Candidates for Intra-Articular Administration Therapeutics and Therapies of Osteoarthritis
Abstract
1. Introduction
2. Target Biological Pathways for DMOADs
3. The Advantage of IA Delivery in OA Treatment
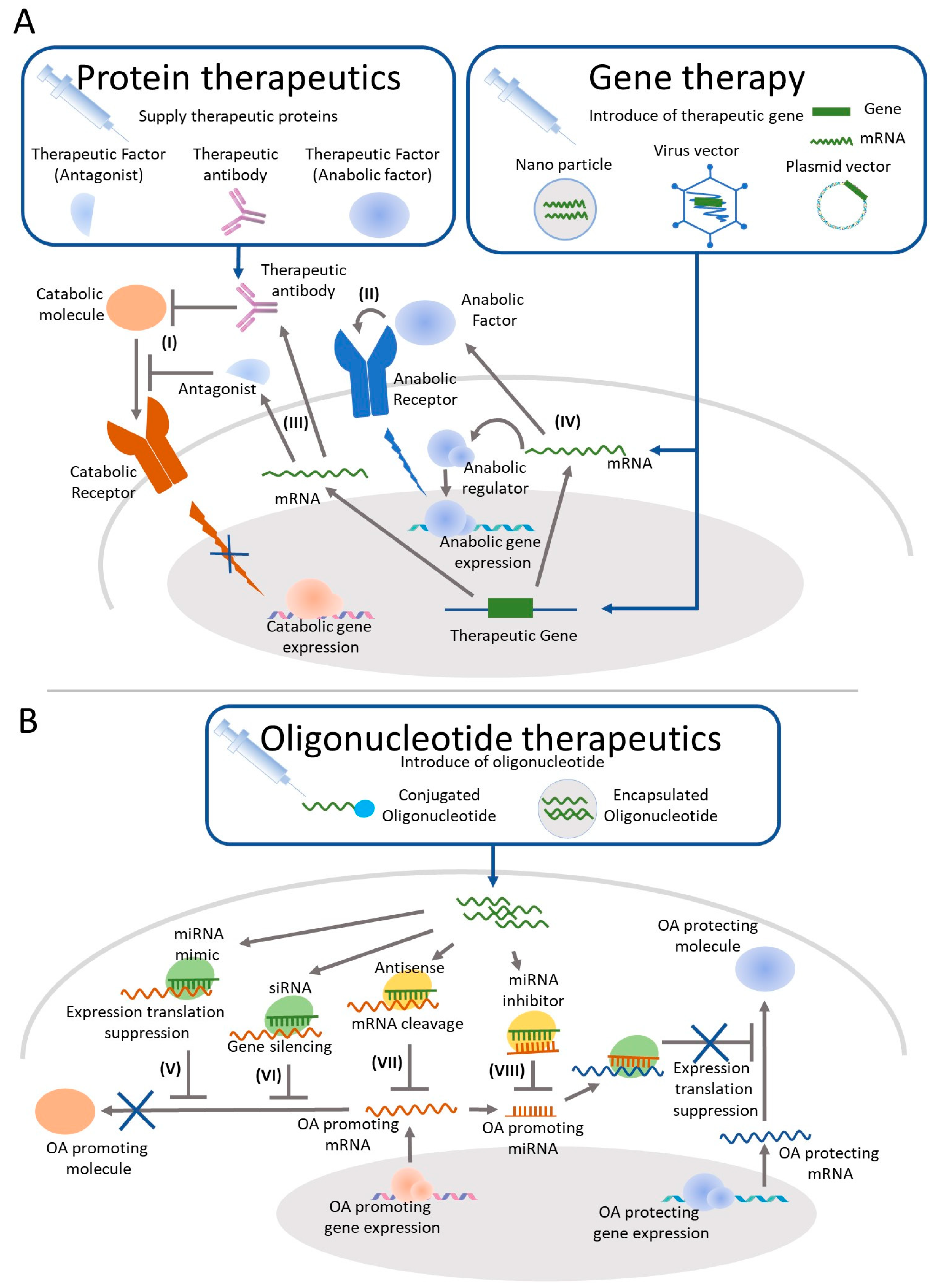
4. OA Treatment by IA Injection of Protein Therapeutics
| Study Name (ClinicalTrials.gov 1) | Mode of Action Biologicals | Study Phase Outcome Measures | Study Identifier Current Status 1 (Completion Year) |
|---|---|---|---|
| Biologic Therapy to Prevent Osteoarthritis After ACL Injury | Inhibit IL-1β IL-1 Receptor antagonist r-metHuIL-1ra (Anakinra) | Early Phase 1 cytokine level/Knee pain and function/marker level | NCT03968913 [22] Not yet recruiting |
| Study of Safety, Tolerability, Preliminary Efficacy of Intra-articular LNA043 Injections in Patients with Articular Cartilage Lesions and Knee Osteoarthritis | Assist cartilage repair. A modified human angiopoietin-like 3 protein | Phase 2 MRI/AEs/protein level/antibodies/Others | NCT03275064 [23] 2017~Recruiting |
| A Study to Investigate the Safety and Effectiveness of Different Doses of Sprifermin in Participants with Osteoarthritis of the Knee (FORWARD) | Assist cartilage repair Fibroblast growth factor 18 (Sprifermin) | Phase 2 MRI/WOMAC/PGA/mJSW/Protein level | NCT01919164 [24] Completed (2019) has results |
| Dose Finding Study of Bone Morphogenetic Protein 7 (BMP-7) in Subjects with Osteoarthritis (OA) of the Knee | Assist cartilage repair Bone Morphogenetic Protein 7 (BMP-7/OP-1) | Phase 2 WOMAC | NCT01111045 [25] Completed (2011) |
| To Determine the Safety, Tolerability, Pharmacokinetics and Effect on Pain of a Single Intra-articular Administration of Canakinumab in Patients with Osteoarthritis in the Knee | Inhibit IL-1β humanized monoclonal antibody to interleukin-1β (Canakinumab) | Phase 2 AEs/VAS/WOMAC/Others | NCT01160822 [26] Completed (2010) has results |
| Treatment of Knee Osteoarthritis with Intra-Articular Infliximab | Inhibit TNFα chimeric monoclonal antibody to TNF-α (Infliximab) | Phase 4 Cellular infiltrates/Effusion/WOMAC/Others | NCT01144143 [27] Completed (2011) has results |
| Study of Intra-articular DLX105 Applied to Patients with Severely Painful Osteoarthritis of the Knee | Inhibit TNFα a single-chain (scFv) antibody fragment against TNF-α (DLX105) | Phase 1/2 AEs/VAS/WOMAC | NCT00819572 [28] Completed (2010) |
5. Gene Therapy for OA Treatment by IA
| Study Name (ClinicalTrials.gov 1) | Mode of Action Biologicals | Study Phase Outcome Measures | Study Identifier Current Status 1 (Completion Year) |
|---|---|---|---|
| Safety of Intra-Articular Sc-rAAV2.5IL-1Ra in Subjects with Moderate Knee OA (AAVIL-1Ra) | Inhibit IL-1β sc-rAAV2.5IL-1Ra | Phase 1 AEs | NCT02790723 [61] 2019~Recruiting |
| Study to Evaluate the Safety and Tolerability of FX201 in Patients with Osteoarthritis of the Knee | Inhibit IL-1β humantakinogene hadenovec IL-1Ra (FX201) | Phase 1 AEs/biodistribution | NCT04119687 [62] 2020~Recruiting |
| Efficacy and Safety of XT-150 in Osteoarthritis of the Knee | Supply IL-10 plasmid DNA with a variant of human IL-10 transgene (XT-150) | Phase 2 KOOS/WOMAC /Others | NCT04124042 [63] 2020~Recruiting |
| A Single Dose Clinical Trial to Study the Safety of ART-I02 in Patients with Arthritis | Supply IFN-β Recombinant AAV type 2/5 containing a hIFN-b gene (ART-I02) | Phase 1 AEs/clinical scores distribution/immune response /Others | NCT02727764 [64] Active (2022) |
6. Oligonucleotide Therapeutics as a Candidate for OA Treatment by IA Injection
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dillon, C.F.; Rasch, E.K.; Gu, Q.; Hirsch, R. Prevalence of Knee Osteoarthritis in the United States: Arthritis Data from the Third National Health and Nutrition Examination Survey 1991-94. J. Rheumatol. 2006, 33, 2271–2279. [Google Scholar]
