Collagen I Modifies Connexin-43 Hemichannel Activity via Integrin α2β1 Binding in TGFβ1-Evoked Renal Tubular Epithelial Cells
Abstract
1. Introduction
2. Results
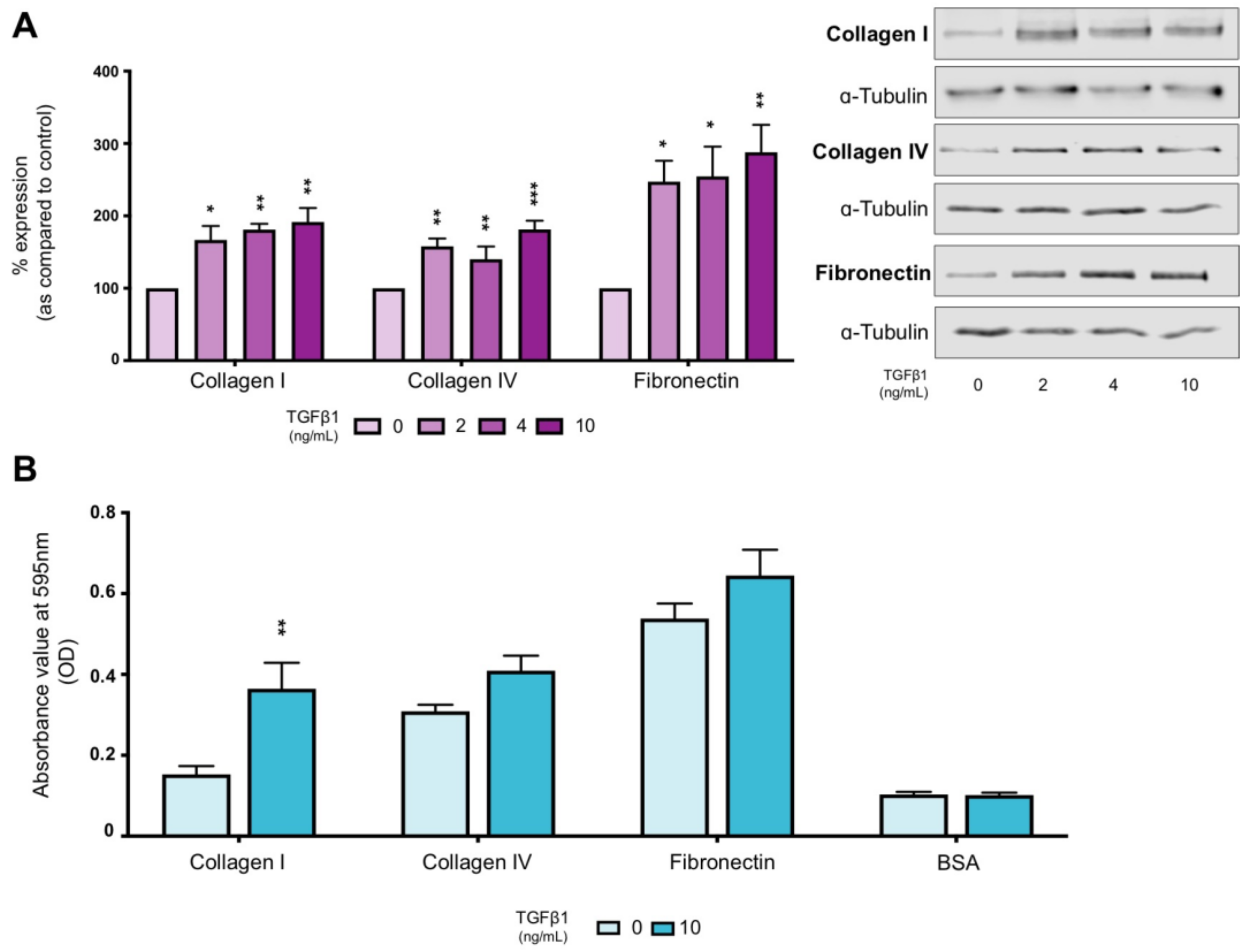
2.1. TGFβ1 Increases Expression of ECM Proteins and Alters Cell–Substrate Interactions
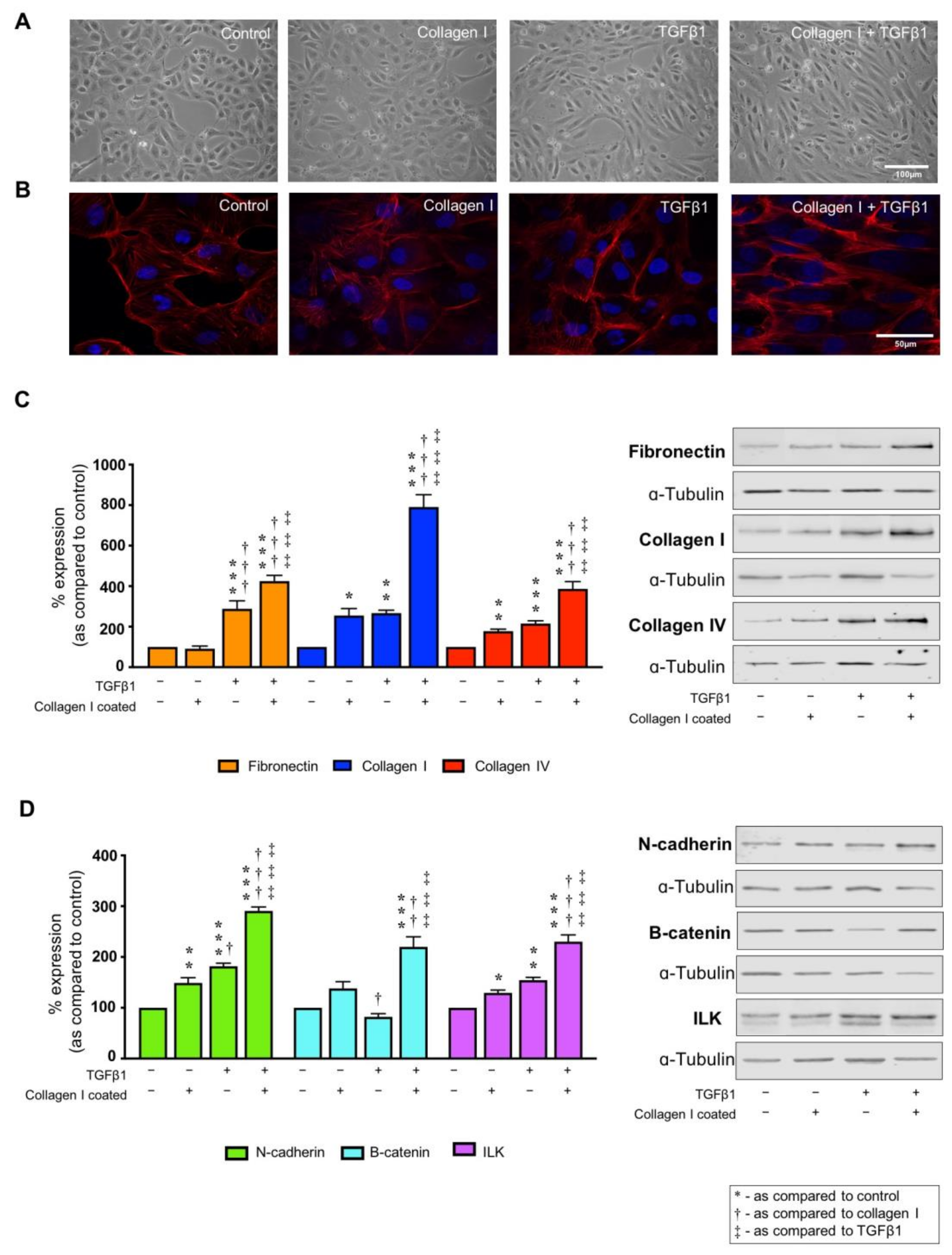
2.2. Collagen I and TGFβ1 Stimulate Aberrant Cell Morphology and Cytoskeletal Reorganization
2.3. TGFβ1 Alters Markers of Tubular Injury, an Effect Exacerbated by Co-Culture on Collagen I
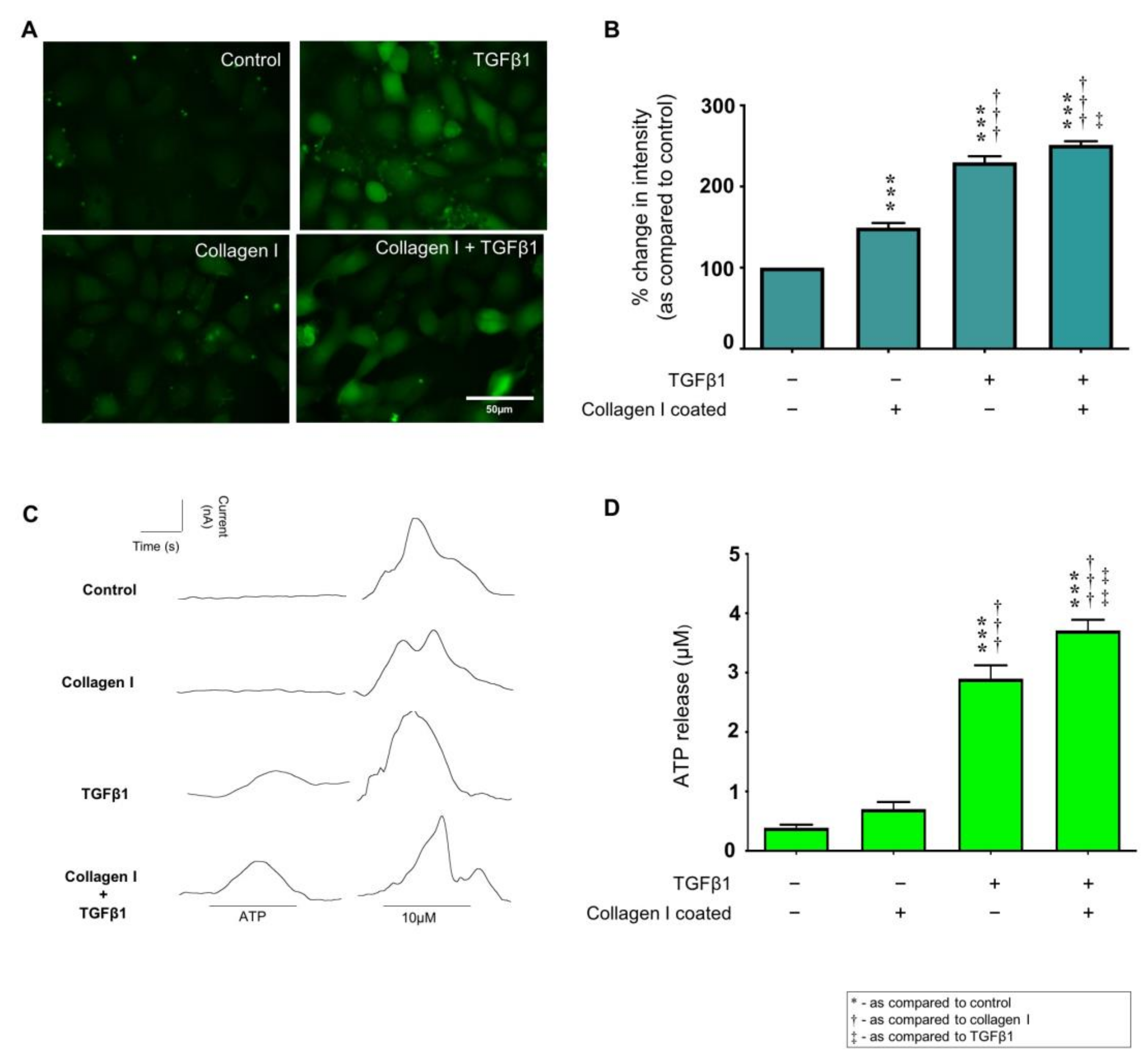
2.4. TGFβ1 Exacerbates Collagen I Induced Hemichannel Activity and Cx43 Mediated ATP Release
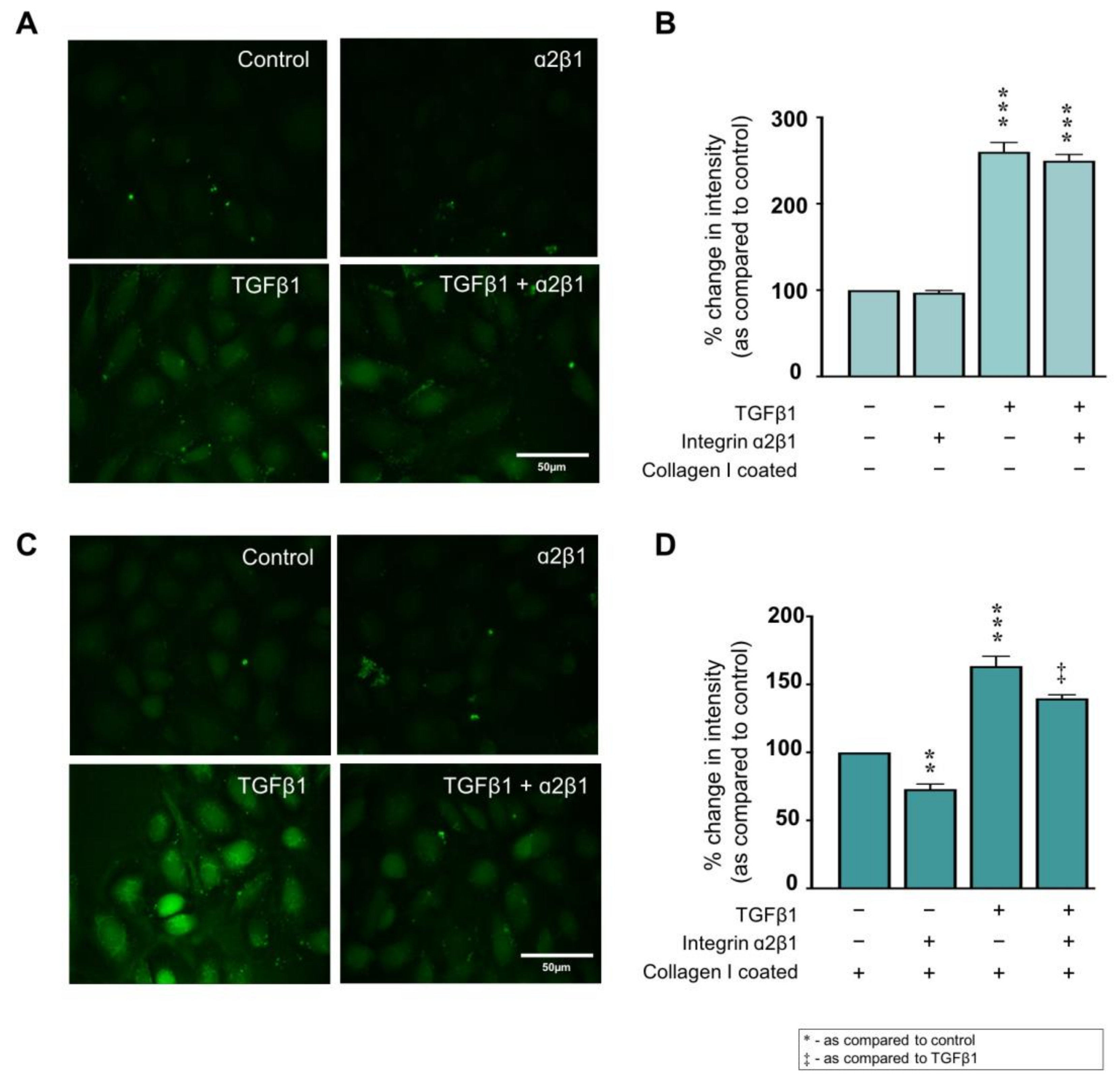
2.5. Collagen I ± TGFβ1-Evoked Hemichannel Activity Is Partially Mediated via Integrin α2β1 Binding
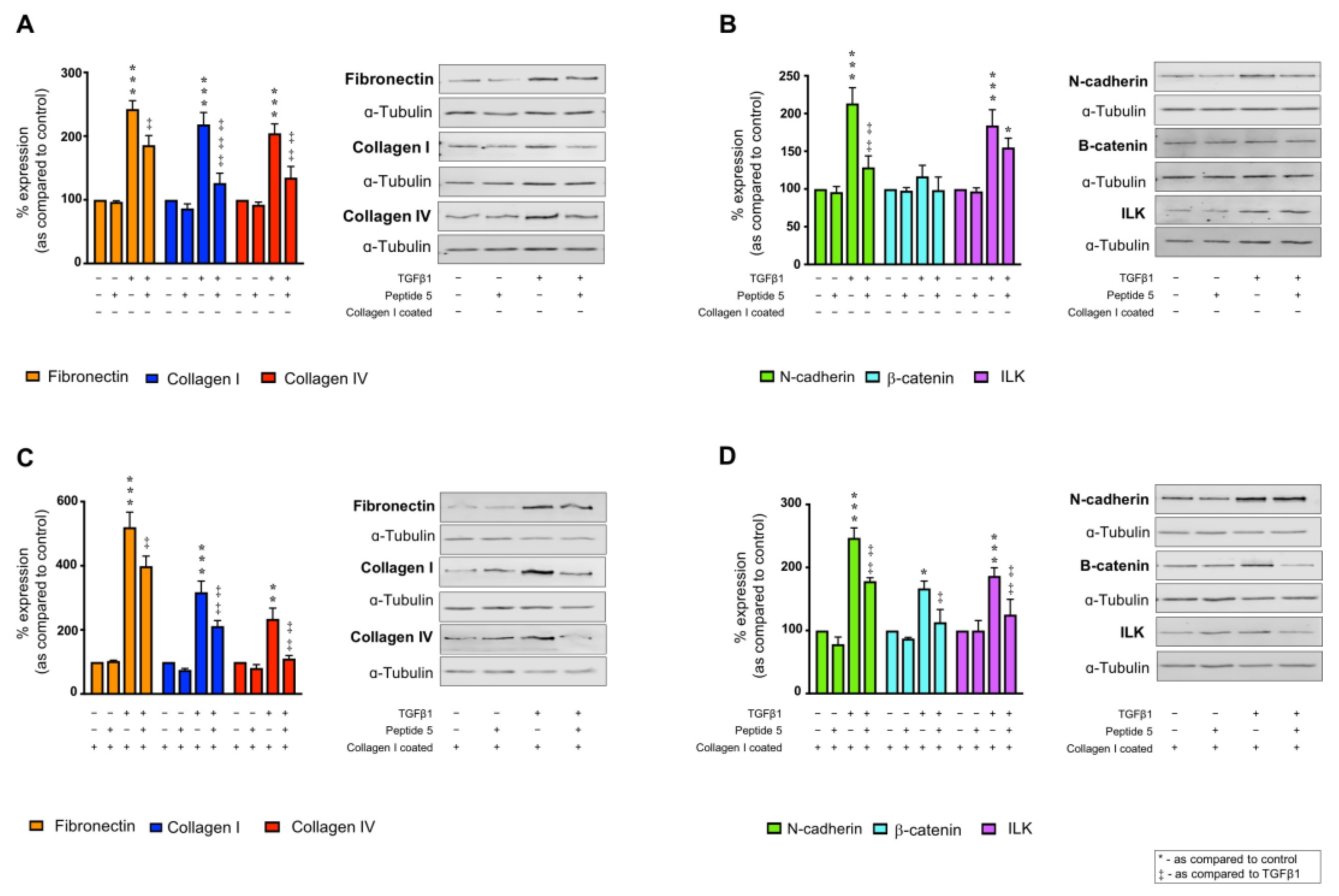
2.6. Collagen I Partly Regulates Markers of Tubular Injury through Integrin α2β1-Mediated Signal Transduction
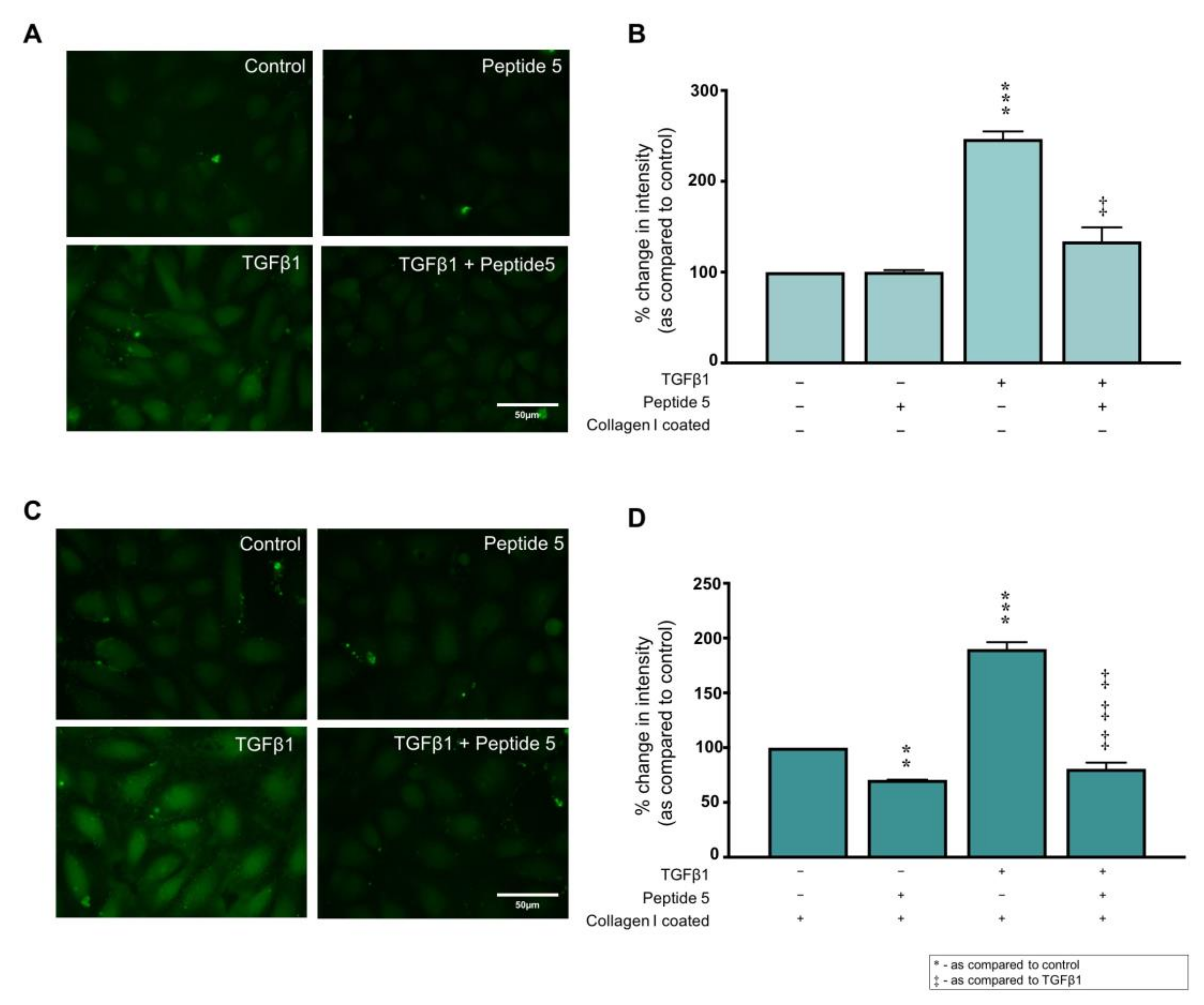
2.7. Peptide 5 Negates Collagen I ± TGFβ1-Induced Cx43 Hemichannel Activity
2.8. Peptide 5 Negates TGFβ1 ± Collagen I-Induced Tubular Injury
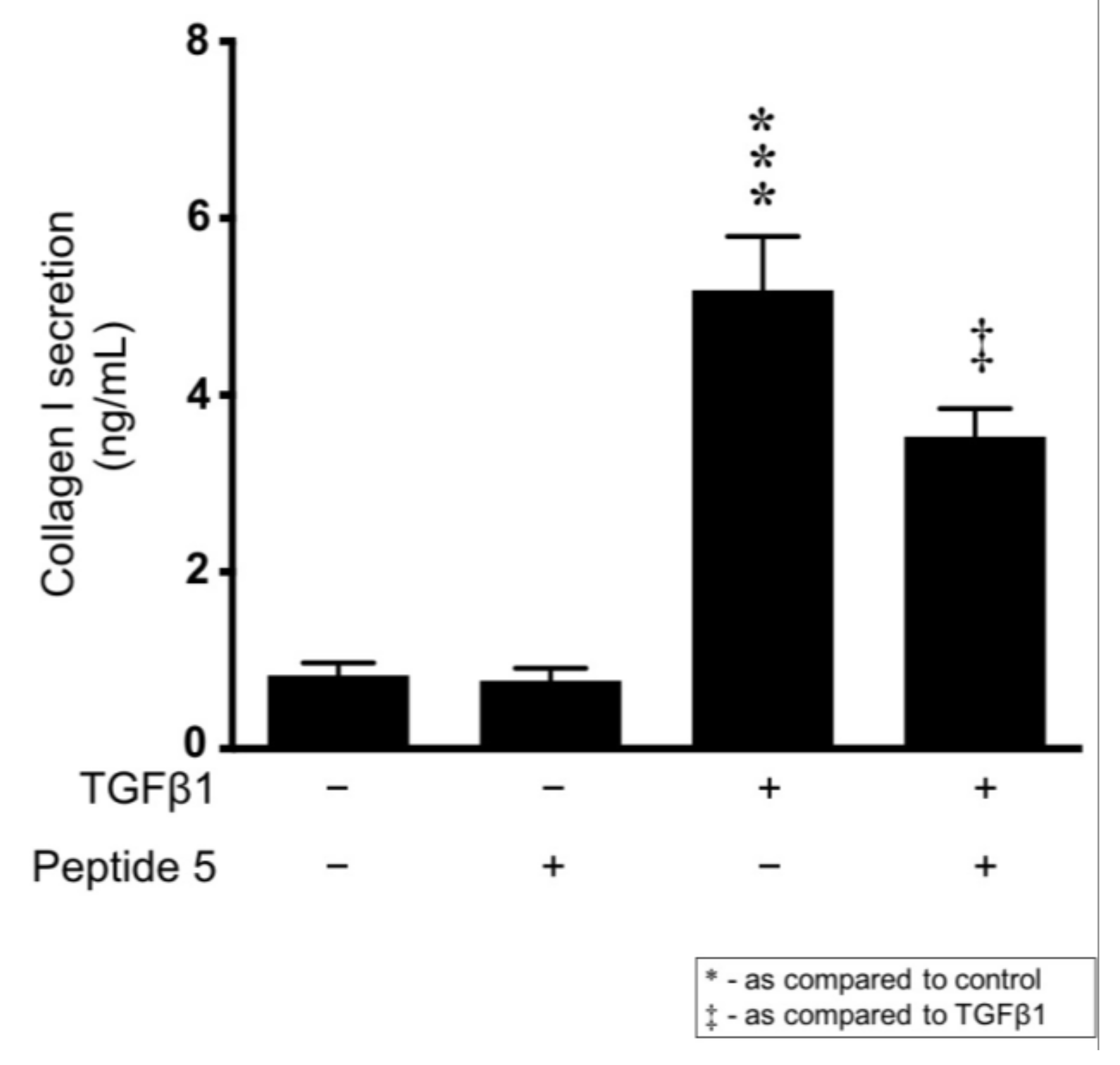
2.9. Peptide 5 Reduces TGFβ1-Induced Collagen I Secretion
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatment
4.3. Immunocytochemistry
4.4. Western Blotting
4.5. Cell Adhesion Array
4.6. Collagen I Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Carboxyfluorescein Dye Uptake Assay
4.8. ATP Biosensing
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- Bello, A.K.; Levin, A.; Lunney, M.; Osman, M.A.; Ye, F.; Ashuntantang, G.E.; Bellorin-Font, E.; Benghanem Gharbi, M.; Davison, S.N.; Ghnaimat, M.; et al. Status of care for end stage kidney disease in countries and regions worldwide: International cross sectional survey. BMJ 2019, 367, 38. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Liu, B.C.; Tang, T.T.; Lv, L.L.; Lan, H.Y. Renal tubule injury: A driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yamasaki, R.; Yamaguchi, H.; Nagata, S.; Une, H.; Cui, Y.; Masaki, K.; Nakamuta, Y.; Iinuma, K.; Watanabe, M.; et al. Oligodendroglial connexin 47 regulates neuroinflammation upon autoimmune demyelination in a novel mouse model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 2160–2169. [Google Scholar] [CrossRef]
- Hills, C.; Price, G.W.; Wall, M.J.; Kaufmann, T.J.; Chi-Wai Tang, S.; Yiu, W.H.; Squires, P.E. Transforming Growth Factor Beta 1 Drives a Switch in Connexin Mediated Cell-to-Cell Communication in Tubular Cells of the Diabetic Kidney. Cell. Physiol. Biochem. 2018, 45, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Mugisho, O.O.; Green, C.R.; Kho, D.T.; Zhang, J.; Graham, E.S.; Acosta, M.L.; Rupenthal, I.D. The inflammasome pathway is amplified and perpetuated in an autocrine manner through connexin43 hemichannel mediated ATP release. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 385–393. [Google Scholar] [CrossRef]
