The Affinity of Carboplatin to B-Vitamins and Nucleobases
Abstract
1. Introduction
1.1. Theoretical Studies
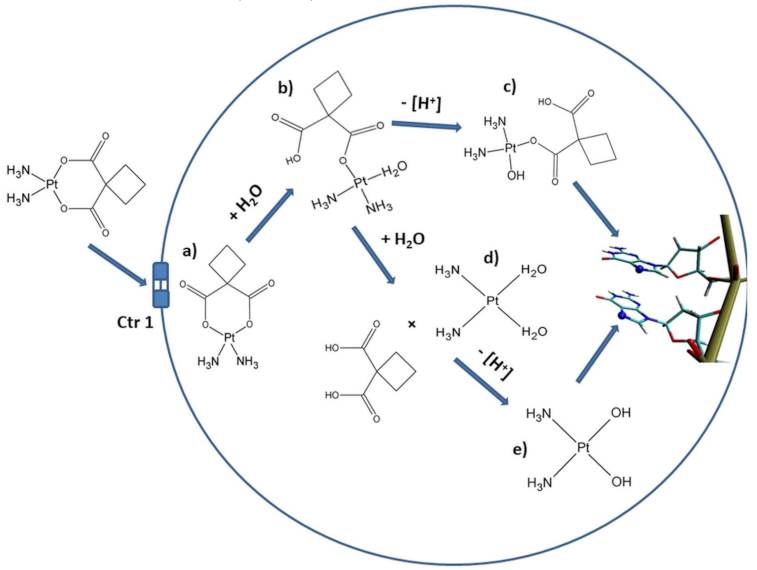
1.2. The Pathway of Carboplatin and Anticancer Activity
1.3. The Motivation of Work
2. Results
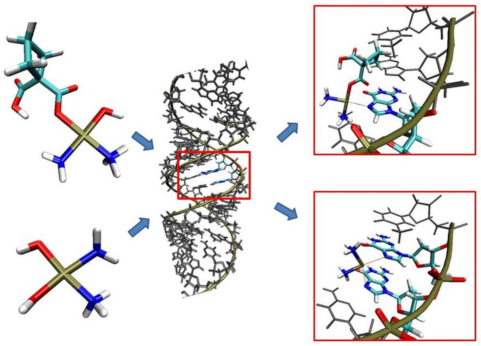
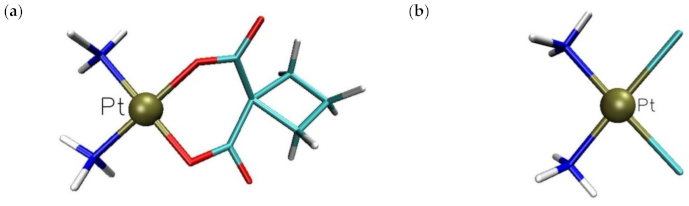
2.1. In Silico Study
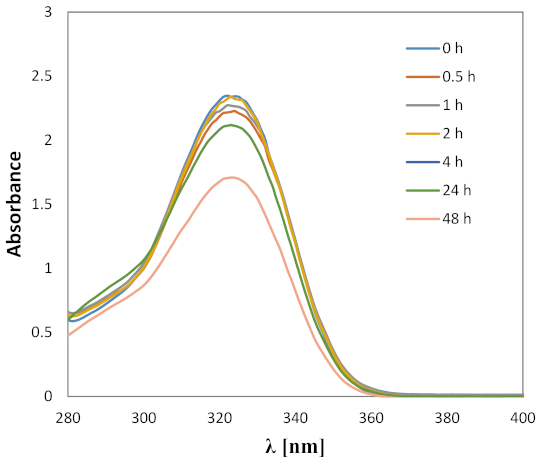
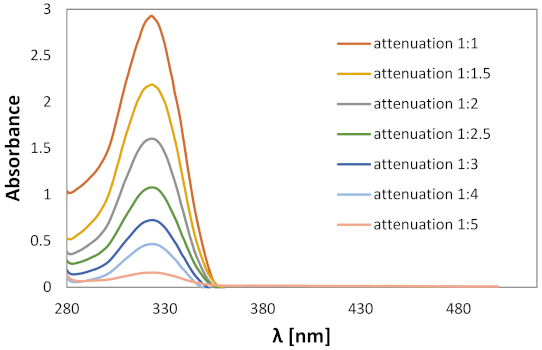
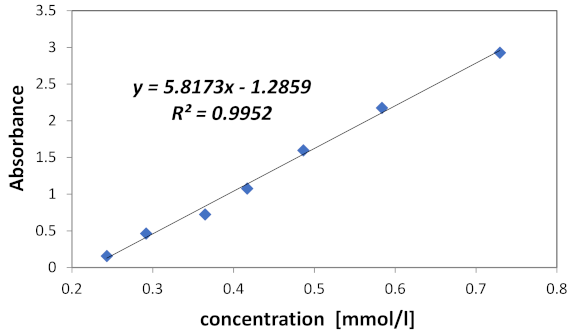
2.2. Experimental Analysis
3. Discussion
4. Materials and Methods
4.1. Gibbs Free Energy Calculation
4.2. Spectroscopic Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, G.Y.; Woodward, N.; Coward, J.I.G. Cisplatin versus carboplatin: Comparative review of therapeutic management in solid malignancies. Crit. Rev. Oncol. Hematol. 2016, 102, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; VanCamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, M.A.; Alonso, C.; Pérez, J.M. Biochemical modulation of cisplatin mechanisms of action: Enhancement of antitumor activity and circumvention of drug resistance. Chem. Rev. 2003, 103, 645–662. [Google Scholar] [CrossRef]
- Rugo, H.S.; Olopade, O.I.; DeMichele, A.; Yau, C.; van’t Veer, L.J.; Buxton, M.B.; Hogarth, M.; Hylton, N.M.; Paoloni, M.; Perlmutter, J.; et al. Adaptive Randomization of Veliparib–Carboplatin Treatment in Breast Cancer. N. Engl. J. Med. 2016. [Google Scholar] [CrossRef]
- Abotaleb, M.; Kubatka, P.; Caprnda, M.; Varghese, E.; Zolakova, B.; Zubor, P.; Opatrilova, R.; Kruzliak, P.; Stefanicka, P.; Büsselberg, D. Chemotherapeutic agents for the treatment of metastatic breast cancer: An update. Biomed. Pharmacother. 2018, 101, 458–477. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Witucka, A.; Pakuła, M.; Uruski, P.; Begier-Krasińska, B.; Niklas, A.; Tykarski, A.; Książek, K. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell. Mol. Life Sci. 2019, 76, 681–697. [Google Scholar] [CrossRef]
- Van Zyl, B.; Tang, D.; Bowden, N.A. Biomarkers of platinum resistance in ovarian cancer: What can we use to improve treatment. Endocr. Relat. Cancer 2018, 25, R303–R318. [Google Scholar] [CrossRef]
- Hah, S.S.; Stivers, K.M.; De Vere White, R.W.; Henderson, P.T. Kinetics of carboplatin-DNA binding in genomic DNA and bladder cancer cells as determined by accelerator mass spectrometry. Chem. Res. Toxicol. 2006, 19, 622–626. [Google Scholar] [CrossRef]