- Yoshimura, N.; Muraki, S.; Oka, H.; Mabuchi, A.; En-Yo, Y.; Yoshida, M.; Saika, A.; Yoshida, H.; Suzuki, T.; Yamamoto, S.; et al. Prevalence of Knee Osteoarthritis, Lumbar Spondylosis, and Osteoporosis in Japanese Men and Women: The Research on Osteoarthritis/Osteoporosis against Disability Study. J. Bone Miner. Metab. 2009, 27, 620–628. [Google Scholar] [CrossRef]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current Evidence on Risk Factors for Knee Osteoarthritis in Older Adults: A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Vina, E.R.; Kwoh, C.K. Epidemiology of Osteoarthritis: Literature Update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI Guidelines for the Non-Surgical Management of Knee, Hip, and Polyarticular Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in Osteoarthritis: Current Understanding with Therapeutic Implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Rai, M.F.; Pham, C.T. Intra-Articular Drug Delivery Systems for Joint Diseases. Curr. Opin. Pharmacol. 2018, 40, 67–73. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheumatol. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of Inflammation in the Pathogenesis of Osteoarthritis: Latest Findings and Interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5, 1–13. [Google Scholar] [CrossRef]
- de Lange-Brokaar, B.J.E.; Ioan-Facsinay, A.; van Osch, G.J.V.M.; Zuurmond, A.-M.; Schoones, J.; Toes, R.E.M.; Huizinga, T.W.J.; Kloppenburg, M. Synovial Inflammation, Immune Cells and Their Cytokines in Osteoarthritis: A Review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef]
- Sohn, D.H.; Sokolove, J.; Sharpe, O.; Erhart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriacchi, T.P.; et al. Plasma Proteins Present in Osteoarthritic Synovial Fluid Can Stimulate Cytokine Production via Toll-like Receptor 4. Arthritis Res. Ther. 2012, 14, R7. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Goldring, M.B. Chondrogenesis, Chondrocyte Differentiation, and Articular Cartilage Metabolism in Health and Osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Lester, C.; Drapp, R.; Hu, D.Z.; Glimcher, L.H.; Jones, D. Tetraspanin CD9 and Ectonucleotidase CD73 Identify an Osteochondroprogenitor Population with Elevated Osteogenic Properties. Development 2015, 142, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, S.; Bonovas, S.; Lytras, T.; Pecoraro, V.; González-Lorenzo, M.; Bastiampillai, A.J.; Gabrielli, E.M.; Lonati, A.C.; Moja, L.; Cinquini, M.; et al. Risk of Infections Using Anti-TNF Agents in Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis: A Systematic Review and Meta-Analysis. Expert Opin. Drug Saf. 2016, 15, 11–34. [Google Scholar] [CrossRef]
- Clinicaltraials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00110916 (accessed on 31 January 2021).
- Chevalier, X.; Goupille, P.; Beaulieu, A.D.; Burch, F.X.; Bensen, W.G.; Conrozier, T.; Loeuille, D.; Kivitz, A.J.; Silver, D.; Appleton, B.E. Intraarticular Injection of Anakinra in Osteoarthritis of the Knee: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Rheumatol. 2009, 61, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Pelletier, J.-P. A Single Injection of Anakinra for Treating Knee OA? Nat. Rev. Rheumatol. 2009, 5, 363–364. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03968913 (accessed on 31 January 2021).
- Clinicaltraials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03275064 (accessed on 25 January 2021).
- Clinicaltraials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01919164 (accessed on 31 January 2021).
- Clinicaltraials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01111045 (accessed on 31 January 2021).
- Clinicaltraials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01160822 (accessed on 31 January 2021).
- Clinicaltraials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01144143 (accessed on 31 January 2021).
- Clinicaltraials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00819572 (accessed on 31 January 2021).
