Human Somatostatin SST4 Receptor Transgenic Mice: Construction and Brain Expression Pattern Characterization
Abstract
1. Introduction
2. Results
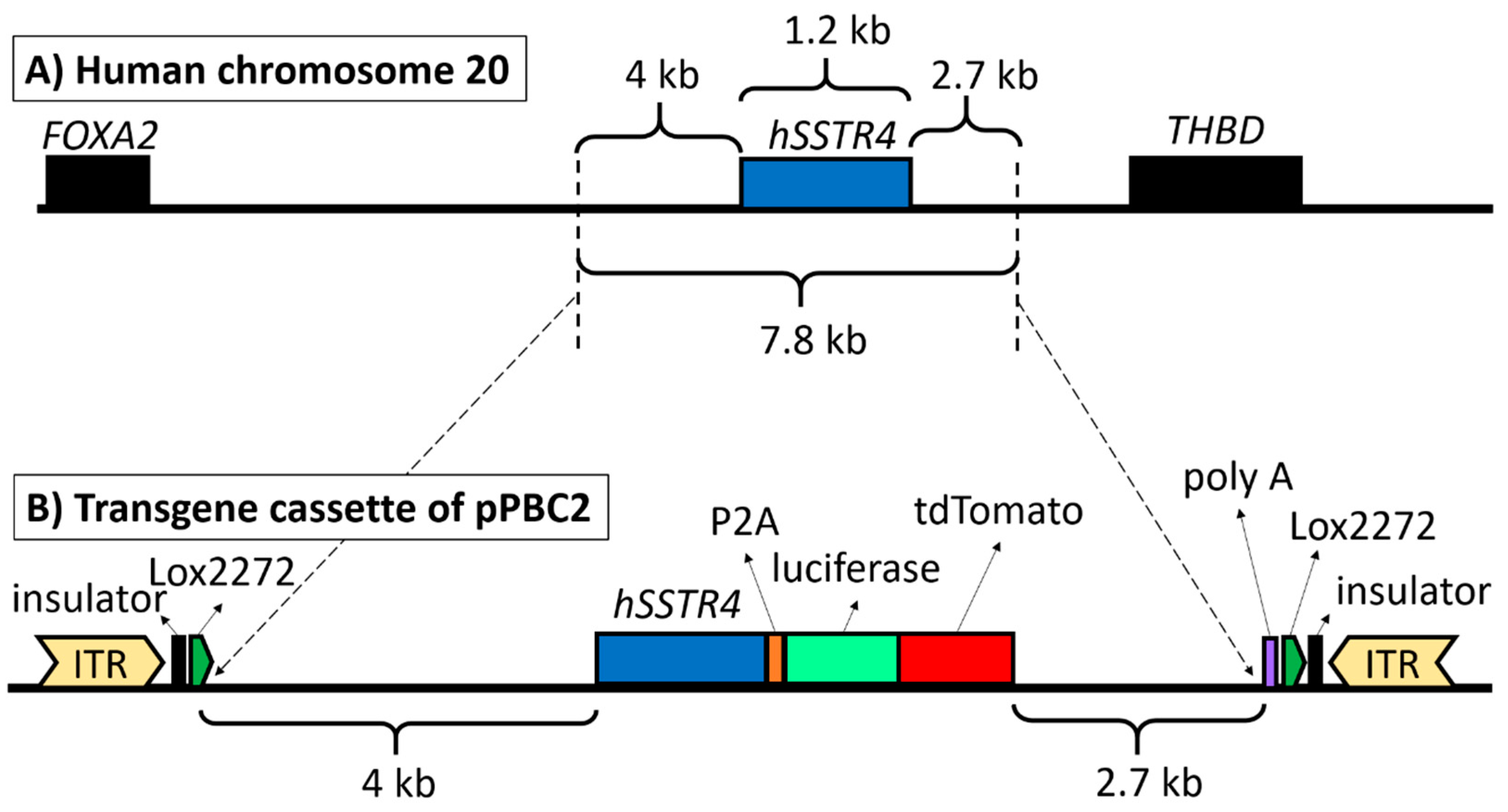
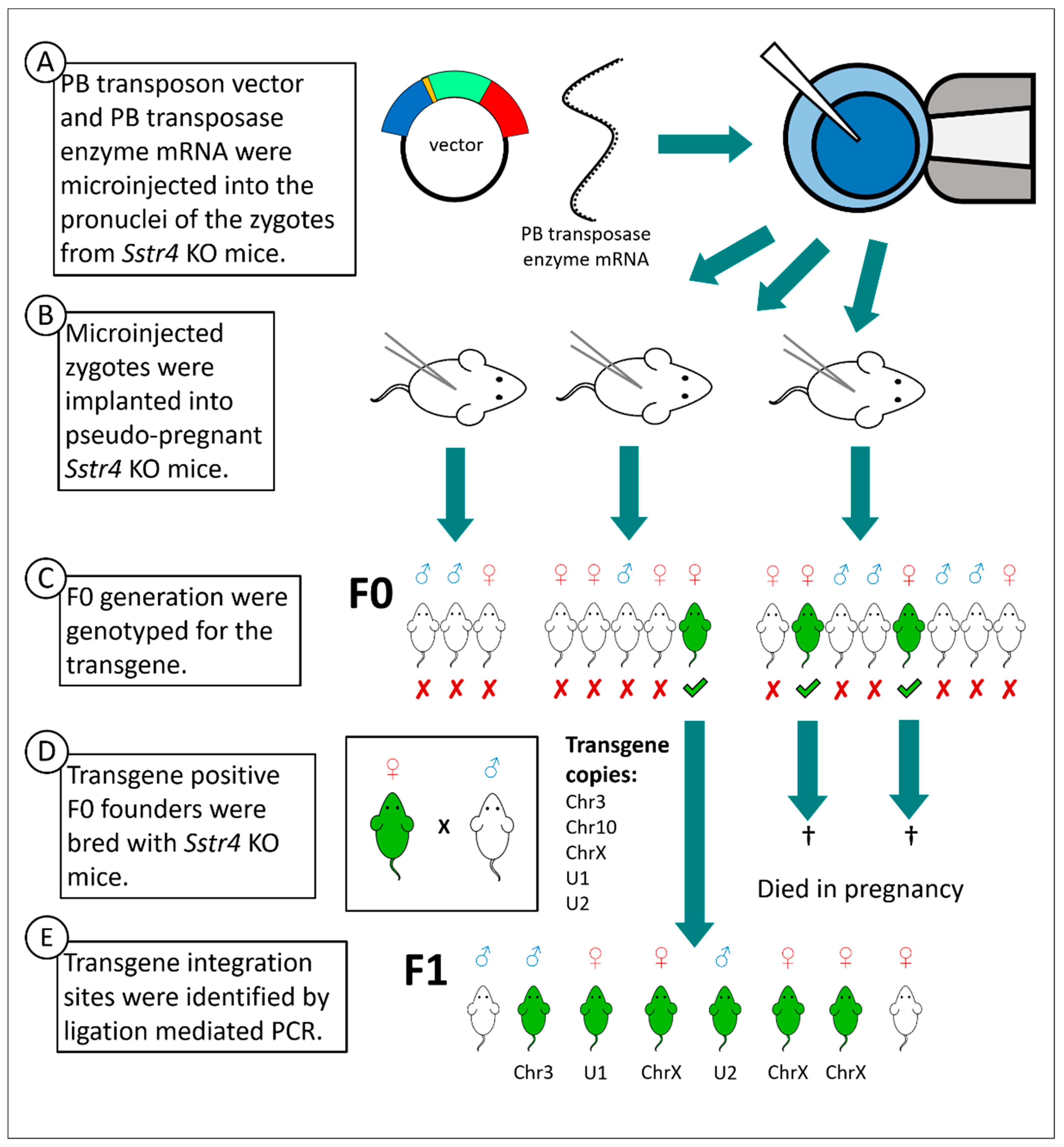
2.1. Vector Construction and Transgenesis
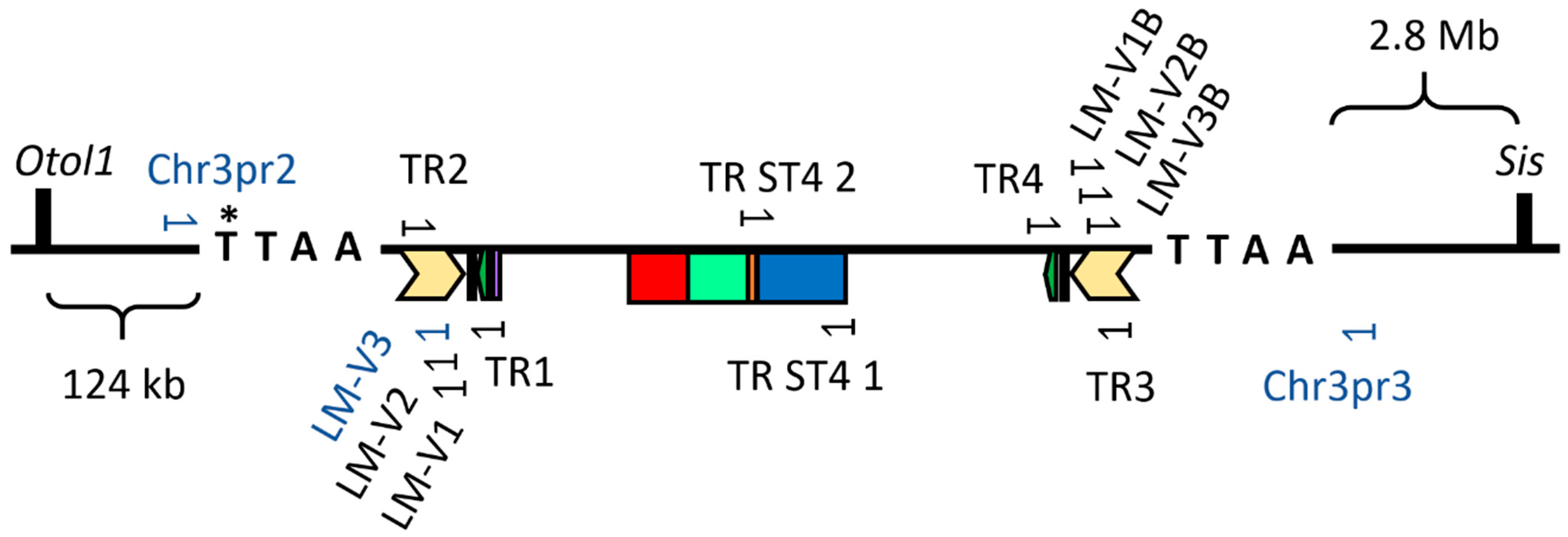
2.2. Integration Sites of the hSSTR4 Transgene
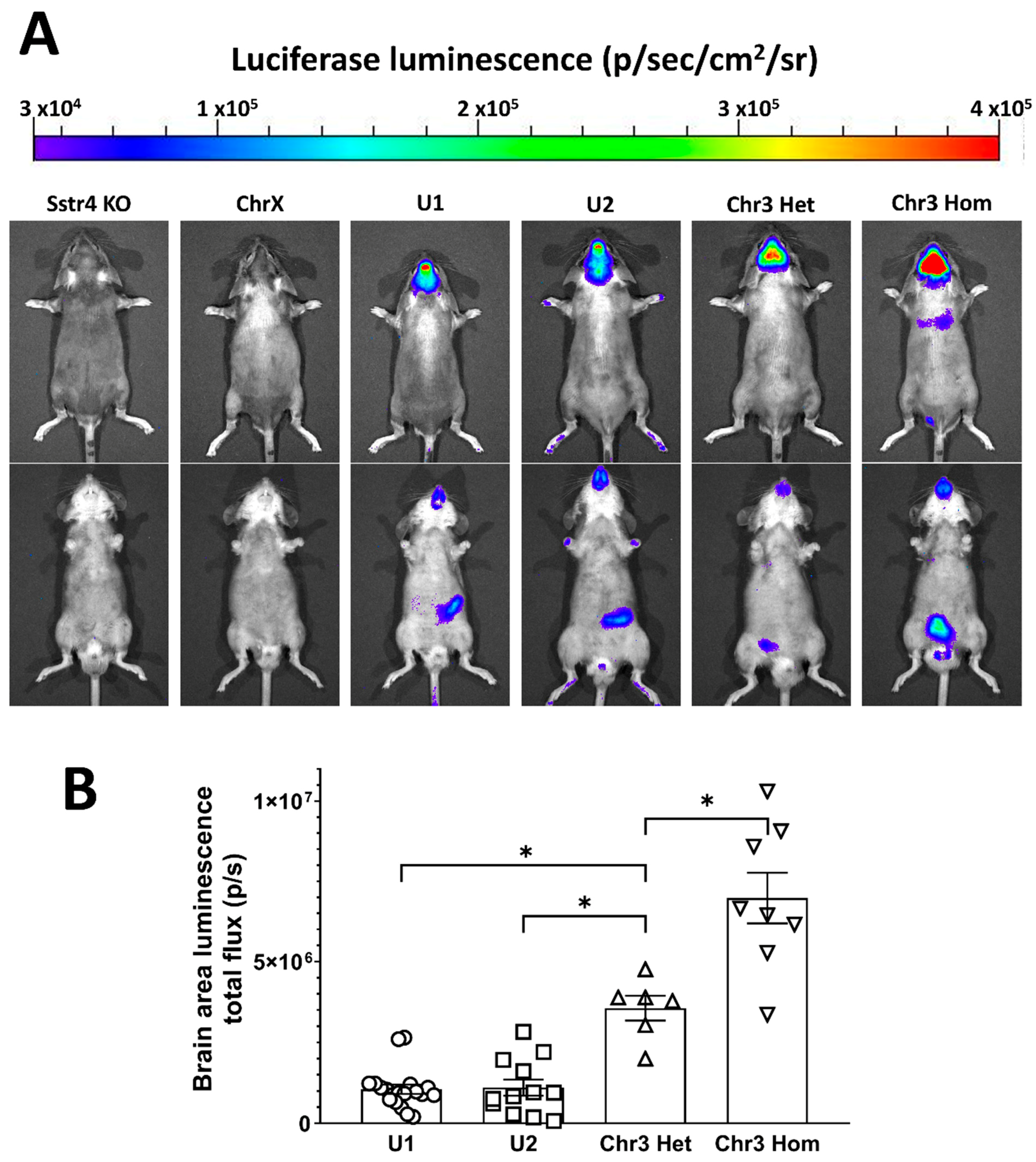
2.3. Distinct Expression Pattern of the hSSTR4-Related Luciferase by Luminescent In Vivo Optical Imaging in Various Mouse Lines
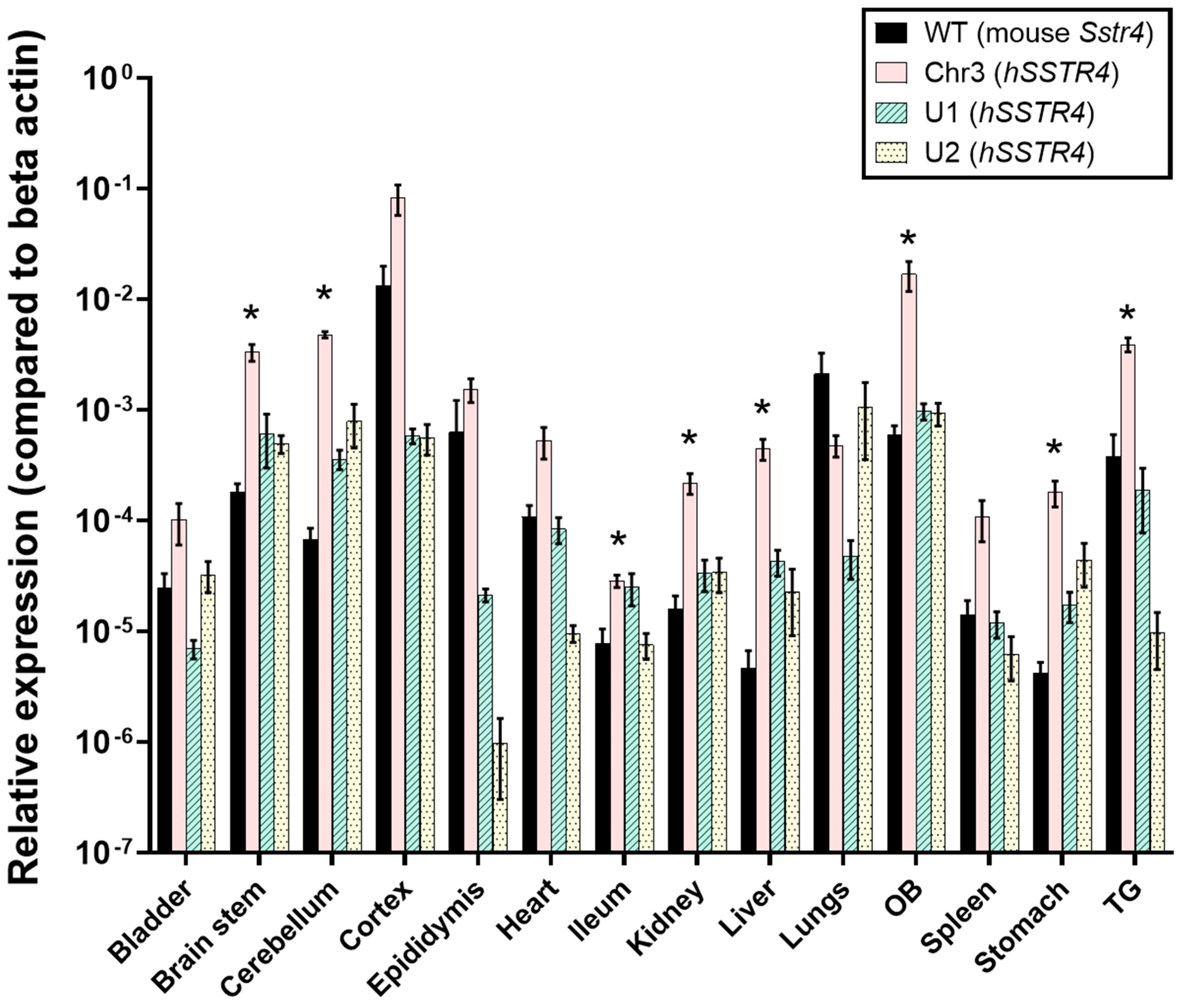
2.4. hSSTR4 Expression Level Assessed by RT-qPCR
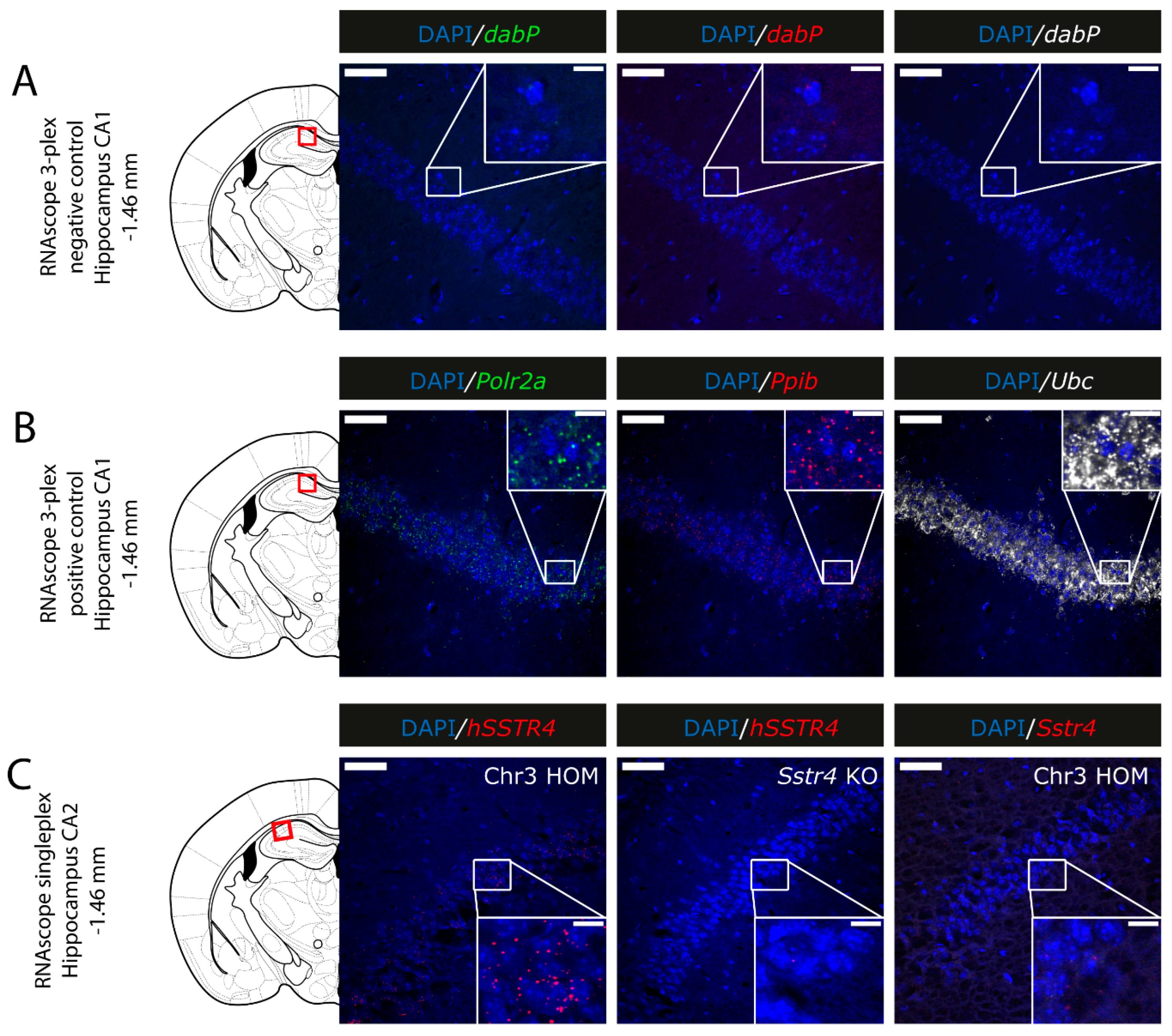
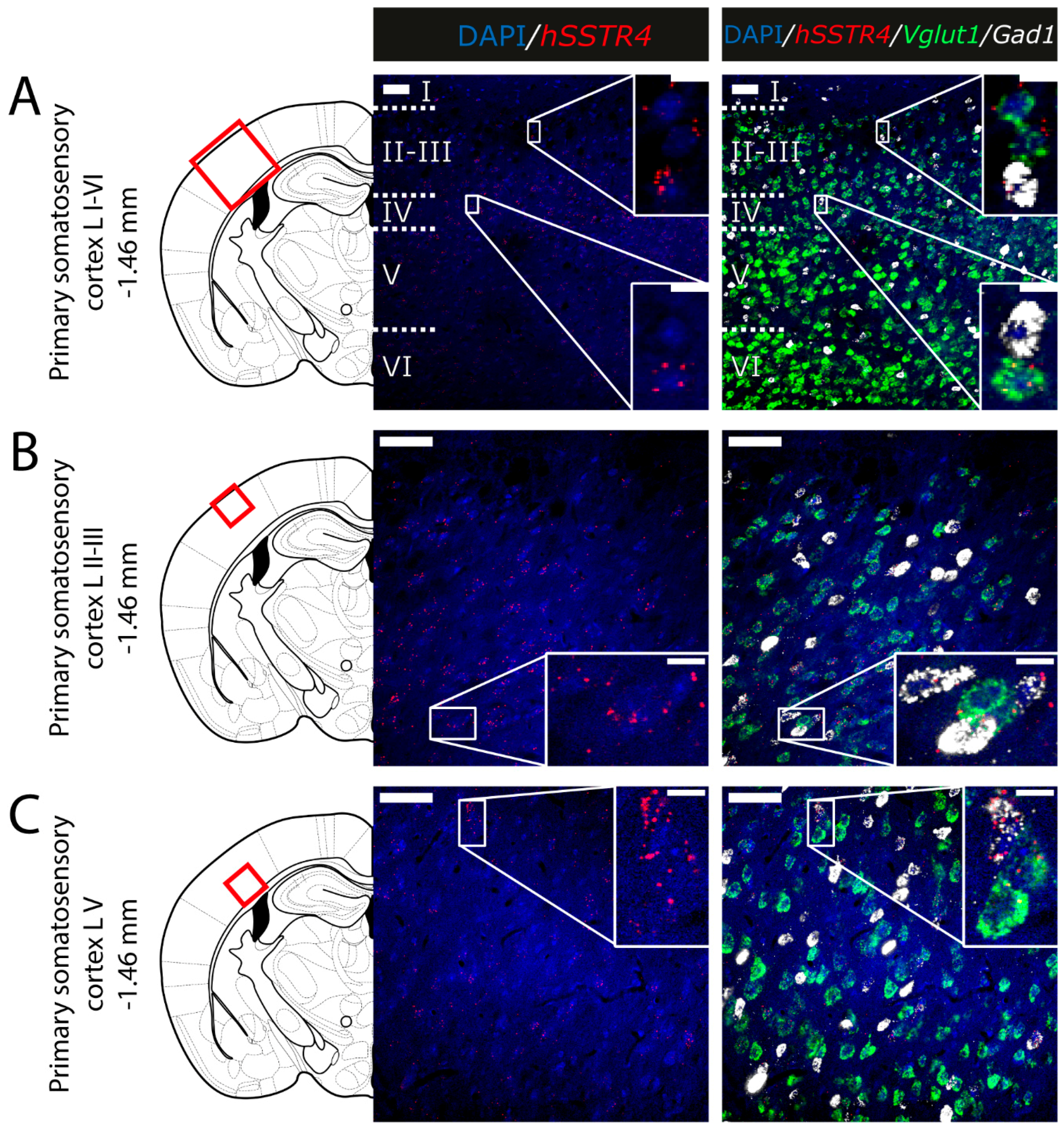
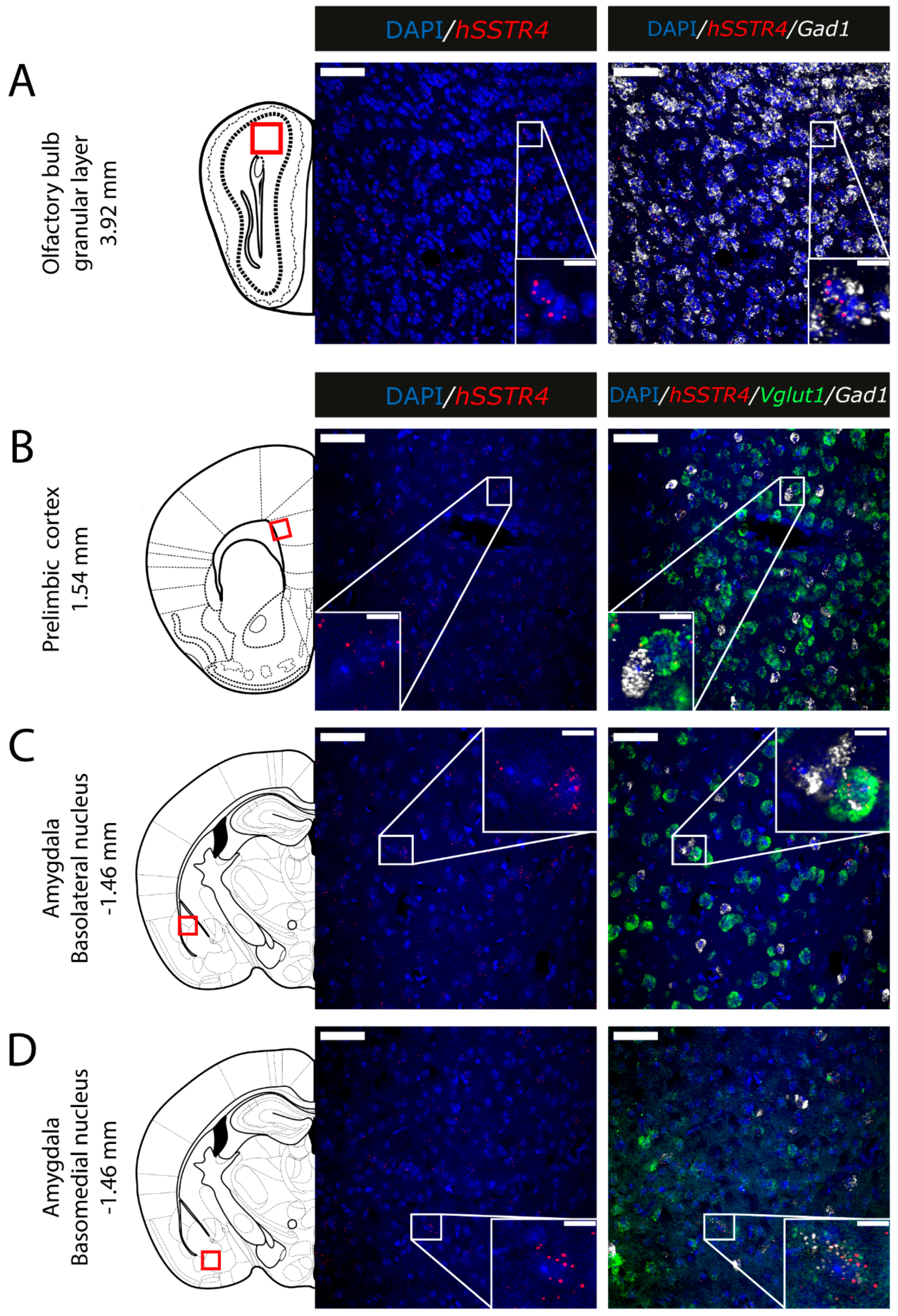
2.5. Expression of hSSTR4 mRNA in Different Types of Neurons in the Brain
3. Discussion
4. Materials and Methods
4.1. PiggyBac Transposon Vector
4.2. Animals
4.3. Transgenesis
4.4. Ligation-Mediated PCR
4.5. Detecting hSSTR4 Expression by In Vivo Optical Imaging of the Luciferase Enzyme and tdTomato
4.6. Investigating hSSTR4-Linked tdTomato Expression in the Mouse Brain by Confocal Microscopy
4.7. Measuring Organ-Specific hSSTR4 Expression by RT-qPCR
4.8. Characterizing hSSTR4 Expressing Neurons in the Mouse Brain by RNAscope In Situ Hybridization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLA | basolateral nucleus of the amygdala |
| BMA | basomedial nucleus of the amygdala |
| CA1 | Cornu Ammonis 1 region of the hippocampus |
| CA2 | Cornu Ammonis 2 region of the hippocampus |
| Ct value | threshold cycle value (in RT-qPCR) |
| dabP | gene of the bacterial D site of albumin promoter (albumin D-box) binding protein |
| DAPI | 4′,6-diamidino-2-phenylindole, a blue-fluorescent DNA stain |
| DNA | deoxyribonucleic acid |