- Kim, Y.; Griffin, J.M.; Nor, M.N.M.; Zhang, J.; Freestone, P.S.; Danesh-Meyer, H.V.; Rupenthal, I.D.; Acosta, M.; Nicholson, L.F.B.; O’Carroll, S.J.; et al. Tonabersat Prevents Inflammatory Damage in the Central Nervous System by Blocking Connexin43 Hemichannels. Neurotherapeutics 2017, 14, 1148–1165. [Google Scholar] [CrossRef]
- Bosco, D.; Haefliger, J.A.; Meda, P. Connexins: Key mediators of endocrine function. Physiol. Rev. 2011, 91, 1393–1445. [Google Scholar] [CrossRef]
- Müller, C.E.; Baqi, Y.; Namasivayam, V. Agonists and antagonists for purinergic receptors. Methods Mol. Biol. 2020, 2041, 45–64. [Google Scholar]
- Price, G.W.; Chadjichristos, C.E.; Kavvadas, P.; Tang, S.C.W.; Yiu, W.H.; Green, C.R.; Potter, J.A.; Siamantouras, E.; Squires, P.E.; Hills, C.E. Blocking Connexin-43 mediated hemichannel activity protects against early tubular injury in experimental chronic kidney disease. Cell Commun. Signal. 2020, 18, 79. [Google Scholar] [CrossRef]
- Menzies, R.I.; Booth, J.W.R.; Mullins, J.J.; Bailey, M.A.; Tam, F.W.K.; Norman, J.T.; Unwin, R.J. Hyperglycemia-induced Renal P2X7 Receptor Activation Enhances Diabetes-related Injury. EBioMedicine 2017, 19, 73–83. [Google Scholar] [CrossRef]
- Therkildsen, J.R.; Christensen, M.G.; Tingskov, S.J.; Wehmöller, J.; Nørregaard, R.; Praetorius, H.A. Lack of P2X7 Receptors Protects against Renal Fibrosis after Pyelonephritis with α-Hemolysin–Producing Escherichia coli. Am. J. Pathol. 2019, 189, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Knight, G.E. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Siamantouras, E.; Price, G.W.; Potter, J.A.; Hills, C.E.; Squires, P.E. Purinergic receptor (P2X7) activation reduces cell–cell adhesion between tubular epithelial cells of the proximal kidney. Nanomedicine Nanotechnology. Biol. Med. 2019, 22, 102108. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Wang, K.; Liang, X.; Wang, W.; Hu, X.; Huang, Z.; Wang, Y. Aerobic Exercise Ameliorates Myocardial Inflammation, Fibrosis and Apoptosis in High-Fat-Diet Rats by Inhibiting P2X7 Purinergic Receptors. Front. Physiol. 2019, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.S.; Cui, Z.Y.; Sun, P.; Piao, H.Q.; Han, X.; Song, J.; Wang, G.; Zheng, S.; Dong, X.X.; Gao, L.; et al. Rutin mitigates hepatic fibrogenesis and inflammation through targeting TLR4 and P2X7 receptor signaling pathway in vitro and in vivo. J. Funct. Foods 2020, 64, 103700. [Google Scholar] [CrossRef]
- Górecki, D.C. P2X7 purinoceptor as a therapeutic target in muscular dystrophies. Curr. Opin. Pharmacol. 2019, 47, 40–45. [Google Scholar] [CrossRef]
- Cicko, S.; Köhler, T.C.; Ayata, C.K.; Müller, T.; Ehrat, N.; Meyer, A.; Hossfeld, M.; Zech, A.; Di Virgilio, F.; Idzko, M. Extracellular ATP is a danger signal activating P2X7 Receptor in a LPS mediated inflammation (ARDS/ALI). Oncotarget 2018, 9, 30635–30648. [Google Scholar] [CrossRef]