- Quartier, P.; Alexeeva, E.; Constantin, T.; Chasnyk, V.; Wulffraat, N.; Palmblad, K.; Wouters, C.; I Brunner, H.; Marzan, K.; Schneider, R.; et al. Tapering Canakinumab Monotherapy in Patients with Systemic Juvenile Idiopathic Arthritis in Clinical Remission: Results from a Phase IIIb/IV Open-Label, Randomized Study. Arthritis Rheumatol. 2021, 73, 336–346. [Google Scholar] [CrossRef]
- Schieker, M.; Conaghan, P.G.; Mindeholm, L.; Praestgaard, J.; Solomon, D.H.; Scotti, C.; Gram, H.; Thuren, T.; Roubenoff, R.; Ridker, P.M. Effects of Interleukin-1β Inhibition on Incident Hip and Knee Replacement: Exploratory Analyses From a Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Intern. Med. 2020, 173, 509–515. [Google Scholar] [CrossRef]
- Caron, M.M.J.; Emans, P.J.; Cremers, A.; Surtel, D.A.M.; Coolsen, M.M.E.; van Rhijn, L.W.; Welting, T.J.M. Hypertrophic Differentiation during Chondrogenic Differentiation of Progenitor Cells Is Stimulated by BMP-2 but Suppressed by BMP-7. Osteoarthr. Cartil. 2013, 21, 604–613. [Google Scholar] [CrossRef]
- Hunter, D.J.; Pike, M.C.; Jonas, B.L.; Kissin, E.; Krop, J.; McAlindon, T. Phase 1 Safety and Tolerability Study of BMP-7 in Symptomatic Knee Osteoarthritis. BMC Musculoskelet. Disord. 2010, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, V.; Aspera-Werz, R.H.; Tendulkar, G.; Reumann, M.K.; Freude, T.; Breitkopf-Heinlein, K.; Dooley, S.; Pscherer, S.; Ochs, B.G.; Flesch, I.; et al. BMP9 a Possible Alternative Drug for the Recently Withdrawn BMP7? New Perspectives for (Re-)Implementation by Personalized Medicine. Arch. Toxicol. 2017, 91, 1353–1366. [Google Scholar] [CrossRef]
- Ellsworth, J.L.; Berry, J.; Bukowski, T.; Claus, J.; Feldhaus, A.; Holderman, S.; Holdren, M.S.; Lum, K.D.; Moore, E.E.; Raymond, F.; et al. Fibroblast Growth Factor-18 Is a Trophic Factor for Mature Chondrocytes and Their Progenitors. Osteoarthr. Cartil. 2002, 10, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Saito, T.; Chang, S.H.; Kobayashi, H.; Ladel, C.H.; Guehring, H.; Chung, U.; Kawaguchi, H. Identification of Fibroblast Growth Factor-18 as a Molecule to Protect Adult Articular Cartilage by Gene Expression Profiling. J. Biol. Chem. 2014, 289, 10192–10200. [Google Scholar] [CrossRef]
- Dahlberg, L.E.; Aydemir, A.; Muurahainen, N.; Gühring, H.; Fredberg Edebo, H.; Krarup-Jensen, N.; Ladel, C.H.; Jurvelin, J.S. A First-in-Human, Double-Blind, Randomised, Placebo-Controlled, Dose Ascending Study of Intra-Articular RhFGF18 (Sprifermin) in Patients with Advanced Knee Osteoarthritis. Clin. Exp. Rheumatol. 2016, 34, 445–450. [Google Scholar]
- Hochberg, M.C.; Guermazi, A.; Guehring, H.; Aydemir, A.; Wax, S.; Fleuranceau-Morel, P.; Reinstrup Bihlet, A.; Byrjalsen, I.; Ragnar Andersen, J.; Eckstein, F. Effect of Intra-Articular Sprifermin vs Placebo on Femorotibial Joint Cartilage Thickness in Patients with Osteoarthritis: The FORWARD Randomized Clinical Trial. JAMA 2019, 322, 1360–1370. [Google Scholar] [CrossRef]
- Brett, A.; Bowes, M.A.; Conaghan, P.G.; Ladel, C.; Guehring, H.; Moreau, F.; Eckstein, F. Automated MRI Assessment Confirms Cartilage Thickness Modification in Patients with Knee Osteoarthritis: Post-Hoc Analysis from a Phase II Sprifermin Study. Osteoarthr. Cartil. 2020, 28, 1432–1436. [Google Scholar] [CrossRef]
- Eckstein, F.; Kraines, J.L.; Aydemir, A.; Wirth, W.; Maschek, S.; Hochberg, M.C. Intra-Articular Sprifermin Reduces Cartilage Loss in Addition to Increasing Cartilage Gain Independent of Location in the Femorotibial Joint: Post-Hoc Analysis of a Randomised, Placebo-Controlled Phase II Clinical Trial. Ann. Rheum. Dis. 2020, 79, 525–528. [Google Scholar] [CrossRef]
- Roemer, F.W.; Kraines, J.; Aydemir, A.; Wax, S.; Hochberg, M.C.; Crema, M.D.; Guermazi, A. Evaluating the Structural Effects of Intra-Articular Sprifermin on Cartilage and Non-Cartilaginous Tissue Alterations, Based on SqMRI Assessment over 2 Years. Osteoarthr. Cartil. 2020, 28, 1229–1234. [Google Scholar] [CrossRef]
- Scotti, C.; Gimbel, J.; Laurent, D.; Madar, A.; Peters, T.; Zhang, Y.; Polus, F.; Beste, M.; Vostiar, I.; Choudhury, S.; et al. LNA043, A Novel Cartilage Regenerative Treatment for Osteoarthritis. Arthritis Rheumatol. 2020, 72 (Suppl. 19), 1485. [Google Scholar]
- Candela, M.E.; Yasuhara, R.; Iwamoto, M.; Enomoto-Iwamoto, M. Resident Mesenchymal Progenitors of Articular Cartilage. Matrix Biol. 2014, 39, 44–49. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent Mesenchymal Stem Cells from Adult Human Synovial Membrane. Arthritis Rheumatol. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Ok, S.M.; Kim, J.H.; Kim, J.S.; Jeong, E.G.; Park, Y.M.; Jeon, H.M.; Heo, J.Y.; Ahn, Y.W.; Yu, S.N.; Park, H.R.; et al. Local Injection of Growth Hormone for Temporomandibular Joint Osteoarthritis. Yonsei Med. J. 2020, 61, 331–340. [Google Scholar] [CrossRef]
