The Antipsychotic Risperidone Alters Dihydroceramide and Ceramide Composition and Plasma Membrane Function in Leukocytes In Vitro and In Vivo
Abstract
1. Introduction
2. Results
2.1. Patients Characteristics
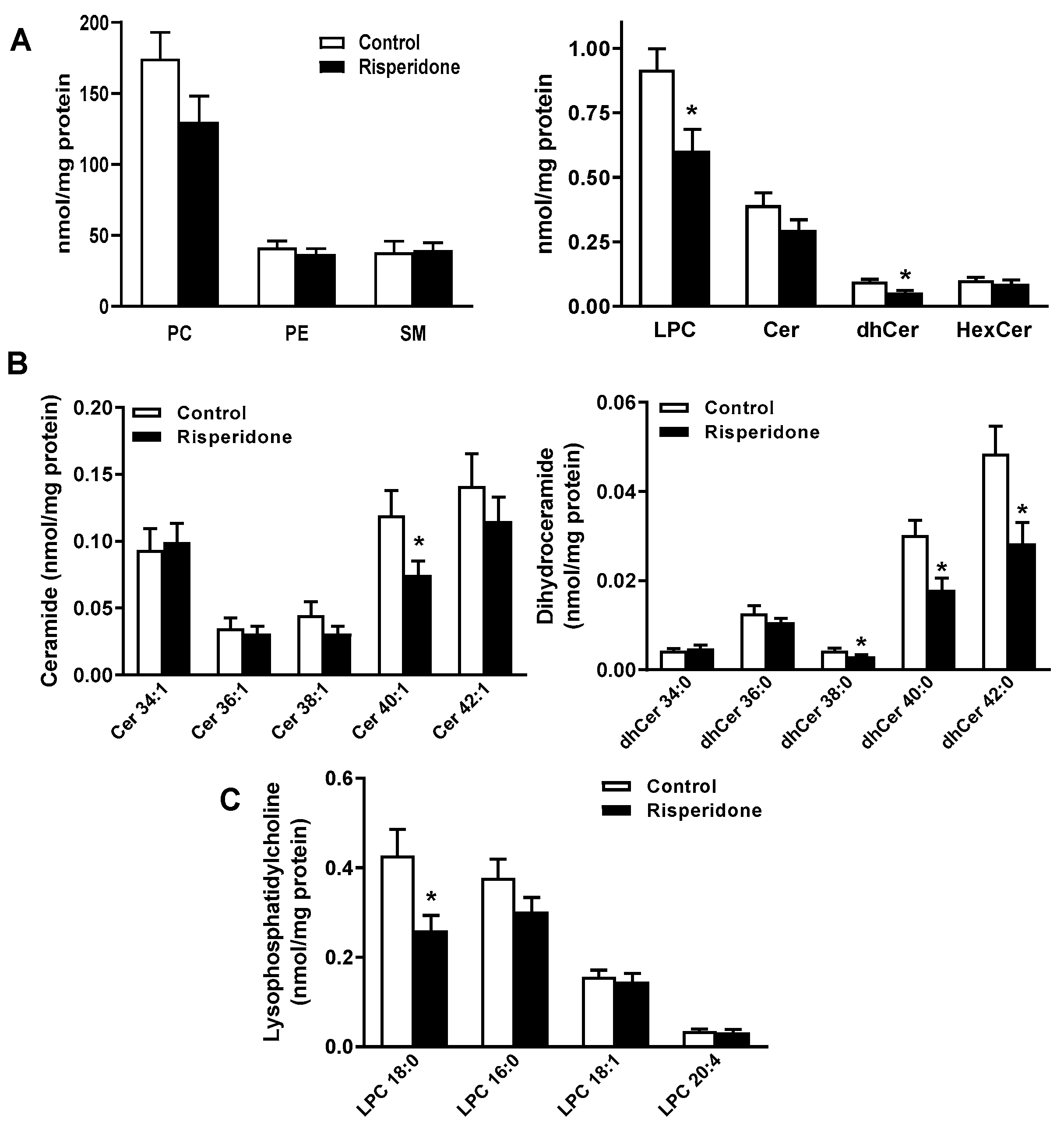
2.2. Phospholipid and Sphingolipid Composition in PBMCs
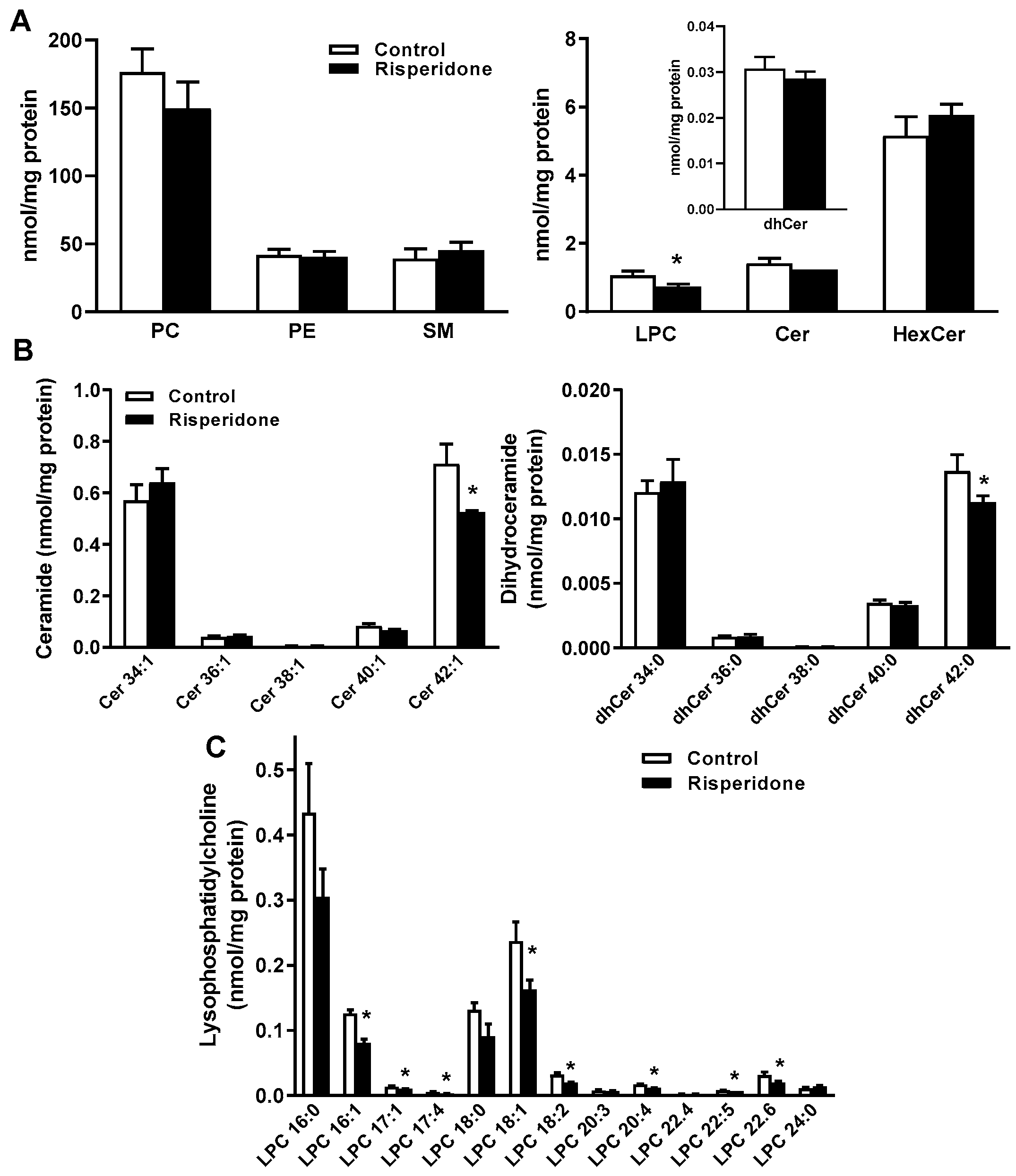
2.3. Phospholipid and Sphingolipid Composition in Human Monocyte THP-1 Cells
2.4. Risperidone Disrupts Lipid Rafts (Detergent-Resistant Membranes [DRMs]) in PBMCs and Monocyte THP-1 Cells
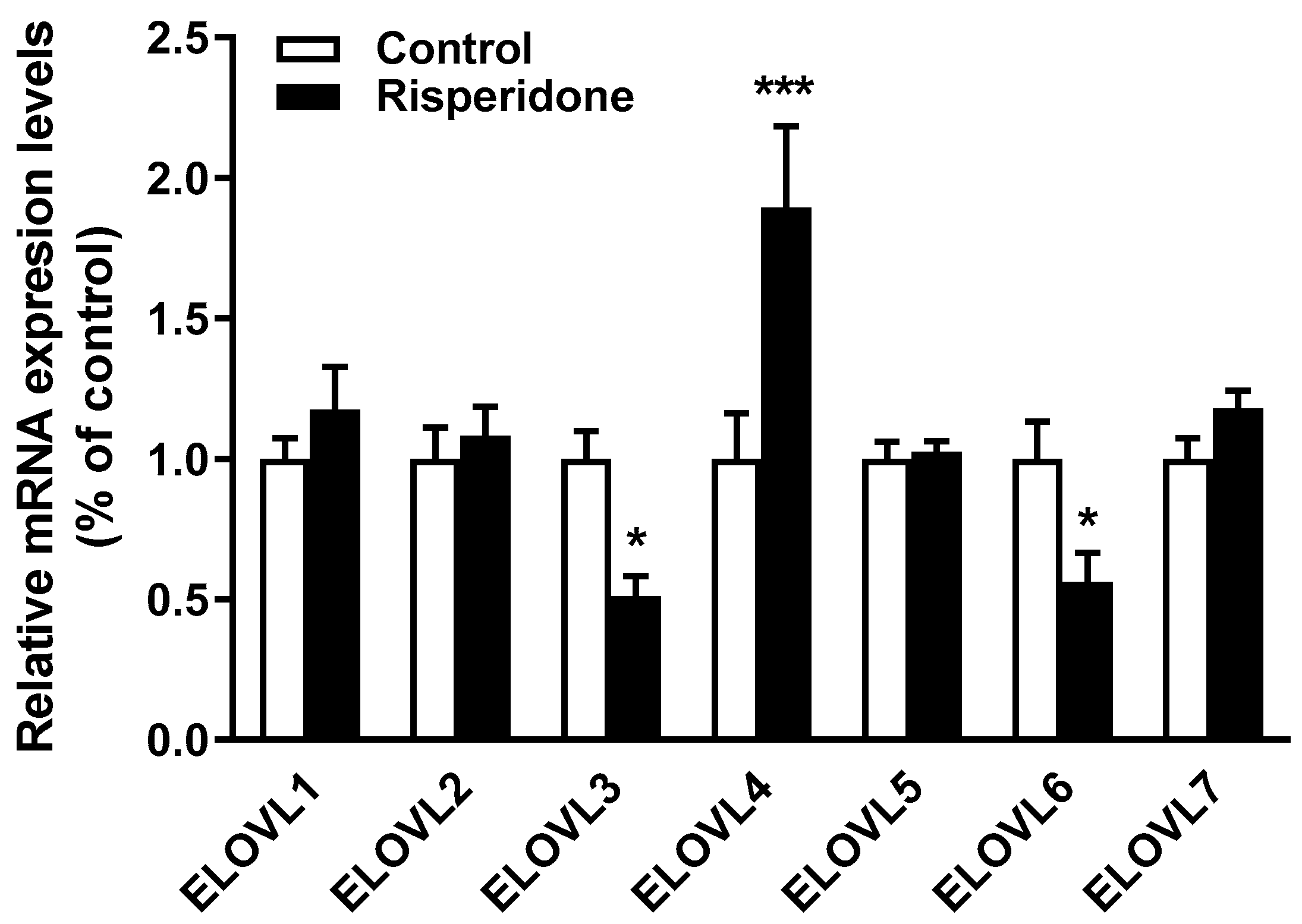
2.5. Effects of Risperidone on the Gene Expression of Fatty Acid Elongases
3. Discussion
Limitations
4. Materials and Methods
4.1. Participants
4.2. Biochemical Parameters
4.3. Isolation of PBMCs
4.4. Cell Culture
4.5. Lipidomic Analysis
4.6. Analysis of Detergent-Resistant Membranes by Flow Cytometry
4.7. RNA Isolation and Quantitative RT-PCR (qRT-PCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaar, S.J.; Natesan, S.; McCutcheon, R.; Howes, O.D. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology 2020, 172, 107704. [Google Scholar] [CrossRef]
- Chacko, E.; Boyd, S.; Murphy, R. Metabolic side effects of atypical antipsychotics in older adults. Int. Psychogeriatr. 2018, 30, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Dagerman, K.S.; Insel, P. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. JAMA 2005, 294, 1934–1943. [Google Scholar] [CrossRef]
- Ballard, C.; Waite, J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, CD003476. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chen, H.; Johnson, M.L.; Aparasu, R.R. Comparative risk of cerebrovascular adverse events in community-dwelling older adults using risperidone, olanzapine and quetiapine: A multiple propensity score-adjusted retrospective cohort study. Drugs Aging 2012, 29, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Lanctot, K.L. Do atypical antipsychotics cause stroke? CNS Drugs 2005, 19, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Canfran-Duque, A.; Casado, M.E.; Pastor, O.; Sanchez-Wandelmer, J.; de la Pena, G.; Lerma, M.; Mariscal, P.; Bracher, F.; Lasuncion, M.A.; Busto, R. Atypical antipsychotics alter cholesterol and fatty acid metabolism in vitro. J. Lipid Res. 2013, 54, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Vantaggiato, C.; Panzeri, E.; Citterio, A.; Orso, G.; Pozzi, M. Antipsychotics Promote Metabolic Disorders Disrupting Cellular Lipid Metabolism and Trafficking. Trends Endocrinol. Metab. TEM 2019, 30, 189–210. [Google Scholar] [CrossRef]
- Sanchez-Wandelmer, J.; Hernandez-Pinto, A.M.; Cano, S.; Davalos, A.; de la Pena, G.; Puebla-Jimenez, L.; Arilla-Ferreiro, E.; Lasuncion, M.A.; Busto, R. Effects of the antipsychotic drug haloperidol on the somastostatinergic system in SH-SY5Y neuroblastoma cells. J. Neurochem. 2009, 110, 631–640. [Google Scholar] [CrossRef]
- Sanchez-Wandelmer, J.; Davalos, A.; de la Pena, G.; Cano, S.; Giera, M.; Canfran-Duque, A.; Bracher, F.; Martin-Hidalgo, A.; Fernandez-Hernando, C.; Lasuncion, M.A.; et al. Haloperidol disrupts lipid rafts and impairs insulin signaling in SH-SY5Y cells. Neuroscience 2010, 167, 143–153. [Google Scholar] [CrossRef]
- Kristiana, I.; Sharpe, L.J.; Catts, V.S.; Lutze-Mann, L.H.; Brown, A.J. Antipsychotic drugs upregulate lipogenic gene expression by disrupting intracellular trafficking of lipoprotein-derived cholesterol. Pharm. J. 2010, 10, 396–407. [Google Scholar] [CrossRef]
- Canfran-Duque, A.; Pastor, O.; Reina, M.; Lerma, M.; Cruz-Jentoft, A.J.; Lasuncion, M.A.; Busto, R. Curcumin Mitigates the Intracellular Lipid Deposit Induced by Antipsychotics In Vitro. PLoS ONE 2015, 10, e0141829. [Google Scholar] [CrossRef]
- Reverter, M.; Rentero, C.; Garcia-Melero, A.; Hoque, M.; Vila de Muga, S.; Alvarez-Guaita, A.; Conway, J.R.; Wood, P.; Cairns, R.; Lykopoulou, L.; et al. Cholesterol Regulates Syntaxin 6 Trafficking at trans-Golgi Network Endosomal Boundaries. Cell Rep. 2014, 7, 883–897. [Google Scholar] [CrossRef]