- Brabec, V.; Kasparkova, J. Modifications of DNA by platinum complexes. Drug Resist. Updat. 2005, 8, 131–146. [Google Scholar] [CrossRef]
- Gullo, J.J.; Litterst, C.L.; Maguire, P.J.; Sikic, B.I.; Hoth, D.F.; Woolley, P.V. Pharmacokinetics and protein binding of cis-dichlorodiammine platinum (II) administered as a one hour or as a twenty hour infusion. Cancer Chemother. Pharmacol. 1980. [Google Scholar] [CrossRef]
- Khalaila, I.; Bergamo, A.; Bussy, F.; Sava, G.; Dyson, P.J. The role of cisplatin and NAMI-A plasma-protein interactions in relation to combination therapy. Int. J. Oncol. 2006. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; Narendran, A.; Gailer, J. Comparative hydrolysis and plasma protein binding of cis-platin and carboplatin in human plasma in vitro. Metallomics 2011. [Google Scholar] [CrossRef]
- Unger, F.T.; Klasen, H.A.; Tchartchian, G.; de Wilde, R.L.; Witte, I. DNA damage induced by cis- and carboplatin as indicator for in vitro sensitivity of ovarian carcinowma cells. BMC Cancer 2009. [Google Scholar] [CrossRef]
- Carozzi, V.; Chiorazzi, A.; Canta, A.; Oggioni, N.; Gilardini, A.; Rodriguez-Menendez, V.; Avezza, F.; Crippa, L.; Ceresa, C.; Nicolini, G.; et al. Effect of the chronic combined administration of cisplatin and paclitaxel in a rat model of peripheral neurotoxicity. Eur. J. Cancer 2009, 45, 656–665. [Google Scholar] [CrossRef]
- McWhinney, S.R.; Goldberg, R.M.; McLeod, H.L. Platinum neurotoxicity pharmacogenetics. Mol. Cancer Ther. 2009, 8, 10–16. [Google Scholar] [CrossRef]
- Shahzad, M.M.K.; Lopez-Berestein, G.; Sood, A.K. Novel strategies for reversing platinum resistance. Drug Resist. Updat. 2009, 12, 148–152. [Google Scholar] [CrossRef]
- Burger, H.; Loos, W.J.; Eechoute, K.; Verweij, J.; Mathijssen, R.H.J.; Wiemer, E.A.C. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist. Updat. 2011, 14, 22–34. [Google Scholar] [CrossRef]
- Kang, S.; Ju, W.; Jae, W.K.; Park, N.H.; Song, Y.S.; Seung, C.K.; Park, S.Y.; Kang, S.B.; Lee, H.P. Association between excision repair cross-complementation group 1 polymorphism and clinical outcome of platinum-based chemotherapy in patients with epithelial ovarian cancer. Exp. Mol. Med. 2006, 38, 320–324. [Google Scholar] [CrossRef]
