β Cell GHS-R Regulates Insulin Secretion and Sensitivity
Abstract
1. Introduction
2. Results
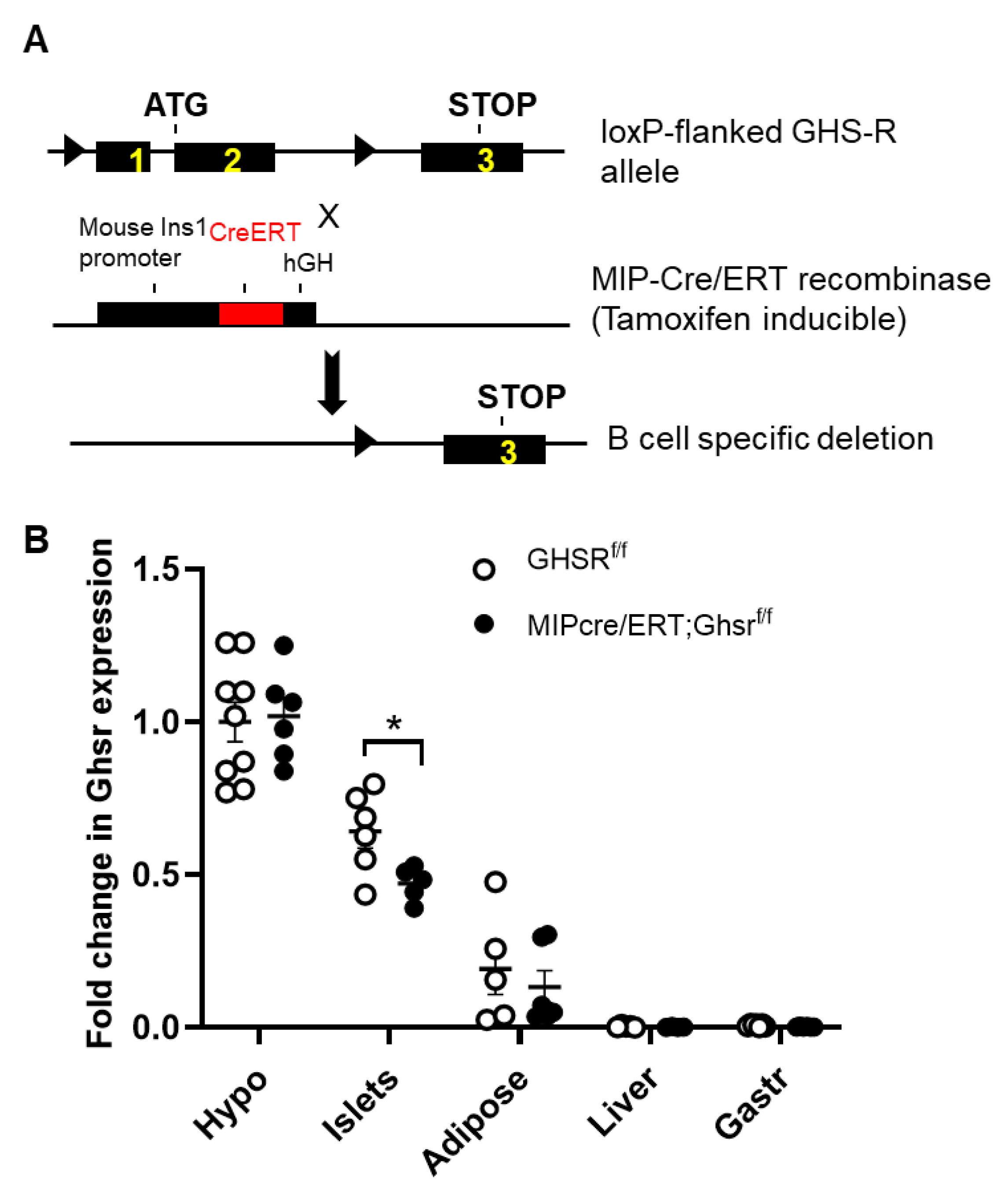
2.1. Validation of GHS-R Localization in Pancreatic Islets and Generation of MIP-Cre/ERT;Ghsrf/f Mice
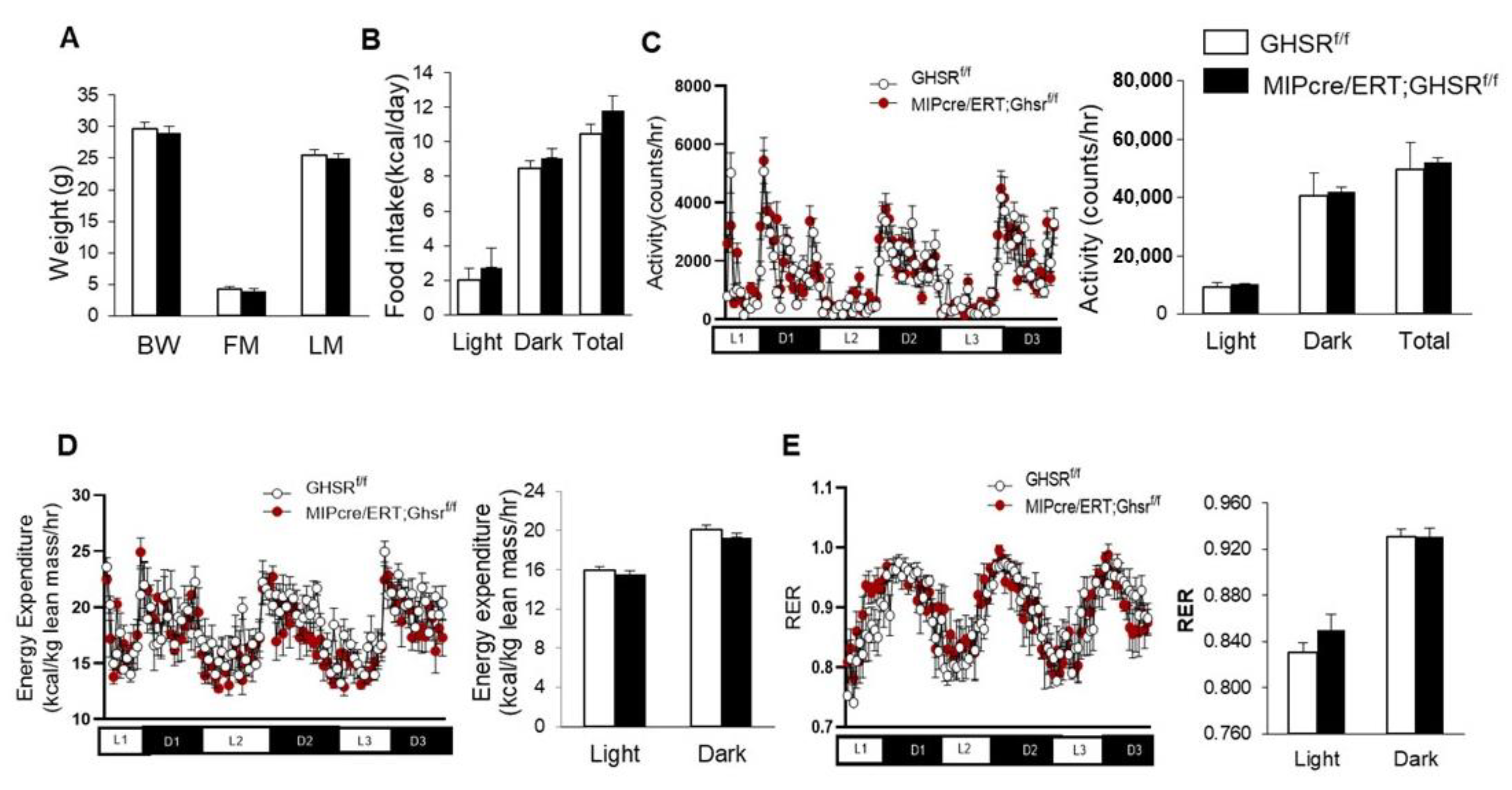
2.2. β-Cell-Specific GHS-R Deletion Had No Effects on Energy Homeostasis
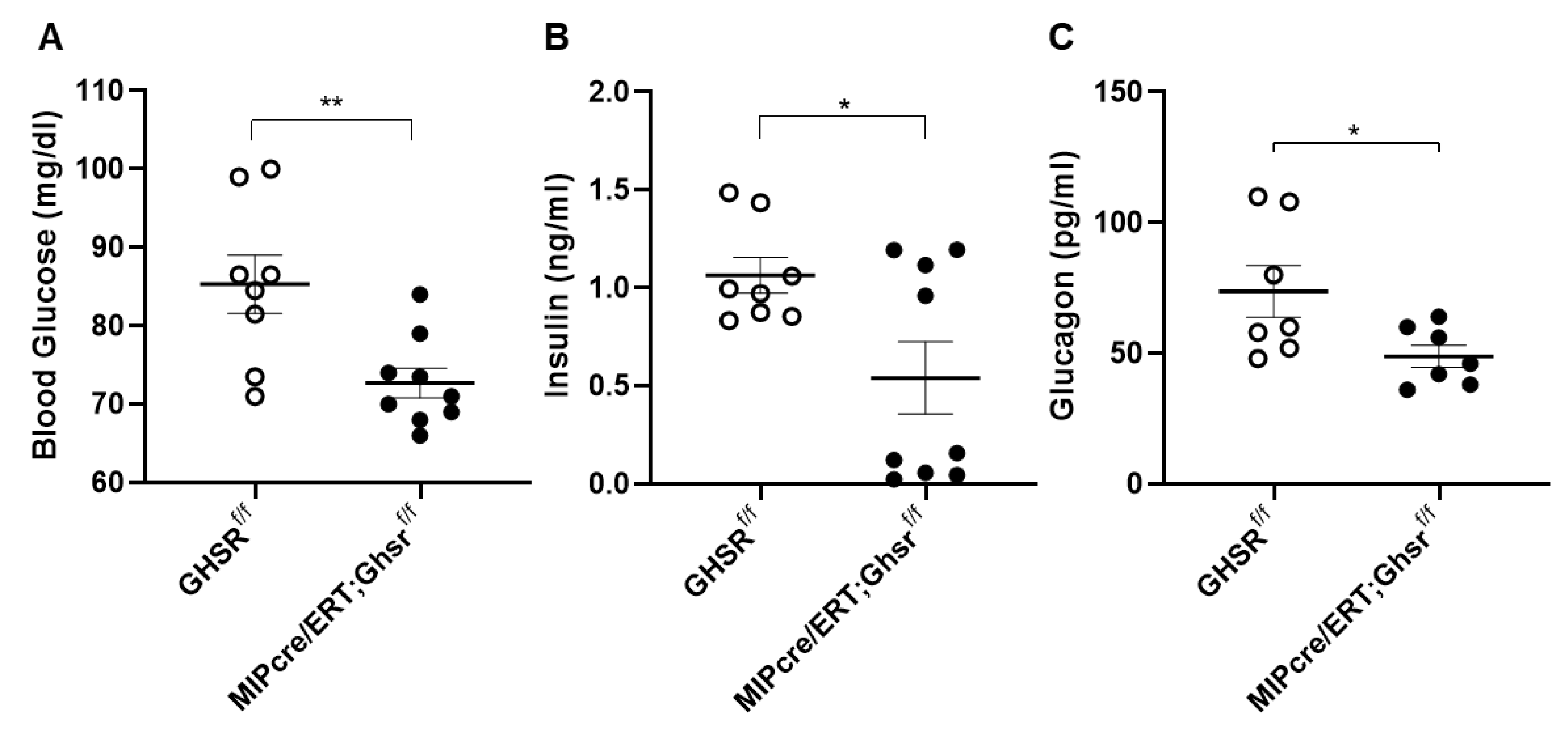
2.3. β-Cell-Specific GHS-R Deletion Regulated Glucose Homeostasis
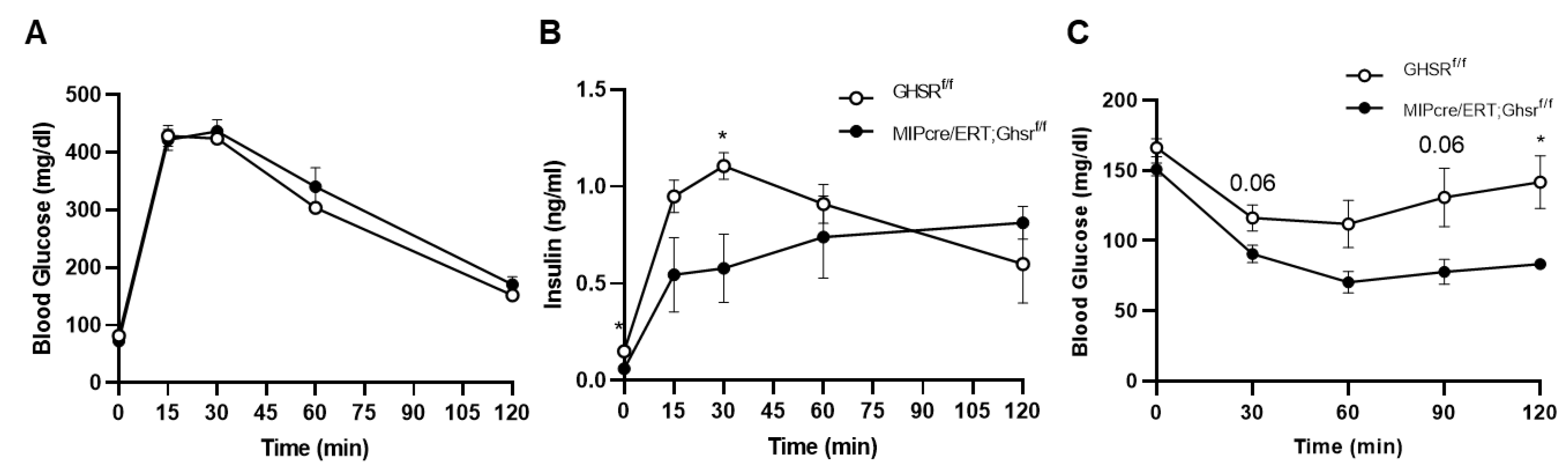
2.4. GHS-R in β Cells Attenuated Glucose-Stimulated Insulin Secretion In Vivo and Ex Vivo
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Real-Time RT-PCR
4.3. Body Composition and Indirect Calorimetry Analysis
4.4. Glucose Tolerance Test (GTT), Glucose-Stimulated Insulin Secretion (GSIS), and Insulin Tolerance Test (ITT)
4.5. Blood Glucose, Insulin, Glucagon Measurement
4.6. Immunofluorescence Staining of Pancreatic Islets
4.7. Ex Vivo Glucose-Stimulated Insulin Secretion (GSIS)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderwald-Stadler, M.; Krebs, M.; Promintzer, M.; Mandl, M.; Bischof, M.G.; Nowotny, P.; Kastenbauer, T.; Luger, A.; Prager, R.; Anderwald, C. Plasma obestatin is lower at fasting and not suppressed by insulin in insulin-resistant humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1393–E1398. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Wang, P.; Zheng, H.; Smith, R.G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 4679–4684. [Google Scholar] [CrossRef]
- Granata, R.; Baragli, A.; Settanni, F.; Scarlatti, F.; Ghigo, E. Unraveling the role of the ghrelin gene peptides in the endocrine pancreas. J. Mol. Endocrinol. 2010, 45, 107–118. [Google Scholar] [CrossRef]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002, 87, 2988. [Google Scholar] [CrossRef]
