Effects of Antifreeze Protein III on Sperm Cryopreservation of Pacific Abalone, Haliotis discus hannai
Abstract
1. Introduction
2. Results
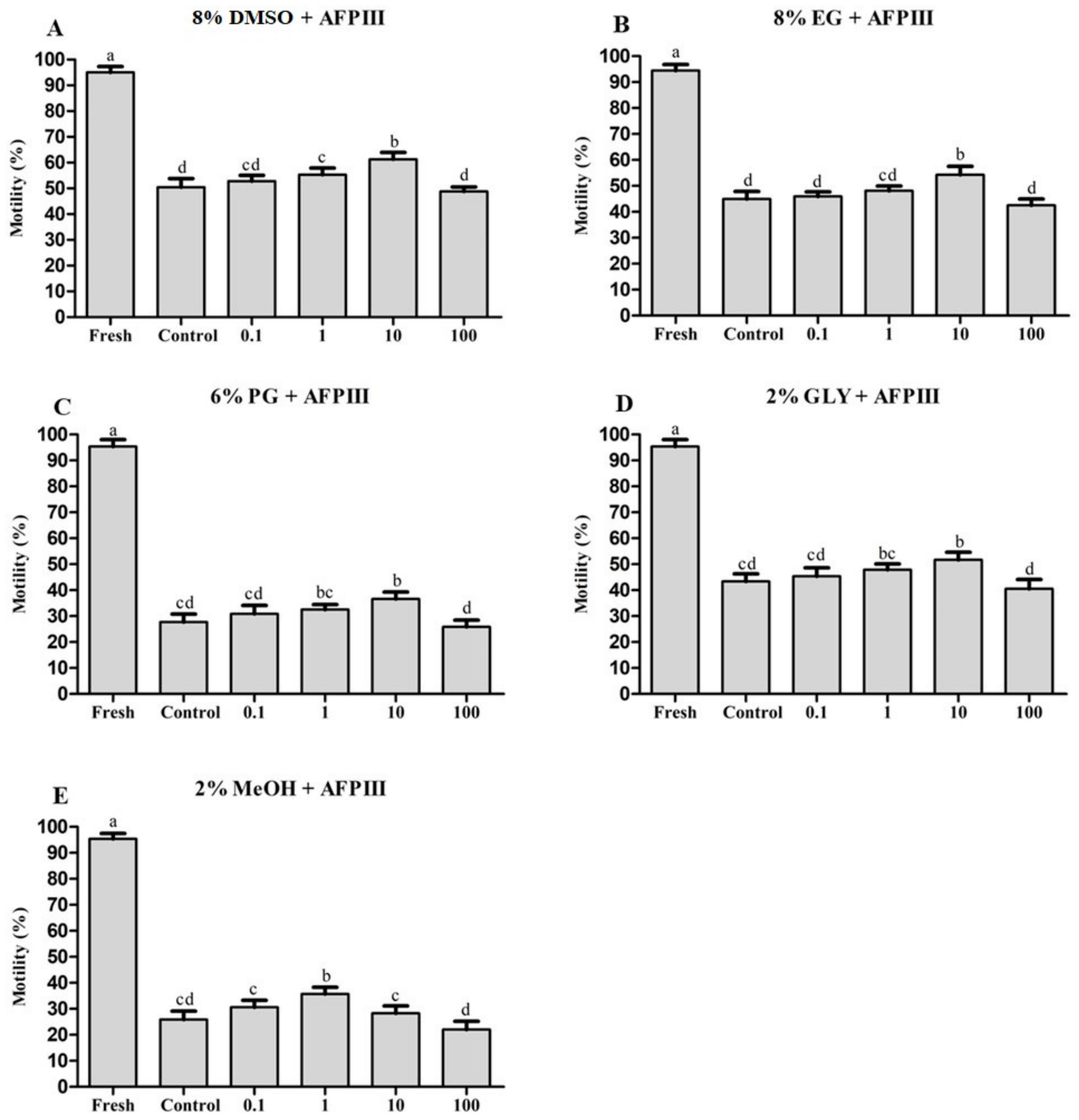
2.1. Effects of AFPIII on Sperm Cryopreservation
2.1.1. Combined Effects of AFPIII and DMSO on Post-Thaw Sperm Motility
2.1.2. Combined Effects of AFPIII and EG on Post-Thaw Sperm Motility
2.1.3. Combined Effects of AFPIII and PG on Post-Thaw Sperm Motility
2.1.4. Combined Effects of AFPIII and GLY on Post-Thaw Sperm Motility
2.1.5. Combined Effects of AFPIII and MeOH on Post-Thaw Sperm Motility
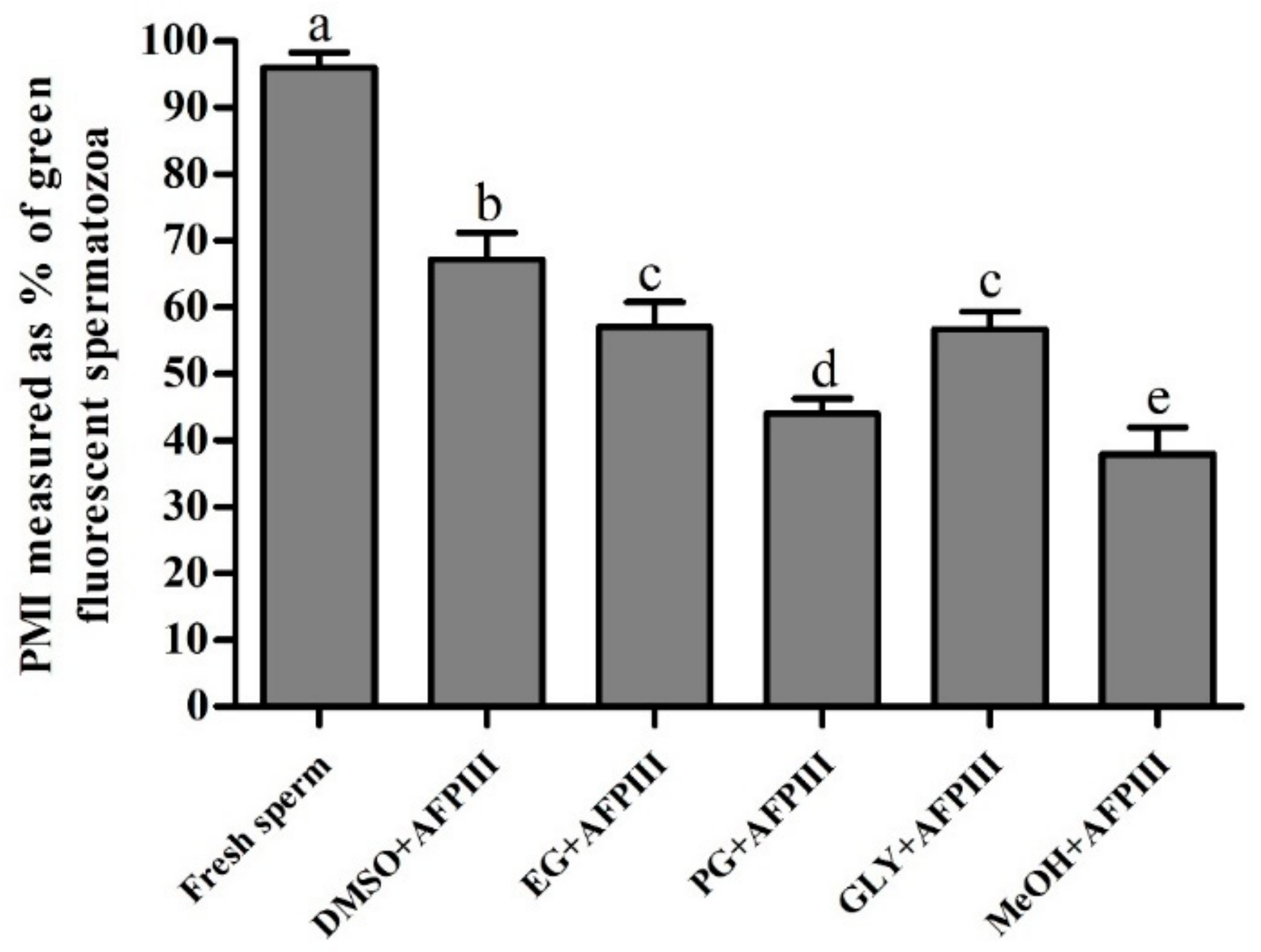
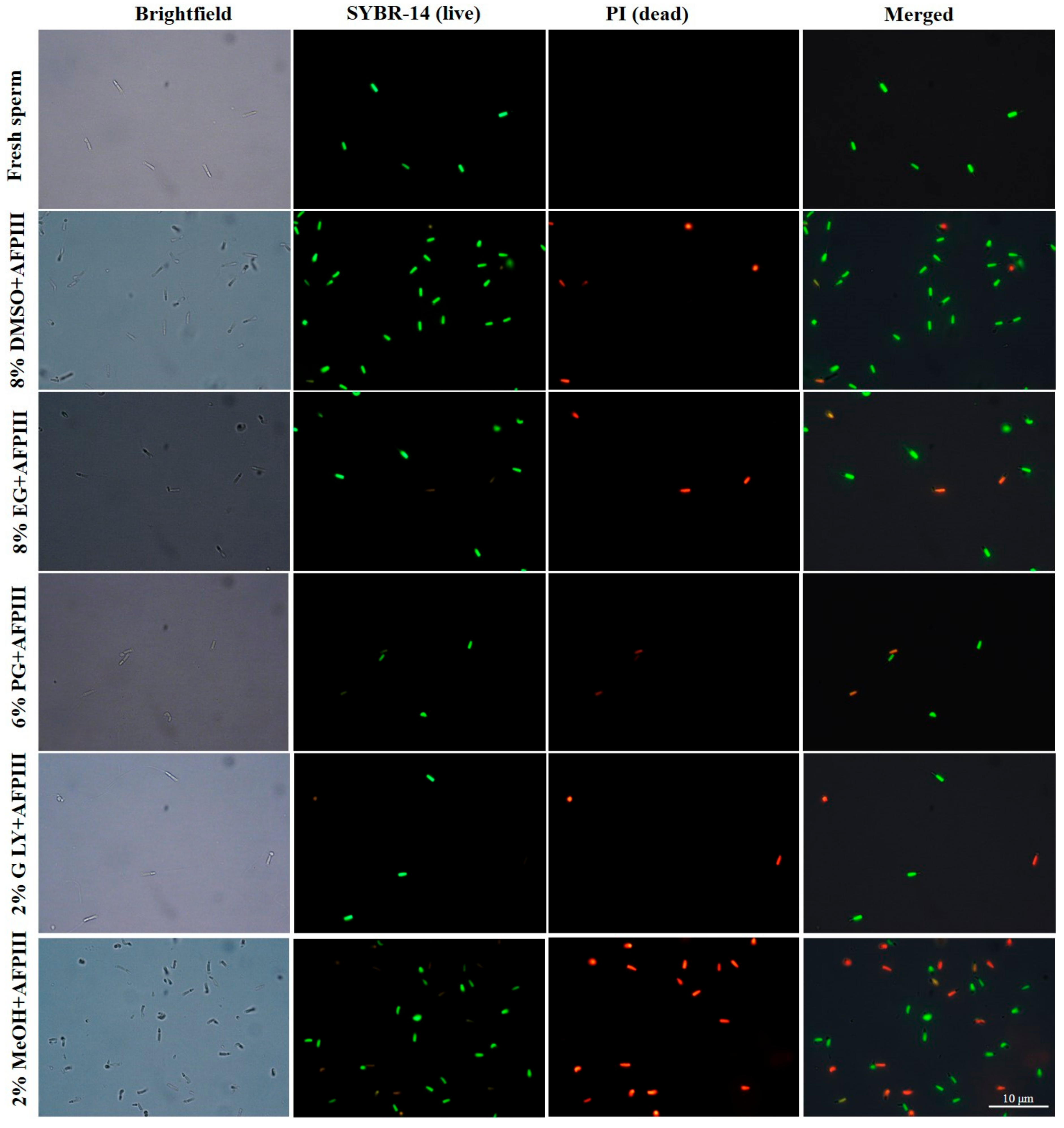
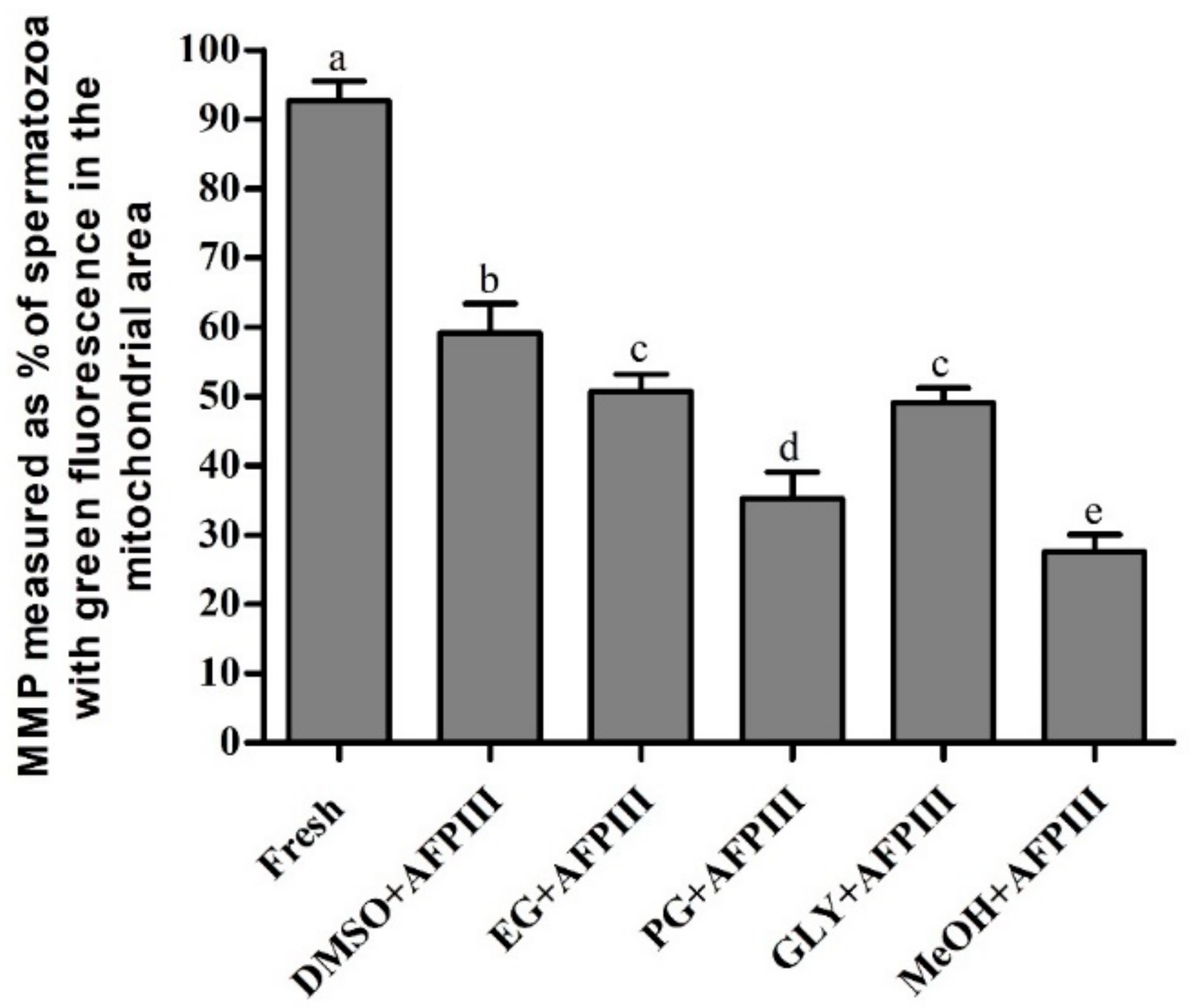
2.2. Fluorescent Technique for Assessing PMI, AI, and MMP of Cryopreserved Sperm
2.2.1. Plasma Membrane Integrity (PMI)
2.2.2. Acrosome Integrity (AI)
2.2.3. Mitochondrial Membrane Potential (MMP)
2.3. DNA Integrity of Fresh and Cryopreserved Sperm
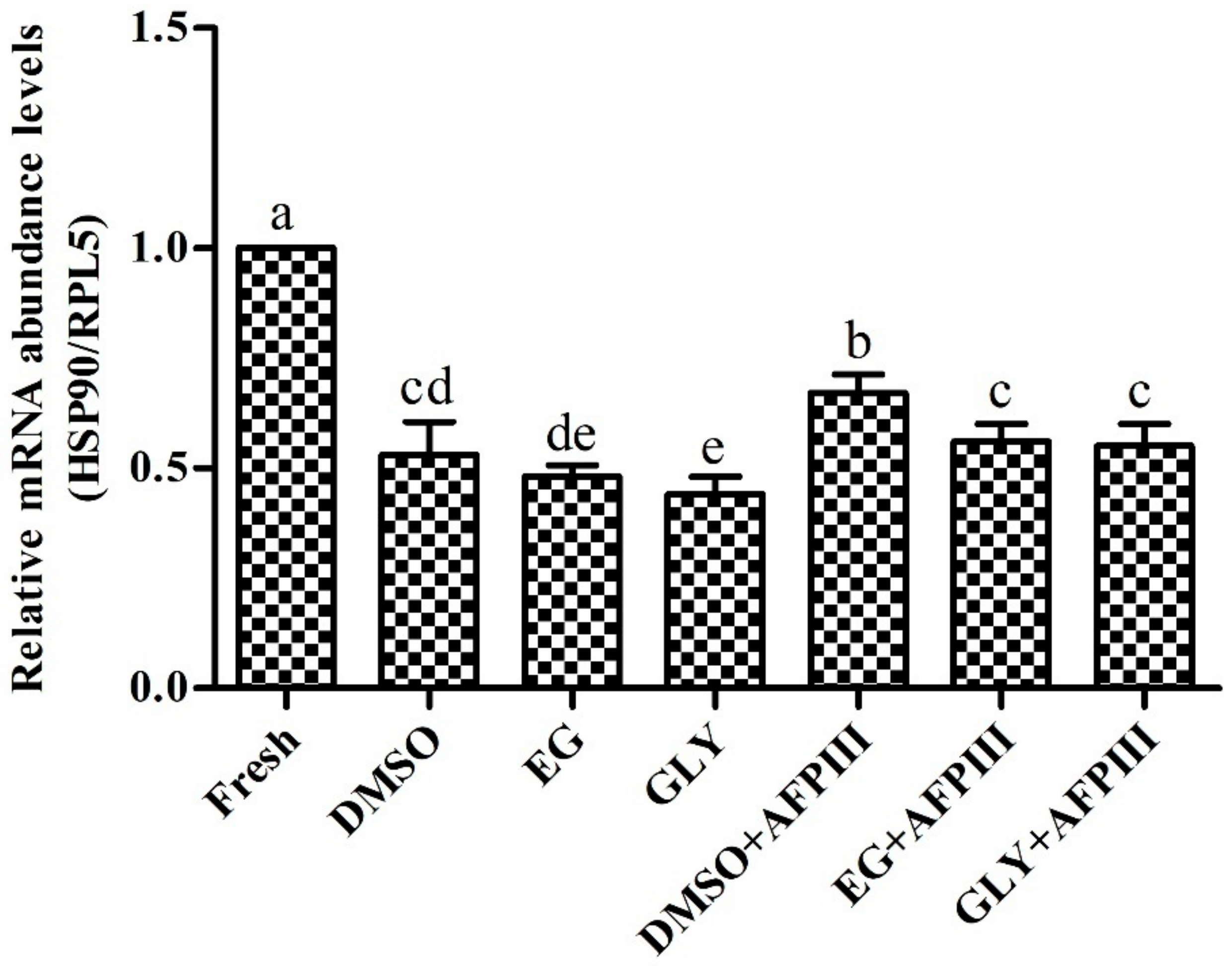
2.4. Expression Analysis of HSP90 mRNA Transcript
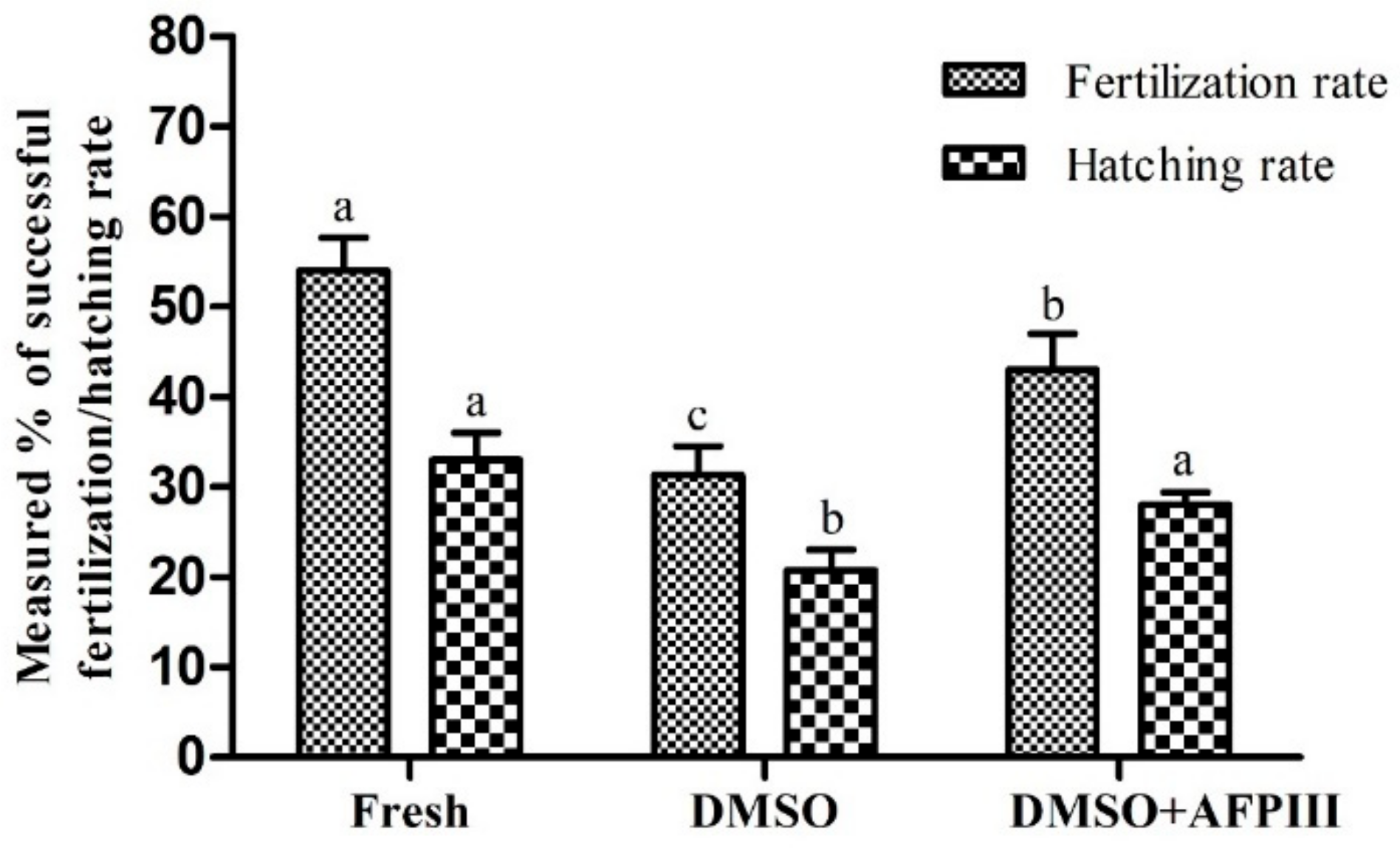
2.5. Fertility and Hatchability
2.6. Correlation Among Sperm Quality Parameters
3. Discussion
4. Materials and Methods
4.1. Ethics Statements
4.2. Experimental Reagents
4.3. Apparatus Arrangement
4.4. Collection of Experimental Animals
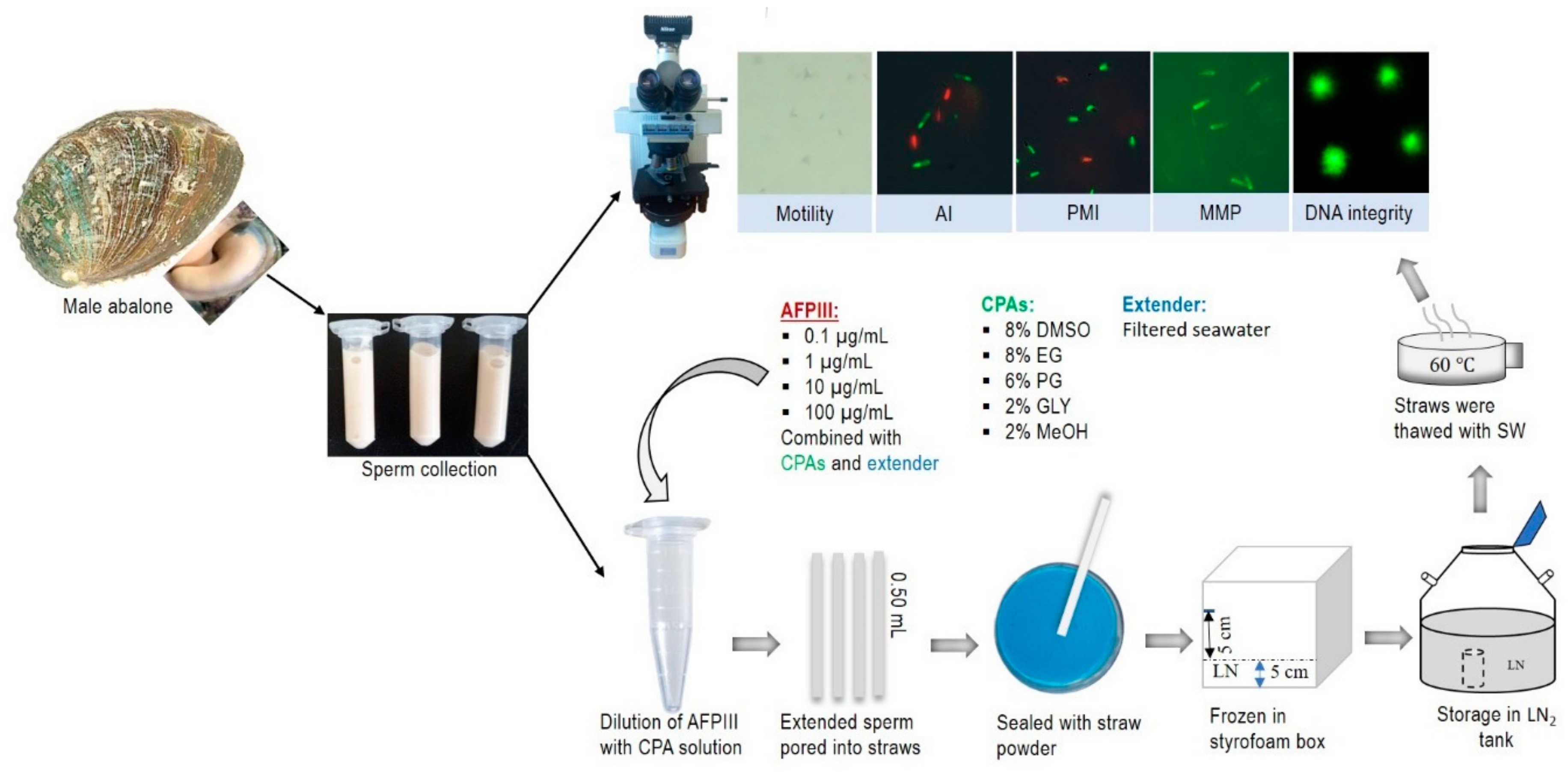
4.5. Sperm Collection and Handling
4.6. Quality Evaluation of Fresh Sperm
4.7. Cryopreservation Protocol
4.8. Effects of AFPIII on Sperm Cryopreservation
4.9. Fluorescent Technique to Assess PMI, AI, and MMP of Cryopreserved Sperm
4.10. Comet Assay to Detect Sperm DNA Integrity of Fresh and Cryopreserved Sperm
4.11. RNA Extraction and cDNA Synthesis
4.12. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.13. Fertility Test
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suleria, H.A.R.; Masci, P.P.; Gobe, G.C.; Osborne, S.A. Therapeutic potential of abalone and status of bioactive molecules: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1742–1748. [Google Scholar] [CrossRef]
- Kim, S.C.; Hossen, S.; Kho, K.H. Cryopreservation of sperm from farmed Pacific abalone, Haliotis discus hannai. Cryobiology 2020, 94, 49–56. [Google Scholar] [CrossRef]