- Angioli, R.; Plotti, F.; Luvero, D.; Aloisi, A.; Guzzo, F.; Capriglione, S.; Terranova, C.; De Cicco Nardone, C.; Benedetti-Panici, P. Feasibility and safety of carboplatin plus paclitaxel as neoadjuvant chemotherapy for locally advanced cervical cancer: A pilot study. Tumor Biol. 2014, 35, 2741–2746. [Google Scholar] [CrossRef]
- Perez, E.A.; Suman, V.J.; Fitch, T.R.; Mailliard, J.A.; Ingle, J.N.; Cole, J.T.; Veeder, M.H.; Flynn, P.J.; Walsh, D.J.; Addo, F.K. A phase II trial of docetaxel and carboplatin as first-line chemotherapy for metastatic breast cancer: NCCTG study N9932. Oncology 2005, 69, 117–121. [Google Scholar] [CrossRef]
- Lim, K.H.; Lee, H.Y.; Song, S.Y. Efficacy and feasibility of gemcitabine and carboplatin as first-line chemotherapy in elderly patients with advanced non-small cell lung cancer. Chin. Med. J. 2013, 126, 4644–4648. [Google Scholar]
- Howell, S.B.; Safaei, R.; Larson, C.A.; Sailor, M.J. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol. Pharmacol. 2010, 77, 887–894. [Google Scholar] [CrossRef]
- Larson, C.A.; Blair, B.G.; Safaei, R.; Howell, S.B. The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol. Pharmacol. 2009. [Google Scholar] [CrossRef]
- Holzer, A.K.; Manorek, G.H.; Howell, S.B. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol. Pharmacol. 2006, 70, 1390–1394. [Google Scholar] [CrossRef]
- Blair, B.G.; Larson, C.; Safaei, R.; Howell, S.B. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of cisplatin and carboplatin. Clin. Cancer Res. 2009. [Google Scholar] [CrossRef]
- Shabalin, I.; Dauter, Z.; Jaskolski, M.; Minor, W.; Wlodawer, A. Crystallography and chemistry should always go together: A cautionary tale of protein complexes with cisplatin and carboplatin. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Alexander, S.M.; Wilson, J.J.; Lippard, S.J. Oxidative halogenation of cisplatin and carboplatin: Synthesis, spectroscopy, and crystal and molecular structures of Pt(iv) prodrugs. Dalt. Trans. 2015. [Google Scholar] [CrossRef]
- Shi, L.; Nishioka, W.K.; Th’ng, J.; Bradbury, E.M.; Litchfield, D.W.; Greenberg, A.H. Premature p34cdc2 activation required for apoptosis. Science 1994, 263, 1143–1145. [Google Scholar] [CrossRef]
- Sarmah, A.; Roy, R.K. Understanding the preferential binding interaction of aqua-cisplatins with nucleobase guanine over adenine: A density functional reactivity theory based approach. RSC Adv. 2013, 3, 2822. [Google Scholar] [CrossRef]
