Molecular and Immunological Identification of Low Allergenic Fruits among Old and New Apple Varieties
Abstract
1. Introduction
2. Results
2.1. Mal d 1
2.2. Mal d 1.06A
2.3. Mal d 1.01
2.4. Mal d 1.06A vs. Mal d 1.01
2.5. Mal d 1 Protein
2.6. Mal d 2.01
2.7. Mal d 3.01
2.8. Mal d 4.01
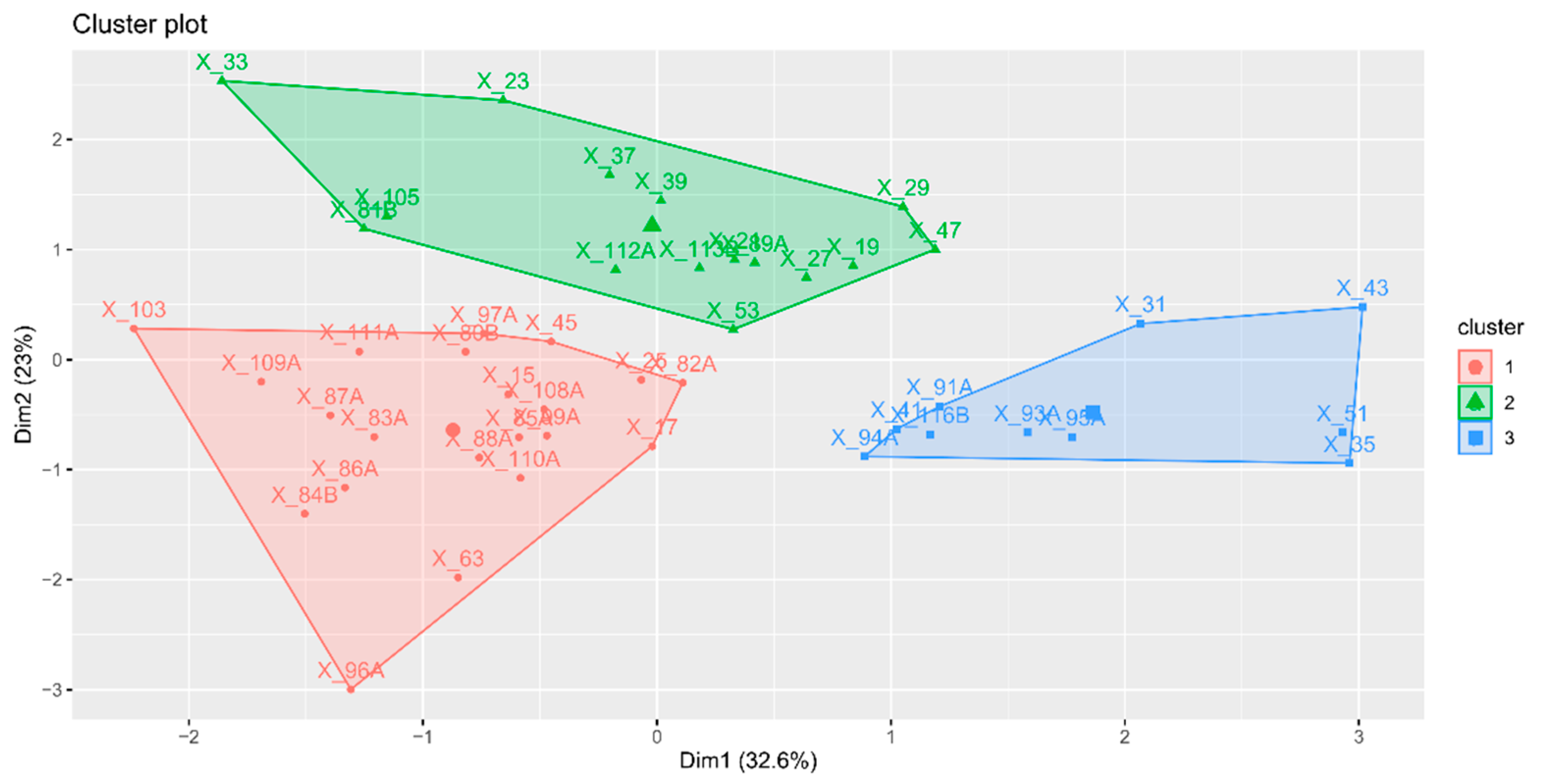
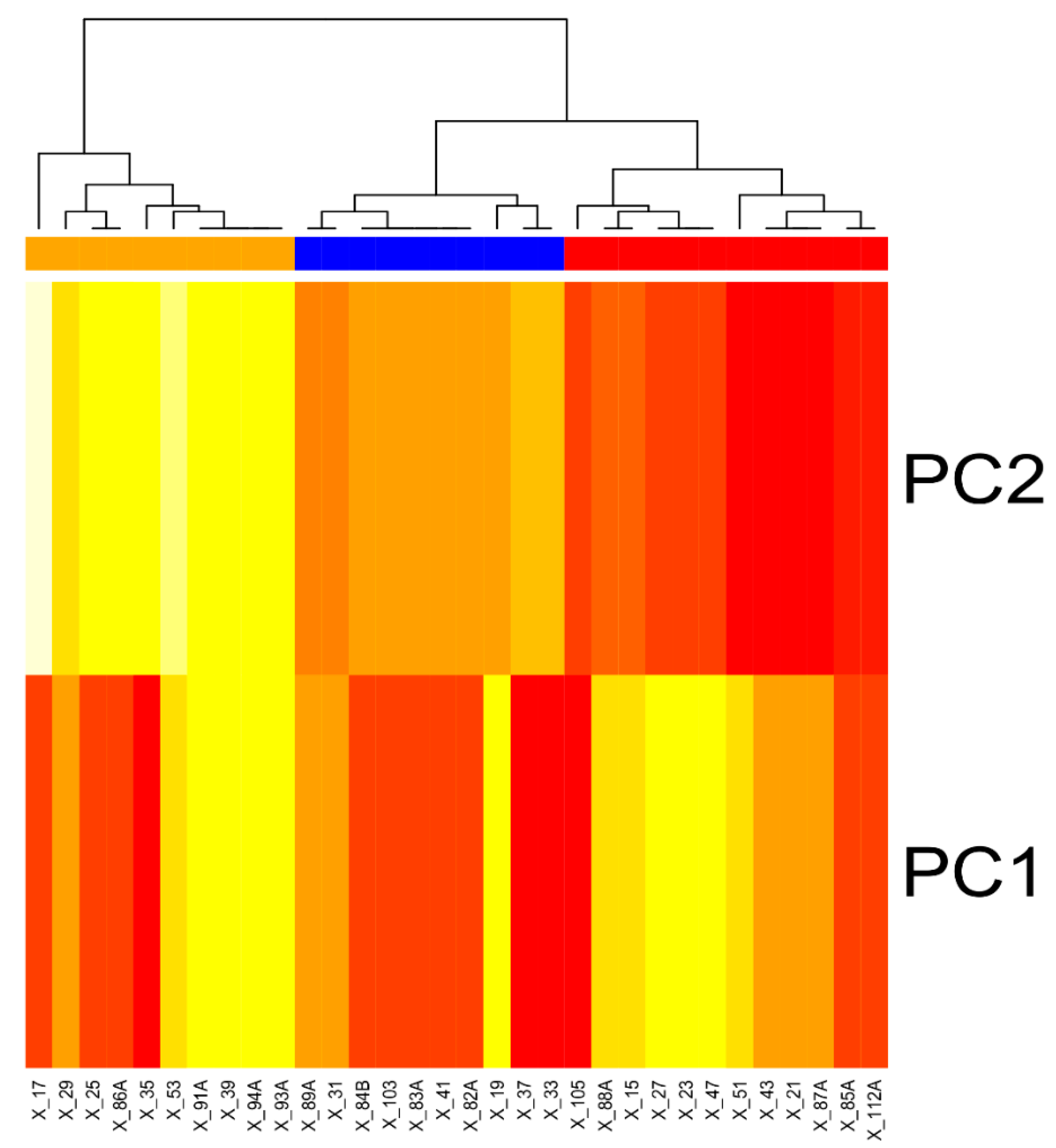
2.9. Hierarchical Classification on Principal Components (HCPC)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Human Samples
4.3. RNA Extraction
4.4. cDNA Synthesis
4.5. Gene Expression
4.6. Extraction of Apple Proteins
4.7. Protein Slot Blotting
4.8. ELISA
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, A.K.G.; Venkatesh, Y.P. An overview of fruit allergy and the causative allergens. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 180–187. [Google Scholar]
- Gao, Z.; Weg, E.W.V.; De Matos, C.I.; Arens, P.; Bolhaar, S.T.H.P.; Knulst, A.C.; Li, Y.; Hoffmann-Sommergruber, K.; Gilissen, L.J.W.J. Assessment of allelic diversity in intron-containing Mal d 1 genes and their association to apple allergenicity. BMC Plant Biol. 2008. [Google Scholar] [CrossRef]
- WHO/IUIS Allergen Nomenclature Home Page. Available online: http://www.allergen.org/ (accessed on 8 July 2020).