- Canfran-Duque, A.; Barrio, L.C.; Lerma, M.; de la Pena, G.; Serna, J.; Pastor, O.; Lasuncion, M.A.; Busto, R. First-Generation Antipsychotic Haloperidol Alters the Functionality of the Late Endosomal/Lysosomal Compartment in Vitro. Int. J. Mol. Sci. 2016, 17, 404. [Google Scholar] [CrossRef]
- Kaddurah-Daouk, R.; McEvoy, J.; Baillie, R.A.; Lee, D.; Yao, J.K.; Doraiswamy, P.M.; Krishnan, K.R. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol. Psychiatry 2007, 12, 934–945. [Google Scholar] [CrossRef]
- Burghardt, K.J.; Ward, K.M.; Sanders, E.J.; Howlett, B.H.; Seyoum, B.; Yi, Z. Atypical Antipsychotics and the Human Skeletal Muscle Lipidome. Metabolites 2018, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Weston-Green, K.; Babic, I.; de Santis, M.; Pan, B.; Montgomery, M.K.; Mitchell, T.; Huang, X.F.; Nealon, J. Disrupted sphingolipid metabolism following acute clozapine and olanzapine administration. J. Biomed. Sci. 2018, 25, 40. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, J.; Wang, D.; Si, D.; Liu, Y.; Zhong, L.; Yin, Y. Unbiased lipidomic profiling reveals metabolomic changes during the onset and antipsychotics treatment of schizophrenia disease. Metab. Off. J. Metab. Soc. 2018, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Leppik, L.; Parksepp, M.; Janno, S.; Koido, K.; Haring, L.; Vasar, E.; Zilmer, M. Profiling of lipidomics before and after antipsychotic treatment in first-episode psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.C.; Aguilera-Albesa, S.; Pujol, A. Ceramide signalling in inherited and multifactorial brain metabolic diseases. Neurobiol. Dis. 2020, 143, 105014. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Z.; Orso, E.; Werner, T.; Klunemann, H.H.; Schmitz, G. Monocyte cholesterol homeostasis correlates with the presence of detergent resistant membrane microdomains. Cytom. Part A J. Int. Soc. Anal. Cytol. 2007, 71, 486–494. [Google Scholar] [CrossRef]