- Black, L.M.; Lever, J.M.; Agarwal, A. Renal Inflammation and Fibrosis: A Double-edged Sword. J. Histochem. Cytochem. 2019, 67, 663–681. [Google Scholar] [CrossRef]
- Kleiser, S.; Nyström, A. Interplay between cell-surface receptors and extracellular matrix in skin. Biomolecules 2020, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Provenzano, P.P. Bringing order to the matrix. Nat. Mater. 2020, 19, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Wullweber, A.; Strick, R.; Lange, F.; Sikic, D.; Taubert, H.; Wach, S.; Wullich, B.; Bertz, S.; Weyerer, V.; Stoehr, R.; et al. Bladder tumor subtype commitment occurs in carcinoma in-situ driven by key signaling pathways including ECM remodeling. Cancer Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chery, D.R.; Han, B.; Li, Q.; Zhou, Y.; Heo, S.J.; Kwok, B.; Chandrasekaran, P.; Wang, C.; Qin, L.; Lu, X.L.; et al. Early changes in cartilage pericellular matrix micromechanobiology portend the onset of post-traumatic osteoarthritis. Acta Biomater. 2020, 111, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Munsterman, I.D.; Kendall, T.J.; Khelil, N.; Popa, M.; Lomme, R.; Drenth, J.P.H.; Tjwa, E.T.T.L. Extracellular matrix components indicate remodelling activity in different fibrosis stages of human non-alcoholic fatty liver disease. Histopathology 2018, 73, 612–621. [Google Scholar] [CrossRef]
- Bihlet, A.R.; Karsdal, M.A.; Sand, J.M.B.; Leeming, D.J.; Roberts, M.; White, W.; Bowler, R. Biomarkers of extracellular matrix turnover are associated with emphysema and eosinophilic-bronchitis in COPD. Respir. Res. 2017, 18. [Google Scholar] [CrossRef]
- McNair, A.J.; Wilson, K.S.; Martin, P.E.; Welsh, D.J.; Dempsie, Y. Connexin 43 plays a role in proliferation and migration of pulmonary arterial fibroblasts in response to hypoxia. Pulm. Circ. 2020, 10, 204589402093713. [Google Scholar] [CrossRef]
- Valls-Lacalle, L.; Consegal, M.; Ruiz-Meana, M.; Benito, B.; Inserte, J.; Barba, I.; Ferreira-González, I.; Rodríguez-Sinovas, A. Connexin 43 deficiency is associated with reduced myocardial scar size and attenuated tgfβ1 signaling after transient coronary occlusion in conditional knock-out mice. Biomolecules 2020, 10, 651. [Google Scholar] [CrossRef]
- Luther, J.; Gala, M.K.; Borren, N.; Masia, R.; Goodman, R.P.; Moeller, I.H.; DiGiacomo, E.; Ehrlich, A.; Warren, A.; Yarmush, M.L.; et al. Hepatic connexin 32 associates with nonalcoholic fatty liver disease severity. Hepatol. Commun. 2018, 2, 786–797. [Google Scholar] [CrossRef]
- Price, G.W.; Potter, J.A.; Williams, B.M.; Cliff, C.L.; Squires, P.E.; Hills, C.E. Connexin-mediated cell communication in the kidney: A potential therapeutic target for future intervention of diabetic kidney disease? Exp. Physiol. 2020, 105, 219–229. [Google Scholar] [CrossRef]