- DiGruccio, M.R.; Mawla, A.M.; Donaldson, C.J.; Noguchi, G.M.; Vaughan, J.; Cowing-Zitron, C.; van der Meulen, T.; Huising, M.O. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol. Metab. 2016, 5, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, A.E.; Svendsen, B.; Lam, B.Y.; Yeo, G.S.; Holst, J.J.; Reimann, F.; Gribble, F.M. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 2016, 59, 2156–2165. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Baldanzi, G.; Filigheddu, N.; Cutrupi, S.; Catapano, F.; Bonissoni, S.; Fubini, A.; Malan, D.; Baj, G.; Granata, R.; Broglio, F.; et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J. Cell Biol. 2002, 159, 1029–1037. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Q.; Li, M.; Wang, W.; Cui, C.; Zhang, J. Ghrelin inhibits AGEs-induced apoptosis in human endothelial cells involving ERK1/2 and PI3K/Akt pathways. Cell Biochem. Funct. 2011, 29, 149–155. [Google Scholar] [CrossRef]
- Chacko, Effect of ghrelin on glucose regulation in mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302. [CrossRef]
- Lin, L.; Saha, P.K.; Ma, X.; Henshaw, I.O.; Shao, L.; Chang, B.H.; Buras, E.D.; Tong, Q.; Chan, L.; McGuinness, O.P.; et al. Ablation of ghrelin receptor reduces adiposity and improves insulin sensitivity during aging by regulating fat metabolism in white and brown adipose tissues. Aging Cell 2011, 10, 996–1010. [Google Scholar] [CrossRef]
- Zigman, J.M.; Nakano, Y.; Coppari, R.; Balthasar, N.; Marcus, J.N.; Lee, C.E.; Jones, J.E.; Deysher, A.E.; Waxman, A.R.; White, R.D.; et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Investig. 2005, 115, 3564–3572. [Google Scholar] [CrossRef]
