Role of Scaffolds, Subchondral, Intra-Articular Injections of Fresh Autologous Bone Marrow Concentrate Regenerative Cells in Treating Human Knee Cartilage Lesions: Different Approaches and Different Results
Abstract
1. Introduction
2. Intra-Articular BMAC Injections Targeting Cartilage and Synovitis
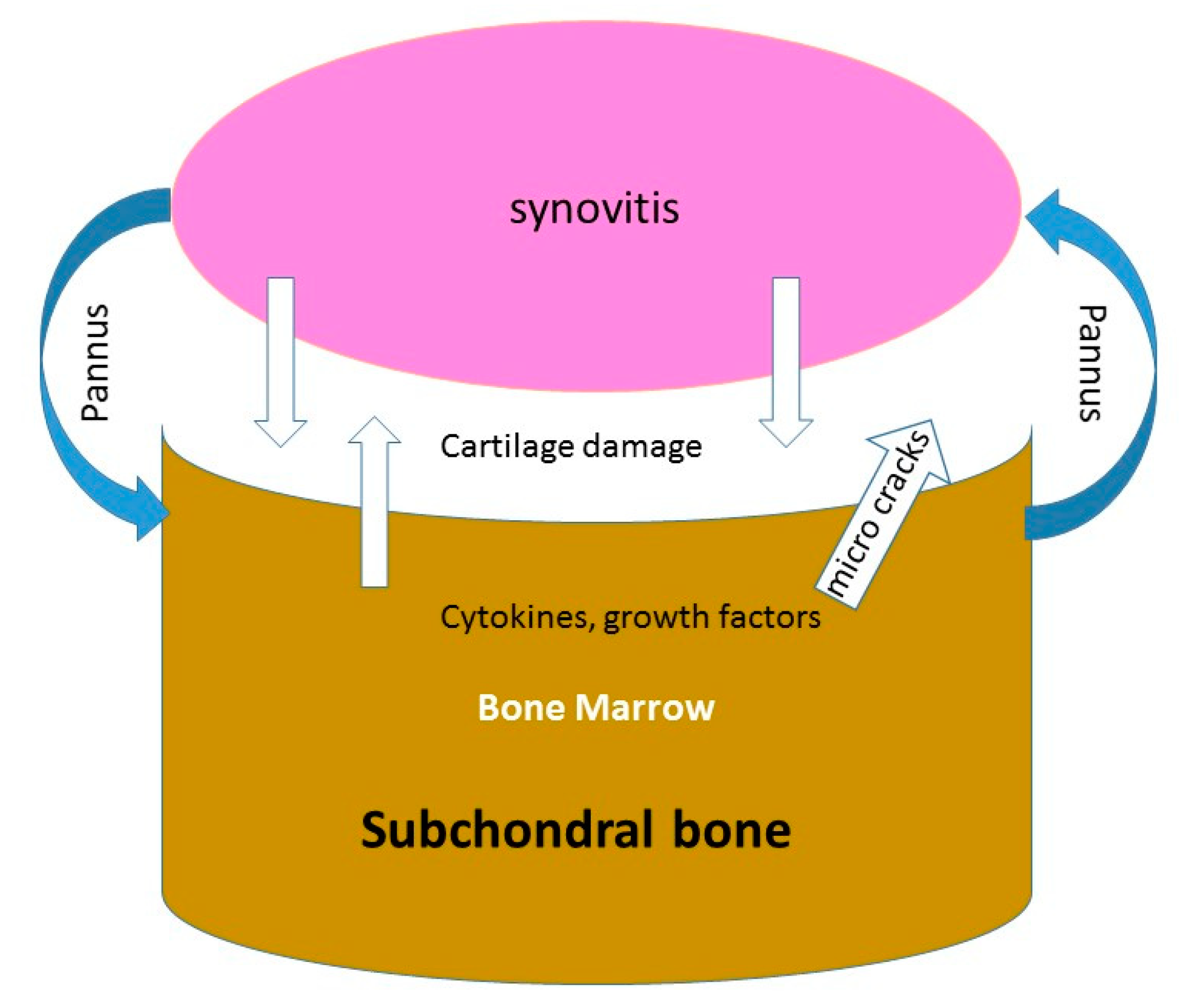
2.1. Destructions of the Cartilage and Occurrence of Inflammatory Synoviopathy
2.2. The Rational of Using BMAC for Intra-Articular Pathology
2.3. Results of Clinical Studies Using Intra-Articular BMAC
- -
- Autologous bone marrow-derived stem cells vs. saline: In a prospective, single-blind, placebo-controlled trial, 25 patients with bilateral knee pain from bilateral osteoarthritis were randomized by Shapiro [31] to receive BMAC into one knee and saline placebo into the other. Bone marrow was aspirated from the iliac crests and concentrated. BMAC or saline were injected into each arthritic knee thereby utilizing each patient as his own control. VAS pain scores in both knees decreased significantly from baseline at 6 months. However, pain relief, although dramatic, did not differ significantly between treated knees.
- -
- Autologous bone-marrow-derived stem cells vs. exercise: In this study, Centeno [32] performed an injection of BMAC versus exercise therapy in 48 patients. Patients who received BMAC injection had better Knee Society Score (KSS) compared to control group after 3 months. Better results were found for BMAC injections against exercise therapy.
- -
- Autologous bone-marrow-derived stem cells vs. hyaluronic acid: Goncars et al. [33] investigated the effect of a single injection with autologous bone-marrow-derived mononuclear cells versus three injections of HA performed 1 week apart. After 12 months, clinical scores improved significantly in both groups. KSS (Knee Society Score) improved in both groups as well and there was no statistically significant difference between these 2 groups.
- -
- Autologous bone-marrow-derived stem cells vs. PRP: Anz [34] evaluated a total of 90 participants with symptomatic knees OA (Kellgren-Lawrence grades 1–3). Patients were randomized into 2 study groups: PRP and BMAC. All clinical scores for both the PRP and BMAC groups significantly improved from baseline to 1 month after the injection. These improvements were sustained for 12 months after the injection, with no difference between PRP and BMAC at any time point.