- Kavvadas, P.; Abed, A.; Poulain, C.; Authier, F.; Labéjof, L.P.; Calmont, A.; Afieri, C.; Prakoura, N.; Dussaule, J.C.; Chatziantoniou, C.; et al. Decreased expression of connexin 43 blunts the progression of experimental GN. J. Am. Soc. Nephrol. 2017, 28, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Holt, S.G.; Smith, E.R. Progression of Tubulointerstitial Fibrosis and the Chronic Kidney Disease Phenotype—Role of Risk Factors and Epigenetics. Front. Pharmacol. 2017, 8, 520. [Google Scholar] [CrossRef] [PubMed]
- Abed, A.; Toubas, J.; Kavvadas, P.; Authier, F.; Cathelin, D.; Alfieri, C.; Boffa, J.J.; Dussaule, J.C.; Chatziantoniou, C.; Chadjichristos, C.E. Targeting connexin 43 protects against the progression of experimental chronic kidney disease in mice. Kidney Int. 2014, 86, 768–779. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2015, 7, 684–696. [Google Scholar] [CrossRef]
- Wang, Z.; Stuckey, D.J.; Murdoch, C.E.; Camelliti, P.; Lip, G.Y.H.; Griffin, M. Cardiac fibrosis can be attenuated by blocking the activity of transglutaminase 2 using a selective small-molecule inhibitor. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.U.B.; Karsdal, M.A.; Brockbank, S.; Cruwys, S.; Rønnow, S.; Leeming, D.J. Tissue turnover of collagen type I, III and elastin is elevated in the PCLS model of IPF and can be restored back to vehicle levels using a phosphodiesterase inhibitor. Respir. Res. 2016, 17. [Google Scholar] [CrossRef]
- Bülow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cyto-Chem. 2019, 67, 643–661. [Google Scholar] [CrossRef]
- Rasmussen, D.G.K.; Boesby, L.; Nielsen, S.H.; Tepel, M.; Birot, S.; Karsdal, M.A.; Kamper, A.L.; Genovese, F. Collagen turnover profiles in chronic kidney disease. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Lopes, T.G.; de Souza, M.L.; da Silva, V.D.; dos Santos, M.; da Silva, W.I.C.; Itaquy, T.P.; Garbin, H.I.; Veronese, F.V. Markers of renal fibrosis: How do they correlate with podocyte damage in glomerular diseases? PLoS ONE 2019, 14, e0217585. [Google Scholar] [CrossRef]
- García-Vega, L.; O’Shaughnessy, E.M.; Jan, A.; Bartholomew, C.; Martin, P.E. Connexin 26 and 43 play a role in regulating proinflammatory events in the epidermis. J. Cell. Physiol. 2019, 234, 15594–15606. [Google Scholar] [CrossRef]
- Perera, L.M.B.; Sekiguchi, A.; Uchiyama, A.; Uehara, A.; Fujiwara, C.; Yamazaki, S.; Yokoyama, Y.; Ogino, S.; Torii, R.; Hosoi, M.; et al. The Regulation of Skin Fibrosis in Systemic Sclerosis by Extracellular ATP via P2Y 2 Purinergic Receptor. J. Invest. Dermatol. 2019, 139, 890–899. [Google Scholar] [CrossRef]
- Muncie, J.M.; Weaver, V.M. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. In Current Topics in Developmental Biology; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 130, pp. 1–37. [Google Scholar]
- Zhang, Y.; Reif, G.; Wallace, D.P. Extracellular matrix, integrins, and focal adhesion signaling in polycystic kidney disease. Cell. Signal. 2020, 72, 109646. [Google Scholar] [CrossRef]