- Lubis, A.M.T.; Wonggokusuma, E.; Marsetio, A.F. Intra-Articular Recombinant Human Growth Hormone In-jection Compared with Hyaluronic Acid and Placebo for an Osteoarthritis Model of New Zealand Rabbits. Knee Surg. Relat. Res. 2019, 31, 44–53. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Pancoast, J.R.; Cai, L.; Vannelli, T.; Dong, J.Z.; Lee, R.T.; Patwari, P. Targeted Delivery to Carti-lage Is Critical for in Vivo Efficacy of Insulin-like Growth Factor 1 in a Rat Model of Osteoarthritis. Arthritis Rheumatol. 2014, 66, 1247–1255. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, W.; Dai, C.; Huang, W.; Liu, F.; Shan, W.; Cheng, C.; Xu, J.; Yin, Z.; He, W. The CRD of Frizzled 7 Exhibits Chondroprotective Effects in Osteoarthritis via Inhibition of the Canonical Wnt3a/β-Catenin Signal-ing Pathway. Int. Immunopharmacol. 2020, 82, 106367. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, S.; Wu, M.; Zhao, Y.; Han, W.; Yu, Y. The Therapeutic Effect of RhMK on Osteoarthritis in Mice, Induced by Destabilization of the Medial Meniscus. Biol. Pharm. Bull. 2014, 37, 1803–1810. [Google Scholar] [CrossRef]
- van Helvoort, E.M.; Popov-Celeketic, J.; Eijkelkamp, N.; Coeleveld, K.; Tryfonidou, M.A.; Wijne, C.D.; Hack, C.E.; Lafeber, F.P.J.G.; Mastbergen, S.C. Canine IL4-10 Fusion Protein Provides Disease Modifying Activity in a Canine Model of OA; an Exploratory Study. PLoS ONE 2019, 14, e0219587. [Google Scholar] [CrossRef]
- Steen-Louws, C.; Popov-Celeketic, J.; Mastbergen, S.C.; Coeleveld, K.; Hack, C.E.; Eijkelkamp, N.; Tryfonidou, M.; Spruijt, S.; van Roon, J.A.G.; Lafeber, F.P.J.G. IL4-10 Fusion Protein Has Chondroprotective, An-ti-Inflammatory and Potentially Analgesic Effects in the Treatment of Osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1127–1135. [Google Scholar] [CrossRef]
- Chang, J.C.; Christiansen, B.A.; Murugesh, D.K.; Sebastian, A.; Hum, N.R.; Collette, N.M.; Hatsell, S.; Econo-mides, A.N.; Blanchette, C.D.; Loots, G.G. SOST/Sclerostin Improves Posttraumatic Osteoarthritis and Inhibits MMP2/3 Expression After Injury. J. Bone Miner. Res. 2018, 33, 1105–1113. [Google Scholar] [CrossRef]
- Wei, J.-L.; Fu, W.; Ding, Y.-J.; Hettinghouse, A.; Lendhey, M.; Schwarzkopf, R.; Kennedy, O.D.; Liu, C.-J. Progranulin Derivative Atsttrin Protects against Early Osteoarthritis in Mouse and Rat Models. Arthritis Res. Ther. 2017, 19, 280. [Google Scholar] [CrossRef]
- Parrish, W.R.; Byers, B.A.; Su, D.; Geesin, J.; Herzberg, U.; Wadsworth, S.; Bendele, A.; Story, B. Intra-Articular Therapy with Recombinant Human GDF5 Arrests Disease Progression and Stimulates Cartilage Repair in the Rat Medial Meniscus Transection (MMT) Model of Osteoarthritis. Osteoarthr. Cartil. 2017, 25, 554–560. [Google Scholar] [CrossRef]
- Waller, K.A.; Chin, K.E.; Jay, G.D.; Zhang, L.X.; Teeple, E.; McAllister, S.; Badger, G.J.; Schmidt, T.A.; Fleming, B.C. Intra-Articular Recombinant Human Proteoglycan 4 Mitigates Cartilage Damage After Destabilization of the Medial Meniscus in the Yucatan Minipig. Am. J. Sports Med. 2017, 45, 1512–1521. [Google Scholar] [CrossRef]
- Jay, G.D.; Fleming, B.C.; Watkins, B.A.; McHugh, K.A.; Anderson, S.C.; Zhang, L.X.; Teeple, E.; Waller, K.A.; Elsaid, K.A. Prevention of Cartilage Degeneration and Restoration of Chondroprotection by Lubricin Tribo-supplementation in the Rat Following Anterior Cruciate Ligament Transection. Arthritis Rheumatol. 2010, 62, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Chiusaroli, R.; Visentini, M.; Galimberti, C.; Casseler, C.; Mennuni, L.; Covaceuszach, S.; Lanza, M.; Ugolini, G.; Caselli, G.; Rovati, L.C.; et al. Targeting of ADAMTS5′s Ancillary Domain with the Recombinant MAb CRB0017 Ameliorates Disease Progression in a Spontaneous Murine Model of Osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Chang, C.-C.; Shieh, M.-J.; Wang, J.-P.; Chen, Y.-T.; Young, T.-H.; Hung, S.-C. Hypoxia Enhances Chondrogenesis and Prevents Terminal Differentiation through PI3K/Akt/FoxO Dependent Anti-Apoptotic Effect. Sci. Rep. 2013, 3, 2683. [Google Scholar] [CrossRef]
- Ulrich-Vinther, M.; Duch, M.R.; Søballe, K.; O’Keefe, R.J.; Schwarz, E.M.; Pedersen, F.S. In Vivo Gene Delivery to Articular Chondrocytes Mediated by an Adeno-Associated Virus Vector. J. Orthop. Res. 2004, 22, 726–734. [Google Scholar] [CrossRef]
- Watson, R.S.; Broome, T.A.; Levings, P.P.; Rice, B.L.; Kay, J.D.; Smith, A.D.; Gouze, E.; Gouze, J.-N.; Dacanay, E.A.; Hauswirth, W.W.; et al. ScAAV-Mediated Gene Transfer of Interleukin-1-Receptor Antagonist to Synovium and Articular Cartilage in Large Mammalian Joints. Gene Ther. 2013, 20, 670–677. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Gene Delivery to Joints by Intra-Articular Injection. Hum. Gene Ther. 2018, 29, 2–14. [Google Scholar] [CrossRef]
- Clinicaltraials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02790723 (accessed on 31 January 2021).
- Clinicaltraials.gov. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04119687 (accessed on 21 February 2021).
- Clinicaltraials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04124042 (accessed on 31 January 2021).
- Clinicaltraials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02727764 (accessed on 31 January 2021).