- -
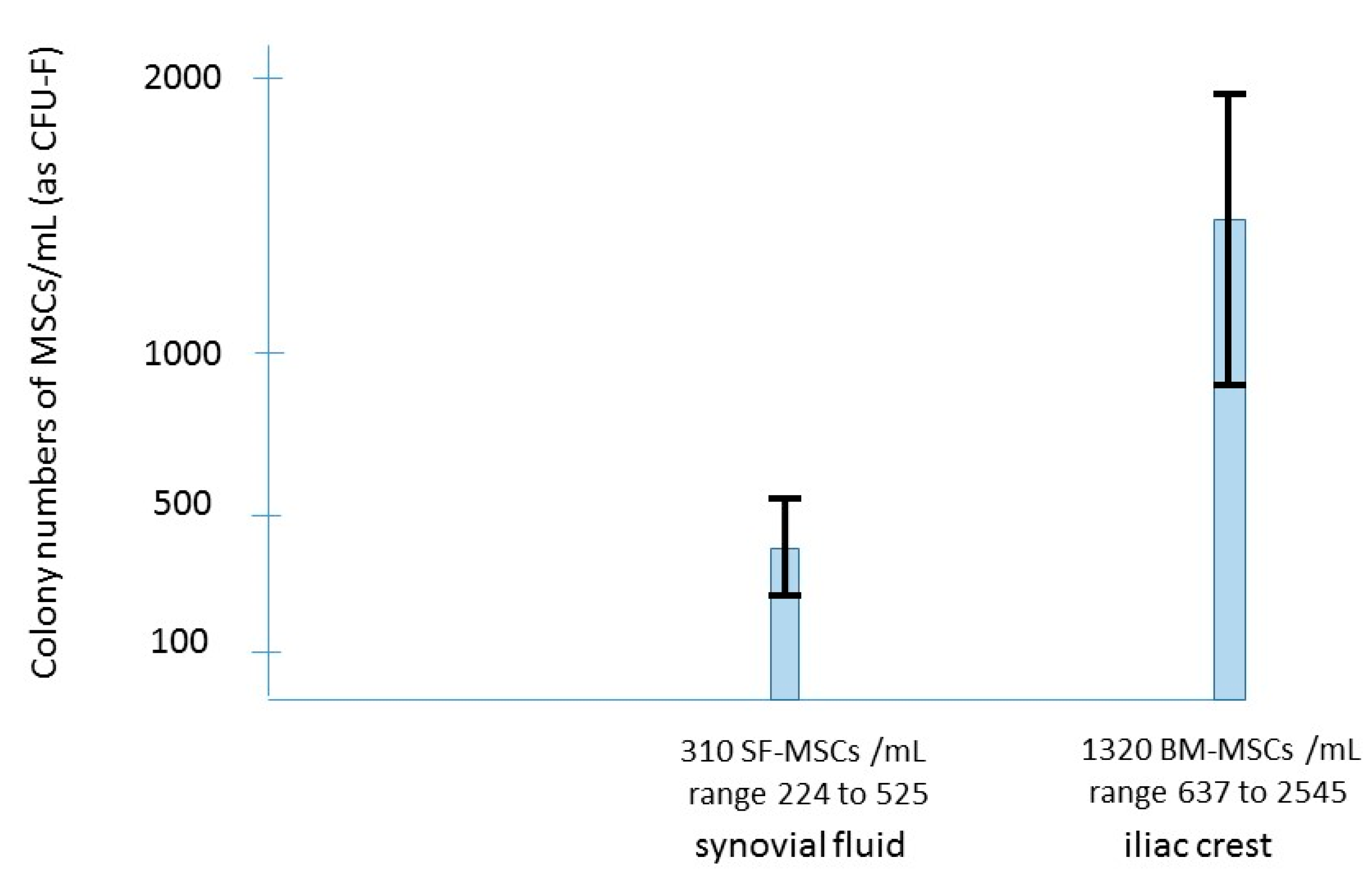
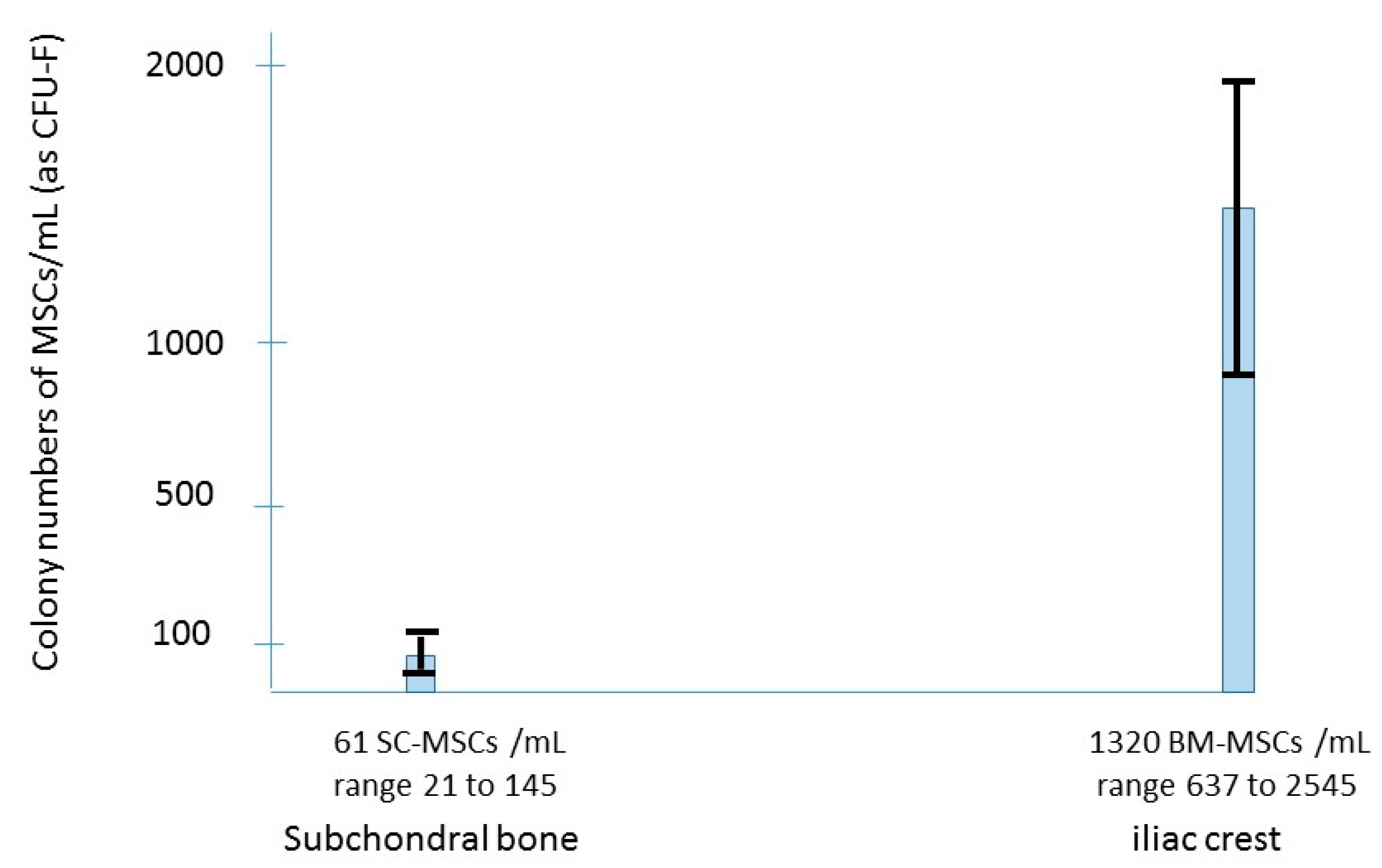
- Autologous bone-marrow-derived stem cells injected intra-articularly versus subchondral bone injection: in a randomized controlled clinical trial performed between 2000 and 2005 in 120 knees of 60 patients with a similar osteoarthritis grade, Hernigou [26] injected the same amount of BMAC in the joint of one knee and in the subchondral bone of the contralateral knee. Concerning the side with an intra-articular injection, 20 mL containing average 5727 MSCs/mL (range 2740 to 7540) were injected in the joint; the relative increased, compared to baseline number in the joint before injections) ranged from 5-fold to 30-fold, depending of the OA severity and age. Effusions were seen for several (<15) days after the procedure in both knees; these were anticipated findings, and probably these initial effusions were likely to be a residual of the 20-mL infiltration performed in each knee. The mean overall changes in knee scores (improved until 2-year follow up) from 52 points ±15 to 64 points ±21. However, with radiographs and MRI, no improvement was observed for joint space, bone marrow lesions and synovitis.