- Acosta-Salmón, H.; Jerry, D.R.; Southgate, P.C. Effects of cryoprotectant agents and freezing protocol on motility of black-lip pearl oyster (Pinctada margaritifera L.) spermatozoa. Cryobiology 2007, 54, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, T.; Robinson, N.; Qin, J.; Li, X. Cryopreservation of sperm in farmed Australian greenlip abalone Haliotis laevigata. Cryobiology 2014, 68, 185–193. [Google Scholar] [CrossRef]
- Mosca, F.; Madeddu, M.; Sayed, A.A.; Zaniboni, L.; Iaffaldano, N.; Cerolini, S. Combined effect of permeant and non-permeant cryoprotectants on the quality of frozen/thawed chicken sperm. Cryobiology 2016, 73, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Holt, W.V. Basic aspects of frozen semen storage. Anim. Reprod. Sci. 2000, 62, 3–22. [Google Scholar] [CrossRef]
- Swain, J.E.; Smith, G.D. Cryoprotectants. In Fertility Cryopreservation; Chian, R.C., Quinn, P., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 24–38. [Google Scholar]
- Brum, A.M.; Sabeur, K.; Ball, B.A. Apoptotic-like changes in equine spermatozoa separated by density-gradient centrifugation or after cryopreservation. Theriogenology 2008, 69, 1041–1055. [Google Scholar] [CrossRef]
- Xin, M.; Tučková, V.; Rodina, M.; Kholodnyy, V.; Dadras, H.; Boryshpolets, S.; Shaliutina-Kolešová, A.; Linhart, O. Effects of antifreeze proteins on cryopreserved sterlet (Acipenser ruthenus) sperm motility variables and fertilization capacity. Anim. Reprod. Sci. 2018, 196, 143–149. [Google Scholar] [CrossRef]
- Soni, Y.; Talluri, T.R.; Kumar, A.; Ravi, S.K.; Mehta, J.S.; Tripathi, B.N. Effects of different concentration and combinations of cryoprotectants on sperm quality, functional integrity in three Indian horse breeds. Cryobiology 2019, 86, 52–57. [Google Scholar] [CrossRef]
- Zilli, L.; Schiavone, R.; Zonno, V.; Storelli, C.; Vilella, S. Evaluation of DNA damage in Dicentrarchus labrax sperm following cryopreservation. Cryobiology 2003, 47, 227–235. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Woods, L.C. Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to striped bass (Morone saxatilis) sperm. Cryobiology 2004, 48, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Cagnon, N.; Sabido, O.; Sion, B.; Grizard, G.; Durand, P.; Levy, R. Kinetics of occurrence of some features of apoptosis during the cryopreservation process of bovine spermatozoa. Hum. Reprod. 2007, 22, 380–388. [Google Scholar] [CrossRef]