- Saraswati, S.; Lietman, C.D.; Li, B.; Mathew, S.; Zent, R.; Young, P.P. Small proline-rich repeat 3 is a novel co-ordinator of PDGFRβ and integrin β1 crosstalk to augment proliferation and matrix synthesis by cardiac fibroblasts. FASEB J. 2020, 34, 7885–7904. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Alam, J.; Liu, H.; Erusappan, P.M.; Hoschuetzky, H.; Molven, A.; Parajuli, H.; Cukierman, E.; Costea, D.E.; Lu, N.; et al. α11β1 integrin is induced in a subset of cancer- associated fibroblasts in desmoplastic tumor stroma and mediates in vitro cell migration. Cancers 2019, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, C.; Wang, Q.; Cai, L.; Du, W.; Li, X.; Zhou, X.; Xie, J. Extracellular Matrix Elasticity Regulates Osteocyte Gap Junction Elongation: Involvement of Paxillin in Intracellular Signal Transduction. Cell. Physiol. Biochem. 2018, 51, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Lagos-Cabré, R.; Kong, M.; Cárdenas, A.; Burgos-Bravo, F.; Schneider, P.; Quest, A.F.G.; Leyton, L. Integrin-mediated transactivation of P2X7R via hemichannel-dependent ATP release stimulates astrocyte migration. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2175–2188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, X.; Pi, C.; Cai, L.; Liu, Y.; Du, W.; Yang, W.; Xie, J. Osteoporosis-decreased extracellular matrix stiffness impairs connexin 43-mediated gap junction intercellular communication in osteocytes. Acta Biochim. Biophys. Sin. 2020, 52, 517–526. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, D.; Du, W.; Zou, J.; Li, X.; Xie, J. Substrate mechanics dictate cell-cell communication by gap junctions in stem cells from human apical papilla. Acta Biomater. 2020, 107, 178–193. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.J.; Ramey, A.N.; Rashid, M.; Boyan, B.D.; Schwartz, Z. Integrin-α7 signaling regulates connexin 43, M-cadherin, and myoblast fusion. Am. J. Physiol. Physiol. 2019, 316, C876–C887. [Google Scholar] [CrossRef] [PubMed]
- Borza, C.M.; Su, Y.; Chen, X.; Yu, L.; Mont, S.; Chetyrkin, S.; Voziyan, P.; Hudson, B.G.; Billings, P.C.; Jo, H.; et al. Inhibition of Integrin a2b1 Ameliorates Glomerular Injury. J. Am. Soc. Nephrol. 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Milojkovic Kerklaan, B.; Slater, S.; Flynn, M.; Greystoke, A.; Witteveen, P.O.; Megui-Roelvink, M.; de Vos, F.; Dean, E.; Reyderman, L.; Ottesen, L.; et al. A phase I, dose escalation, pharmacodynamic, pharmacokinetic, and food-effect study of α2 integrin inhibitor E7820 in patients with advanced solid tumors. Invest. New Drugs 2016, 34, 329–337. [Google Scholar] [CrossRef]
- Ojalill, M.; Parikainen, M.; Rappu, P.; Aalto, E.; Jokinen, J.; Virtanen, N.; Siljamäki, E.; Heino, J. Integrin α2β1 decelerates proliferation, but promotes survival and invasion of prostate cancer cells. Oncotarget 2018, 9, 32435–32447. [Google Scholar] [CrossRef]