- Nixon, A.J.; Grol, M.W.; Lang, H.M.; Ruan, M.Z.C.; Stone, A.; Begum, L.; Chen, Y.; Dawson, B.; Gannon, F.; Plutizki, S.; et al. Disease-Modifying Osteoarthritis Treatment with Interleukin-1 Receptor Antagonist Gene Therapy in Small and Large Animal Models. Arthritis Rheumatol. 2018, 70, 1757–1768. [Google Scholar] [CrossRef]
- Stone, A.; Grol, M.W.; Ruan, M.Z.C.; Dawson, B.; Chen, Y.; Jiang, M.-M.; Song, I.-W.; Jayaram, P.; Cela, R.; Gannon, F.; et al. Combinatorial Prg4 and Il-1ra Gene Therapy Protects Against Hyperalgesia and Cartilage Degeneration in Post-Traumatic Osteoarthritis. Hum. Gene Ther. 2019, 30, 225–235. [Google Scholar] [CrossRef]
- Nathwani, A.C.; Gray, J.T.; Ng, C.Y.C.; Zhou, J.; Spence, Y.; Waddington, S.N.; Tuddenham, E.G.D.; Kemball-Cook, G.; McIntosh, J.; Boon-Spijker, M.; et al. Self-Complementary Adeno-Associated Virus Vectors Containing a Novel Liver-Specific Human Factor IX Expression Cassette Enable Highly Efficient Transduction of Murine and Nonhuman Primate Liver. Blood 2006, 107, 2653–2661. [Google Scholar] [CrossRef]
- Wang, G.; Evans, C.H.; Benson, J.M.; Hutt, J.A.; Seagrave, J.; Wilder, J.A.; Grieger, J.C.; Samulski, R.J.; Terse, P.S. Safety and Biodistribution Assessment of Sc-RAAV2.5IL-1Ra Administered via Intra-Articular Injection in a Mono-Iodoacetate-Induced Osteoarthritis Rat Model. Mol. Ther. Methods Clin. Dev. 2016, 3, 15052. [Google Scholar] [CrossRef]
- Watson Levings, R.S.; Broome, T.A.; Smith, A.D.; Rice, B.L.; Gibbs, E.P.; Myara, D.A.; Hyddmark, E.V.; Nasri, E.; Zarezadeh, A.; Levings, P.P.; et al. Gene Therapy for Osteoarthritis: Pharmacokinetics of Intra-Articular Self-Complementary Adeno-Associated Virus Interleukin-1 Receptor Antagonist Delivery in an Equine Model. Hum. Gene Ther. Clin. Dev. 2018, 29, 90–100. [Google Scholar] [CrossRef]
- Katsikis, P.D.; Chu, C.Q.; Brennan, F.M.; Maini, R.N.; Feldmann, M. Immunoregulatory Role of Interleukin 10 in Rheumatoid Arthritis. J. Exp. Med. 1994, 179, 1517–1527. [Google Scholar] [CrossRef]
- Moroguchi, A.; Ishimura, K.; Okano, K.; Wakabayashi, H.; Maeba, T.; Maeta, H. Interleukin-10 Suppresses Proliferation and Remodeling of Extracellular Matrix of Cultured Human Skin Fibroblasts. Eur. Surg. Res. 2004, 36, 39–44. [Google Scholar] [CrossRef]
- Broeren, M.G.A.; de Vries, M.; Bennink, M.B.; Arntz, O.J.; van Lent, P.L.E.M.; van der Kraan, P.M.; van den Berg, W.B.; van den Hoogen, F.H.J.; Koenders, M.I.; van de Loo, F.A.J. Suppression of the Inflammatory Response by Disease-Inducible Interleukin-10 Gene Therapy in a Three-Dimensional Micromass Model of the Human Synovial Membrane. Arthritis Res. Ther. 2016, 18. [Google Scholar] [CrossRef]
- Watkins, L.R.; Chavez, R.A.; Landry, R.; Fry, M.; Green-Fulgham, S.M.; Coulson, J.D.; Collins, S.D.; Glover, D.K.; Rieger, J.; Forsayeth, J.R. Targeted Interleukin-10 Plasmid DNA Therapy in the Treatment of Osteoarthritis: Toxicology and Pain Efficacy Assessments. Brain Behav. Immun. 2020, 90, 155–166. [Google Scholar] [CrossRef]
- Aalbers, C.J.; Bevaart, L.; Loiler, S.; de Cortie, K.; Wright, J.F.; Mingozzi, F.; Tak, P.P.; Vervoordeldonk, M.J. Preclinical Potency and Biodistribution Studies of an AAV 5 Vector Expressing Human Interferon-β (ART-I02) for Local Treatment of Patients with Rheumatoid Arthritis. PLoS ONE 2015, 10, e0130612. [Google Scholar] [CrossRef]
- Bevaart, L.; Aalbers, C.J.; Vierboom, M.P.M.; Broekstra, N.; Kondova, I.; Breedveld, E.; Hauck, B.; Wright, J.F.; Tak, P.P.; Vervoordeldonk, M.J. Safety, Biodistribution, and Efficacy of an AAV-5 Vector Encoding Human Interferon-Beta (ART-I02) Delivered via Intra-Articular Injection in Rhesus Monkeys with Collagen-Induced Arthritis. Hum. Gene Ther. Clin. Dev. 2015, 26, 103–112. [Google Scholar] [CrossRef]
- Ruan, M.Z.; Cerullo, V.; Cela, R.; Clarke, C.; Lundgren-Akerlund, E.; Barry, M.A.; Lee, B.H. Treatment of Osteo-arthritis Using a Helper-Dependent Adenoviral Vector Retargeted to Chondrocytes. Mol. Ther. Methods Clin. Dev. 2016, 3, 16008. [Google Scholar] [CrossRef][Green Version]
- Ortved, K.; Wagner, B.; Calcedo, R.; Wilson, J.; Schaefer, D.; Nixon, A. Humoral and Cell-Mediated Immune Response, and Growth Factor Synthesis after Direct Intraarticular Injection of RAAV2-IGF-I and RAAV5-IGF-I in the Equine Middle Carpal Joint. Hum. Gene Ther. 2015, 26, 161–171. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.-P. The Current State and Future Directions of RNAi-Based Therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. MRNA-Based Therapeutics--Developing a New Class of Drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Bennett, C.F.; Krainer, A.R.; Cleveland, D.W. Antisense Oligonucleotide Therapies for Neurodegenerative Diseases. Annu. Rev. Neurosci. 2019, 42, 385–406. [Google Scholar] [CrossRef]
- Heo, Y.-A. Golodirsen: First Approval. Drugs 2020, 80, 329–333. [Google Scholar] [CrossRef]
- Ashizawa, A.T.; Cortes, J. Liposomal Delivery of Nucleic Acid-Based Anticancer Therapeutics: BP-100-1.01. Expert Opin. Drug Deliv. 2015, 12, 1107–1120. [Google Scholar] [CrossRef]
- Falzarano, M.S.; Passarelli, C.; Ferlini, A. Nanoparticle Delivery of Antisense Oligonucleotides and Their Application in the Exon Skipping Strategy for Duchenne Muscular Dystrophy. Nucleic Acid. Ther. 2014, 24, 87–100. [Google Scholar] [CrossRef]
- Mäe, M.; Andaloussi, S.E.; Lehto, T.; Langel, U. Chemically Modified Cell-Penetrating Peptides for the Delivery of Nucleic Acids. Expert Opin. Drug Deliv. 2009, 6, 1195–1205. [Google Scholar] [CrossRef]