- Figueroa, E.; Valdebenito, I.; Farias, J.G. Technologies used in the study of sperm function in cryopreserved fish spermatozoa. Aquac. Res. 2016, 47, 1691–1705. [Google Scholar] [CrossRef]
- Xin, M.; Sterba, J.; Shaliutina-Kolesova, A.; Dzyuba, B.; Lieskovska, J.; Boryshpolets, S.; Siddique, M.A.M.; Kholodnyy, V.; Lebeda, I.; Linhart, O. Protective role of antifreeze proteins on sterlet (Acipenser ruthenus) sperm during cryopreservation. Fish Physiol. Biochem. 2018, 44, 1527–1533. [Google Scholar] [CrossRef]
- Steele, E.K.; McClure, N.; Lewis, S.E. Comparison of the effects of two methods of cryopreservation on testicular sperm DNA. Fertil. Steril. 2000, 74, 450–453. [Google Scholar] [CrossRef]
- Balamurugan, R.; Prapaporn, W.; Munuswamy, N. Sperm activation and effects of cryopreservation on motility, ultrastructure and DNA integrity in grey mullet Mugil cephalus. Aquac. Rep. 2019, 14, 100204. [Google Scholar] [CrossRef]
- Virro, M.R.; Larson-Cook, K.L.; Evenson, D.P. Sperm chromatin structure assay (SCSA®) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil. Steril. 2004, 81, 1289–1295. [Google Scholar] [CrossRef]
- Balasuriya, A.; Speyer, B.; Serhal, P.; Doshi, A.; Harper, J.C. Sperm chromatin dispersion test in the assessment of DNA fragmentation and aneuploidy in human spermatozoa. Reprod. Biomed. Online 2011, 22, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Masaki, J.; Agarwal, A. Sperm DNA fragmentation analysis using the TUNEL assay. In Spermatogenesis; Humana Press: Totowa, NJ, USA, 2013; Volume 927, pp. 121–136. [Google Scholar]
- Gwo, J.C.; Wu, C.Y.; Chang, W.S.P.; Cheng, H.Y. Evaluation of damage in Pacific oyster (Crassostrea gigas) spermatozoa before and after cryopreservation using comet assay. Cryoletters 2003, 24, 171–180. [Google Scholar]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef]
- Riesco, M.F.; Félix, F.; Matias, D.; Joaquim, S.; Suquet, M.; Cabrita, E. Comparative study on cellular and molecular responses in oyster sperm revealed different susceptibilities to cryopreservation. Aquaculture 2019, 498, 223–229. [Google Scholar] [CrossRef]