| FOXA2 | gene of the human forkhead box A2 |
| GABA | gamma-aminobutyric acid |
| Gad1 | gene of the mouse glutamate decarboxylase 1 |
| Het | heterozygote/heterozygous, carrying different alleles of the gene |
| Hom | homozygote/homozygous, carrying identical alleles of the gene |
| hSSTR4 | gene of human somatostatin receptor subtype 4 |
| i.p. | intraperitoneally |
| KO | knock-out, an artificially created nonfunctional variant of the gene |
| LM-PCR | ligation-mediated polymerase chain reaction |
| mRNA | messenger ribonucleic acid |
| NCBI | National Center for Biotechnology Information |
| OB | olfactory bulb |
| Otol1 | gene of the mouse otolin 1 |
| PB | piggyBac, a type of transposon system |
| PBS | phosphate-buffered saline |
| pcDNA | plasmid cloning DNA |
| PCR | polymerase chain reaction |
| Pir | piriform cortex |
| Polr2a | gene of the mouse RNA polymerase II subunit A |
| polyA | polyadenylation signal sequence |
| Ppib | gene of the mouse peptidylprolyl isomerase B |
| PrL | prelimbic cortex |
| RT | room temperature |
| RT-qPCR | reverse transcription quantitative polymerase chain reaction |
| S1 | primary somatosensory cortex |
| Sis | gene of the mouse sucrase-isomaltase |
| SNP | single-nucleotide polymorphism |
| SST1-5 | somatostatin receptor subtype 1-5 |
| Sstr4 | gene of mouse somatostatin receptor subtype 4 |
| SV | seminal vesicle |
| tdTomato | tandem dimer Tomato, a red fluorescent reporter protein |
| TG | trigeminal ganglion |
| THBD | gene of the human thrombomodulin |
| Ubc | gene of the mouse ubiquitin C |
| Vglut1 | gene of the mouse vesicular glutamate transporter 1 |
| WT | wild type |
Appendix A
References