- Gombos, I.; Bacso, Z.; Detre, C.; Nagy, H.; Goda, K.; Andrasfalvy, M.; Szabo, G.; Matko, J. Cholesterol sensitivity of detergent resistance: A rapid flow cytometric test for detecting constitutive or induced raft association of membrane proteins. Cytom. Part A J. Int. Soc. Anal. Cytol. 2004, 61, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.; Nagy, P.; Balogh, A.; Szollosi, J.; Matko, J. Cytometry of raft and caveola membrane microdomains: From flow and imaging techniques to high throughput screening assays. Cytom. Part A J. Int. Soc. Anal. Cytol. 2008, 73, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Sassa, T.; Kihara, A. Metabolism of very long-chain Fatty acids: Genes and pathophysiology. Biomol. Ther. 2014, 22, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.C.; Reggiori, A.; Paletta, S.; Di Pace, C.; Altamura, A.C. Paliperidone for the treatment of schizophrenia and schizoaffective disorders—A drug safety evaluation. Expert Opin. Drug Saf. 2017, 16, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Smesny, S.; Schmelzer, C.E.; Hinder, A.; Kohler, A.; Schneider, C.; Rudzok, M.; Schmidt, U.; Milleit, B.; Milleit, C.; Nenadic, I.; et al. Skin ceramide alterations in first-episode schizophrenia indicate abnormal sphingolipid metabolism. Schizophr. Bull. 2013, 39, 933–941. [Google Scholar] [CrossRef] [PubMed]
- McClay, J.L.; Vunck, S.A.; Batman, A.M.; Crowley, J.J.; Vann, R.E.; Beardsley, P.M.; van den Oord, E.J. Neurochemical Metabolomics Reveals Disruption to Sphingolipid Metabolism Following Chronic Haloperidol Administration. J. Neuroimmune Pharmacol. 2015, 10, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Pewzner-Jung, Y.; Park, H.; Laviad, E.L.; Silva, L.C.; Lahiri, S.; Stiban, J.; Erez-Roman, R.; Brugger, B.; Sachsenheimer, T.; Wieland, F.; et al. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J. Biol. Chem. 2010, 285, 10902–10910. [Google Scholar] [CrossRef]
- Pinto, S.N.; Silva, L.C.; Futerman, A.H.; Prieto, M. Effect of ceramide structure on membrane biophysical properties: The role of acyl chain length and unsaturation. Biochim. Biophys. Acta 2011, 1808, 2753–2760. [Google Scholar] [CrossRef]