- Zhang, X.G.; Hu, S.; Han, C.; Zhu, Q.C.; Yan, G.J.; Hu, J.H. Association of heat shock protein 90 with motility of post-thawed sperm in bulls. Cryobiology 2015, 70, 164–169. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kasimanickam, R.; Arangasamy, A.; Saberivand, A.; Stevenson, J.S.; Kastelic, J.P. Association between mRNA abundance of functional sperm function proteins and fertility of Holstein bulls. Theriogenology 2012, 78, 2007–2019. [Google Scholar] [CrossRef] [PubMed]
- Valcarce, D.G.; Cartón-García, F.; Herráez, M.P.; Robles, V. Effect of cryopreservation on human sperm messenger RNAs crucial for fertilization and early embryo development. Cryobiology 2013, 67, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Beirão, J.; Zilli, L.; Vilella, S.; Cabrita, E.; Schiavone, R.; Herráez, M.P. Improving sperm cryopreservation with antifreeze proteins: Effect on gilthead seabream (Sparus aurata) plasma membrane lipids. Biol. Reprod. 2012, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zilli, L.; Beirão, J.; Schiavone, R.; Herraez, M.P.; Gnoni, A.; Vilella, S. Comparative proteome analysis of cryopreserved flagella and head plasma membrane proteins from sea bream spermatozoa: Effect of antifreeze proteins. PLoS ONE 2014, 9, e99992. [Google Scholar] [CrossRef]
- Zilli, L.; Bianchi, A.; Sabbagh, M.; Pecoraro, L.; Schiavone, R.; Vilella, S. Development of sea bream (Sparus aurata) semen vitrification protocols. Theriogenology 2018, 110, 103–109. [Google Scholar] [CrossRef]
- Shaliutina-Kolešová, A.; Dietrich, M.; Xian, M.; Nian, R. Seminal plasma transferrin effects on cryopreserved common carp Cyprinus carpio sperm and comparison with bovine serum albumin and antifreeze proteins. Anim. Reprod. Sci. 2019, 204, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Abed-Elmdoust, A.; Farahmand, H.; Mojazi-Amiri, B.; Rafiee, G.; Rahimi, R. Novel droplet vitrification combined with fish antifreeze protein type III enhances cryoprotection of semen in wild endangered Persian sturgeon Acipenser persicus (Borodin, 1897). Aquac. Res. 2015, 46, 2392–2397. [Google Scholar] [CrossRef]
- Prathalingam, N.S.; Holt, W.V.; Revell, S.G.; Mirczuk, S.; Fleck, R.A.; Watson, P.F. Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze-thaw. Theriogenology 2006, 66, 1894–1900. [Google Scholar] [CrossRef]