- Takahara, P.M.; Rosenzweig, A.C.; Frederick, C.A.; Lippard, S.J. Crystal structure of double-stranded DNA containing the major adduct of the anticancer drug cisplatin. Nature 1995, 377, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H.; Caudill, M.A. Biochemical, Physiological, and Molecular Aspects of Human Nutrition; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018; ISBN 9781455746293. [Google Scholar]
- Szefler, B.; Czeleń, P.; Szczepanik, A.; Cysewski, P. Does the Affinity of Cisplatin to B-Vitamins Impair the Therapeutic Effect in the Case of Patients with Lung Cancer-consuming Carrot or Beet Juice? Anticancer. Agents Med. Chem. 2019, 19, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Diet., Nutrition, and Cancer; The National Academies Press: Washington, DC, USA, 1982; ISBN 978-0-309-03280-3. [Google Scholar]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Patterson, R.E.; Kristal, A.R.; Thornquist, M.; King, I.; Shattuck, A.L.; Evans, I.; Satia-Abouta, J.; Littman, A.J.; Potter, J.D. VITamins and Lifestyle Cohort Study: Study Design and Characteristics of Supplement Users. Am. J. Epidemiol. 2004. [Google Scholar] [CrossRef]
- Kennedy, D. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Zielińska-Dawidziak, M.; Grajek, K.; Olejnik, A.; Czaczyk, K.; Grajek, W. Transport of high concentration of thiamin, riboflavin and pyridoxine across intestinal epithelial cells Caco-2. J. Nutr. Sci. Vitaminol. (Tokyo) 2008, 54, 423–429. [Google Scholar] [CrossRef]
- Vandemark, N.L.; Salisbury, G.W. The concentration of some B vitamins in bull semen. J. Biol. Chem. 1944, 156, 289–291. [Google Scholar] [CrossRef]
- de Broe, M.E.; Wedeen, R.P. Prevention of cisplatin nephrotoxicity. Eur. J. Cancer Clin. Oncol. 1986, 22, 1029–1031. [Google Scholar] [CrossRef]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02, 2016; Gaussian Inc.: Wallingford CT, USA, 2016. [Google Scholar]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. Implicit Solvation Models: Equilibria, Structure, Spectra, and Dynamics. Chem. Rev. 1999, 99, 2161–2200. [Google Scholar] [CrossRef]
- Marten, B.; Kim, K.; Cortis, C.; Friesner, R.A.; Murphy, R.B.; Ringnalda, M.N.; Sitkoff, D.; Honig, B. New model for calculation of solvation free energies: Correction of self-consistent reaction field continuum dielectric theory for short-range hydrogen-bonding effects. J. Phys. Chem. 1996, 100, 11775–11788. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]