- Tamarina, N.A.; Roe, M.W.; Philipson, L. Characterization of mice expressing Ins1 gene promoter driven CreERT recombinase for conditional gene deletion in pancreatic beta-cells. ISLETS 2014, 6, e27685. [Google Scholar] [CrossRef] [PubMed]
- Wicksteed, B.; Brissova, M.; Yan, W.; Opland, D.M.; Plank, J.L.; Reinert, R.B.; Dickson, L.M.; Tamarina, N.A.; Philipson, L.H.; Shostak, A.; et al. Conditional gene targeting in mouse pancreatic ss-Cells: Analysis of ectopic Cre transgene expression in the brain. Diabetes 2010, 59, 3090–3098. [Google Scholar] [CrossRef]
- Dezaki, K.; Sone, H.; Koizumi, M.; Nakata, M.; Kakei, M.; Nagai, H.; Hosoda, H.; Kangawa, K.; Yada, T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 2006, 55, 3486–3493. [Google Scholar] [CrossRef] [PubMed]
- Dezaki, K.; Kakei, M.; Yada, T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: Novel signal transduction of ghrelin. Diabetes 2007, 56, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.C.; Sakata, I.; Kohno, D.; Perello, M.; Osborne-Lawrence, S.; Repa, J.J.; Zigman, J.M. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol. Endocrinol. 2011, 25, 1600–1611. [Google Scholar] [CrossRef]
- Gauna, C.; Delhanty, P.J.; Hofland, L.J.; Janssen, J.A.; Broglio, F.; Ross, R.J.; Ghigo, E.; van der Lely, A.J. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J. Clin. Endocrinol. Metab. 2005, 90, 1055–1060. [Google Scholar] [CrossRef]
- Ma, X.; Lin, L.; Qin, G.; Lu, X.; Fiorotto, M.; Dixit, V.D.; Sun, Y. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PLoS ONE 2011, 6, e16391. [Google Scholar] [CrossRef]
- Thompson, N.M.; Gill, D.A.; Davies, R.; Loveridge, N.; Houston, P.A.; Robinson, I.C.; Wells, T. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 2004, 145, 234–242. [Google Scholar] [CrossRef]