- Robles, V.; Cabrita, E.; Anel, L.; Herráez, M.P. Microinjection of the antifreeze protein type III (AFPIII) in turbot (Scophthalmus maximus) embryos: Toxicity and protein distribution. Aquaculture 2006, 261, 1299–1306. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.H.; Koo, B.W. Marine antifreeze proteins: Structure, function, and application to cryopreservation as a potential cryoprotectant. Mar. Drugs 2017, 15, 27. [Google Scholar] [CrossRef]
- Qadeer, S.; Khan, M.A.; Ansari, M.S.; Rakha, B.A.; Ejaz, R.; Husna, A.U.; Ashiq, M.; Iqbal, R.; Ullah, N.S. Akhter. Evaluation of antifreeze protein III for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2014, 148, 26–31. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Mattioli, M.; Devries, A.L. The effect of antifreeze glycopeptides on membrane potential changes at hypothermic temperatures. Biochem. Biophys. Res. Commun. 1990, 173, 1369–1374. [Google Scholar] [CrossRef]
- Abed-Elmdoust, A.; Farahmand, H.; Mojazi-Amiri, B.; Rafiee, G.; Rahimi, R. Metabolic changes in droplet vitrified semen of wild endangered Persian sturgeon Acipenser persicus (Borodin, 1997). Cryobiology 2017, 76, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, S.; Khan, M.A.; Ansari, M.S.; Rakha, B.A.; Ejaz, R.; Iqbal, R.; Younis, M.; Ullah, N.; DeVries, A.L.; Akhter, S. Efficiency of antifreeze glycoproteins for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2015, 157, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.T.M.; Carvalho, J.D.O.; Dode, M.A.N. Techniques for sperm evaluation using fluorescent probes. Semin. Ciênc. Agrár. 2015, 36, 4365–4376. [Google Scholar] [CrossRef]
- Agnihotri, S.K.; Agrawal, A.K.; Hakim, B.A.; Vishwakarma, A.L.; Narender, T.; Sachan, R.; Sachdev, M. Mitochondrial membrane potential (MMP) regulates sperm motility. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 953–960. [Google Scholar] [CrossRef]
- Wang, S.; Duan, Y.; Yan, Y.; Adar, C.; Braslavsky, I.; Chen, B.; Huang, T.; Qiu, S.; Li, X.; Inglis, B.M.; et al. Improvement of sperm cryo-survival of cynomolgus macaque (Macaca fascicularis) by commercial egg-yolk-free freezing medium with type III antifreeze protein. Anim. Reprod. Sci. 2019, 210, 106177. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. The effects of cold shock on sperm cell membranes. In Effects of Low Temperatures on Biological Membranes; Morris, G.J., Clarke, A., Eds.; Academic Press: London, UK, 1981; pp. 189–218. [Google Scholar]
