asparuhova, M.B.; Stähli, A.; Guldener, K.; Sculean, A. A Novel Volume-Stable Collagen Matrix Induces Changes in the Behavior of Primary Human Oral Fibroblasts, Periodontal Ligament, and Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 4051. https://doi.org/10.3390/ijms22084051
Asparuhova MB, Stähli A, Guldener K, Sculean A. A Novel Volume-Stable Collagen Matrix Induces Changes in the Behavior of Primary Human Oral Fibroblasts, Periodontal Ligament, and Endothelial Cells. International Journal of Molecular Sciences. 2021; 22(8):4051. https://doi.org/10.3390/ijms22084051
Chicago/Turabian StyleAsparuhova, Maria B., Alexandra Stähli, Kevin Guldener, and Anton Sculean. 2021. "A Novel Volume-Stable Collagen Matrix Induces Changes in the Behavior of Primary Human Oral Fibroblasts, Periodontal Ligament, and Endothelial Cells" International Journal of Molecular Sciences 22, no. 8: 4051. https://doi.org/10.3390/ijms22084051
APA StyleAsparuhova, M. B., Stähli, A., Guldener, K., & Sculean, A. (2021). A Novel Volume-Stable Collagen Matrix Induces Changes in the Behavior of Primary Human Oral Fibroblasts, Periodontal Ligament, and Endothelial Cells. International Journal of Molecular Sciences, 22(8), 4051. https://doi.org/10.3390/ijms22084051