- Delhanty, P.J.; van der Eerden, B.C.; van der Velde, M.; Gauna, C.; Pols, H.A.; Jahr, H.; Chiba, H.; van der Lely, A.J.; van Leeuwen, J.P. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J. Endocrinol. 2006, 188, 37–47. [Google Scholar] [CrossRef]
- Filigheddu, N.; Gnocchi, V.F.; Coscia, M.; Cappelli, M.; Porporato, P.E.; Taulli, R.; Traini, S.; Baldanzi, G.; Chianale, F.; Cutrupi, S.; et al. Ghrelin and Des-Acyl Ghrelin Promote Differentiation and Fusion of C2C12 Skeletal Muscle Cells. Mol. Biol. Cell 2007, 18, 986–994. [Google Scholar] [CrossRef]
- Sun, Y.; Asnicar, M.; Saha, P.K.; Chan, L.; Smith, R.G. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006, 3, 379–386. [Google Scholar] [CrossRef]
- Sun, Y.; Butte, N.F.; Garcia, J.M.; Smith, R.G. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology 2008, 149, 843–850. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.; Lin, L.; Qin, G.; Pereira, F.A.; Haymond, M.W.; Butte, N.F.; Sun, Y. Ablation of ghrelin receptor in leptin-deficient ob/ob mice has paradoxical effects on glucose homeostasis when compared with ablation of ghrelin in ob/ob mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E422–E431. [Google Scholar] [CrossRef]
- Pradhan, G.; Wu, C.S.; Han Lee, J.; Kanikarla, P.; Guo, S.; Yechoor, V.K.; Samson, S.L.; Sun, Y. Obestatin stimulates glucose-induced insulin secretion through ghrelin receptor GHS-R. Sci. Rep. 2017, 7, 979. [Google Scholar] [CrossRef]
- Lee, J.H.; Lin, L.; Xu, P.; Saito, K.; Wei, Q.; Meadows, A.G.; Bongmba, O.Y.; Pradhan, G.; Zheng, H.; Xu, Y.; et al. Neuronal Deletion of Ghrelin Receptor Almost Completely Prevents Diet-Induced Obesity. Diabetes 2016, 65, 2169–2178. [Google Scholar] [CrossRef]
- Aamodt, K.I.; Powers, A.C. Signals in the pancreatic islet microenvironment influence β-cell proliferation. Diabetes Obes. Metab. 2017, 19, 124–136. [Google Scholar] [CrossRef]