- Ebner, C.; Birkner, T.; Valenta, R.; Rumpold, H.; Breitenbach, M.; Scheiner, O.; Kraft, D. Common epitopes of birch pollen and apples-Studies by western and northern blot. J. Allergy Clin. Immunol. 1991. [Google Scholar] [CrossRef]
- Sancho, A.I.; Foxall, R.; Rigby, N.M.; Browne, T.; Zuidmeer, L.; Van Ree, R.; Waldron, K.W.; Mills, E.N.C. Maturity and storage influence on the apple (Malus domestica) allergen Mal d 3, a nonspecific lipid transfer protein. J. Agric. Food Chem. 2006. [Google Scholar] [CrossRef]
- Gao, Z.S.; Van De Weg, W.E.; Schaart, J.G.; Van Arkel, G.; Breiteneder, H.; Hoffmann-Sommergruber, K.; Gilissen, L.J.W.J. Genomic characterization and linkage mapping of the apple allergen genes Mal d 2 (thaumatin-like protein) and Mal d 4 (profilin). Theor. Appl. Genet. 2005. [Google Scholar] [CrossRef]
- Botton, A.; Lezzer, P.; Dorigoni, A.; Barcaccia, G.; Ruperti, B.; Ramina, A. Genetic and environmental factors affecting allergen-related gene expression in apple fruit (Malus domestica L. Borkh). J. Agric. Food Chem. 2008. [Google Scholar] [CrossRef] [PubMed]
- Bolhaar, S.T.H.P.; Van De Weg, W.E.; Van Ree, R.; Gonzalez-Mancebo, E.; Zuidmeer, L.; Bruijnzeel-Koomen, C.A.F.M.; Fernandez-Rivas, M.; Jansen, J.; Hoffmann-Sommergruber, K.; Knulst, A.C.; et al. In vivo assessment with prick-to-prick testing and double-blind, placebo-controlled food challenge of allergenicity of apple cultivars. J. Allergy Clin. Immunol. 2005. [Google Scholar] [CrossRef]
- Matthes, A.; Schmitz-Eiberger, M. Apple (Malus domestica L. Borkh.) allergen Mal d 1: Effect of cultivar, cultivation system, and storage conditions. J. Agric. Food Chem. 2009. [Google Scholar] [CrossRef] [PubMed]
- Kootstra, H.S.; Vlieg-Boerstra, B.J.; Dubois, A.E.J. Assessment of the reduced allergenic properties of the Santana apple. Ann. Allergy Asthma Immunol. 2007. [Google Scholar] [CrossRef]
- Vieths, S.; Jankiewicz, A.; Schöning, B.; Aulepp, H. Apple allergy: The IgE-binding potency of apple strains is related to the occurrence of the 18-kDa allergen. Allergy 1994, 49, 262–271. [Google Scholar] [CrossRef]
- Pagliarani, G.; Paris, R.; Arens, P.; Tartarini, S.; Ricci, G.; Smulders, M.M.J.; van de Weg, W.E. A qRT-PCR assay for the expression of all Mal d 1 isoallergen genes. BMC Plant Biol. 2013. [Google Scholar] [CrossRef]
- Schmitz-Eiberger, M.; Matthes, A. Effect of harvest maturity, duration of storage and shelf life of apples on the allergen Mal d 1, polyphenoloxidase activity and polyphenol content. Food Chem. 2011. [Google Scholar] [CrossRef]
- Pühringer, H.; Moll, D.; Hoffmann-Sommergruber, K.; Watillon, B.; Katinger, H.; Da Câmara Machado, M.L. The promoter of an apple Ypr10 gene, encoding the major allergen Mal d 1, is stress- and pathogen-inducible. Plant Sci. 2000, 152, 35–50. [Google Scholar] [CrossRef]
- Yang, X.T.; Song, J.; Campbell-Palmer, L.; Walker, B.; Zhang, Z. Allergen related gene expression in apple fruit is differentially controlled by ethylene during ripening. Postharvest Biol. Technol. 2012. [Google Scholar] [CrossRef]