| Author | Study Design | Comparison | Nb of Knees | Age | Follow-Up | MRI |
|---|---|---|---|---|---|---|
| Shapiro [31] | blinded RCT | BMAC vs. saline | 25/25 | 60(42–68) | 1 year | No |
| Goncars [33] | Un-blinded RCT | BMAC vs. HA | 28/28 | 53 ± 15 | 1 year | No |
| Centeno [32] | Un-blinded RCT | BMAC vs. exercise | 26/22 | 54 ± 9/57 ± 8 | 2 years | No |
| Anz [34] | Un-blinded RCT | BMAB vs. PRP | 45/41 | 56 ± 11/52 ± 12 | 1 year | No |
| Hernigou [26] | Blinded RCT | BMAC | 60/60 | 76(62–87) | 15 years | Yes |
| Intra-articular vs. subchondral | ||||||
2.4. Proposed Mechanisms of Action of BMAC Injected in the Joint
3. Subchondral Injections BMAC Without Scaffold
3.1. The Subchondral Bone Damage in OA
3.2. The Rational of Using BMAC for Subchondral Pathology
3.3. Results of Clinical Studies
- -
- In a prospective randomized controlled clinical trial [16] carried out in 60 knees of 30 young patients who presented bilateral OA, one knee received a total knee arthroplasty (TKA) and the other knee received a subchondral bone marrow graft. At a follow up of average of 12 years (range 8 to 16 years), six (out of 30) TKA knees needed subsequent surgery versus only 1 with cell therapy. Knees with cell therapy had improvement on cartilage and bone marrow lesions observed at the site of bone marrow subchondral injection.
- -
- In another prospective study, Hernigou et al. [45] included 140 adults aged 65 to 90 years. These 140 patients (mean age 75.4 ± 14.2 years) planned to undergo staged-bilateral total knee arthroplasty (TKA) for medial osteoarthritis, had “comparable” pain in both knees, and accepted randomization of the knees for surgery. They received TKA on one side and a subchondral injection of MSCs (from iliac bone marrow concentrate) on the contralateral knee during the same anesthetic. This study showed that subchondral bone marrow concentrate (as compared with TKA) had a sufficient effect on pain to postpone or avoid the TKA in the contralateral joint of patients with bilateral osteoarthritis.
- -
- In a third randomized controlled clinical trial performed between 2000 and 2005, 60 patients with a similar osteoarthritis grade [26] received BMAC in the subchondral bone of one knee while the joint of the contralateral knee received intra-articular BMAC.
3.4. Proposed Mechanism of Action BMAC Injected in the Subchondral Bone
4. Scaffolds Loaded with BMAC as Beginning of Cartilage Engineering
4.1. The Rationale to Remove the Subchondral Bone in Osteoarthritis
4.2. Scaffolds: Towards Biocartilage
4.3. Rationale to Load Scaffolds with BMAC
4.4. Clinical Results in Long Term
4.5. Proposed Mechanism of Scaffolds Loaded with BMAC to Produce Hyaline Cartilage
5. Discussion: Balancing the Different Methods of Treatment
5.1. Merits of and Demerits of Intra-Articular BMACs Injections
5.2. Merits of and Demerits of Subchondral BMACs Injections
5.3. Merits of and Demerits of Scaffolds Loaded with BMACs
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caplan, A.I. Review: Mesenchymal Stem Cells: Cell-Based Reconstructive Therapy in Orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef]
- Chen, F.H.; Tuan, R.S. Mesenchymal Stem Cells in Arthritic Diseases. Arthritis Res. Ther. 2008, 10, 223. [Google Scholar] [CrossRef]
- Kuçi, S.; Henschler, R.; Müller, I.; Biagi, E.; Meisel, R. Basic Biology and Clinical Application of Multipotent Mesenchymal Stromal Cells: From Bench to Bedside. Stem Cells Int. 2012, 2012, 185943. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in Transplants of Bone Marrow Cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar]
- Pagani, S.; Borsari, V.; Veronesi, F.; Ferrari, A.; Cepollaro, S.; Torricelli, P.; Filardo, G.; Fini, M. Increased Chondrogenic Potential of Mesenchymal Cells From Adipose Tissue Versus Bone Marrow-Derived Cells in Osteoarthritic In Vitro Models. J. Cell Physiol. 2017, 232, 1478–1488. [Google Scholar] [CrossRef]
- Turner, L.; Knoepfler, P. Selling Stem Cells in the USA: Assessing the Direct-to-Consumer Industry. Cell Stem Cell 2016, 19, 154–157. [Google Scholar] [CrossRef]