- Baraban, S.C.; Tallent, M.K. Interneuron Diversity Series: Interneuronal Neuropeptides—Endogenous Regulators of Neuronal Excitability. Trends Neurosci. 2004, 27, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Helyes, Z.; Pinter, E.; Sandor, K.; Elekes, K.; Banvolgyi, A.; Keszthelyi, D.; Szoke, E.; Toth, D.M.; Sandor, Z.; Kereskai, L.; et al. Impaired Defense Mechanism against Inflammation, Hyperalgesia, and Airway Hyperreactivity in Somatostatin 4 Receptor Gene-Deleted Mice. Proc. Natl. Acad. Sci. USA 2009, 106, 13088–13093. [Google Scholar] [CrossRef] [PubMed]
- Sándor, K.; Elekes, K.; Szabó, Á.; Pintér, E.; Engström, M.; Wurster, S.; Szolcsányi, J.; Helyes, Z. Analgesic Effects of the Somatostatin Sst4 Receptor Selective Agonist J-2156 in Acute and Chronic Pain Models. Eur. J. Pharmacol. 2006, 539, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Pintér, E.; Pozsgai, G.; Hajna, Z.; Helyes, Z.; Szolcsányi, J. Neuropeptide Receptors as Potential Drug Targets in the Treatment of Inflammatory Conditions. Br. J. Clin. Pharmacol. 2014, 77, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, N.; Maeda, N.; Yamaguchi, I.; Satoh, M. Possible Involvement of Brain Somatostatin in the Memory Formation of Rats and the Cognitive Enhancing Action of FR121196 in Passive Avoidance Task. Brain Res. 1994, 642, 11–19. [Google Scholar] [CrossRef]
- Tuboly, G.; Vecsei, L. Somatostatin and Cognitive Function in Neurodegenerative Disorders. Mini Rev. Med. Chem. 2013, 13, 34–46. [Google Scholar] [CrossRef]
- Martel, G.; Dutar, P.; Epelbaum, J.; Viollet, C.P. Somatostatinergic Systems: An Update on Brain Functions in Normal and Pathological Aging. Front. Endocrinol. 2012, 3, 154. [Google Scholar] [CrossRef][Green Version]