- Granata, R.; Settanni, F.; Biancone, L.; Trovato, L.; Nano, R.; Bertuzzi, F.; Destefanis, S.; Annunziata, M.; Martinetti, M.; Catapano, F.; et al. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: Involvement of 3′,5′-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology 2007, 148, 512–529. [Google Scholar] [PubMed]
- Kageyama, H.; Funahashi, H.; Hirayama, M.; Takenoya, F.; Kita, T.; Kato, S.; Sakurai, J.; Lee, E.Y.; Inoue, S.; Date, Y.; et al. Morphological analysis of ghrelin and its receptor distribution in the rat pancreas. Regul. Pept. 2005, 126, 67–71. [Google Scholar] [CrossRef]
- Yin, T.C.; Bauchle, C.J.; Rouault, A.A.J.; Stephens, S.B.; Sebag, J.A. The Insulinostatic Effect of Ghrelin Requires MRAP2 Expression in delta Cells. iScience 2020, 23, 101216. [Google Scholar] [CrossRef]
- Magnuson, M.A.; Osipovich, A.B. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013, 18, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, B.; de Faudeur, G.; Osipovich, A.B.; Goyvaerts, L.; Lemaire, K.; Boesmans, L.; Cauwelier, E.J.; Granvik, M.; Pruniau, V.P.; Van Lommel, L.; et al. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab. 2014, 20, 979–990. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ristow, M.; Lin, X.; White, M.F.; Magnuson, M.A.; Hennighausen, L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J. Biol. Chem. 2006, 281, 2649–2653. [Google Scholar] [CrossRef]
- Oropeza, D.; Jouvet, N.; Budry, L.; Campbell, J.E.; Bouyakdan, K.; Lacombe, J.; Perron, G.; Bergeron, V.; Neuman, J.C.; Brar, H.; et al. Phenotypic Characterization of MIP-CreERT1Lphi Mice With Transgene-Driven Islet Expression of Human Growth Hormone. Diabetes 2015, 64, 3798–3807. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Kim, K.; German, M.S.; Kim, H. Ectopic serotonin production in beta-cell specific transgenic mice. Biochem. Biophys. Res. Commun. 2018, 495, 1986–1991. [Google Scholar] [CrossRef]