- Kim, D.Y. Evaluation of antifreeze proteins on miniature pig sperm viability, DNA damage, and acrosome status during cryopreservation. J. Emb. Trans. 2016, 31, 355–365. [Google Scholar] [CrossRef]
- Bai, C.; Kang, N.; Zhao, J.; Dai, J.; Gao, H.; Chen, Y.; Dong, H.; Huang, C.; Dong, Q. Cryopreservation disrupts lipid rafts and heat shock proteins in yellow catfish sperm. Cryobiology 2019, 87, 32–39. [Google Scholar] [CrossRef]
- Li, K.; Xue, Y.; Chen, A.; Jiang, Y.; Xie, H.; Shi, Q.; Zhang, S.; Ni, Y. Heat shock protein 90 has roles in intracellular calcium homeostasis, protein tyrosine phosphorylation regulation, and progesterone-responsive sperm function in human sperm. PLoS ONE 2014, 9, e115841. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Speltra, E.; Patassini, C.; Pati, M.A.; Garolla, A.; Caretta, N.; Foresta, C. Heat shock protein and heat shock factor expression in sperm: Relation to oligozoospermia and varicocele. J. Urol. 2010, 183, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cui, Y.; Baloch, A.R.; Fan, J.; He, J.; Zhang, Y.; Zheng, H.; Li, G.; Yu, S. Association of heat shock protein 90 with the developmental competence of immature oocytes following Cryotop and solid surface vitrification in yaks (Bos grunniens). Cryobiology 2015, 71, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. Cell. Mol. Life Sci. 2002, 59, 1640–1648. [Google Scholar] [CrossRef]
- Krone, P.H.; Sass, J.B.; Lele, Z. Heat shock protein gene expression during embryonic development of the zebrafish. Cell. Mol. Life Sci. 1997, 53, 122–129. [Google Scholar] [CrossRef]
- Yeyati, P.L.; Bancewicz, R.M.; Maule, J.; Van Heyningen, V. Hsp90 selectively modulates phenotype in vertebrate development. PLoS Genet. 2007, 3, e43. [Google Scholar] [CrossRef]
- Chao, N.H.; Liao, I.C. Cryopreservation of finfish and shellfish gametes and embryos. Aquaculture 2001, 197, 161–189. [Google Scholar] [CrossRef]
- Matteo, D.O.; Langellotti, A.L.; Masullo, P.; Sansone, G. Cryopreservation of the Mediterranean mussel (Mytilus galloprovincialis) spermatozoa. Cryobiology 2009, 58, 145–150. [Google Scholar] [CrossRef]
- Alcay, S.; Ustuner, B.; Aktar, A.; Mulkpinar, E.; Duman, M.; Akkasoglu, M.; Cetinkaya, M. Goat semen cryopreservation with rainbow trout seminal plasma supplemented lecithin-based extenders. Andrologia 2020, 52, e13555. [Google Scholar] [CrossRef]