- Sandoval, K.E.; Farr, S.A.; Banks, W.A.; Crider, A.M.; Morley, J.E.; Witt, K.A. Somatostatin Receptor Subtype-4 Agonist NNC 26-9100 Decreases Extracellular and Intracellular Aβ₁₋₄₂ Trimers. Eur. J. Pharmacol. 2012, 683, 116–124. [Google Scholar] [CrossRef][Green Version]
- Sandoval, K.; Umbaugh, D.; House, A.; Crider, A.; Witt, K. Somatostatin Receptor Subtype-4 Regulates MRNA Expression of Amyloid-Beta Degrading Enzymes and Microglia Mediators of Phagocytosis in Brains of 3xTg-AD Mice. Neurochem. Res. 2019, 44, 2670–2680. [Google Scholar] [CrossRef]
- Lin, L.C.; Sibille, E. Somatostatin, Neuronal Vulnerability and Behavioral Emotionality. Mol. Psychiatry 2015, 20, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Engin, E.; Stellbrink, J.; Treit, D.; Dickson, C.T. Anxiolytic and Antidepressant Effects of Intracerebroventricularly Administered Somatostatin: Behavioral and Neurophysiological Evidence. Neuroscience 2008, 157, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.M.S.; Sibille, E.P.D. Reduced Brain Somatostatin in Mood Disorders: A Common Pathophysiological Substrate and Drug Target? Front. Pharmacol. 2013, 4, 110. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Gaszner, B. Role of Neuropeptides in Anxiety, Stress, and Depression: From Animals to Humans. Neuropeptides 2013, 47, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, J. Somatostatin in the Central Nervous System: Physiology and Pathological Modifications. Prog. Neurobiol. 1986, 27, 63–100. [Google Scholar] [CrossRef]
- Gulyás, A.I.; Hájos, N.; Katona, I.; Freund, T.F. Interneurons Are the Local Targets of Hippocampal Inhibitory Cells Which Project to the Medial Septum. Eur. J. Neurosci. 2003, 17, 1861–1872. [Google Scholar] [CrossRef]
