Effects of Adenine Methylation on the Structure and Thermodynamic Stability of a DNA Minidumbbell
Abstract
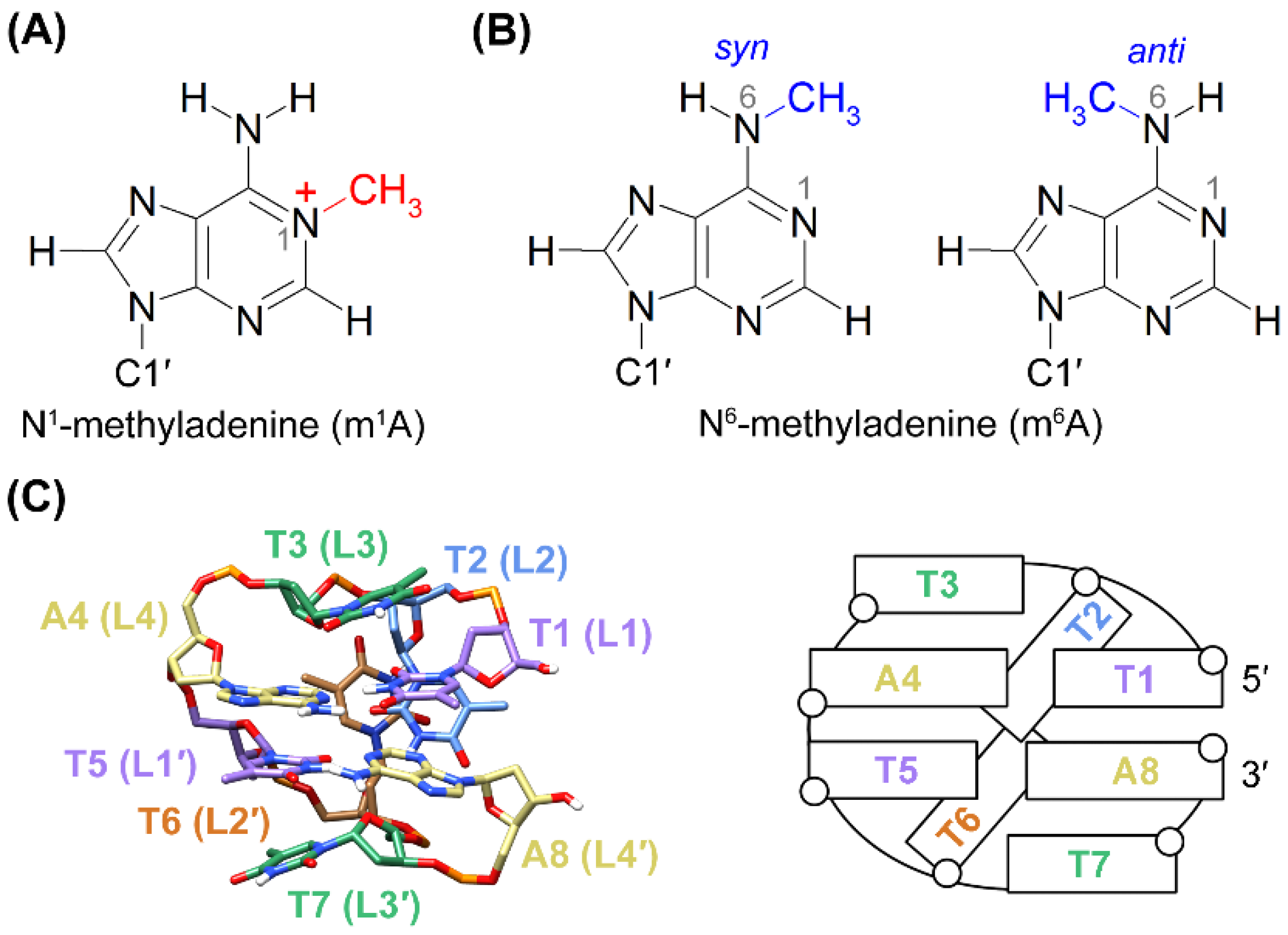
1. Introduction
2. Results
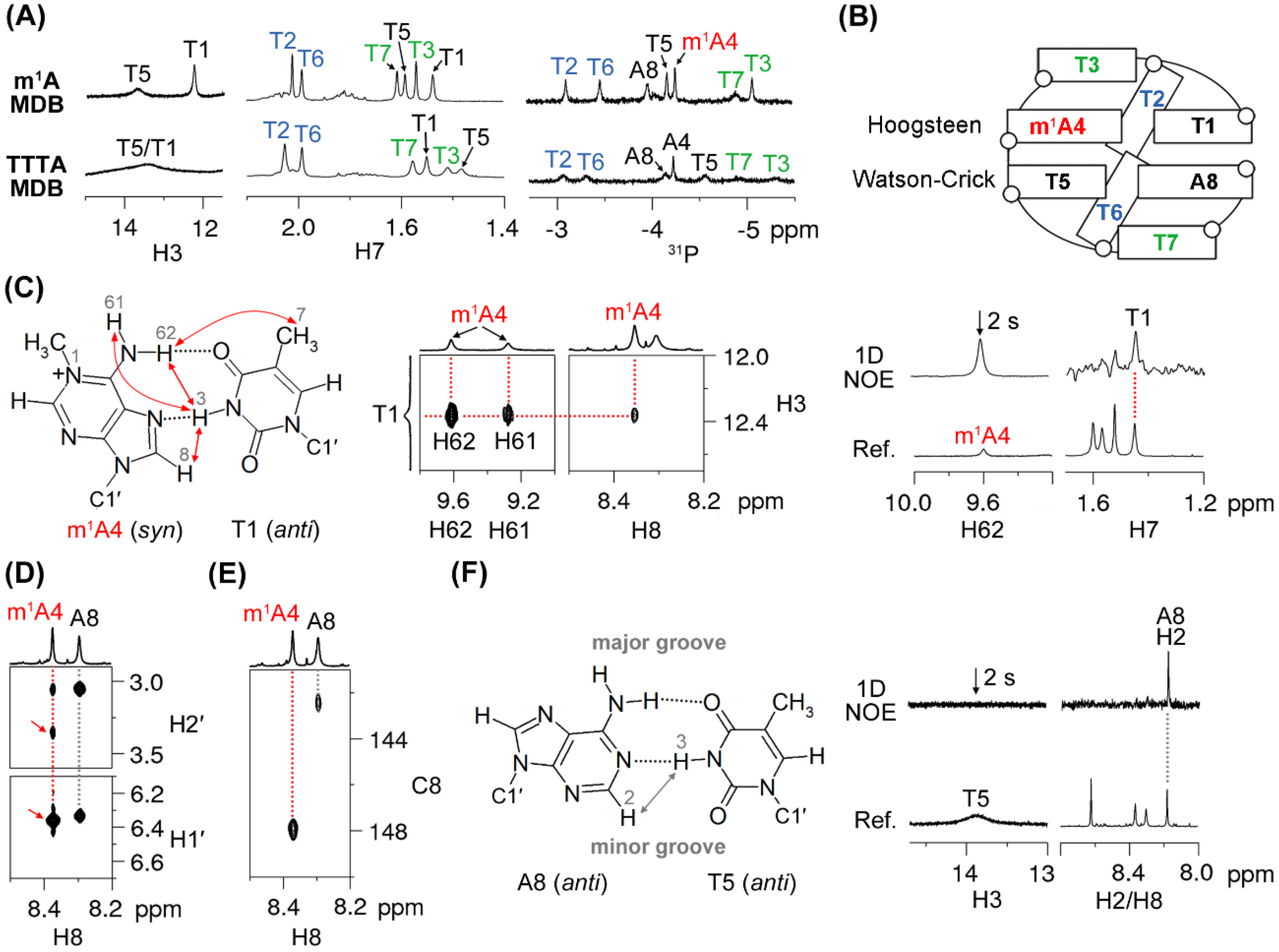
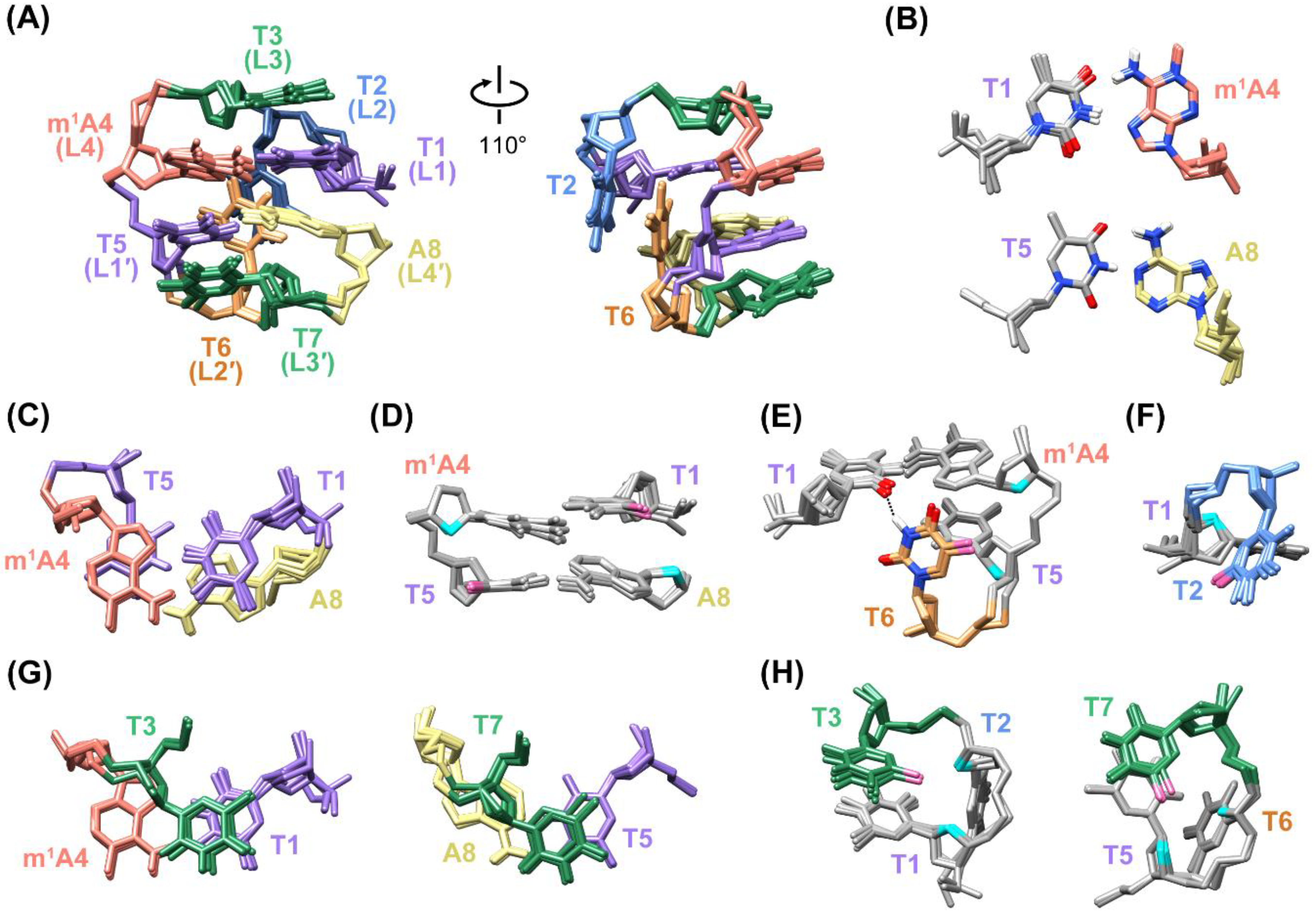
2.1. The 5′-TTTm1ATTTA-3′ MDB Containing a T·m1A Hoogsteen Base Pair
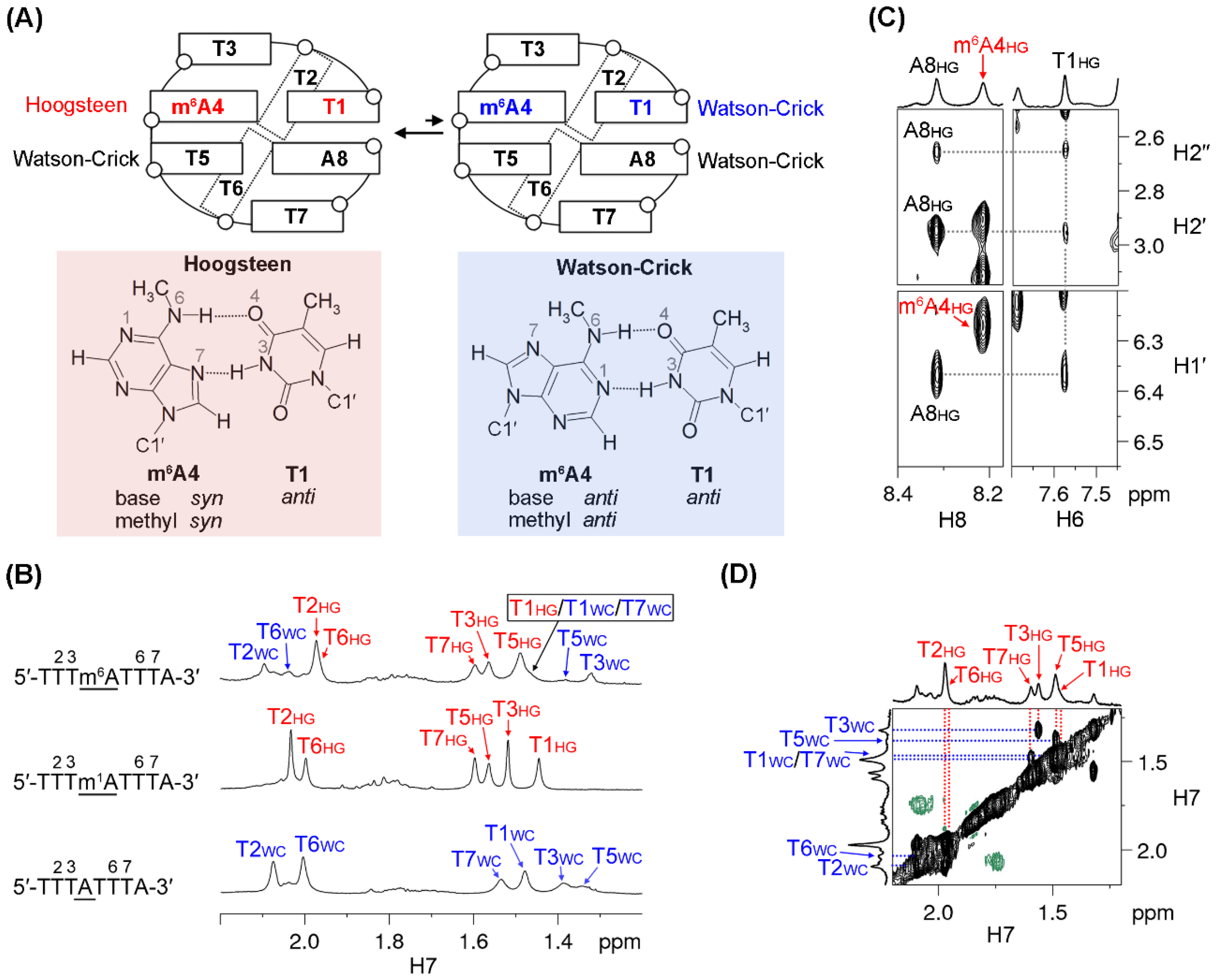
2.2. Conformational Exchange between Two MDB Structures in 5′-TTTm6ATTTA-3′
3. Discussion
4. Materials and Methods
4.1. DNA Samples
4.2. NMR Experiments
4.3. Resonance Assignments
4.4. Extraction of Experimental NMR Restraints
4.5. rMD–rEM Calculations
4.6. Analyses of Calculated Structures
4.7. UV Melting Experiments
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| m1A | N1-methyladenine |
| m6A | N6-methyladenine |
| MDB | minidumbbell |
| Tm | melting temperature |
| NMR | nuclear magnetic resonance |
| NOESY | nuclear Overhauser effect spectroscopy |
| HSQC | heteronuclear single-quantum correlation |
| TOCSY | total correlation spectroscopy |
| HMBC | heteronuclear multiple bond correlation |
| rMD | restrained molecular dynamics |
| rEM | restrained energy minimization |
| UV | ultraviolet |
| ROESY | rotating frame Overhauser effect spectroscopy |
| DSS | 2,2-dimethyl-2-silapentane-5-sulfonate |
| NaPi | sodium phosphate |
| DQF-COSY | double-quantum-filtered correlation spectroscopy |
References
- Schübeler, D. Function and Information Content of DNA Methylation. Nature 2015, 517, 321–326. [Google Scholar] [CrossRef]
- Heyn, H.; Esteller, M. An Adenine Code for DNA: A Second Life for N6-Methyladenine. Cell 2015, 161, 710–713. [Google Scholar] [CrossRef]