- Song, L.; Tuan, R.S. Transdifferentiation Potential of Human Mesenchymal Stem Cells Derived from Bone Marrow. FASEB J. 2004, 18, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Herberts, C.A.; Kwa, M.S.G.; Hermsen, H.P.H. Risk Factors in the Development of Stem Cell Therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Desando, G.; Bartolotti, I.; Cavallo, C.; Schiavinato, A.; Secchieri, C.; Kon, E.; Filardo, G.; Paro, M.; Grigolo, B. Short-Term Homing of Hyaluronan-Primed Cells: Therapeutic Implications for Osteoarthritis Treatment. Tissue Eng. Part C Methods 2018, 24, 121–133. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, C.; Fei, H.; Hu, J.; Cui, W.; Wang, Z.; Li, Z.; Fan, W. Intraarticular Injection Autologous Platelet-Rich Plasma and Bone Marrow Concentrate in a Goat Osteoarthritis Model. J. Orthop. Res. 2018. [Google Scholar] [CrossRef]
- Song, F.; Tang, J.; Geng, R.; Hu, H.; Zhu, C.; Cui, W.; Fan, W. Comparison of the Efficacy of Bone Marrow Mononuclear Cells and Bone Mesenchymal Stem Cells in the Treatment of Osteoarthritis in a Sheep Model. Int. J. Clin. Exp. Pathol. 2014, 7, 1415–1426. [Google Scholar] [PubMed]
- Singh, A.; Goel, S.C.; Gupta, K.K.; Kumar, M.; Arun, G.R.; Patil, H.; Kumaraswamy, V.; Jha, S. The Role of Stem Cells in Osteoarthritis: An Experimental Study in Rabbits. Bone Joint Res. 2014, 3, 32–37. [Google Scholar] [CrossRef]
- Hernigou, J.; Vertongen, P.; Chahidi, E.; Kyriakidis, T.; Dehoux, J.-P.; Crutzen, M.; Boutry, S.; Larbanoix, L.; Houben, S.; Gaspard, N.; et al. Effects of Press-Fit Biphasic (Collagen and HA/ΒTCP) Scaffold with Cell-Based Therapy on Cartilage and Subchondral Bone Repair Knee Defect in Rabbits. Int. Orthop. 2018, 42, 1755–1767. [Google Scholar] [CrossRef]
- Filardo, G.; Perdisa, F.; Roffi, A.; Marcacci, M.; Kon, E. Stem Cells in Articular Cartilage Regeneration. J. Orthop. Surg. Res. 2016, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Karnatzikos, G.; Scotti, C.; Mahajan, V.; Mazzucco, L.; Grigolo, B. One-Step Cartilage Repair with Bone Marrow Aspirate Concentrated Cells and Collagen Matrix in Full-Thickness Knee Cartilage Lesions: Results at 2-Year Follow-Up. Cartilage 2011, 2, 286–299. [Google Scholar] [CrossRef]
- Hernigou, P.; Auregan, J.C.; Dubory, A.; Flouzat-Lachaniette, C.H.; Chevallier, N.; Rouard, H. Subchondral Stem Cell Therapy versus Contralateral Total Knee Arthroplasty for Osteoarthritis Following Secondary Osteonecrosis of the Knee. Int. Orthop. 2018, 42, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Yusup, A.; Kaneko, H.; Liu, L.; Ning, L.; Sadatsuki, R.; Hada, S.; Kamagata, K.; Kinoshita, M.; Futami, I.; Shimura, Y.; et al. Bone Marrow Lesions, Subchondral Bone Cysts and Subchondral Bone Attrition Are Associated with Histological Synovitis in Patients with End-Stage Knee Osteoarthritis: A Cross-Sectional Study. Osteoarthr. Cartil. 2015, 23, 1858–1864. [Google Scholar] [CrossRef]
- Egloff, C.; Hügle, T.; Valderrabano, V. Biomechanics and Pathomechanisms of Osteoarthritis. Swiss Med. Wkly. 2012, 142, w13583. [Google Scholar] [CrossRef]
- Atukorala, I.; Kwoh, C.K.; Guermazi, A.; Roemer, F.W.; Boudreau, R.M.; Hannon, M.J.; Hunter, D.J. Synovitis in Knee Osteoarthritis: A Precursor of Disease? Ann. Rheum. Dis. 2016, 75, 390–395. [Google Scholar] [CrossRef]
- Schueller-Weidekamm, C.; Schueller, G.; Uffmann, M.; Bader, T.R. Does Marathon Running Cause Acute Lesions of the Knee? Evaluation with Magnetic Resonance Imaging. Eur. Radiol. 2006, 16, 2179–2185. [Google Scholar] [CrossRef]
- Loeuille, D.; Chary-Valckenaere, I.; Champigneulle, J.; Rat, A.-C.; Toussaint, F.; Pinzano-Watrin, A.; Goebel, J.C.; Mainard, D.; Blum, A.; Pourel, J.; et al. Macroscopic and Microscopic Features of Synovial Membrane Inflammation in the Osteoarthritic Knee: Correlating Magnetic Resonance Imaging Findings with Disease Severity. Arthritis Rheum. 2005, 52, 3492–3501. [Google Scholar] [CrossRef]
- Roemer, F.W.; Kassim Javaid, M.; Guermazi, A.; Thomas, M.; Kiran, A.; Keen, R.; King, L.; Arden, N.K. Anatomical Distribution of Synovitis in Knee Osteoarthritis and Its Association with Joint Effusion Assessed on Non-Enhanced and Contrast-Enhanced MRI. Osteoarthr. Cartil. 2010, 18, 1269–1274. [Google Scholar] [CrossRef]