- Paniagua-Chávez, C.G.; Jenkins, J.; Segovia, M.; Tiersch, T.R. Assessment of gamete quality for the eastern oyster (Crassostrea virginica) by use of fluorescent dyes. Cryobiology 2006, 53, 128–138. [Google Scholar] [CrossRef]
- Gwo, J.C.; Chen, C.W.; Cheng, H.Y. Semen cryopreservation of small abalone (Haliotis diversicolor supertexa). Theriogenology 2002, 58, 1563–1578. [Google Scholar] [CrossRef]
- Kim, S.C.; Hossen, S.; Kho, K.H. Effects of 3 years of cryopreservation on sperm quality of seven-band grouper, Epinephelus septemfasciatus. Aquac. Res. 2020, 51, 3050–3053. [Google Scholar] [CrossRef]
- Wan, Q.; Whang, I.; Choi, C.Y.; Lee, J.S.; Lee, J. Validation of housekeeping genes as internal controls for studying biomarkers of endocrine-disrupting chemicals in disk abalone by real-time PCR. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Sharker, M.R.; Hossen, S.; Nou, I.S.; Kho, K.H. Characterization of insulin-like growth factor binding protein 7 (igfbp7) and its potential involvement in shell formation and metamorphosis of Pacific abalone, Haliotis discus hannai. Int. J. Mol. Sci. 2020, 21, 6529. [Google Scholar] [CrossRef] [PubMed]
- Sharker, M.R.; Kim, S.C.; Hossen, S.; Kho, K.H. Characterization of insulin-like growth factor binding protein-5 (igfbp-5) gene and its potential roles in ontogenesis in the Pacific abalone, Haliotis discus hannai. Biology 2020, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.E.; Duncan, H.; Hooker, N.; Morse, A. Hydrogen peroxide induces spawning in mollusks, with activation of prostaglandin endoperoxide synthetase. Science 1977, 196, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Uki, N.; Kikuchi, S. Regulation of maturation and spawning of an abalone, Haliotis (Gastropoda) by external environmental factors. Aquaculture 1984, 39, 247–261. [Google Scholar] [CrossRef]
- Leighton, P. Abalone hatchery manual. In Aquaculture Explained; Robinson, G., McGowan, N., Eds.; Bord Iascaigh Mhara: Dublin, Ireland, 2008; Volume 25, pp. 1–77. [Google Scholar]

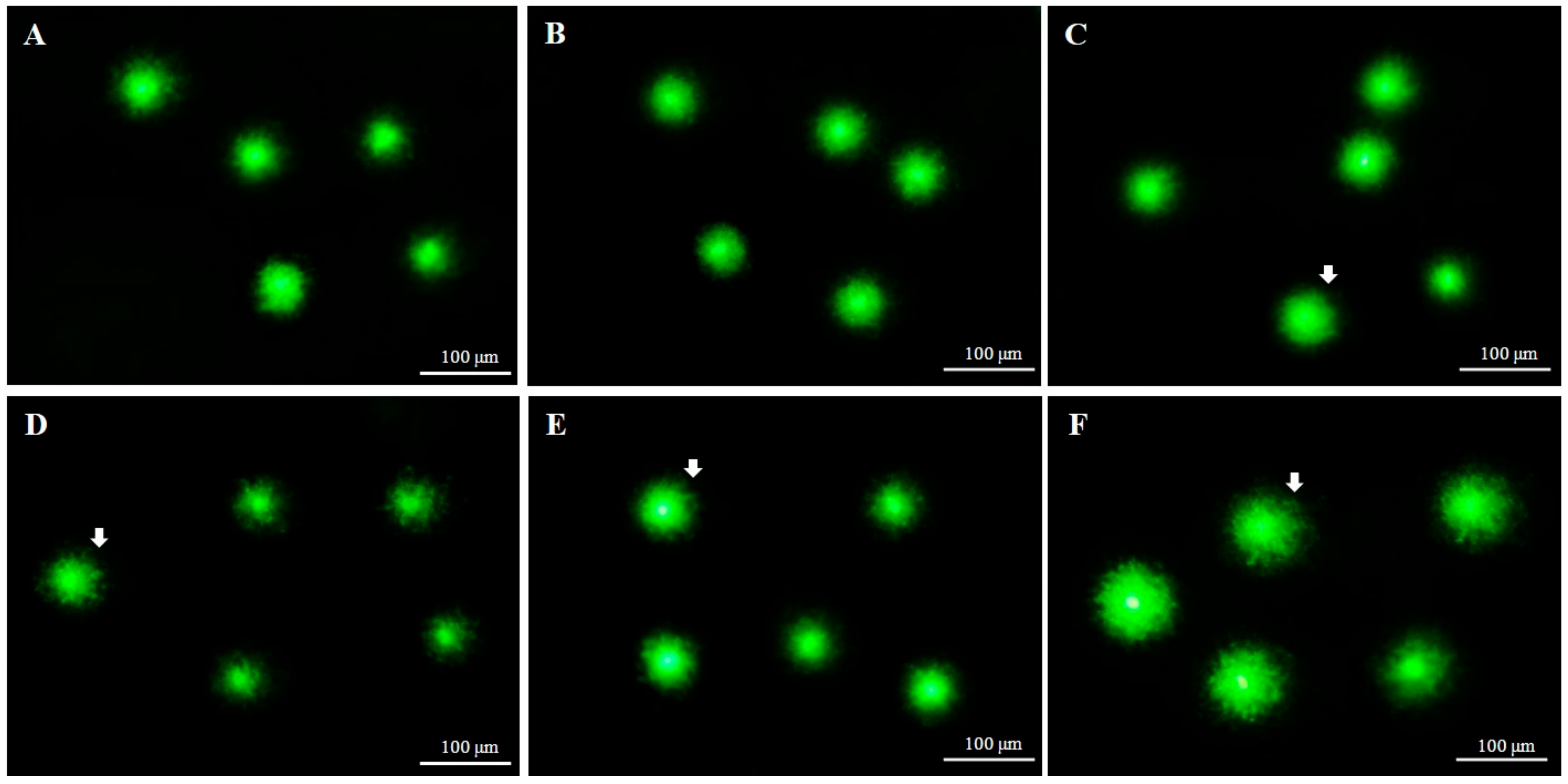
| Parameters | Fresh Sperm | 8% DMSO + AFPIII | 8% EG + AFPIII | 6% PG + AFPIII | 2% GLY + AFPIII | 2% MeOH + AFPIII |
|---|---|---|---|---|---|---|
| Head length (μ) | 50.2 ± 4.3 c | 59.4 ± 8.1 a | 59.6 ± 6.4 a | 57.7 ± 5.7 b | 58.5 ± 4.8 ab | 60.2 ± 6.2 a |