- Sedgwick, B.; Bates, P.A.; Paik, J.; Jacobs, S.C.; Lindahl, T. Repair of Alkylated DNA: Recent Advances. DNA Repair 2007, 6, 429–442. [Google Scholar] [CrossRef]
- Delaney, J.C.; Essigmann, J.M. Mutagenesis, Genotoxicity, and Repair of 1-methyladenine, 3-alkylcytosines, 1-methylguanine, and 3-methylthymine in alkB Escherichia coli. Proc. Natl. Acad. Sci. USA 2004, 101, 14051–14056. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Calvo, J.A.; Samson, L.D. Balancing Repair and Tolerance of DNA Damage Caused by Alkylating Agents. Nat. Rev. Cancer 2012, 12, 104–120. [Google Scholar] [CrossRef]
- Vanyushin, B.F.; Belozersky, A.N.; Kokurina, N.A.; Kadirova, D.X. 5-Methylcytosine and 6-Methylaminopurine in Bacterial DNA. Nature 1968, 218, 1066–1067. [Google Scholar] [CrossRef] [PubMed]
- Wion, D.; Casadesús, J. N6-methyl-adenine: An Epigenetic Signal for DNA–protein Interactions. Nat. Rev. Microbiol. 2006, 4, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Koziol, M.J.; Bradshaw, C.R.; Allen, G.E.; Costa, A.S.H.; Frezza, C.; Gurdon, J.B. Identification of Methylated Deoxyadenosines in Vertebrates Reveals Diversity in DNA Modifications. Nat. Struct. Mol. Biol. 2016, 23, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, Y.; Luo, G.-Z.; Wang, X.; Yue, Y.; Wang, X.; Zong, X.; Chen, K.; Yin, H.; Fu, Y.; et al. Abundant DNA 6mA Methylation during Early Embryogenesis of Zebrafish and Pig. Nat. Commun. 2016, 7, 13052. [Google Scholar] [CrossRef]
- Yao, B.; Cheng, Y.; Wang, Z.; Li, Y.; Chen, L.; Huang, L.; Zhang, W.; Chen, D.; Wu, H.; Tang, B.; et al. DNA N6-methyladenine is Dynamically Regulated in the Mouse Brain Following Environmental Stress. Nat. Commun. 2017, 8, 1122. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lai, W.; Li, Y.; Chen, S.; Liu, B.; Zhang, N.; Mo, J.; Lyu, C.; Zheng, J.; Du, Y.-R.; et al. N6-methyladenine is Incorporated into Mammalian Genome by DNA Polymerase. Cell Res. 2021, 31, 94–97. [Google Scholar] [CrossRef]