- Jones, E.A.; Crawford, A.; English, A.; Henshaw, K.; Mundy, J.; Corscadden, D.; Chapman, T.; Emery, P.; Hatton, P.; McGonagle, D. Synovial Fluid Mesenchymal Stem Cells in Health and Early Osteoarthritis: Detection and Functional Evaluation at the Single-Cell Level. Arthritis Rheum. 2008, 58, 1731–1740. [Google Scholar] [CrossRef]
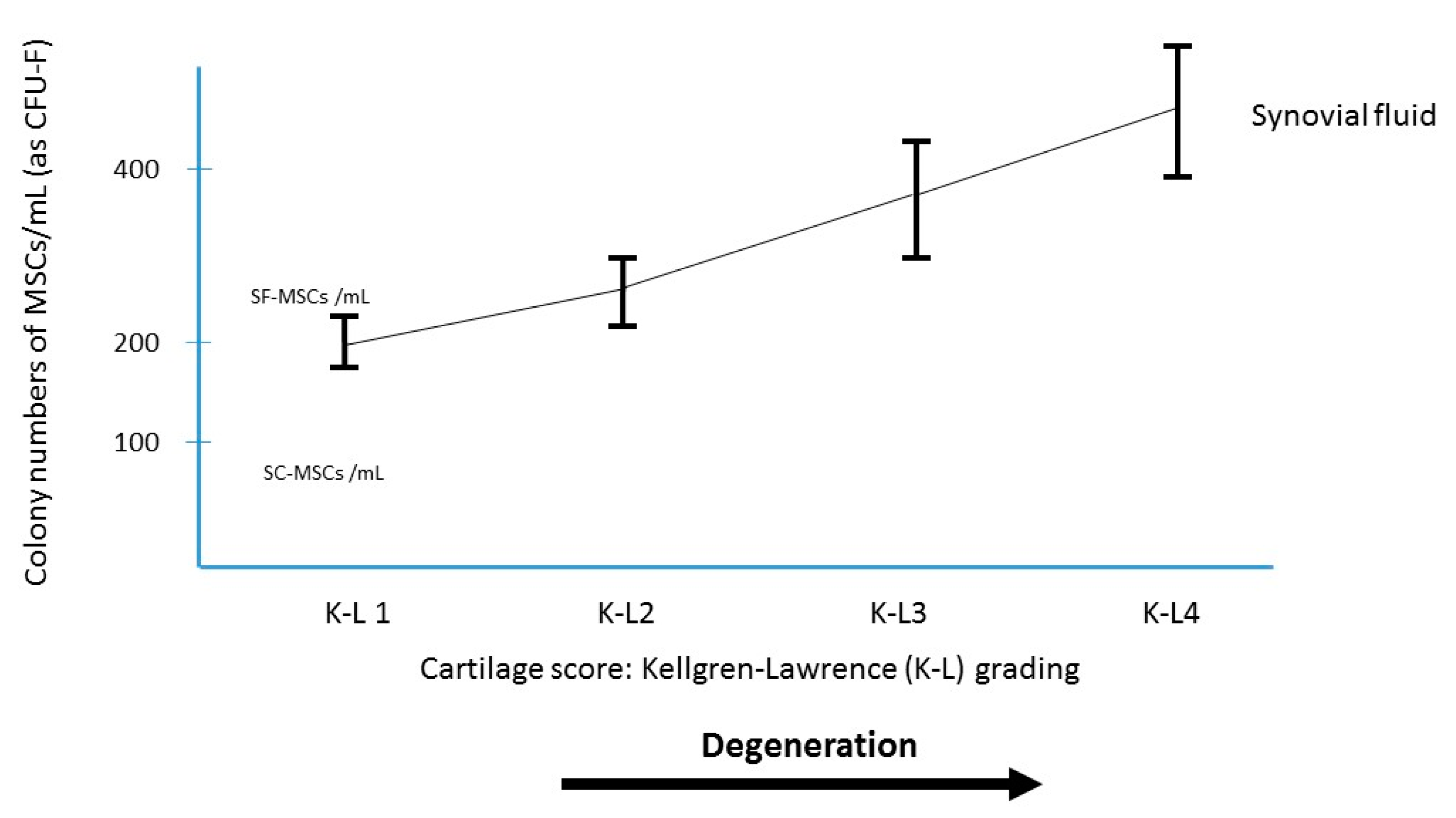
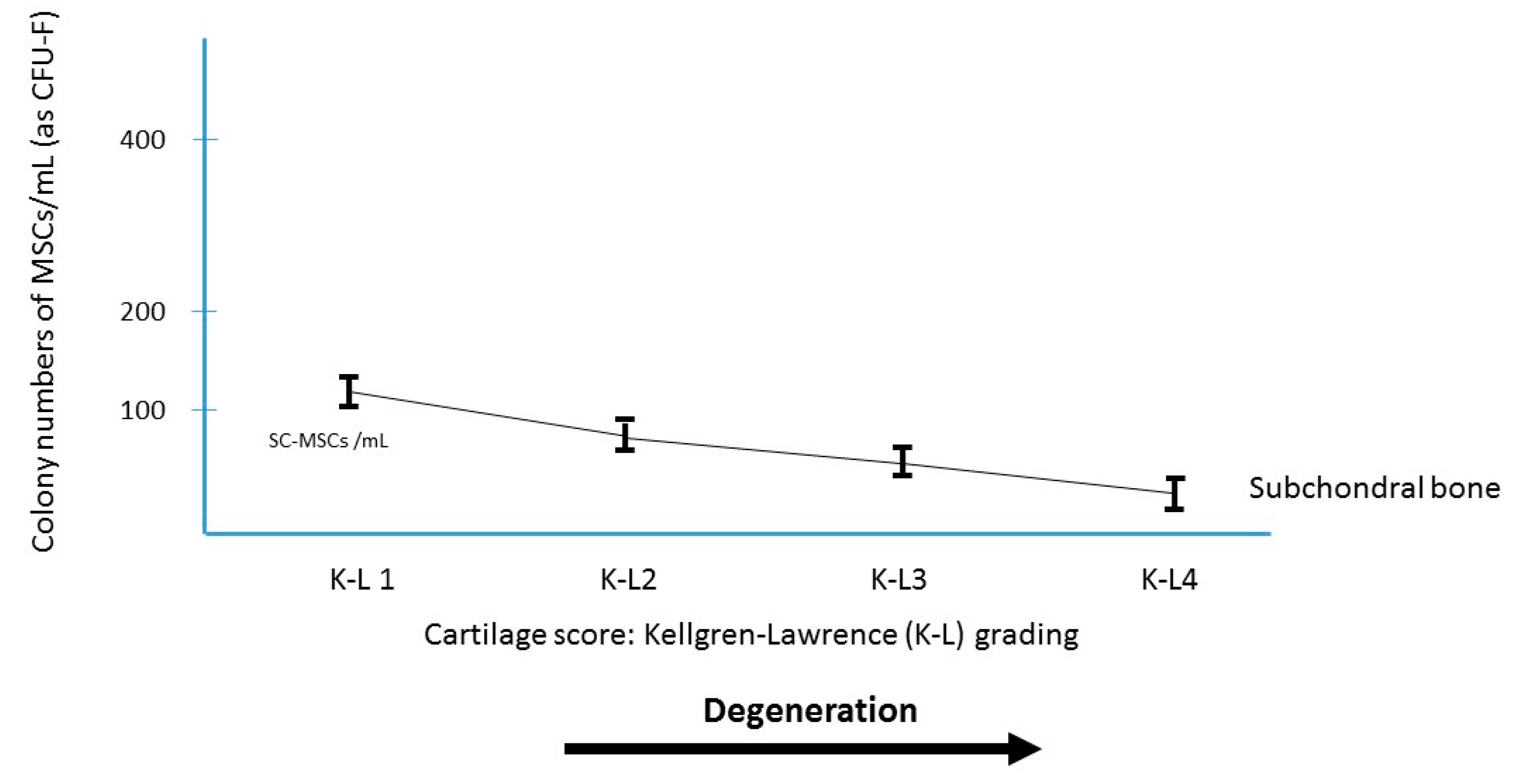
- Sekiya, I.; Ojima, M.; Suzuki, S.; Yamaga, M.; Horie, M.; Koga, H.; Tsuji, K.; Miyaguchi, K.; Ogishima, S.; Tanaka, H.; et al. Human Mesenchymal Stem Cells in Synovial Fluid Increase in the Knee with Degenerated Cartilage and Osteoarthritis. J. Orthop. Res. 2012, 30, 943–949. [Google Scholar] [CrossRef]
- Davies, B.M.; Snelling, S.J.B.; Quek, L.; Hakimi, O.; Ye, H.; Carr, A.; Price, A.J. Identifying the Optimum Source of Mesenchymal Stem Cells for Use in Knee Surgery. J. Orthop. Res. 2017, 35, 1868–1875. [Google Scholar] [CrossRef]
- Hernigou, P.; Bouthors, C.; Bastard, C.; Flouzat Lachaniette, C.H.; Rouard, H.; Dubory, A. Subchondral Bone or Intra-Articular Injection of Bone Marrow Concentrate Mesenchymal Stem Cells in Bilateral Knee Osteoarthritis: What Better Postpone Knee Arthroplasty at Fifteen Years? A Randomized Study. Int. Orthop. 2021, 45, 391–399. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Mautner, K.; Bowers, R.; Easley, K.; Fausel, Z.; Robinson, R. Functional Outcomes Following Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate Injections for Symptomatic Knee Osteoarthritis. Stem Cells Transl. Med. 2019, 8, 1149–1156. [Google Scholar] [CrossRef]
- Shaw, B.; Darrow, M.; Derian, A. Short-Term Outcomes in Treatment of Knee Osteoarthritis With 4 Bone Marrow Concentrate Injections. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 1179544118781080. [Google Scholar] [CrossRef]
- Themistocleous, G.S.; Chloros, G.D.; Kyrantzoulis, I.M.; Georgokostas, I.A.; Themistocleous, M.S.; Papagelopoulos, P.J.; Savvidou, O.D. Effectiveness of a Single Intra-Articular Bone Marrow Aspirate Concentrate (BMAC) Injection in Patients with Grade 3 and 4 Knee Osteoarthritis. Heliyon 2018, 4, e00871. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef]
- Centeno, C.; Sheinkop, M.; Dodson, E.; Stemper, I.; Williams, C.; Hyzy, M.; Ichim, T.; Freeman, M. A Specific Protocol of Autologous Bone Marrow Concentrate and Platelet Products versus Exercise Therapy for Symptomatic Knee Osteoarthritis: A Randomized Controlled Trial with 2 Year Follow-Up. J. Transl. Med. 2018, 16, 355. [Google Scholar] [CrossRef]
- Goncars, V.; Jakobsons, E.; Blums, K.; Briede, I.; Patetko, L.; Erglis, K.; Erglis, M.; Kalnberzs, K.; Muiznieks, I.; Erglis, A. The Comparison of Knee Osteoarthritis Treatment with Single-Dose Bone Marrow-Derived Mononuclear Cells vs. Hyaluronic Acid Injections. Medicina 2017, 53, 101–108. [Google Scholar] [CrossRef]
- Anz, A.W.; Hubbard, R.; Rendos, N.K.; Everts, P.A.; Andrews, J.R.; Hackel, J.G. Bone Marrow Aspirate Concentrate Is Equivalent to Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis at 1 Year: A Prospective, Randomized Trial. Orthop. J. Sports Med. 2020, 8, 2325967119900958. [Google Scholar] [CrossRef]
- Montaseri, A.; Busch, F.; Mobasheri, A.; Buhrmann, C.; Aldinger, C.; Rad, J.S.; Shakibaei, M. IGF-1 and PDGF-Bb Suppress IL-1β-Induced Cartilage Degradation through down-Regulation of NF-ΚB Signaling: Involvement of Src/PI-3K/AKT Pathway. PLoS ONE 2011, 6, e28663. [Google Scholar] [CrossRef]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the Cannabinoid Receptor System in Synovial Tissue and Fluid in Patients with Osteoarthritis and Rheumatoid Arthritis. Arthritis Res. Ther. 2008, 10, R43. [Google Scholar] [CrossRef]
- Hayashi, D.; Englund, M.; Roemer, F.W.; Niu, J.; Sharma, L.; Felson, D.T.; Crema, M.D.; Marra, M.D.; Segal, N.A.; Lewis, C.E.; et al. Knee Malalignment Is Associated with an Increased Risk for Incident and Enlarging Bone Marrow Lesions in the More Loaded Compartments: The MOST Study. Osteoarthr. Cartil. 2012, 20, 1227–1233. [Google Scholar] [CrossRef]