- Beuning, L.L.; Bowen, J.H.; Persson, H.A.; Barraclough, D.; Bulley, S.; MacRae, E.A. Characterisation of Mal d 1-related genes in Malus. Plant Mol. Biol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Gau, A.E.; Koutb, M.; Pitrowski, M.; Kloppstech, K. Accumulation of pathogenesis-related proteins in the apoplast of a susceptible cultivar of apple (Malus domestica cv. Elstar) after infection by Venturia inaequalis and constitutive expression of PR genes in the resistant cultivar Remo. Eur. J. Plant Pathol. 2004, 110, 703–711. [Google Scholar] [CrossRef]
- Hsieh, L.S.; Moos, M.; Lin, Y. Characterization of apple 18 and 31 kD allergens by microsequencing and evaluation of their content during storage and ripening. J. Allergy Clin. Immunol. 1995, 96, 960–970. [Google Scholar] [CrossRef]
- Borges, J.P.; Jauneau, A.; Brulé, C.; Culerrier, R.; Barre, A.; Didier, A.; Rougé, P. The lipid transfer proteins (LTP) essentially concentrate in the skin of Rosaceae fruits as cell surface exposed allergens. Plant Physiol. Biochem. 2006. [Google Scholar] [CrossRef]
- Kader, J.C. Lipid-transfer proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996. [Google Scholar] [CrossRef]
- Fernández-Rivas, M.; Bolhaar, S.; González-Mancebo, E.; Asero, R.; van Leeuwen, A.; Bohle, B.; Ma, Y.; Ebner, C.; Rigby, N.; Sancho, A.I.; et al. Apple allergy across Europe: How allergen sensitization profiles determine the clinical expression of allergies to plant foods. J. Allergy Clin. Immunol. 2006. [Google Scholar] [CrossRef]
- Machesky, L.M.; Poland, T.D. Profilin as a potential mediator of membrane-cytoskeleton communication. Trends Cell Biol. 1993. [Google Scholar] [CrossRef]
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahlstrom, J.; Lindner, T.; Sigurdardottir, S.T.; et al. The prevalence of plant food allergies: A systematic review. J. Allergy Clin. Immunol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Zuidmeer, L.; Van Leeuwen, W.A.; Budde, I.K.; Breiteneder, H.; Ma, Y.; Mills, C.; Sancho, A.I.; Meulenbroek, E.J.; Van De Weg, E.; Gilissen, L.; et al. Allergenicity assessment of apple cultivars: Hurdles in quantifying labile fruit allergens. Int. Arch. Allergy Immunol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Marzban, G.; Puehringer, H.; Dey, R.; Brynda, S.; Ma, Y.; Martinelli, A.; Zaccarini, M.; Van Der Weg, E.; Housley, Z.; Kolarich, D.; et al. Localisation and distribution of the major allergens in apple fruits. Plant Sci. 2005. [Google Scholar] [CrossRef]
- Vlieg-Boerstra, B.J.; Van De Weg, W.E.; Van Der Heide, S.; Kerkhof, M.; Arens, P.; Heijerman-Peppelman, G.; Dubois, A.E.J. Identification of low allergenic apple cultivars using skin prick tests and oral food challenges. Allergy Eur. J. Allergy Clin. Immunol. 2011. [Google Scholar] [CrossRef]