- Engel, J.D. Mechanism of the Dimroth Rearrangement in Adenosine. Biochem. Biophys. Res. Commun. 1975, 64, 581–586. [Google Scholar] [CrossRef]
- Engel, J.D.; von Hippel, P.H. Effects of Methylation on the Stability of Nucleic Acid Conformations. Studies at the Polymer Level. J. Biol. Chem. 1978, 253, 927–934. [Google Scholar] [CrossRef]
- Yang, H.; Zhan, Y.; Fenn, D.; Chi, L.M.; Lam, S.L. Effect of 1-Methyladenine on Double-helical DNA Structures. FEBS Lett. 2008, 582, 1629–1633. [Google Scholar] [CrossRef]
- Yang, H.; Lam, S.L. Effect of 1-Methyladenine on Thermodynamic Stabilities of Double-Helical DNA Structures. FEBS Lett. 2009, 583, 1548–1553. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Yi, C.; Jian, X.; Zheng, G.; He, C. Structure Determination of DNA Methylation Lesions N1-meA and N3-meC in Duplex DNA Using a Cross-linked Protein-DNA System. Nucleic Acids Res. 2010, 38, 4415–4425. [Google Scholar] [CrossRef]
- Sathyamoorthy, B.; Shi, H.; Zhou, H.; Xue, Y.; Rangadurai, A.; Merriman, D.K.; Al-Hashimi, H.M. Insights into Watson–Crick/Hoogsteen Breathing Dynamics and Damage Repair from the Solution Structure and Dynamic Ensemble of DNA Duplexes Containing m1A. Nucleic Acids Res. 2017, 45, 5586–5601. [Google Scholar] [CrossRef]
- Laddachote, S.; Nagata, M.; Yoshida, W. Destabilisation of the c-kit1 G-quadruplex Structure by N6-Methyladenosine Modification. Biochem. Biophys. Res. Commun. 2020, 524, 472–476. [Google Scholar] [CrossRef]