- Vieira, C.R.; Munoz-Olaya, J.M.; Sot, J.; Jimenez-Baranda, S.; Izquierdo-Useros, N.; Abad, J.L.; Apellaniz, B.; Delgado, R.; Martinez-Picado, J.; Alonso, A.; et al. Dihydrosphingomyelin impairs HIV-1 infection by rigidifying liquid-ordered membrane domains. Chem. Biol. 2010, 17, 766–775. [Google Scholar] [CrossRef]
- Turpin-Nolan, S.M.; Bruning, J.C. The role of ceramides in metabolic disorders: When size and localization matters. Nat. Rev. Endocrinol. 2020, 16, 224–233. [Google Scholar] [CrossRef]
- Park, J.W.; Park, W.J.; Kuperman, Y.; Boura-Halfon, S.; Pewzner-Jung, Y.; Futerman, A.H. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology 2013, 57, 525–532. [Google Scholar] [CrossRef]
- Liu, Y.; Chan, D.K.Y.; Thalamuthu, A.; Wen, W.; Jiang, J.; Paradise, M.; Lee, T.; Crawford, J.; Wai Kin Wong, M.; Hua Xu, Y.; et al. Plasma lipidomic biomarker analysis reveals distinct lipid changes in vascular dementia. Comput. Struct. Biotechnol. J. 2020, 18, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Wang, Y.; Duan, X.; Wenk, M.R.; Kalaria, R.N.; Chen, C.P.; Lai, M.K.; Shui, G. Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia. Neurobiol. Aging 2014, 35, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- McGrath, E.R.; Himali, J.J.; Xanthakis, V.; Duncan, M.S.; Schaffer, J.E.; Ory, D.S.; Peterson, L.R.; DeCarli, C.; Pase, M.P.; Satizabal, C.L.; et al. Circulating ceramide ratios and risk of vascular brain aging and dementia. Ann. Clin. Transl. Neurol. 2020, 7, 160–168. [Google Scholar] [CrossRef]
- Anderson, N.; Borlak, J. Drug-induced phospholipidosis. FEBS Lett. 2006, 580, 5533–5540. [Google Scholar] [CrossRef]
- Garcia-Canaveras, J.C.; Castell, J.V.; Donato, M.T.; Lahoz, A. A metabolomics cell-based approach for anticipating and investigating drug-induced liver injury. Sci. Rep. 2016, 6, 27239. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Maekawa, K.; Ishikawa, M.; Senoo, Y.; Urata, M.; Murayama, M.; Nakatsu, N.; Yamada, H.; Saito, Y. Glucosylceramide and lysophosphatidylcholines as potential blood biomarkers for drug-induced hepatic phospholipidosis. Toxicol. Sci. 2014, 141, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Stanley, J.A.; Reddy, R.D.; Keshavan, M.S.; Pettegrew, J.W. Correlations between peripheral polyunsaturated fatty acid content and in vivo membrane phospholipid metabolites. Biol. Psychiatry 2002, 52, 823–830. [Google Scholar] [CrossRef]
- Peters, B.D.; Machielsen, M.W.; Hoen, W.P.; Caan, M.W.; Malhotra, A.K.; Szeszko, P.R.; Duran, M.; Olabarriaga, S.D.; de Haan, L. Polyunsaturated fatty acid concentration predicts myelin integrity in early-phase psychosis. Schizophr. Bull. 2013, 39, 830–838. [Google Scholar] [CrossRef]
- Gonzalez, C.E.; Venkatraman, V.K.; An, Y.; Landman, B.A.; Davatzikos, C.; Ratnam Bandaru, V.V.; Haughey, N.J.; Ferrucci, L.; Mielke, M.M.; Resnick, S.M. Peripheral sphingolipids are associated with variation in white matter microstructure in older adults. Neurobiol. Aging 2016, 43, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, F.; Fernandez-Suarez, M.E.; Alonso, R.; Alonso, M.; Vazquez, C.; Pastor, O.; Mata, P.; Lasuncion, M.A.; Gomez-Coronado, D. Clinically used selective oestrogen receptor modulators increase LDL receptor activity in primary human lymphocytes. Br. J. Pharmacol. 2015, 172, 1379–1394. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Pastor, O.; Guzman-Lafuente, P.; Serna, J.; Munoz-Hernandez, M.; Lopez Neyra, A.; Garcia-Rozas, P.; Garcia-Seisdedos, D.; Alcazar, A.; Lasuncion, M.A.; Busto, R.; et al. A comprehensive evaluation of omega-3 fatty acid supplementation in cystic fibrosis patients using lipidomics. J. Nutr. Biochem. 2019, 63, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Ahrends, R. Adaptation of Skyline for Targeted Lipidomics. J. Proteome Res. 2016, 15, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Serna, J.; Garcia-Seisdedos, D.; Alcazar, A.; Lasuncion, M.A.; Busto, R.; Pastor, O. Quantitative lipidomic analysis of plasma and plasma lipoproteins using MALDI-TOF mass spectrometry. Chem. Phys. Lipids 2015, 189, 7–18. [Google Scholar] [CrossRef]