- Englund, M.; Guermazi, A.; Roemer, F.W.; Yang, M.; Zhang, Y.; Nevitt, M.C.; Lynch, J.A.; Lewis, C.E.; Torner, J.; Felson, D.T. Meniscal Pathology on MRI Increases the Risk for Both Incident and Enlarging Subchondral Bone Marrow Lesions of the Knee: The MOST Study. Ann. Rheum. Dis. 2010, 69, 1796–1802. [Google Scholar] [CrossRef]
- Hunter, D.J.; Zhang, Y.; Niu, J.; Goggins, J.; Amin, S.; LaValley, M.P.; Guermazi, A.; Genant, H.; Gale, D.; Felson, D.T. Increase in Bone Marrow Lesions Associated with Cartilage Loss: A Longitudinal Magnetic Resonance Imaging Study of Knee Osteoarthritis. Arthritis Rheum. 2006, 54, 1529–1535. [Google Scholar] [CrossRef]
- Lisignoli, G.; Toneguzzi, S.; Piacentini, A.; Cristino, S.; Grassi, F.; Cavallo, C.; Facchini, A. CXCL12 (SDF-1) and CXCL13 (BCA-1) Chemokines Significantly Induce Proliferation and Collagen Type I Expression in Osteoblasts from Osteoarthritis Patients. J. Cell Physiol. 2006, 206, 78–85. [Google Scholar] [CrossRef]
- Massicotte, F.; Lajeunesse, D.; Benderdour, M.; Pelletier, J.-P.; Hilal, G.; Duval, N.; Martel-Pelletier, J. Can Altered Production of Interleukin-1beta, Interleukin-6, Transforming Growth Factor-Beta and Prostaglandin E(2) by Isolated Human Subchondral Osteoblasts Identify Two Subgroups of Osteoarthritic Patients. Osteoarthr. Cartil. 2002, 10, 491–500. [Google Scholar] [CrossRef]
- Paredes, Y.; Massicotte, F.; Pelletier, J.-P.; Martel-Pelletier, J.; Laufer, S.; Lajeunesse, D. Study of the Role of Leukotriene B()4 in Abnormal Function of Human Subchondral Osteoarthritis Osteoblasts: Effects of Cyclooxygenase and/or 5-Lipoxygenase Inhibition. Arthritis Rheum. 2002, 46, 1804–1812. [Google Scholar] [CrossRef]
- Hilal, G.; Martel-Pelletier, J.; Pelletier, J.P.; Ranger, P.; Lajeunesse, D. Osteoblast-like Cells from Human Subchondral Osteoarthritic Bone Demonstrate an Altered Phenotype in Vitro: Possible Role in Subchondral Bone Sclerosis. Arthritis Rheum. 1998, 41, 891–899. [Google Scholar] [CrossRef]
- Aspden, R.M.; Scheven, B.A.; Hutchison, J.D. Osteoarthritis as a Systemic Disorder Including Stromal Cell Differentiation and Lipid Metabolism. Lancet 2001, 357, 1118–1120. [Google Scholar] [CrossRef]
- Hernigou, P.; Delambre, J.; Quiennec, S.; Poignard, A. Human Bone Marrow Mesenchymal Stem Cell Injection in Subchondral Lesions of Knee Osteoarthritis: A Prospective Randomized Study versus Contralateral Arthroplasty at a Mean Fifteen Year Follow-Up. Int. Orthop. 2021, 45, 365–373. [Google Scholar] [CrossRef]
- Hügle, T.; Geurts, J. What Drives Osteoarthritis?-Synovial versus Subchondral Bone Pathology. Rheumatology 2017, 56, 1461–1471. [Google Scholar] [CrossRef]
- Burr, D.B.; Radin, E.L. Microfractures and Microcracks in Subchondral Bone: Are They Relevant to Osteoarthrosis? Rheum. Dis. Clin. North. Am. 2003, 29, 675–685. [Google Scholar] [CrossRef]
- Villanueva, A.R.; Longo, J.A.; Weiner, G. Staining and Histomorphometry of Microcracks in the Human Femoral Head. Biotech. Histochem. 1994, 69, 81–88. [Google Scholar] [CrossRef]
- Sokoloff, L. Microcracks in the Calcified Layer of Articular Cartilage. Arch. Pathol. Lab. Med. 1993, 117, 191–195. [Google Scholar]
- Clark, J.M. The Structure of Vascular Channels in the Subchondral Plate. J. Anat. 1990, 171, 105–115. [Google Scholar]
- Endres, M.; Neumann, K.; Häupl, T.; Erggelet, C.; Ringe, J.; Sittinger, M.; Kaps, C. Synovial Fluid Recruits Human Mesenchymal Progenitors from Subchondral Spongious Bone Marrow. J. Orthop. Res. 2007, 25, 1299–1307. [Google Scholar] [CrossRef]