- Wada, R.; Yoshida, W. Thermal Stability Changes in Telomeric G-Quadruplex Structures Due to N6-Methyladenosine Modification. Epigenomes 2021, 5, 5. [Google Scholar] [CrossRef]
- Nikolova, E.N.; Kim, E.; Wise, A.A.; O’Brien, P.J.; Andricioaei, I.; Al-Hashimi, H.M. Transient Hoogsteen Base Pairs in Canonical Duplex DNA. Nature 2011, 470, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.D.; Von Hippel, P.H. Effects of Methylation on the Stability of Nucleic Acid Conformations. Monomer Level. Biochemistry 1974, 13, 4143–4158. [Google Scholar] [CrossRef] [PubMed]
- Roost, C.; Lynch, S.R.; Batista, P.J.; Qu, K.; Chang, H.Y.; Kool, E.T. Structure and Thermodynamics of N6-Methyladenosine in RNA: A Spring-Loaded Base Modification. J. Am. Chem. Soc. 2015, 137, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Lam, S.L. Minidumbbell: A New Form of Native DNA Structure. J. Am. Chem. Soc. 2016, 138, 12534–12540. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Lam, S.L. Minidumbbell Structures Formed by ATTCT Pentanucleotide Repeats in Spinocerebellar Ataxia Type 10. Nucleic Acids Res. 2020, 48, 7557–7568. [Google Scholar] [CrossRef]
- Guo, P.; Lam, S.L. Unusual Structures of TTTA Repeats in icaC Gene of Staphylococcus Aureus. FEBS Lett. 2015, 589, 1296–1300. [Google Scholar] [CrossRef]
- Guo, P.; Lam, S.L. New Insights into the Genetic Instability in CCTG Repeats. FEBS Lett. 2015, 589, 3058–3063. [Google Scholar] [CrossRef]
- Wijmenga, S.S.; van Buuren, B.N.M. The Use of NMR Methods for Conformational Studies of Nucleic Acids. Prog. Nucl. Magn. Reson. Spectrosc. 1998, 32, 287–387. [Google Scholar] [CrossRef]
- van Dongen, M.J.P.; Wijmenga, S.S.; Eritja, R.; Azorín, F.; Hilbers, C.W. Through-bond Correlation of Adenine H2 and H8 Protons in Unlabeled DNA Fragments by HMBC Spectroscopy. J. Biomol. NMR 1996, 8, 207–212. [Google Scholar] [CrossRef][Green Version]