- Vester, B.; Wengel, J. LNA (Locked Nucleic Acid): High-Affinity Targeting of Complementary RNA and DNA. Biochemistry 2004, 43, 13233–13241. [Google Scholar] [CrossRef]
- Dhillon, S. Viltolarsen: First Approval. Drugs 2020, 80, 1027–1031. [Google Scholar] [CrossRef]
- Hair, P.; Cameron, F.; McKeage, K. Mipomersen Sodium: First Global Approval. Drugs 2013, 73, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Nusinersen: First Global Approval. Drugs 2017, 77, 473–479. [Google Scholar] [CrossRef]
- Hoy, S.M. Patisiran: First Global Approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef]
- Keam, S.J. Inotersen: First Global Approval. Drugs 2018, 78, 1371–1376. [Google Scholar] [CrossRef]
- Paik, J.; Duggan, S. Volanesorsen: First Global Approval. Drugs 2019, 79, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.M.; Balfour, J.A. Fomivirsen. Drugs 1999, 57, 375–380. [Google Scholar] [CrossRef]
- Scott, L.J. Givosiran: First Approval. Drugs 2020, 80, 335–339. [Google Scholar] [CrossRef]
- Scott, L.J.; Keam, S.J. Lumasiran: First Approval. Drugs 2021. [Google Scholar] [CrossRef]
- Syed, Y.Y. Eteplirsen: First Global Approval. Drugs 2016, 76, 1699–1704. [Google Scholar] [CrossRef]
- Steinberg, J.; Ritchie, G.R.S.; Roumeliotis, T.I.; Jayasuriya, R.L.; Clark, M.J.; Brooks, R.A.; Binch, A.L.A.; Shah, K.M.; Coyle, R.; Pardo, M.; et al. Integrative Epigenomics, Transcriptomics and Proteomics of Patient Chon-drocytes Reveal Genes and Pathways Involved in Osteoarthritis. Sci. Rep. 2017, 7, 8935. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ning, Y.; Zhou, B.; Yang, L.; Wang, Y.; Guo, X. Integrated Bioinformatics Analysis of the Osteoarthri-tis-associated MicroRNA Expression Signature. Mol. Med. Rep. 2018, 17, 1833–1838. [Google Scholar] [CrossRef]
- Peffers, M.; Liu, X.; Clegg, P. Transcriptomic Signatures in Cartilage Ageing. Arthritis Res. Ther. 2013, 15, R98. [Google Scholar] [CrossRef]
- Malemud, C.J. MicroRNAs and Osteoarthritis. Cells 2018, 7, 72. [Google Scholar] [CrossRef]
- Sondag, G.R.; Haqqi, T.M. The Role of MicroRNAs and Their Targets in Osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
- Wu, C.; Tian, B.; Qu, X.; Liu, F.; Tang, T.; Qin, A.; Zhu, Z.; Dai, K. MicroRNAs Play a Role in Chondrogenesis and Osteoarthritis (Review). Int. J. Mol. Med. 2014, 34, 13–23. [Google Scholar] [CrossRef]
- Ji, M.-L.; Jiang, H.; Wu, F.; Geng, R.; Ya, L.K.; Lin, Y.C.; Xu, J.H.; Wu, X.T.; Lu, J. Precise Targeting of MiR-141/200c Cluster in Chondrocytes Attenuates Osteoarthritis Development. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- Tian, L.; Su, Z.; Ma, X.; Wang, F.; Guo, Y. Inhibition of MiR-203 Ameliorates Osteoarthritis Cartilage Degrada-tion in the Postmenopausal Rat Model: Involvement of Estrogen Receptor α. Hum. Gene Ther. Clin. Dev. 2019, 30, 160–168. [Google Scholar] [CrossRef]
- Wang, X.-B.; Zhao, F.-C.; Yi, L.-H.; Tang, J.-L.; Zhu, Z.-Y.; Pang, Y.; Chen, Y.-S.; Li, D.-Y.; Guo, K.-J.; Zheng, X. MicroRNA-21-5p as a Novel Therapeutic Target for Osteoarthritis. Rheumatology 2019. [Google Scholar] [CrossRef]
- Endisha, H.; Datta, P.; Sharma, A.; Nakamura, S.; Rossomacha, E.; Younan, C.; Ali, S.A.; Tavallaee, G.; Lively, S.; Potla, P.; et al. MicroRNA-34a-5p Promotes Joint Destruction During Osteoarthritis. Arthritis Rheumatol. 2020. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Zhang, H.; Shao, Y.; Chen, Z.; Feng, X.; Fang, H.; Zhao, C.; Pan, J.; Zhang, H.; et al. MiR-146b Accelerates Osteoarthritis Progression by Targeting Alpha-2-Macroglobulin. Aging 2019, 11, 6014–6028. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Rampersaud, Y.R.; Nakamura, S.; Sharma, A.; Zeng, F.; Rossomacha, E.; Ali, S.A.; Krawetz, R.; Haroon, N.; Perruccio, A.V.; et al. MicroRNA-181a-5p Antisense Oligonucleotides Attenuate Osteoarthritis in Facet and Knee Joints. Ann. Rheum. Dis. 2019, 78, 111–121. [Google Scholar] [CrossRef]
- Lian, W.-S.; Ko, J.-Y.; Wu, R.-W.; Sun, Y.-C.; Chen, Y.-S.; Wu, S.-L.; Weng, L.-H.; Jahr, H.; Wang, F.-S. Mi-croRNA-128a Represses Chondrocyte Autophagy and Exacerbates Knee Osteoarthritis by Disrupting Atg12. Cell Death Dis. 2018, 9, 919. [Google Scholar] [CrossRef]
- Baek, D.; Lee, K.-M.; Park, K.W.; Suh, J.W.; Choi, S.M.; Park, K.H.; Lee, J.W.; Kim, S.-H. Inhibition of MiR-449a Promotes Cartilage Regeneration and Prevents Progression of Osteoarthritis in In Vivo Rat Models. Mol. Ther. Nucleic Acids 2018, 13, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Zhang, X.; Shao, Z.; Zhao, F.; Hu, X.; Ao, Y. Intra-Articular Delivery of Anti-Hif-2α SiRNA by Chondro-cyte-Homing Nanoparticles to Prevent Cartilage Degeneration in Arthritic Mice. Gene Ther. 2015, 22, 439–448. [Google Scholar] [CrossRef]
- Hoshi, H.; Akagi, R.; Yamaguchi, S.; Muramatsu, Y.; Akatsu, Y.; Yamamoto, Y.; Sasaki, T.; Takahashi, K.; Sasho, T. Effect of Inhibiting MMP13 and ADAMTS5 by Intra-Articular Injection of Small Interfering RNA in a Sur-gically Induced Osteoarthritis Model of Mice. Cell Tissue Res. 2017, 368, 379–387. [Google Scholar] [CrossRef]