- Chung, C.H.; Chang, C.H.; Hsu, C.C.; Lin, K.T.; Peng, H.C.; Huang, T.F. Aggretin Venom Polypeptide as a Novel Anti-angiogenesis Agent by Targeting Integrin alpha2beta1. Sci. Rep. 2017, 7, 43612. [Google Scholar] [CrossRef]
- Pozzi, A.; Zent, R. Integrins in Kidney Disease. J. Am. Soc. Nephrol. 2013, 24, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, S.J.; Alkadhi, M.; Nicholson, L.F.B.; Green, C.R. Connexin43 mimetic peptides reduce swelling, astrogliosis, and neuronal cell death after spinal cord injury. Cell Commun. Adhes. 2008, 15, 27–42. [Google Scholar] [CrossRef]
- Yang, P.; Davidson, J.O.; Fowke, T.M.; Galinsky, R.; Wassink, G.; Karunasinghe, R.N.; Prasad, J.D.; Rana-singhe, S.; Green, C.R.; Bennet, L.; et al. Connexin hemichannel mimetic peptide attenuates cortical inter-neuron loss and perineuronal net disruption following cerebral ischemia in near-term fetal sheep. Int. J. Mol. Sci. 2020, 21, 6475. [Google Scholar] [CrossRef]
- Guo, C.X.; Nor, M.N.M.; Danesh-Meyer, H.V.; Vessey, K.A.; Fletcher, E.L.; O’Carroll, S.J.; Acosta, M.L.; Green, C.R. Connexin43 mimetic peptide improves retinal function and reduces inflammation in a light-damaged albino rat model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3961–3973. [Google Scholar] [CrossRef]
- Mao, Y.; Nguyen, T.; Tonkin, R.S.; Lees, J.G.; Warren, C.; O’Carroll, S.J.; Nicholson, L.F.B.; Green, C.R.; Moalem-Taylor, G.; Gorrie, C.A. Characterisation of Peptide5 systemic administration for treating traumatic spinal cord injured rats. Exp. Brain Res. 2017, 235, 3033–3048. [Google Scholar] [CrossRef]
- Kim, Y.; Griffin, J.M.; Harris, P.W.R.; Chan, S.H.C.; Nicholson, L.F.B.; Brimble, M.A.; O’Carroll, S.J.; Green, C.R. Characterizing the mode of action of extracellular Connexin43 channel blocking mimetic peptides in an in vitro ischemia injury model. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.M.; Guo, C.X.; Rupenthal, I.D.; Chen, Y.S.; Green, C.R.; Acosta, M.L. Sustained connexin43 mimetic peptide release from loaded nanoparticles reduces retinal and choroidal photodamage. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3682–3693. [Google Scholar] [CrossRef]
- Louie, H.H.; Shome, A.; Kuo, C.Y.; Rupenthal, I.D.; Green, C.R.; Mugisho, O.O. Connexin43 hemichannel block inhibits NLRP3 inflammasome activation in a human retinal explant model of diabetic retinopathy. Exp. Eye Res. 2021, 202. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Schnaper, H.W. The Tubulointerstitial Pathophysiology of Progressive Kidney Disease. Adv. Chronic Kidney Dis. 2017, 24, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lyon, H.; Shome, A.; Rupenthal, I.D.; Green, C.R.; Mugisho, O.O. Tonabersat inhibits connexin43 hemichannel opening and inflammasome activation in an in vitro retinal epithelial cell model of diabetic retinopathy. Int. J. Mol. Sci. 2021, 22, 298. [Google Scholar] [CrossRef]