- Shenk, M.F.; Maas, M.P.; van der Smulders, M.J.M.; Gilissen, L.J.W.J.; Fischer, A.R.H.; Lans, I.A. van der Jacobsen, E.; Frewer, L.J. Consumer attitudes towards hypoallergenic apples that alleviate mild apple allergy. Food Qual. Prefer. 2011, 22, 83–91. [Google Scholar] [CrossRef]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An optimised grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006. [Google Scholar] [CrossRef]
- Gao, Z.S.; Van De Weg, W.E.; Schaart, J.G.; Schouten, H.J.; Tran, D.H.; Kodde, L.P.; Van Der Meer, I.M.; Van Der Geest, A.H.M.; Kodde, J.; Breiteneder, H.; et al. Genomic cloning and linkage mapping of the Mal d 1 (PR-10) gene family in apple (Malus domestica). Theor. Appl. Genet. 2005. [Google Scholar] [CrossRef]
- Wiersma, P.A.; Zhang, H.; Lu, C.; Quail, A.; Toivonen, P.M.A. Survey of the expression of genes for ethylene synthesis and perception during maturation and ripening of “Sunrise” and “Golden Delicious” apple fruit. Postharvest Biol. Technol. 2007. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Lekanne Deprez, R.H.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003. [Google Scholar] [CrossRef]
- Meng, C.; Helm, D.; Frejno, M.; Kuster, B. moCluster: Identifying Joint Patterns Across Multiple Omics Data Sets. J. Proteome Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R.; Walther, G.; Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Ser. B Stat. Methodol. 2001. [Google Scholar] [CrossRef]
| Old Cultivars | New Cultivars | |||||
|---|---|---|---|---|---|---|
| Tissue | Gene | Organic | All New | Organic | Intensive | Integrated |
| Flesh | Mal d 1.06A | 3.43 | 2.90 | 2.74 | 3.04 | 2.99 |
| Mal d 1.01 | 3.61 | 3.37 | 3.44 (g) | 3.38 | 2.95 (g) | |
| Mal d 2.01 | 4.12 (b,d,p) | 3.69 (d) | 3.35 (a,b,c) | 4.01 (c) | 3.66 (a) | |
| Mal d 3.01 | 3.05 (e) | 2.88 | 3.13 | 2.77 | 2.61 | |
| Mal d 4.01 | 3.30 | 3.23 | 3.29 | 3.2 | 2.96 | |
| Skin | Mal d 1.06A | 3.90 (h) | 3.33 | 3.40 | 3.51 | 2.93 |
| Mal d 1.01 | 4.32 (h) | 3.78 | 3.91 | 4.01 | 2.9 | |
| Mal d 2.01 | 3.56 (p) | 3.31 | 3.05 | 3.67 | 2.96 | |
| Mal d 3.01 | 4.97 (e) | 4.54 | 4.94 | 4.96 | 3.02 | |
| Mal d 4.01 | 2.81 | 2.89 | 2.85 | 2.93 | 2.78 | |
| Average | Mal d 1.06A | 3.67 (f,l) | 3.12 (l) | 3.07 | 3.28 | 2.96 |
| Mal d 1.01 | 3.97 (f,n) | 3.57 (n) | 3.68 (m) | 3.70 | 2.93 (m) | |
| Mal d 2.01 | 3.84 (j,k) | 3.50 (j) | 3.20 (i,k) | 3.84 (i) | 3.31 | |
| Mal d 3.01 | 3.99 | 3.71 | 4.04 (r) | 3.86 (r) | 2.81 (r) | |
| Mal d 4.01 | 3.05 | 3.06 | 3.07 | 3.10 | 2.87 | |
| Apple Cultivars | Mal d 1 Content (µg/g FW 1) | |
|---|---|---|
| Old organic farming | Kantówka Gdańska | 0.3 |
| Kosztela | 0.6 | |
| Antonovka Usual | 1.8 | |
| Sztetyna | 1.9 | |
| Rumianka from Alma-Ata | 2 | |
| Reinette Coulon | 2.5 | |
| Żeleźniak | 3.5 | |
| Kandil Sinap | 4.6 | |
| Bukówka | 7.3 | |
| Emperor Alexander Apple | 7.6 | |
| Jonathan | 7.7 | |
| Antonovka One and a Half Pound | 7.7 | |
| Grochówka | 8.2 | |
| Oberland Raspberry Apple | 10 | |
| Winter Banana | 12.9 | |
| Jakub Lebel | 17.5 | |