| % DNA in head | 96.6 ± 2.8 a | 96.4 ± 2.9 a | 94.9 ± 4.2 b | 94.0 ± 4.6 b | 94.6 ± 4.3 b | 92.4 ± 5.4 c |
| Tail length (μ) | 28.0 ± 5.2 c | 29.7 ± 9.3 c | 32.4 ± 7.0 b | 33.8 ± 6.8 ab | 32.0 ± 4.9 b | 35.5 ± 13.6 a |
| % DNA in tail | 3.4 ± 2.8 c | 3.6 ± 2.9 c | 5.1 ± 4.2 b | 6.0 ± 4.6 b | 5.4 ± 4.3 b | 7.6 ± 5.4 a |
| Tail moment (μ) | 0.6 ± 0.5 c | 0.8 ± 0.6 c | 1.1 ± 0.9 b | 1.2 ± 0.9 b | 1.1 ± 0.9 b | 1.5 ± 1.1 a |
| Olive tail moment (μ) | 2.1 ± 1.3 d | 2.7 ± 2.0 d | 5.6 ± 3.6 c | 7.3 ± 4.1 b | 5.8 ± 3.8 c | 11.2 ± 6.1 a |
| Extent tail moment (μ) | 94.0 ± 2.6 e | 107.1 ± 7.4 d | 169.4 ± 6.3 c | 204.8 ± 6.12 b | 171.4 ± 4.1 c | 267.2 ± 14.9 a |
| AI (%) | PMI (%) | MMP (%) | DNA Fragmentation (%) | Fertilization (%) | Hatching (%) | |
|---|---|---|---|---|---|---|
| Motility (%) | 0.990 ** | 0.994 ** | 0.990 ** | −0.858 ** | 0.903 * | 0.848 * |
| AI (%) | 0.991 ** | 0.992 ** | −0.855 ** | 0.925 ** | 0.839 * | |
| PMI (%) | 0.994 ** | −0.894 ** | 0.925 ** | 0.854 * | ||
| MMP (%) | −0.885 ** | 0.935 ** | 0.853 * | |||
| DNA fragmentation (%) | −0.870 * | −0.792 * | ||||
| Fertilization (%) | 0.957 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossen, S.; Sharker, M.R.; Cho, Y.; Sukhan, Z.P.; Kho, K.H. Effects of Antifreeze Protein III on Sperm Cryopreservation of Pacific Abalone, Haliotis discus hannai. Int. J. Mol. Sci. 2021, 22, 3917. https://doi.org/10.3390/ijms22083917
Hossen S, Sharker MR, Cho Y, Sukhan ZP, Kho KH. Effects of Antifreeze Protein III on Sperm Cryopreservation of Pacific Abalone, Haliotis discus hannai. International Journal of Molecular Sciences. 2021; 22(8):3917. https://doi.org/10.3390/ijms22083917
Chicago/Turabian StyleHossen, Shaharior, Md. Rajib Sharker, Yusin Cho, Zahid Parvez Sukhan, and Kang Hee Kho. 2021. "Effects of Antifreeze Protein III on Sperm Cryopreservation of Pacific Abalone, Haliotis discus hannai" International Journal of Molecular Sciences 22, no. 8: 3917. https://doi.org/10.3390/ijms22083917
APA StyleHossen, S., Sharker, M. R., Cho, Y., Sukhan, Z. P., & Kho, K. H. (2021). Effects of Antifreeze Protein III on Sperm Cryopreservation of Pacific Abalone, Haliotis discus hannai. International Journal of Molecular Sciences, 22(8), 3917. https://doi.org/10.3390/ijms22083917