- Acosta, M.; Mat Nor, M.; Guo, C.; Mugisho, O.; Coutinho, F.; Rupenthal, I.; Green, C. Connexin therapeutics: Blocking connexin hemichannel pores is distinct from blocking pannexin channels or gap junctions. Neural Regen. Res. 2021, 16, 482–488. [Google Scholar] [CrossRef]
- Coutinho, F.P.; Green, C.R.; Acosta, M.L.; Rupenthal, I.D. Xentry-Gap19 inhibits Connexin43 hemichannel opening especially during hypoxic injury. Drug Deliv. Transl. Res. 2020, 10, 751–765. [Google Scholar] [CrossRef]
- Kuo, C.; Green, C.R.; Rupenthal, I.D.; Mugisho, O.O. Connexin43 hemichannel block protects against retinal pigment epithelial cell barrier breakdown. Acta Diabetol. 2020, 57, 13–22. [Google Scholar] [CrossRef]
- Tonkin, R.S.; Bowles, C.; Perera, C.J.; Keating, B.A.; Makker, P.G.S.; Duffy, S.S.; Lees, J.G.; Tran, C.; Don, A.S.; Fath, T.; et al. Attenuation of mechanical pain hypersensitivity by treatment with Peptide5, a connexin-43 mimetic peptide, involves inhibition of NLRP3 inflammasome in nerve-injured mice. Exp. Neurol. 2018, 300, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Prakoura, N.; Kavvadas, P.; Chadjichristos, C.E. Connexin 43: A new therapeutic target against chronic kidney disease. Cell. Physiol. Biochem. 2018, 49, 998–1009. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor–ß in tissue fibrosis. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Hinz, B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef]
- Bon, H.; Hales, P.; Lumb, S.; Holdsworth, G.; Johnson, T.; Qureshi, O.; Twomey, B.M. Spontaneous Extracellular Matrix Accumulation in a Human in vitro Model of Renal Fibrosis Is Mediated by αV Integrins. Nephron 2019, 142, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.; Dadu, E.; Nykvist, P.; Käpylä, J.; White, D.J.; Ivaska, J.; Vehviläinen, P.; Reunanen, H.; Larjava, H.; Häkkinen, L.; et al. Integrin-mediated cell adhesion to type I collagen fibrils. J. Biol. Chem. 2004, 279, 31956–31963. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Wei, W.C.; Wang, Y.K.; Lin, S.C.; Sung, J.M.; Tang, M.J. Transforming growth factor-β1 induces Smad3-dependent β1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am. J. Pathol. 2010, 177, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dedhar, S. Integrin-linked kinase (ILK) and its interactors: A new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J. Cell Biol. 2001, 155, 505–510. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Dai, C.; Stolz, D.B.; Wang, D.; Liu, Y. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J. Am. Soc. Nephrol. 2009, 20, 1907–1918. [Google Scholar] [CrossRef]