| Gloria Mundi | 20.9 | |
| Gray French Reinette | 23.5 | |
| Reinette de Canada | 28.8 | |
| Berner Rose | 37.7 | |
| Median | 7.65 | |
| New organic farming | Gala | 1.3 |
| Golden Delicious | 1.8 | |
| Jonagored | 5.8 | |
| Idared | 6 | |
| Santana | 8.5 | |
| Trinity I (x Gold Millennium) | 12.7 | |
| Gold Millennium | 13.2 | |
| Trinity II (x Ligol) | 15 | |
| Median | 7.25 | |
| New intensive farming | Gold Millennium | 2.3 |
| Gala | 2.4 | |
| Idared | 2.4 | |
| Golden Delicious | 5.8 | |
| Jonagored | 9.5 | |
| Trinity | 13.6 | |
| Median | 4.1 | |
| Median in new | 5.9 | |
| Mal d 1.06A | Mal d 1.01 | Mal d 2.01 | Mal d 3.01 | Mal d 4.01 | |
|---|---|---|---|---|---|
| Pearson’s r | 0.5211 | 0.6129 | 0.3461 | 0.4012 | 0.6175 |
| p-value | 0.038 | 0.012 | 0.189 | 0.123 | 0.011 |
| Mal d1 (µg/g FW 1) | Serum 1 | Serum 2 | Serum 3 | Serum 4 | ||
|---|---|---|---|---|---|---|
| Expression of Mal d 1.06A | Pearson’s r coeff. | 0.38 | 0.4 | 0.34 | 0.4 | 0.37 |
| p-value | 0.036 | 0.027 | ns | 0.028 | 0.046 | |
| Mal d1 (µg/g FW) | Pearson’s r coeff. | - | 0.24 | 0.25 | 0.22 | 0.39 |
| p-value | - | ns | ns | ns | 0.031 |
| Type of Varieties | Sample Name | Cultivar Name | Cultivation Method | Sample Origin |
|---|---|---|---|---|
| Old | X_15 | Rumianka from Alma-Ata | organic | Bolestraszyce |
| X_17 | Sztetyna | organic | Bolestraszyce | |
| X_19 | Gloria Mundi | organic | Bolestraszyce | |
| X_21 | Kosztela | organic | Bolestraszyce | |
| X_23 | Reinette Coulon | organic | Bolestraszyce | |
| X_25 | Emperor Alexander Apple | organic | Bolestraszyce | |
| X_27 | Kantówka Gdańska (Danzinger Kantapfel) | organic | Bolestraszyce | |
| X_29 | Żeleźniak (Rother Eiserapfel) | organic | Bolestraszyce | |
| X_31 | Jonathan | organic | Bolestraszyce | |
| X_33 | Reinette de Canada | organic | Bolestraszyce | |
| X_35 | Oberland Raspberry Apple (Callville d’Automne Raye) | organic | Bolestraszyce | |
| X_37 | Bukówka | organic | Bolestraszyce | |
| X_39 | Jakub Lebel | organic | Bolestraszyce | |
| X_41 | Winter Banana | organic | Bolestraszyce | |
| X_43 | Kandil Sinap | organic | Bolestraszyce | |
| X_45 | Parker’s Pippin | organic | Bolestraszyce | |
| X_47 | Gray French Reinette | organic | Bolestraszyce | |
| X_51 | Grochówka (Grosser Bohnapfel) | organic | Bolestraszyce | |
| X_53 | Berner Rose | organic | Bolestraszyce | |
| X_103 | Antonovka Usual | organic | Bolestraszyce | |
| X_105 | Antonovka One and a Half Pound | organic | Bolestraszyce | |
| Modern | X_80B | Jonagored | organic | Wojciechow |
| X_81B | Jonagored | intensive | Wojciechow | |
| X_82A | Gold Millennium | organic | Wojciechow | |
| X_83A | Gold Millennium | intensive | Wojciechow | |
| X_84B | Gala | organic | Wojciechow | |
| X_85A | Gala | intensive | Wojciechow | |
| X_86A | Idared | organic | Wojciechow | |
| X_87A | Idared | intensive | Wojciechow | |
| X_88A | Golden Delicious | organic | Wojciechow | |