- Blommers, M.J.J.; Walters, J.A.L.I.; Haasnoot, C.A.G.; Aelen, J.M.A.; Van der Marel, G.A.; Van Boom, J.H.; Hilbers, C.W. Effects of Base Sequence on the Loop Folding in DNA Hairpins. Biochemistry 1989, 28, 7491–7498. [Google Scholar] [CrossRef]
- Markley, J.L.; Bax, A.; Arata, Y.; Hilbers, C.W.; Kaptein, R.; Sykes, B.D.; Wright, P.E.; Wüthrich, K. Recommendations for the Presentation of NMR Structures of Proteins and Nucleic Acids—IUPAC-IUBMB-IUPAB Inter-Union Task Group on the Standardization of Data Bases of Protein and Nucleic Acid Structures Determined by NMR Spectroscopy. J. Biomol. NMR 1998, 12, 1–23. [Google Scholar] [CrossRef]
- Lam, S.L.; Chi, L.M. Use of Chemical Shifts for Structural Studies of Nucleic Acids. Prog. Nucl. Magn. Reson. Spectrosc. 2010, 56, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Lam, S.L.; Lee, H.K.; Guo, P. Rational design of a reversible Mg2+/EDTA-controlled molecular switch based on a DNA minidumbbell. Chem. Commun. 2020, 56, 10127–10130. [Google Scholar] [CrossRef]
- Onofrio, A.; Parisi, G.; Punzi, G.; Todisco, S.; Di Noia, M.A.; Bossis, F.; Turi, A.; De Grassi, A.; Pierri, C.L. Distance-dependent Hydrophobic–hydrophobic Contacts in Protein Folding Simulations. Phys. Chem. Chem. Phys. 2014, 16, 18907–18917. [Google Scholar] [CrossRef]
- Wüthrich, K. NMR of Proteins and Nucleic Acids; Wiley: Hoboken, NJ, USA, 1986. [Google Scholar]
- Greenfield, N.J. Using Circular Dichroism Collected as a Function of Temperature to Determine the Thermodynamics of Protein Unfolding and Binding Interactions. Nat. Protoc. 2006, 1, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Lam, S.L. The Competing Mini-dumbbell Mechanism: New Insights into CCTG Repeat Expansion. Signal Transduct. Target. Ther. 2016, 1, 16028. [Google Scholar] [CrossRef] [PubMed]