- Tomioka, R.; Okamoto, K.; Furuta, T.; Fujiyama, F.; Iwasato, T.; Yanagawa, Y.; Obata, K.; Kaneko, T.; Tamamaki, N. Demonstration of Long-Range GABAergic Connections Distributed throughout the Mouse Neocortex. Eur. J. Neurosci. 2005, 21, 1587–1600. [Google Scholar] [CrossRef]
- Szolcsányi, J. Forty Years in Capsaicin Research for Sensory Pharmacology and Physiology. Neuropeptides 2004, 38, 377–384. [Google Scholar] [CrossRef]
- Thán, M.; Németh, J.; Szilvássy, Z.; Pintér, E.; Helyes, Z.; Szolcsányi, J. Systemic Anti-Inflammatory Effect of Somatostatin Released from Capsaicin-Sensitive Vagal and Sciatic Sensory Fibres of the Rat and Guinea-Pig. Eur. J. Pharmacol. 2000, 399, 251–258. [Google Scholar] [CrossRef]
- Helyes, Z.; Pintér, E.; Németh, J.; Sándor, K.; Elekes, K.; Szabó, A.; Pozsgai, G.; Keszthelyi, D.; Kereskai, L.; Engström, M.; et al. Effects of the Somatostatin Receptor Subtype 4 Selective Agonist J-2156 on Sensory Neuropeptide Release and Inflammatory Reactions in Rodents. Br. J. Pharmacol. 2006, 149, 405–415. [Google Scholar] [CrossRef]
- Markovics, A.; Szőke, É.; Sándor, K.; Börzsei, R.; Bagoly, T.; Kemény, Á.; Elekes, K.; Pintér, E.; Szolcsányi, J.; Helyes, Z. Comparison of the Anti-Inflammatory and Anti-Nociceptive Effects of Cortistatin-14 and Somatostatin-14 in Distinct In Vitro and In Vivo Model Systems. J. Mol. Neurosci. 2012, 46, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zeyda, T.; Johnson, B.; Hochgeschwender, U.; de Lecea, L.; Tallent, M.K. Somatostatin Receptor Subtype 4 Couples to the M-Current to Regulate Seizures. J. Neurosci. 2008, 28, 3567–3576. [Google Scholar] [CrossRef]
- Scheich, B.; Gaszner, B.; Kormos, V.; László, K.; Ádori, C.; Borbély, É.; Hajna, Z.; Tékus, V.; Bölcskei, K.; Ábrahám, I.; et al. Somatostatin Receptor Subtype 4 Activation Is Involved in Anxiety and Depression-like Behavior in Mouse Models. Neuropharmacology 2016, 101, 204–215. [Google Scholar] [CrossRef]