| Control (n = 10) | Risperidone (n = 10) | |
|---|---|---|
| Age | 88.5 ± 2.6 | 88.5 ± 2.3 |
| Gender: male/female | 5/5 | 5/5 |
| Risperidone mean daily dose (mg) | — | 1.05 ± 0.15 |
| Self-caring (n) | 6/10 | 1/10 |
| Restricted diet (n) | 0/10 | 0/10 |
| Oral supplements (n) | 0/10 | 1/10 |
| Co-medication (n): | ||
| Antidepressants | 2/10 | 2/10 |
| Anxiolytic, sedatives and hypnotics | 1/10 | 3/10 |
| Anti-dementia drugs | 3/10 | 3/10 |
| Anithypertensives | 8/10 | 6/10 |
| Anticoagulants | 7/10 | 6/10 |
| Diabetes drugs | 2/10 | 2/10 |
| Drugs used for respiratory diseases | 5/10 | 3/10 |
| Analgesics | 7/10 | 8/10 |
| Control | Risperidone | |
|---|---|---|
| Glucose (mg/dL) | 100.3 ± 7.9 | 103.3 ± 14.7 |
| Creatinine (mg/dL) | 1.1 ± 0.2 | 0.8 ± 0.1 |
| Uric acid (mg/dL) | 5.6 ± 0.7 | 4.8 ± 0.8 |
| Sodium (mM/L) | 139.5 ± 1.4 | 138.7 ± 0.7 |
| Potassium (mM/L) | 4.2 ± 0.2 | 4.2 ± 0.2 |
| Chlorine (mM/L) | 104.2 ± 1.9 | 103.3 ± 1.1 |
| Calcium (mg/dL) | 8.8 ± 0.3 | 8.6 ± 0.2 |
| Total protein (g/dL) | 6.2 ± 0.3 | 5.6 ± 0.2 |
| Total billirubin (mg/dL) | 0.5 ± 0.1 | 0.5 ± 0.1 |
| AST (U/L) | 14.5 ± 1.6 | 21.7 ± 2.4 * |
| ALT (U/L) | 11.5 ± 1.4 | 20.3 ± 3.8 * |
| γGT (U/L) | 30.1 ± 6.1 | 23.4 ± 2.8 |
| Lactate dehydrogenase (U/L) | 163.2 ± 6.7 | 194.4 ± 28.6 |
| Alkaline phosphatase (U/L) | 79.9 ± 11.3 | 78.3 ± 5.9 |
| C-reactive protein (mg/dL) | 30.2 ± 13.4 | 85.7 ± 37.7 |
| TSH (mU/L) | 1.6 ± 0.2 | 2.5 ± 1.2 |
| Total cholesterol (mg/dL) | 159.5 ± 15.1 | 155.5 ± 20.5 |
| Cholesterol-HDL (mg/dL) | 33.9 ± 3.0 | 36.1 ± 6.9 |
| Cholesterol-LDL (mg/dL) | 99.6 ± 12.2 | 98.2 ± 14.0 |
| Triglycerides (mg/dL) | 128.4 ± 11.4 | 103.7 ± 16.7 |
| Red blood cells (106/µL) | 3.8 ± 0.2 | 3.6 ± 0.3 |
| Hemoglobin (g/dL) | 11.5 ± 0.5 | 10.9 ± 0.9 |
| Hematocrit (%) | 33.7 ± 1.4 | 32.3 ± 2.6 |
| Mean corpuscular volume (fl) | 90.2 ± 2.0 | 90.6 ± 1.8 |
| Platelets (103/µL) | 231.6 ± 35.8 | 249.1 ± 24.4 |
| White blood cells (103/µL) | 8.1 ± 0.7 | 9.7 ± 1.3 |