- Guo, Q.; Robson-Doucette, C.A.; Allister, E.M.; Wheeler, M.B. Inducible Deletion of UCP2 in Pancreatic b-Cells Enhances Insulin Secretion. Can. J. Diabetes 2012, 36, 237–243. [Google Scholar] [CrossRef]
- Mosleh, E.; Ou, K.; Haemmerle, M.W.; Tembo, T.; Yuhas, A.; Carboneau, B.A.; Townsend, S.E.; Bosma, K.J.; Gannon, M.; O’Brien, R.M.; et al. Ins1-Cre and Ins1-CreER Gene Replacement Alleles Are Susceptible To Silencing By DNA Hypermethylation. Endocrinology 2020, 161. [Google Scholar] [CrossRef]
- Minami, A.; Iseki, M.; Kishi, K.; Wang, M.; Ogura, M.; Furukawa, N.; Hayashi, S.; Yamada, M.; Obata, T.; Takeshita, Y.; et al. Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes 2003, 52, 2657–2665. [Google Scholar] [CrossRef]
- McClain, D.A.; Abraham, D.; Rogers, J.; Brady, R.; Gault, P.; Ajioka, R.; Kushner, J.P. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia 2006, 49, 1661–1669. [Google Scholar] [CrossRef]
- Nieuwenhuizen, A.G.; Karlsson, S.; Fridolf, T.; Ahren, B. Mechanisms underlying the insulinostatic effect of peptide YY in mouse pancreatic islets. Diabetologia 1994, 37, 871–878. [Google Scholar] [CrossRef]
- Silvestre, R.A.; Egido, E.M.; Hernandez, R.; Marco, J. Characterization of the insulinostatic effect of urotensin II: A study in the perfused rat pancreas. Regul. Pept. 2009, 153, 37–42. [Google Scholar] [CrossRef]
- Manning, S.; Batterham, R.L. The role of gut hormone peptide YY in energy and glucose homeostasis: Twelve years on. Annu. Rev. Physiol. 2014, 76, 585–608. [Google Scholar] [CrossRef]