- Nakagawa, R.; Akagi, R.; Yamaguchi, S.; Enomoto, T.; Sato, Y.; Kimura, S.; Ogawa, Y.; Sadamasu, A.; Ohtori, S.; Sasho, T. Single vs. Repeated Matrix Metalloproteinase-13 Knockdown with Intra-Articular Short Interfering RNA Administration in a Murine Osteoarthritis Model. Connect. Tissue Res. 2019, 60, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, S.-J.; Liu, R.; Zhan, J.-F.; Tan, C.; Fang, Y.-F.; Chen, Y.; Yu, B. Inhibition of YAP with SiRNA Pre-vents Cartilage Degradation and Ameliorates Osteoarthritis Development. J. Mol. Med. 2019, 97, 103–114. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Pang, Y.; Wang, J.; Wan, Y.; Zhu, C.; Yin, Z. Abnormal Thyroid Hormone Receptor Signaling in Osteoarthritic Osteoblasts Regulates Microangiogenesis in Subchondral Bone. Life Sci. 2019, 239, 116975. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Zhang, H.; Gao, W.; Lu, M.; Liu, W.; Li, Y.; Yin, Z. Forkhead Box C1 Promotes the Pathology of Osteoarthritis by Upregulating β-Catenin in Synovial Fibroblasts. FEBS J. 2020, 287, 3065–3087. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, Y.; Nakasa, T.; Shoji, T.; Hamanishi, M.; Shimizu, R.; Kamei, N.; Usman, M.A.; Ochi, M. In-tra-Articular Injection of Synthetic MicroRNA-210 Accelerates Avascular Meniscal Healing in Rat Medial Meniscal Injured Model. Arthritis Res. Ther. 2014, 16. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Z.; Pan, Y.; Ma, J.; Miao, X.; Qi, X.; Zhou, H.; Jia, L. MiR-26a and MiR-26b Mediate Osteoarthritis Progression by Targeting FUT4 via NF-ΚB Signaling Pathway. Int. J. Biochem. Cell Biol. 2018, 94, 79–88. [Google Scholar] [CrossRef]
- Hu, G.; Zhao, X.; Wang, C.; Geng, Y.; Zhao, J.; Xu, J.; Zuo, B.; Zhao, C.; Wang, C.; Zhang, X. MicroRNA-145 At-tenuates TNF-α-Driven Cartilage Matrix Degradation in Osteoarthritis via Direct Suppression of MKK4. Cell Death Dis. 2017, 8, e3140. [Google Scholar] [CrossRef]
- Si, H.-B.; Zeng, Y.; Liu, S.-Y.; Zhou, Z.-K.; Chen, Y.-N.; Cheng, J.-Q.; Lu, Y.-R.; Shen, B. Intra-Articular Injection of MicroRNA-140 (MiRNA-140) Alleviates Osteoarthritis (OA) Progression by Modulating Extracellular Ma-trix (ECM) Homeostasis in Rats. Osteoarthr. Cartil. 2017, 25, 1698–1707. [Google Scholar] [CrossRef]
- Brown, S.; Kumar, S.; Sharma, B. Intra-Articular Targeting of Nanomaterials for the Treatment of Osteoarthri-tis. Acta Biomater. 2019, 93, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Almagro, J.C.; Daniels-Wells, T.R.; Perez-Tapia, S.M.; Penichet, M.L. Progress and Challenges in the Design and Clinical Development of Antibodies for Cancer Therapy. Front. Immunol. 2017, 8, 1751. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The Impact of PEGylation on Biological Therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Y.; Wu, L.; Maharjan, A.; Regmi, B.; Zhang, J.; Gui, S. In Situ Hexagonal Liquid Crystal for In-tra-Articular Delivery of Sinomenine Hydrochloride. Biomed. Pharmacother. 2019, 117, 108993. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in Oligonucleotide Drug Delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Geary, R.S.; Norris, D.; Yu, R.; Bennett, C.F. Pharmacokinetics, Biodistribution and Cell Uptake of Antisense Oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 46–51. [Google Scholar] [CrossRef]
- Zhang, X.; Prasadam, I.; Fang, W.; Crawford, R.; Xiao, Y. Chondromodulin-1 Ameliorates Osteoarthritis Pro-gression by Inhibiting HIF-2α Activity. Osteoarthr. Cartil. 2016, 24, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; You, H.; Yuan, X.; Zhao, W.; Li, W.; Guo, X. Protective Effect of Lentivirus-Mediated SiRNA Targeting ADAMTS-5 on Cartilage Degradation in a Rat Model of Osteoarthritis. Int. J. Mol. Med. 2013, 31, 1222–1228. [Google Scholar] [CrossRef]
- Ji, Q.; Xu, X.; Kang, L.; Xu, Y.; Xiao, J.; Goodman, S.B.; Zhu, X.; Li, W.; Liu, J.; Gao, X.; et al. Hematopoietic PBX-Interacting Protein Mediates Cartilage Degeneration during the Pathogenesis of Osteoarthritis. Nat. Commun. 2019, 10, 313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Crawford, R.; Xiao, Y. Inhibition of Vascular Endothelial Growth Factor with ShRNA in Chondro-cytes Ameliorates Osteoarthritis. J. Mol. Med. (Berl.) 2016, 94, 787–798. [Google Scholar] [CrossRef]
- Peng, J.-S.; Chen, S.-Y.; Wu, C.-L.; Chong, H.-E.; Ding, Y.-C.; Shiau, A.-L.; Wang, C.-R. Amelioration of Experi-mental Autoimmune Arthritis Through Targeting of Synovial Fibroblasts by Intraarticular Delivery of Mi-croRNAs 140-3p and 140-5p. Arthritis Rheumatol. 2016, 68, 370–381. [Google Scholar] [CrossRef]
- Song, J.; Kim, D.; Chun, C.-H.; Jin, E.-J. MiR-370 and MiR-373 Regulate the Pathogenesis of Osteoarthritis by Modulating One-Carbon Metabolism via SHMT-2 and MECP-2, Respectively. Aging Cell 2015, 14, 826–837. [Google Scholar] [CrossRef]
- Yan, S.; Wang, M.; Zhao, J.; Zhang, H.; Zhou, C.; Jin, L.; Zhang, Y.; Qiu, X.; Ma, B.; Fan, Q. MicroRNA-34a Af-fects Chondrocyte Apoptosis and Proliferation by Targeting the SIRT1/P53 Signaling Pathway during the Pathogenesis of Osteoarthritis. Int. J. Mol. Med. 2016, 38, 201–209. [Google Scholar] [CrossRef]
- Anguela, X.M.; High, K.A. Entering the Modern Era of Gene Therapy. Annu. Rev. Med. 2019, 70, 273–288. [Google Scholar] [CrossRef]
- Brown, T.D.; Johnston, R.C.; Saltzman, C.L.; Marsh, J.L.; Buckwalter, J.A. Posttraumatic Osteoarthritis: A First Estimate of Incidence, Prevalence, and Burden of Disease. J. Orthop. Trauma 2006, 20, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal Models of Osteoarthritis: Classification, Up-date, and Measurement of Outcomes. J. Orthop. Surg. Res. 2016, 11. [Google Scholar] [CrossRef]
- Bijlsma, J.W.J.; Berenbaum, F.; Lafeber, F.P.J.G. Osteoarthritis: An Update with Relevance for Clinical Practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Juras, V.; Chang, G.; Regatte, R.R. Current Status of Functional MRI of Osteoarthritis for Diagnosis and Prog-nosis. Curr. Opin. Rheumatol. 2020, 32, 102–109. [Google Scholar] [CrossRef]
| Protein | OA Animal Models | References | |
|---|---|---|---|
| GH | Growth hormone | Rat TMJ-MIA model | [44] |
| Rabbit collagenase injection model | [45] | ||