- Lychagin, A.; Lipina, M.; Garkavi, A.; Islaieh, O.; Timashev, P.; Ashmore, K.; Kon, E. Intraosseous Injections of Platelet Rich Plasma for Knee Bone Marrow Lesions Treatment: One Year Follow-Up. Int. Orthop. 2021, 45, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Bai, Y.; Wang, J.; Zhang, H.; Liu, H.; Ma, S. Comparison of Hyaluronic Acid and PRP Intra-Articular Injection with Combined Intra-Articular and Intraosseous PRP Injections to Treat Patients with Knee Osteoarthritis. Clin. Rheumatol. 2018, 37, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Delgado, D.; Sánchez, P.; Muiños-López, E.; Paiva, B.; Granero-Moltó, F.; Prósper, F.; Pompei, O.; Pérez, J.C.; Azofra, J.; et al. Combination of Intra-Articular and Intraosseous Injections of Platelet Rich Plasma for Severe Knee Osteoarthritis: A Pilot Study. Biomed. Res. Int. 2016, 2016, 4868613. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Delgado, D.; Pompei, O.; Pérez, J.C.; Sánchez, P.; Garate, A.; Bilbao, A.M.; Fiz, N.; Padilla, S. Treating Severe Knee Osteoarthritis with Combination of Intra-Osseous and Intra-Articular Infiltrations of Platelet-Rich Plasma: An Observational Study. Cartilage 2019, 10, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Fiz, N.; Guadilla, J.; Padilla, S.; Anitua, E.; Sánchez, P.; Delgado, D. Intraosseous Infiltration of Platelet-Rich Plasma for Severe Knee Osteoarthritis. Arthrosc. Tech. 2014, 3, e713–e717. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Martel-Pelletier, J.; Lascau-Coman, V.; Moldovan, F.; Jovanovic, D.; Raynauld, J.P.; Pelletier, J.P. Collagenase-1 and Collagenase-3 Synthesis in Normal and Early Experimental Osteoarthritic Canine Cartilage: An Immunohistochemical Study. J. Rheumatol. 1998, 25, 1585–1594. [Google Scholar]
- Moldovan, F.; Pelletier, J.P.; Hambor, J.; Cloutier, J.M.; Martel-Pelletier, J. Collagenase-3 (Matrix Metalloprotease 13) Is Preferentially Localized in the Deep Layer of Human Arthritic Cartilage in Situ: In Vitro Mimicking Effect by Transforming Growth Factor Beta. Arthritis Rheum. 1997, 40, 1653–1661. [Google Scholar] [CrossRef]
- Moldovan, F.; Pelletier, J.P.; Mineau, F.; Dupuis, M.; Cloutier, J.M.; Martel-Pelletier, J. Modulation of Collagenase 3 in Human Osteoarthritic Cartilage by Activation of Extracellular Transforming Growth Factor Beta: Role of Furin Convertase. Arthritis Rheum. 2000, 43, 2100–2109. [Google Scholar] [CrossRef]
- Häuselmann, H.J.; Aydelotte, M.B.; Schumacher, B.L.; Kuettner, K.E.; Gitelis, S.H.; Thonar, E.J. Synthesis and Turnover of Proteoglycans by Human and Bovine Adult Articular Chondrocytes Cultured in Alginate Beads. Matrix 1992, 12, 116–129. [Google Scholar] [CrossRef]
- Wong, M.; Siegrist, M.; Wang, X.; Hunziker, E. Development of Mechanically Stable Alginate/Chondrocyte Constructs: Effects of Guluronic Acid Content and Matrix Synthesis. J. Orthop. Res. 2001, 19, 493–499. [Google Scholar] [CrossRef]
- Ragan, P.M.; Chin, V.I.; Hung, H.H.; Masuda, K.; Thonar, E.J.; Arner, E.C.; Grodzinsky, A.J.; Sandy, J.D. Chondrocyte Extracellular Matrix Synthesis and Turnover Are Influenced by Static Compression in a New Alginate Disk Culture System. Arch. Biochem. Biophys. 2000, 383, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Gigant-Huselstein, C.; Dumas, D.; Hubert, P.; Baptiste, D.; Dellacherie, E.; Mainard, D.; Netter, P.; Payan, E.; Stoltz, J.F. Influence of Mechanical Stress on Extracellular Matrixes Synthesized by Chondrocytes Seeded onto Alginate and Hyaluronate-Based 3d Biosystems. J. Mech. Med. Biol. 2003, 03, 59–70. [Google Scholar] [CrossRef]
- Miralles, G.; Baudoin, R.; Dumas, D.; Baptiste, D.; Hubert, P.; Stoltz, J.F.; Dellacherie, E.; Mainard, D.; Netter, P.; Payan, E. Sodium Alginate Sponges with or without Sodium Hyaluronate: In Vitro Engineering of Cartilage. J. Biomed. Mater. Res. 2001, 57, 268–278. [Google Scholar] [CrossRef]
- Gigante, A.; Calcagno, S.; Cecconi, S.; Ramazzotti, D.; Manzotti, S.; Enea, D. Use of Collagen Scaffold and Autologous Bone Marrow Concentrate as a One-Step Cartilage Repair in the Knee: Histological Results of Second-Look Biopsies at 1 Year Follow-Up. Int. J. Immunopathol. Pharmacol. 2011, 24, 69–72. [Google Scholar] [CrossRef]
- Skowroński, J.; Rutka, M. Osteochondral Lesions of the Knee Reconstructed with Mesenchymal Stem Cells-Results. Ortop Traumatol. Rehabil. 2013, 15, 195–204. [Google Scholar] [CrossRef]
- Gobbi, A.; Whyte, G.P. Long-Term Clinical Outcomes of One-Stage Cartilage Repair in the Knee With Hyaluronic Acid-Based Scaffold Embedded With Mesenchymal Stem Cells Sourced From Bone Marrow Aspirate Concentrate. Am. J. Sports Med. 2019, 47, 1621–1628. [Google Scholar] [CrossRef]