- De Frutos, S.; Luengo, A.; García-Jérez, A.; Hatem-Vaquero, M.; Griera, M.; O’Valle, F.; Rodríguez–Puyol, M.; Rodríguez–Puyol, D.; Calleros, L. Chronic kidney disease induced by an adenine rich diet upregulates integrin linked kinase (ILK) and its depletion prevents the disease progression. Biochim. Biophys. Acta Mol. Basis Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mugisho, O.O.; Rupenthal, I.D.; Paquet-Durand, F.; Acosta, M.L.; Green, C.R. Targeting connexin hemichannels to control the inflammasome: The correlation between connexin43 and NLRP3 expression in chronic eye disease. Expert Opin. Ther. Targets 2019, 23, 855–863. [Google Scholar] [CrossRef]
- Hills, C.E.; Siamantouras, E.; Smith, S.W.; Cockwell, P.; Liu, K.-K.; Squires, P.E. TGFβ modulates cell-to-cell communication in early epithelial-to-mesenchymal transition. Diabetologia 2012, 55, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.E.; Kerr, M.I.; Wall, M.J.; Squires, P.E. Visfatin reduces gap junction mediated cell-to-cell communication in proximal tubule-derived epithelial cells. Cell. Physiol. Biochem. 2013, 32, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.A.; Price, G.W.; Cliff, C.L.; Williams, B.M.; Hills, C.E.; Squires, P.E. Carboxyfluorescein dye uptake to measure connexin-mediated hemichannel activity in cultured cells. Bio-Protocol 2021, 11, e3901. [Google Scholar] [CrossRef] [PubMed]
- Price, G.W.; Potter, J.A.; Williams, B.M.; Cliff, C.L.; Wall, M.J.; Hills, C.E.; Squires, P.E. Examining Local Cell-to-Cell Signalling in the Kidney Using ATP Biosensing. In Methods in Molecular Biology; Clifton, N.J., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Squires, P.E.; Price, G.W.; Mouritzen, U.; Potter, J.A.; Williams, B.M.; Hills, C.E. Danegaptide Prevents TGFβ1-Induced Damage in Human Proximal Tubule Epithelial Cells of the Kidney. Int. J. Mol. Sci. 2021, 22, 2809. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potter, J.A.; Price, G.W.; Cliff, C.L.; Green, C.R.; Squires, P.E.; Hills, C.E. Collagen I Modifies Connexin-43 Hemichannel Activity via Integrin α2β1 Binding in TGFβ1-Evoked Renal Tubular Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 3644. https://doi.org/10.3390/ijms22073644
Potter JA, Price GW, Cliff CL, Green CR, Squires PE, Hills CE. Collagen I Modifies Connexin-43 Hemichannel Activity via Integrin α2β1 Binding in TGFβ1-Evoked Renal Tubular Epithelial Cells. International Journal of Molecular Sciences. 2021; 22(7):3644. https://doi.org/10.3390/ijms22073644
Chicago/Turabian StylePotter, Joe A., Gareth W. Price, Chelsy L. Cliff, Colin R. Green, Paul E. Squires, and Claire E. Hills. 2021. "Collagen I Modifies Connexin-43 Hemichannel Activity via Integrin α2β1 Binding in TGFβ1-Evoked Renal Tubular Epithelial Cells" International Journal of Molecular Sciences 22, no. 7: 3644. https://doi.org/10.3390/ijms22073644
APA StylePotter, J. A., Price, G. W., Cliff, C. L., Green, C. R., Squires, P. E., & Hills, C. E. (2021). Collagen I Modifies Connexin-43 Hemichannel Activity via Integrin α2β1 Binding in TGFβ1-Evoked Renal Tubular Epithelial Cells. International Journal of Molecular Sciences, 22(7), 3644. https://doi.org/10.3390/ijms22073644