| X_89A | Golden Delicious | intensive | Wojciechow | |
| X_91A | Trinity | intensive | Wojciechow | |
| X_93A | Trinity I (× Gold Millennium) | organic | Wojciechow | |
| X_94A | Trinity II (× Ligol) | organic | Wojciechow | |
| X_96A | Idared | integrated | Brzezna | |
| X_97A | Gala | integrated | Brzezna | |
| X_99A | Golden Delicious | integrated | Brzezna | |
| X_108 | Golden Delicious 2 | organic | Wojciechow | |
| X_109 | Golden Delicious 2 | intensive | Wojciechow | |
| X_110 | Idared 2 | intensive | Wojciechow | |
| X_111A | Idared 2 | organic | Wojciechow | |
| X_112A | Santana | organic | Bielsko-Biała | |
| X_113B | Golden Delicious 2 | integrated | Brzezna |
| Gene | Primers | Sequence (5′–3′) | Access No. |
|---|---|---|---|
| Mal d 1.01 | Md1-1.01F | AAGCTGAAATCCTTGAAGGAA | AJ417551 |
| Md1-1.01R | GTGCTCTTCCTTGATTTCAATG | ||
| Mal d 1.06A | Mal d 1.06AF | TTGTTGCCAGATGGATGGTC | AY428580 |
| Mal d 1.06AR | TTGATGCTGACAATCTCATT | ||
| Mal d 2.01 | Mal d 2.01 F | GTGTGCCCGGCTCCACTT | AJ243427 |
| Mal d 2.01 R | TTCGAATCACCAAACGCAAG | ||
| Mal d 3.01 | Mal d3.01F | GTGACCAGCAGCCTTGCG | AF221502 |
| Mal d 3.01R | TTCAGGCAGTTGCAAGCAGT | ||
| Mal d 4.01 | Mal d 4.01F | GCTCTGGTGGCGTAACTGTG | AF129426 |
| Mal d 4.01R | CCTGGAGTCAAAGGCTCCTC | ||
| MdUBI | UBI-F | TTGATCTTTGCTGGGAAACAG | CN491263 |
| UBI-R | CACCACCATCATTCAACACC | ||
| MdActin | Actin-F | TGACCGAATGAGCAAGGAAATTACT | CN938023 |
| Actin-R | TACTCAGCTTTGGCAATCCACATC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siekierzynska, A.; Piasecka-Kwiatkowska, D.; Litwinczuk, W.; Burzynska, M.; Myszka, A.; Karpinski, P.; Zygala, E.; Piorecki, N.; Springer, E.; Sozanski, T. Molecular and Immunological Identification of Low Allergenic Fruits among Old and New Apple Varieties. Int. J. Mol. Sci. 2021, 22, 3527. https://doi.org/10.3390/ijms22073527
Siekierzynska A, Piasecka-Kwiatkowska D, Litwinczuk W, Burzynska M, Myszka A, Karpinski P, Zygala E, Piorecki N, Springer E, Sozanski T. Molecular and Immunological Identification of Low Allergenic Fruits among Old and New Apple Varieties. International Journal of Molecular Sciences. 2021; 22(7):3527. https://doi.org/10.3390/ijms22073527
Chicago/Turabian StyleSiekierzynska, Aleksandra, Dorota Piasecka-Kwiatkowska, Wojciech Litwinczuk, Marta Burzynska, Aleksander Myszka, Pawel Karpinski, Elzbieta Zygala, Narcyz Piorecki, Ewa Springer, and Tomasz Sozanski. 2021. "Molecular and Immunological Identification of Low Allergenic Fruits among Old and New Apple Varieties" International Journal of Molecular Sciences 22, no. 7: 3527. https://doi.org/10.3390/ijms22073527
APA StyleSiekierzynska, A., Piasecka-Kwiatkowska, D., Litwinczuk, W., Burzynska, M., Myszka, A., Karpinski, P., Zygala, E., Piorecki, N., Springer, E., & Sozanski, T. (2021). Molecular and Immunological Identification of Low Allergenic Fruits among Old and New Apple Varieties. International Journal of Molecular Sciences, 22(7), 3527. https://doi.org/10.3390/ijms22073527