- Hashimoto, S.; Kubota, N.; Sato, H.; Sasaki, M.; Takamoto, I.; Kubota, T.; Nakaya, K.; Noda, M.; Ueki, K.; Kadowaki, T. Insulin receptor substrate-2 (Irs2) in endothelial cells plays a crucial role in insulin secretion. Diabetes 2015, 64, 876–886. [Google Scholar] [CrossRef]
- Faerch, K.; Vistisen, D.; Pacini, G.; Torekov, S.S.; Johansen, N.B.; Witte, D.R.; Jonsson, A.; Pedersen, O.; Hansen, T.; Lauritzen, T.; et al. Insulin Resistance Is Accompanied by Increased Fasting Glucagon and Delayed Glucagon Suppression in Individuals With Normal and Impaired Glucose Regulation. Diabetes 2016, 65, 3473–3481. [Google Scholar] [CrossRef]
- Adeghate, E.; Ponery, A.S.; Pallot, D.; Parvez, S.H.; Singh, J. Distribution of serotonin and its effect on insulin and glucagon secretion in normal and diabetic pancreatic tissues in rat. Neuro. Endocrinol. Lett. 1999, 20, 315–322. [Google Scholar]
- Gray, S.M.; Page, L.C.; Tong, J. Ghrelin regulation of glucose metabolism. J. Neuroendocr. 2019, 31, e12705. [Google Scholar] [CrossRef]
- Mear, Y.; Enjalbert, A.; Thirion, S. GHS-R1a constitutive activity and its physiological relevance. Front. Neurosci. 2013, 7, 87. [Google Scholar] [CrossRef]
- Kurashina, T.; Dezaki, K.; Yoshida, M.; Sukma Rita, R.; Ito, K.; Taguchi, M.; Miura, R.; Tominaga, M.; Ishibashi, S.; Kakei, M.; et al. The beta-cell GHSR and downstream cAMP/TRPM2 signaling account for insulinostatic and glycemic effects of ghrelin. Sci. Rep. 2015, 5, 14041. [Google Scholar] [CrossRef]
- Carter, J.D.; Dula, S.B.; Corbin, K.L.; Wu, R.; Nunemaker, C.S. A practical guide to rodent islet isolation and assessment. Biol. Proced Online 2009, 11, 3–31. [Google Scholar] [CrossRef]