- Scheich, B.; Csekő, K.; Borbély, É.; Ábrahám, I.; Csernus, V.; Gaszner, B.; Helyes, Z. Higher Susceptibility of Somatostatin 4 Receptor Gene-Deleted Mice to Chronic Stress-Induced Behavioral and Neuroendocrine Alterations. Neuroscience 2017, 346, 320–336. [Google Scholar] [CrossRef]
- Botz, B.; Bölcskei, K.; Helyes, Z. Challenges to Develop Novel Anti-Inflammatory and Analgesic Drugs. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2017, 9, e1427. [Google Scholar] [CrossRef]
- Kántás, B.; Börzsei, R.; Szőke, É.; Bánhegyi, P.; Horváth, Á.; Hunyady, Á.; Borbély, É.; Hetényi, C.; Pintér, E.; Helyes, Z. Novel Drug-Like Somatostatin Receptor 4 Agonists Are Potential Analgesics for Neuropathic Pain. IJMS 2019, 20, 6245. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.A.; Kuo, A.; Khan, N.; Gorham, L.; Nicholson, J.R.; Corradini, L.; Vetter, I.; Smith, M.T. The Somatostatin Receptor-4 Agonist J-2156 Alleviates Mechanical Hypersensitivity in a Rat Model of Breast Cancer Induced Bone Pain. Front. Pharmacol. 2018, 9, 495. [Google Scholar] [CrossRef]
- Park, T.S.W.; Khan, N.; Kuo, A.; Nicholson, J.R.; Corradini, L.; Smith, M.T. J-2156, a Somatostatin Receptor Type 4 Agonist, Alleviates Mechanical Hyperalgesia in a Rat Model of Chronic Low Back Pain. Biomed. Pharmacother. 2019, 117, 109056. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The Neuropathic Pain: An Overview of the Current Treatment and Future Therapeutic Approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 205873841983838. [Google Scholar] [CrossRef] [PubMed]
- Clinical Development Pipeline|Science|Eli Lilly and Company. Available online: https://www.lilly.com/discovery/clinical-development-pipeline (accessed on 2 April 2021).