- Buda, R.; Vannini, F.; Cavallo, M.; Baldassarri, M.; Luciani, D.; Mazzotti, A.; Pungetti, C.; Olivieri, A.; Giannini, S. One-Step Arthroscopic Technique for the Treatment of Osteochondral Lesions of the Knee with Bone-Marrow-Derived Cells: Three Years Results. Musculoskelet. Surg. 2013, 97, 145–151. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Peterson, L.; Vasiliadis, H.S.; Brittberg, M.; Lindahl, A. Autologous Chondrocyte Implantation: A Long-Term Follow-Up. Am. J. Sports Med. 2010, 38, 1117–1124. [Google Scholar] [CrossRef]
- Samuelson, E.M.; Brown, D.E. Cost-Effectiveness Analysis of Autologous Chondrocyte Implantation: A Comparison of Periosteal Patch versus Type I/III Collagen Membrane. Am. J. Sports Med. 2012, 40, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in Mesenchymal Stromal Cells Induces in Vivo Recipient-Mediated Immunomodulation. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- De Witte, S.F.H.; Luk, F.; Sierra Parraga, J.M.; Gargesha, M.; Merino, A.; Korevaar, S.S.; Shankar, A.S.; O’Flynn, L.; Elliman, S.J.; Roy, D.; et al. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells 2018, 36, 602–615. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC Exosomes Mediate Cartilage Repair by Enhancing Proliferation, Attenuating Apoptosis and Modulating Immune Reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
| Author | Study Design | Comparison | Nb of Knees | Age | Follow-Up | MRI |
|---|---|---|---|---|---|---|
| Hernigou [16] | Unblinded RCT | BMAC vs TKA | 30/30 | 28 (18–41) | 12 years | Yes |
| Hernigou [45] | Unblinded RCT | BMAC vs TKA | 140/140 | 75(65–90) | 15 years | Yes |
| Author | Study Design | Scaffold | Nb of Knees | Age | Follow-Up | Biopsy/MRI |
|---|---|---|---|---|---|---|
| Gigante [65] | Retrospective | Collagen | 5 | 43(25–54) | 1 year | second look biopsies |
| Skowronski [66] | Retrospective | Collagen | 21 | 26(17–52) | 5 years | MRI |
| Gobi [67] | Prospective | Hyaluronic Acid | 23 | 48 ± 9 | 8 years | MRI |
| Buda [68] | Retrospective | Hyaluronic Acid | 20 | 35(15–50) | 2 years | Biopsies |
| Technique | In Favor | Against |
|---|---|---|
| Intra-articular | percutaneous | short term efficiency |
| Local anesthesia | ||
| Subchondral | percutaneous | needs fluoroscopy |
| Long term efficiency | ±general anesthesia | |
| Scaffolds | protect cells | open surgery |
| Bioactive material | potential complications |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernigou, J.; Vertongen, P.; Rasschaert, J.; Hernigou, P. Role of Scaffolds, Subchondral, Intra-Articular Injections of Fresh Autologous Bone Marrow Concentrate Regenerative Cells in Treating Human Knee Cartilage Lesions: Different Approaches and Different Results. Int. J. Mol. Sci. 2021, 22, 3844. https://doi.org/10.3390/ijms22083844
Hernigou J, Vertongen P, Rasschaert J, Hernigou P. Role of Scaffolds, Subchondral, Intra-Articular Injections of Fresh Autologous Bone Marrow Concentrate Regenerative Cells in Treating Human Knee Cartilage Lesions: Different Approaches and Different Results. International Journal of Molecular Sciences. 2021; 22(8):3844. https://doi.org/10.3390/ijms22083844
Chicago/Turabian StyleHernigou, Jacques, Pascale Vertongen, Joanne Rasschaert, and Philippe Hernigou. 2021. "Role of Scaffolds, Subchondral, Intra-Articular Injections of Fresh Autologous Bone Marrow Concentrate Regenerative Cells in Treating Human Knee Cartilage Lesions: Different Approaches and Different Results" International Journal of Molecular Sciences 22, no. 8: 3844. https://doi.org/10.3390/ijms22083844
APA StyleHernigou, J., Vertongen, P., Rasschaert, J., & Hernigou, P. (2021). Role of Scaffolds, Subchondral, Intra-Articular Injections of Fresh Autologous Bone Marrow Concentrate Regenerative Cells in Treating Human Knee Cartilage Lesions: Different Approaches and Different Results. International Journal of Molecular Sciences, 22(8), 3844. https://doi.org/10.3390/ijms22083844