- Morsi, M.; Schulze, T.; Fruh, E.; Bruning, D.; Panten, U.; Rustenbeck, I. Fresh and cultured mouse islets differ in their response to nutrient stimulation. Endocr. Connect. 2020, 9, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Betancourt, L.; Smith, R.G. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol. Endocrinol. 2006, 20, 1772–1785. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.; Pradhan, G.; Wu, C.-S.; Allred, C.D.; Guo, S.; Sun, Y. A Simple High Efficiency Protocol for Pancreatic Islet Isolation from Mice. J. Vis. Exp. 2019, e57048. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Garcia, J.M.; Smith, R.G. Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology 2007, 148, 1323–1329. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, G.; Wu, C.-S.; Villarreal, D.; Lee, J.H.; Han, H.W.; Gaharwar, A.; Tian, Y.; Fu, W.; Guo, S.; Smith, R.G.; et al. β Cell GHS-R Regulates Insulin Secretion and Sensitivity. Int. J. Mol. Sci. 2021, 22, 3950. https://doi.org/10.3390/ijms22083950
Pradhan G, Wu C-S, Villarreal D, Lee JH, Han HW, Gaharwar A, Tian Y, Fu W, Guo S, Smith RG, et al. β Cell GHS-R Regulates Insulin Secretion and Sensitivity. International Journal of Molecular Sciences. 2021; 22(8):3950. https://doi.org/10.3390/ijms22083950
Chicago/Turabian StylePradhan, Geetali, Chia-Shan Wu, Daniel Villarreal, Jong Han Lee, Hye Won Han, Akhilesh Gaharwar, Yanan Tian, Wenxian Fu, Shaodong Guo, Roy G. Smith, and et al. 2021. "β Cell GHS-R Regulates Insulin Secretion and Sensitivity" International Journal of Molecular Sciences 22, no. 8: 3950. https://doi.org/10.3390/ijms22083950
APA StylePradhan, G., Wu, C.-S., Villarreal, D., Lee, J. H., Han, H. W., Gaharwar, A., Tian, Y., Fu, W., Guo, S., Smith, R. G., & Sun, Y. (2021). β Cell GHS-R Regulates Insulin Secretion and Sensitivity. International Journal of Molecular Sciences, 22(8), 3950. https://doi.org/10.3390/ijms22083950