- Szőke, É.; Bálint, M.; Hetényi, C.; Markovics, A.; Elekes, K.; Pozsgai, G.; Szűts, T.; Kéri, G.; Őrfi, L.; Sándor, Z.; et al. Small Molecule Somatostatin Receptor Subtype 4 (Sst4) Agonists Are Novel Anti-Inflammatory and Analgesic Drug Candidates. Neuropharmacology 2020, 178, 108198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Crider, A.M.; Ansbro, D.; Hayes, C.; Kontoyianni, M. A Structure-Based Approach to Understanding Somatostatin Receptor-4 Agonism (Sst4). J. Chem. Inf. Model. 2012, 52, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized Mice in Translational Biomedical Research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Manz, M.G.; Di Santo, J.P. Renaissance for Mouse Models of Human Hematopoiesis and Immunobiology. Nat. Immunol. 2009, 10, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.; Greiner, D.L.; Shultz, L.D. Humanized SCID Mouse Models for Biomedical Research. Curr. Top. Microbiol. Immunol. 2008, 324, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Kobayashi, K.; Nakahata, T. NOD/Shi-Scid IL2rgamma(Null) (NOG) Mice More Appropriate for Humanized Mouse Models. Curr. Top. Microbiol. Immunol. 2008, 324, 53–76. [Google Scholar] [CrossRef]
- Legrand, N.; Weijer, K.; Spits, H. Experimental Models to Study Development and Function of the Human Immune System in Vivo. J. Immunol. 2006, 176, 2053–2058. [Google Scholar] [CrossRef]
- Zhang, B.; Duan, Z.; Zhao, Y. Mouse Models with Human Immunity and Their Application in Biomedical Research. J. Cell. Mol. Med. 2009, 13, 1043–1058. [Google Scholar] [CrossRef]
- Devoy, A.; Bunton-Stasyshyn, R.K.A.; Tybulewicz, V.L.J.; Smith, A.J.H.; Fisher, E.M.C. Genomically Humanized Mice: Technologies and Promises. Nat. Rev. Genet. 2012, 13, 14–20. [Google Scholar] [CrossRef]
- Zhu, F.; Nair, R.R.; Fisher, E.M.C.; Cunningham, T.J. Humanising the Mouse Genome Piece by Piece. Nat. Commun. 2019, 10, 1845. [Google Scholar] [CrossRef]
- Lampreht Tratar, U.; Horvat, S.; Cemazar, M. Transgenic Mouse Models in Cancer Research. Front. Oncol. 2018, 8, 268. [Google Scholar] [CrossRef]
- Moriwaki, T.; Abe, S.; Oshimura, M.; Kazuki, Y. Transchromosomic Technology for Genomically Humanized Animals. Exp. Cell Res. 2020, 390, 111914. [Google Scholar] [CrossRef]
- Henderson, C.J.; Kapelyukh, Y.; Scheer, N.; Rode, A.; McLaren, A.; MacLeod, A.K.; Lin, D.; Wright, J.; Stanley, L.A.; Wolf, C.R. An Extensively Humanized Mouse Model to Predict Pathways of Drug Disposition and Drug/Drug Interactions, and to Facilitate Design of Clinical Trials. Drug. Metab. Dispos. 2019, 47, 601–615. [Google Scholar] [CrossRef]
- Ueda, O.; Tateishi, H.; Higuchi, Y.; Fujii, E.; Kato, A.; Kawase, Y.; Wada, N.A.; Tachibe, T.; Kakefuda, M.; Goto, C.; et al. Novel Genetically-Humanized Mouse Model Established to Evaluate Efficacy of Therapeutic Agents to Human Interleukin-6 Receptor. Sci. Rep. 2013, 3, 1196. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Kaur, S.; Li, B.; Panesar, M.; Saha, U.; Davis, C.; Dragoni, I.; Colley, S.; Ritchie, T.; Bevan, S.; et al. Antihyperalgesic Activity of a Novel Nonpeptide Bradykinin B1 Receptor Antagonist in Transgenic Mice Expressing the Human B1 Receptor. Br J. Pharmacol. 2005, 144, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.J.; Budd, P.S.; Keighren, M.; McKie, L. Humanized MC1R Transgenic Mice Reveal Human Specific Receptor Function. Hum. Mol. Genet. 2007, 16, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Kecskés, A.; Pohóczky, K.; Kecskés, M.; Varga, Z.V.; Kormos, V.; Szőke, É.; Henn-Mike, N.; Fehér, M.; Kun, J.; Gyenesei, A.; et al. Characterization of Neurons Expressing the Novel Analgesic Drug Target Somatostatin Receptor 4 in Mouse and Human Brains. Int. J. Mol. Sci. 2020, 21, 7788. [Google Scholar] [CrossRef] [PubMed]
- Mus Musculus Chromosome 3, GRCm39 Reference Primary Assembly C57BL/6J. Available online: http://www.ncbi.nlm.nih.gov/nuccore/CM000996.3 (accessed on 2 April 2021).