| HB-IGF-1 | Humanized insulin like growth factor-1 fusion protein with a heparin-binding domain for targeting to cartilage | Rat MMx model | [46] |
| FzD7 CRD | Recombinant-Frizzled 7-cysteine-rich domain designed to inhibit Wnt3a/β-catenin signaling | Mouse DMM model | [47] |
| rhMidkine | rhMidkine | Mouse DMM model | [48] |
| IL4-10 FP | A fusion protein, the biological activity of IL-4 and IL-10 are preserved | Canine groove model | [49,50] |
| Sclerostin | Mouse tibial compression OA injury model | [51] | |
| Atsttrin | An engineered protein composed of three tumor necrosis factor receptor (TNFR)-binding fragments of progranulin (PGRN) | Rat noninvasive ACL rupture model mouse ACLT model | [52] |
| rhGDF5 | rh growth differentiation factor-5 | Rat MMx model | [53] |
| rhPRG4 | rh lubricin | Yucatan minipigs DMM model | [54] |
| Rat ACLT model | [55] | ||
| CRB0017 | recombinant monoclonal antibodies directed against ADAMTS5 | STR/ort | [56] |
| Mode of Action | Oligonucleotide | Target Gene(s) | Outcomes | References |
|---|---|---|---|---|
| miRNA inhibitor | miRNA inhibitors antisense oligonucleotide | miR-141/200c | Recover SIRT1/modify IL-6/STAT3 pathway/prevent OA in mouse DMM model | [102] |
| miR-203 | Recover Erα/decrease cartilage degradation in postmenopausal OA rats | [103] | ||
| miR-21-5p | Recover FGF18/attenuate the severity of OA in the mouse DMM model | [104] | ||
| miR-34-5a | Protect cartilage in the DMM and high-fat diet/DMM mice | [105] | ||
| miR-146b | Recover α2-macroglobulin/ prevent OA in mouse DMM model | [106] | ||
| miR-181a-5p | Attenuate cartilage destruction, hypertrophic, apoptotic/cell death, and type II collagen breakdown markers in mouse DMM model | [107] | ||
| miR-128a | Recover ATG12/slow articular tissue destruction in rat ACLT model | [108] | ||
| miR-449a | Recover SIRT 1/Prevent cartilage degradation in rat DMM model | [109] | ||
| mRNA inhibition | siRNA for target genes | Hif-2α | Prevent cartilage degeneration in ACLT/DMM mice | [110] |
| Mmp13 | Delay cartilage degradation in mouse DMM model | [111,112] | ||
| Yap | Ameliorate OA development and reduce subchondral bone formation in ACLT mice | [113] | ||
| Thr | Reduce angiogenic activities in subchondral bone ameliorated cartilage degradation in mouse DMM model | [114] | ||
| FoxC1 | Decrease β-catenin, ADAMTS-5, fibronectin, MMP3, and MMP13/decrease cartilage destruction in mouse DMM model | [115] | ||
| miRNA supplement | miR-210 mimic | Not mentioned | Upregulate Col2a1 expression in the meniscus cells and VEGF and FGF2 expression in the synovial cell/enhance repair of the meniscus and prevent cartilage degeneration in rat DMM model | [116] |
| miR-26a/26b mimic | Fut4 | Promote chondrocytes proliferation and inhibit apoptosis/attenuate OA progression in rat ACLT-MMx model | [117] | |
| miR-145 mimic | Mkk4 | Suppress the expression of MMP-3 and MMP-13, as well as p-MKK4, p-c-Jun, and p-ATF2/reduce cartilage destruction in rat MCLT-DMM model | [118] | |
| miR-140 mimic | Not mentioned | Reduce pathological scores and MMP-13 and ADAMTS-5 expression in rat ACLT-MMx model | [119] |
| Protein Therapeutics | Gene Therapy | Oligonucleotide Therapeutics | |
|---|---|---|---|
| Mechanism of action | Supply the required protein | Transduce target gene in cells Gene expression and translation is needed | Transfer to target cells Modulate the function or the fate of target mRNA |
| Application range of targets | For proteins that act extracellularly | Limitation in size of gene | Oligonucleotide sequence can be designed without off target effect |
| Delivery and distribution | Distribute to whole joint by IA | AAV vector provide cell type specific gene transfer | Drug delivery system for target need to be established |
| Retention time | Short Rapidly excreted from the joint | Vector: Continuous expression can be expected when transfected cells and vector retained mRNA: transient protein production | Effect will continue as long as oligonucleotide remain in cytosol |
| Control dosage and time | Possible | Amount of protein depend on transfection efficiency, host cell activity and etc. Promotor design provide regulated induction of protein | Possible |
| Relative manufacturing cost | High Biological manufacturing | High Biological manufacturing | Low Chemical synthesis” |
| Technical establishment and Safety concerns | Established Predictable | Approved mainly on life threatening disease Remain unknown risks? | Approved mainly in specific genetical disorder Remain unknown risks? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyoda, E.; Maehara, M.; Watanabe, M.; Sato, M. Candidates for Intra-Articular Administration Therapeutics and Therapies of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 3594. https://doi.org/10.3390/ijms22073594
Toyoda E, Maehara M, Watanabe M, Sato M. Candidates for Intra-Articular Administration Therapeutics and Therapies of Osteoarthritis. International Journal of Molecular Sciences. 2021; 22(7):3594. https://doi.org/10.3390/ijms22073594
Chicago/Turabian StyleToyoda, Eriko, Miki Maehara, Masahiko Watanabe, and Masato Sato. 2021. "Candidates for Intra-Articular Administration Therapeutics and Therapies of Osteoarthritis" International Journal of Molecular Sciences 22, no. 7: 3594. https://doi.org/10.3390/ijms22073594
APA StyleToyoda, E., Maehara, M., Watanabe, M., & Sato, M. (2021). Candidates for Intra-Articular Administration Therapeutics and Therapies of Osteoarthritis. International Journal of Molecular Sciences, 22(7), 3594. https://doi.org/10.3390/ijms22073594