- Plateau, P.; Gueron, M. Exchangeable Proton NMR without Base-line Distorsion, Using New Strong-pulse Sequences. J. Am. Chem. Soc. 1982, 104, 7310–7311. [Google Scholar] [CrossRef]
- Stott, K.; Stonehouse, J.; Keeler, J.; Hwang, T.-L.; Shaka, A.J. Excitation Sculpting in High-Resolution Nuclear Magnetic Resonance Spectroscopy: Application to Selective NOE Experiments. J. Am. Chem. Soc. 1995, 117, 4199–4200. [Google Scholar] [CrossRef]
- Luy, B.; Marino, J.P. 1H−31P CPMG-Correlated Experiments for the Assignment of Nucleic Acids. J. Am. Chem. Soc. 2001, 123, 11306–11307. [Google Scholar] [CrossRef]
- Saenger, W. Principles of Nucleic Acid Structure; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Hosur, R.V.; Govil, G.; Miles, H.T. Application of Two-dimensional NMR Spectroscopy in the Determination of Solution Conformation of Nucleic Acids. Magn. Reson. Chem. 1988, 26, 927–944. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E., III; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber Biomolecular Simulation Programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Zgarbová, M.; Šponer, J.; Otyepka, M.; Cheatham, T.E.; Galindo-Murillo, R.; Jurečka, P. Refinement of the Sugar–Phosphate Backbone Torsion Beta for AMBER Force Fields Improves the Description of Z- and B-DNA. J. Chem. Theory Comput. 2015, 11, 5723–5736. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kim, E.; Lim, M.; Pak, Y. Computational Probing of Watson–Crick/Hoogsteen Breathing in a DNA Duplex Containing N1-Methylated Adenine. J. Chem. Theory Comput. 2019, 15, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