| Neutrophils (%) | 68.7 ± 3.5 | 72.4 ± 5.2 |
| Lymphocytes (%) | 19.9 ± 3.0 | 19.2 ± 5.0 |
| Monocytes (%) | 8.3 ± 0.5 | 7.2 ± 1.0 |
| Eosinophils (%) | 2.8 ± 0.5 | 2.0 ± 0.3 |
| Basophils (%) | 0.4 ± 0.1 | 0.3 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canfrán-Duque, A.; Pastor, Ó.; García-Seisdedos, D.; Molina, Y.L.; Babiy, B.; Lerma, M.; Sánchez-Castellano, C.; Martínez-Botas, J.; Gómez-Coronado, D.; Lasunción, M.A.; et al. The Antipsychotic Risperidone Alters Dihydroceramide and Ceramide Composition and Plasma Membrane Function in Leukocytes In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 3919. https://doi.org/10.3390/ijms22083919
Canfrán-Duque A, Pastor Ó, García-Seisdedos D, Molina YL, Babiy B, Lerma M, Sánchez-Castellano C, Martínez-Botas J, Gómez-Coronado D, Lasunción MA, et al. The Antipsychotic Risperidone Alters Dihydroceramide and Ceramide Composition and Plasma Membrane Function in Leukocytes In Vitro and In Vivo. International Journal of Molecular Sciences. 2021; 22(8):3919. https://doi.org/10.3390/ijms22083919
Chicago/Turabian StyleCanfrán-Duque, Alberto, Óscar Pastor, David García-Seisdedos, Yessenia L. Molina, Bohdan Babiy, Milagros Lerma, Carmen Sánchez-Castellano, Javier Martínez-Botas, Diego Gómez-Coronado, Miguel A. Lasunción, and et al. 2021. "The Antipsychotic Risperidone Alters Dihydroceramide and Ceramide Composition and Plasma Membrane Function in Leukocytes In Vitro and In Vivo" International Journal of Molecular Sciences 22, no. 8: 3919. https://doi.org/10.3390/ijms22083919
APA StyleCanfrán-Duque, A., Pastor, Ó., García-Seisdedos, D., Molina, Y. L., Babiy, B., Lerma, M., Sánchez-Castellano, C., Martínez-Botas, J., Gómez-Coronado, D., Lasunción, M. A., Cruz-Jentoft, A. J., & Busto, R. (2021). The Antipsychotic Risperidone Alters Dihydroceramide and Ceramide Composition and Plasma Membrane Function in Leukocytes In Vitro and In Vivo. International Journal of Molecular Sciences, 22(8), 3919. https://doi.org/10.3390/ijms22083919