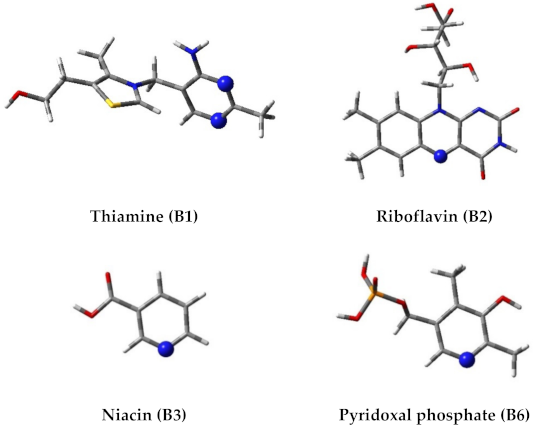
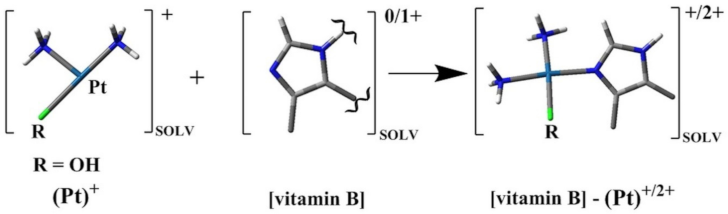
| Number of Reaction | Reaction | ΔGr |
|---|---|---|
| 1 | cis[Pt*]+ + B1(N7) → cis[Pt*~B1(N7)]+ | −18.90 |
| 2 | cis[Pt*]+ + B1(N1) → cis[Pt*~B1(N1)]+ | −20.89 |
| 3 | cis[Pt*]+ + B2(N7) → cis[Pt*~B2(N7)]+ | −3.75 |
| 4 | cis[Pt*]+ + B3(N7) → cis[Pt*~B3(N7)]+ | −24.90 |
| 5 | cis[Pt*]+ + B6(N7) → cis[Pt*~B6(N7)]+- | −15.61 |
| 6 | cis[Pt*]+ + GUA(N7) → cis[Pt*~GUA1(N7)]+ | −28.17 |
| Number of Reaction | Reaction | ΔGr |
|---|---|---|
| 1 | cis[Pt**]2+ + B1(N7) → cis[Pt**~B1(N7)]2+ | −20.23 |
| 2 | cis[Pt**]2+ + B1(N1) → cis[Pt**~B1(N1)]2+ | −22.64 |
| 3 | cis[Pt**]2+ + B2(N7) → cis[Pt**~B2(N7)]2+ | −7.84 |
| 4 | cis[Pt**]2+ + B3(N7) → cis[Pt**~B3(N7)]2+ | −27.28 |
| 5 | cis[Pt**]2+ + B6(N7) → cis[Pt**~B6(N7)]2+ | −24.66 |
| 6 | cis[Pt**]2+ + GUA(N7) → cis~[Pt**~GUA(N7)]2+ | −37.58 |
| Number of Reaction | Reaction | ΔGr |
|---|---|---|
| 1 | carbo[Pt***]+ + B1(N7) → carbo[Pt***~B1(N7)]+ | 149.53 |
| 2 | carbo[Pt***]+ + B1(N1) → carbo[Pt***~B1(N1)]+ | 144.60 |
| 3 | carbo[Pt***]+ + B2(N7) → carbo[Pt***~B2(N7)]+ | 233.04 |
| 4 | carbo[Pt***]+ + B3(N7) → carbo[Pt***~B3(N7)]+ | −6.3 |
| 5 | carbo[Pt***]+ + B6(N7) → carbo[Pt***~B6(N7)]+- | −12.96 |
| 6 | carbo[Pt***]+ + ADE(N7) → carbo[Pt***~ADE(N7)]+ | −26.46 |
| 7 | carbo[Pt***]+ + GUA(N7) → carbo[Pt***~GUA(N7)]+ | −30.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szefler, B.; Czeleń, P.; Krawczyk, P. The Affinity of Carboplatin to B-Vitamins and Nucleobases. Int. J. Mol. Sci. 2021, 22, 3634. https://doi.org/10.3390/ijms22073634
Szefler B, Czeleń P, Krawczyk P. The Affinity of Carboplatin to B-Vitamins and Nucleobases. International Journal of Molecular Sciences. 2021; 22(7):3634. https://doi.org/10.3390/ijms22073634
Chicago/Turabian StyleSzefler, Beata, Przemysław Czeleń, and Przemysław Krawczyk. 2021. "The Affinity of Carboplatin to B-Vitamins and Nucleobases" International Journal of Molecular Sciences 22, no. 7: 3634. https://doi.org/10.3390/ijms22073634
APA StyleSzefler, B., Czeleń, P., & Krawczyk, P. (2021). The Affinity of Carboplatin to B-Vitamins and Nucleobases. International Journal of Molecular Sciences, 22(7), 3634. https://doi.org/10.3390/ijms22073634