| MDB | Sequence | Tm (°C) | ∆H° (kcal·mol−1) | ∆S° (cal·K−1·mol−1) | ∆G°25 °C (kcal·mol−1) |
|---|---|---|---|---|---|
| m1A | 5′-TTTm1ATTTA-3′ | 23.2 ± 0.6 | −19 ± 2 | −64 ± 6 | 0.12 ± 0.04 |
| TTTA b | 5′-TTTATTTA-3′ | 19.2 ± 0.9 | −22 ± 1 | −76 ± 4 | 0.43 ± 0.08 |
| Restraint Satisfaction | |
|---|---|
| Number of distance restraint violation > 0.2 Å | 0 |
| Number of angle restraint violation > 5° | 0 |
| Deviation from ideal geometry | |
| Bond distance (Å) | 0.0097 ± 0.0002 |
| Angle (°) | 2.69 ± 0.08 |
| Heavy atomic RMSD (Å) a | |
| Average pairwise RMSD | 0.62 ± 0.09 |
| RMSD from mean structure | 0.39 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, L.; Lam, S.L.; Lee, H.K.; Guo, P. Effects of Adenine Methylation on the Structure and Thermodynamic Stability of a DNA Minidumbbell. Int. J. Mol. Sci. 2021, 22, 3633. https://doi.org/10.3390/ijms22073633
Wan L, Lam SL, Lee HK, Guo P. Effects of Adenine Methylation on the Structure and Thermodynamic Stability of a DNA Minidumbbell. International Journal of Molecular Sciences. 2021; 22(7):3633. https://doi.org/10.3390/ijms22073633
Chicago/Turabian StyleWan, Liqi, Sik Lok Lam, Hung Kay Lee, and Pei Guo. 2021. "Effects of Adenine Methylation on the Structure and Thermodynamic Stability of a DNA Minidumbbell" International Journal of Molecular Sciences 22, no. 7: 3633. https://doi.org/10.3390/ijms22073633
APA StyleWan, L., Lam, S. L., Lee, H. K., & Guo, P. (2021). Effects of Adenine Methylation on the Structure and Thermodynamic Stability of a DNA Minidumbbell. International Journal of Molecular Sciences, 22(7), 3633. https://doi.org/10.3390/ijms22073633