- Mus Musculus Chromosome 10, GRCm39 Reference Primary Assembly C57BL/6J. Available online: http://www.ncbi.nlm.nih.gov/nuccore/CM001003.3 (accessed on 2 April 2021).
- Mus Musculus Chromosome X, GRCm39 Reference Primary Assembly C57BL/6J. Available online: http://www.ncbi.nlm.nih.gov/nuccore/CM001013.3 (accessed on 2 April 2021).
- Scheer, N.; Snaith, M.; Wolf, C.R.; Seibler, J. Generation and Utility of Genetically Humanized Mouse Models. Drug Discov. Today 2013, 18, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Maillet, M.; Miano, J.M.; Molkentin, J.D. Lost in Transgenesis: A Users Guide for Genetically Manipulating the Mouse in Cardiac Research. Circ. Res. 2012, 111, 761–777. [Google Scholar] [CrossRef]
- Bryda, E.C.; Pearson, M.; Agca, Y.; Bauer, B.A. Method for Detection and Identification of Multiple Chromosomal Integration Sites in Transgenic Animals Created with Lentivirus. BioTechniques 2006, 41, 715–719. [Google Scholar] [CrossRef]
- Caron, P.; Buscail, L.; Beckers, A.; Estève, J.-P.; Igout, A.; Hennen, G.; Susini, C. Expression of Somatostatin Receptor SST4 in Human Placenta and Absence of Octreotide Effect on Human Placental Growth Hormone Concentration during Pregnancy1. J. Clin. Endocrinol. Metab. 1997, 82, 3771–3776. [Google Scholar] [CrossRef]
- Møller, L.N.; Stidsen, C.E.; Hartmann, B.; Holst, J.J. Somatostatin Receptors. Biochim. Biophys. Acta Biomembr. 2003, 1616, 1–84. [Google Scholar] [CrossRef]
- Schwabe, W.; Brennan, M.B.; Hochgeschwender, U. Isolation and Characterization of the Mouse (Mus Musculus) Somatostatin Receptor Type-4-Encoding Gene (MSSTR4). Gene 1996, 168, 233–235. [Google Scholar] [CrossRef]
- Regard, J.B.; Sato, I.T.; Coughlin, S.R. Anatomical Profiling of G Protein-Coupled Receptor Expression. Cell 2008, 135, 561–571. [Google Scholar] [CrossRef] [PubMed]
- SSTR4 Protein Expression Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000132671-SSTR4 (accessed on 21 January 2021).
- Sstr4 MGI Mouse Gene Detail—MGI:105372—Somatostatin Receptor 4. Available online: http://www.informatics.jax.org/marker/MGI:105372 (accessed on 21 January 2021).
- Sstr4 RT-PCR Gene Expression Assay—GXD. Available online: http://www.informatics.jax.org/assay/MGI:1204215 (accessed on 21 January 2021).
- Sstr4 RT-PCR Gene Expression Assay—GXD. Available online: http://www.informatics.jax.org/assay/MGI:1204217 (accessed on 21 January 2021).
- Sstr4 RT-PCR Gene Expression Assay—GXD. Available online: http://www.informatics.jax.org/assay/MGI:1204442 (accessed on 21 January 2021).
- Teschendorf, C.; Warrington, K.H.; Siemann, D.W.; Muzyczka, N. Comparison of the EF-1 Alpha and the CMV Promoter for Engineering Stable Tumor Cell Lines Using Recombinant Adeno-Associated Virus. Anticancer. Res. 2002, 22, 3325–3330. [Google Scholar] [PubMed]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.G.; Palmer, A.E.; Tsien, R.Y. Improved Monomeric Red, Orange and Yellow Fluorescent Proteins Derived from Discosoma Sp. Red Fluorescent Protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Lauf, U.; Lopez, P.; Falk, M.M. Expression of Fluorescently Tagged Connexins: A Novel Approach to Rescue Function of Oligomeric DsRed-Tagged Proteins1. FEBS Lett. 2001, 498, 11–15. [Google Scholar] [CrossRef]
- Palmer, E.; Freeman, T. Investigation Into the Use of C- and N-Terminal GFP Fusion Proteins for Subcellular Localization Studies Using Reverse Transfection Microarrays. Comp. Funct. Genom. 2004, 5, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Nocera, S.; Simon, A.; Fiquet, O.; Chen, Y.; Gascuel, J.; Datiche, F.; Schneider, N.; Epelbaum, J.; Viollet, C. Somatostatin Serves a Modulatory Role in the Mouse Olfactory Bulb: Neuroanatomical and Behavioral Evidence. Front. Behav. Neurosci. 2019, 13, 61. [Google Scholar] [CrossRef]
- Homo Sapiens Somatostatin Receptor 4 (SSTR4), MRNA. Available online: http://www.ncbi.nlm.nih.gov/nuccore/NM_001052.2 (accessed on 2 April 2021).
- Paxinos, G.; Franklin, K. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, 5th ed; Academic Press: Cambridge, MA, USA, 2019; ISBN 978-0-12-816157-9. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, B.; Bölcskei, K.; Kecskés, A.; Kormos, V.; Gaszner, B.; Aczél, T.; Hegedüs, D.; Pintér, E.; Helyes, Z.; Sándor, Z. Human Somatostatin SST4 Receptor Transgenic Mice: Construction and Brain Expression Pattern Characterization. Int. J. Mol. Sci. 2021, 22, 3758. https://doi.org/10.3390/ijms22073758
Nemes B, Bölcskei K, Kecskés A, Kormos V, Gaszner B, Aczél T, Hegedüs D, Pintér E, Helyes Z, Sándor Z. Human Somatostatin SST4 Receptor Transgenic Mice: Construction and Brain Expression Pattern Characterization. International Journal of Molecular Sciences. 2021; 22(7):3758. https://doi.org/10.3390/ijms22073758
Chicago/Turabian StyleNemes, Balázs, Kata Bölcskei, Angéla Kecskés, Viktória Kormos, Balázs Gaszner, Timea Aczél, Dániel Hegedüs, Erika Pintér, Zsuzsanna Helyes, and Zoltán Sándor. 2021. "Human Somatostatin SST4 Receptor Transgenic Mice: Construction and Brain Expression Pattern Characterization" International Journal of Molecular Sciences 22, no. 7: 3758. https://doi.org/10.3390/ijms22073758
APA StyleNemes, B., Bölcskei, K., Kecskés, A., Kormos, V., Gaszner, B., Aczél, T., Hegedüs, D., Pintér, E., Helyes, Z., & Sándor, Z. (2021). Human Somatostatin SST4 Receptor Transgenic Mice: Construction and Brain Expression Pattern Characterization. International Journal of Molecular Sciences, 22(7), 3758. https://doi.org/10.3390/ijms22073758







