Systemic Administration of G-CSF Accelerates Bone Regeneration and Modulates Mobilization of Progenitor Cells in a Rat Model of Distraction Osteogenesis
Abstract
1. Introduction
2. Results
2.1. G-CSF Altered Kinetic Patterns of Mobilization of HSPCs
2.2. G-CSF Had No Effect on the Kinetic Pattern of Mobilization of EPCs
2.3. G-CSF Altered Kinetic Patterns of Mobilization of MSCs
2.4. Trend of Increase in Hydroxyapatite Deposition at 3 Weeks of Consolidation
2.5. Strong Temporal Modification of Vascularization in the Distracted Callus Induced by G-CSF
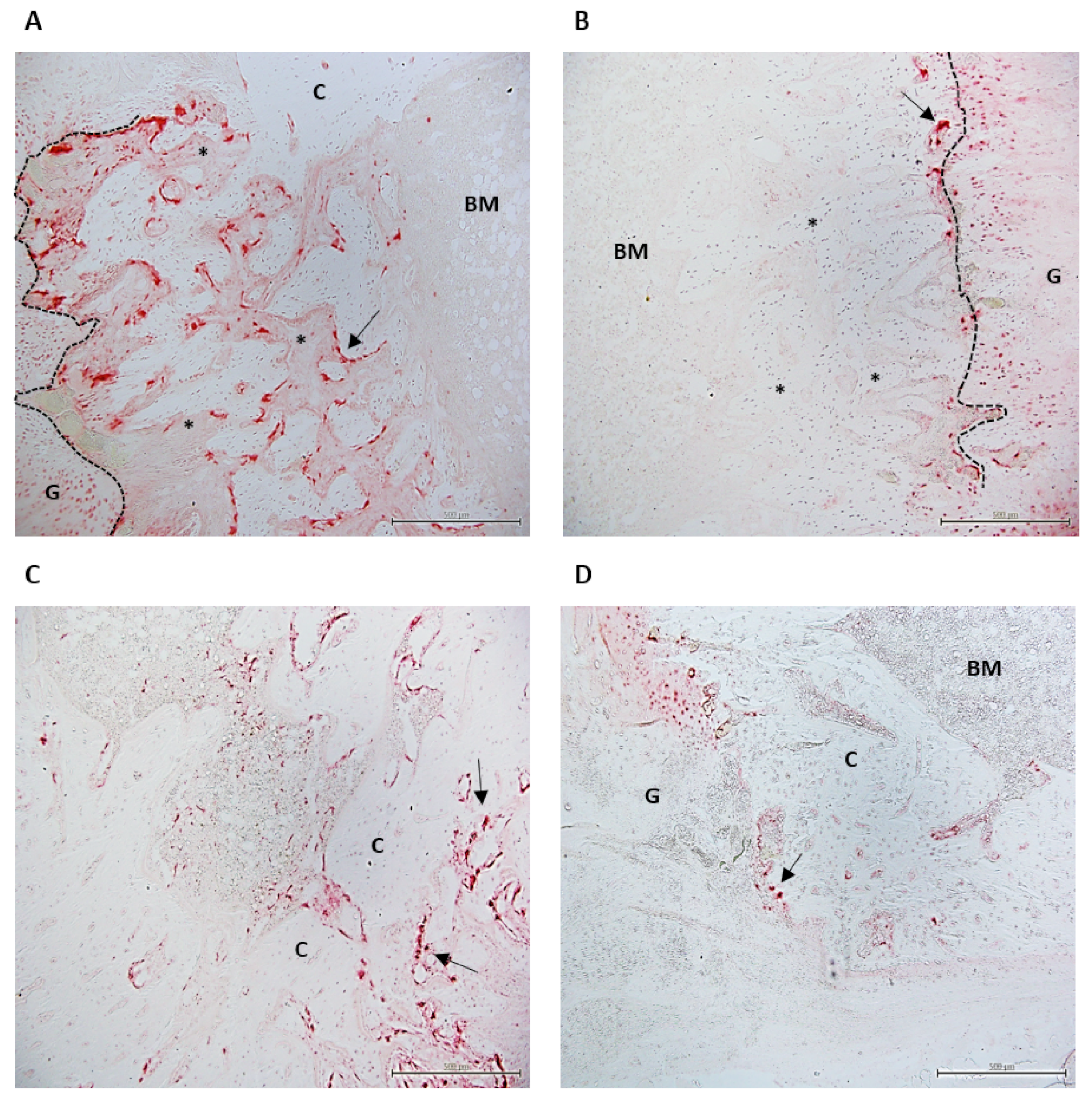
2.6. Histological Study
3. Discussion
4. Materials and Methods
4.1. Animal Model and Mobilizing Agents
4.2. Phenotype Characterization by Flow Cytometry Analysis
4.3. Longitudinal In Vivo Positron Emission Tomography Measurements
4.4. Longitudinal In Vivo Scintigraphy Analyses
4.5. Histological Analysis
4.5.1. Histomorphometric Analysis
4.5.2. TRAP Staining
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Compton, J.; Fragomen, A.; Rozbruch, S.R. Skeletal Repair in Distraction Osteogenesis: Mechanisms and Enhancements. JBJS Rev. 2015, 3, 1. [Google Scholar] [CrossRef]
- Singh, M.; Vashistha, A.; Chaudhary, M.; Kaur, G. Biological Basis of Distraction Osteogenesis—A Review. J. Oral Maxillofac. Surg. Med. and Pathol. 2016, 28. [Google Scholar] [CrossRef]
- Launay, F.; Younsi, R.; Pithioux, M.; Chabrand, P.; Bollini, G.; Jouve, J.-L. Fracture Following Lower Limb Lengthening in Children: A Series of 58 Patients. Orthop. Traumatol. Surg. Res. 2013, 99, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Lascombes, P.; Popkov, D.; Huber, H.; Haumont, T.; Journeau, P. Classification of Complications after Progressive Long Bone Lengthening: Proposal for a New Classification. Orthop. Traumatol. Surg. Res. 2012, 98, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Marsh, D.R.; Shah, S.; Elliott, J.; Kurdy, N. The Ilizarov Method in Nonunion, Malunion and Infection of Fractures. J. Bone Joint Surg. Br. 1997, 79, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Kenwright, J. Fracture after Distraction Osteogenesis. Bone Joint J. 2000, 82, 659–665. [Google Scholar] [CrossRef]
- AI-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular Mechanisms Controlling Bone Formation during Fracture Healing and Distraction Osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef]
- Aronson, J. Temporal and Spatial Increases in Blood Flow During Distraction Osteogenesis. Clin. Orthop. Relat. Res. 1994, 301, 124–131. [Google Scholar] [CrossRef]
- Li, G.; Simpson, A.H.R.W.; Kenwright, J.; Triffitt, J.T. Effect of Lengthening Rate on Angiogenesis during Distraction Osteogenesis: Angiogenesis during Distraction Osteogenesis. J. Orthop. Res. 1999, 17, 362–367. [Google Scholar] [CrossRef]
- Choi, I.H.; Chung, C.Y.; Cho, T.J.; Yoo, W.J. Angiogenesis and Mineralization during Distraction Osteogenesis. J. Korean Med. Sci. 2002, 17, 435. [Google Scholar] [CrossRef]
- Weiss, S.; Zimmermann, G.; Baumgart, R.; Kasten, P.; Bidlingmaier, M.; Henle, P. Systemic Regulation of Angiogenesis and Matrix Degradation in Bone Regeneration—Distraction Osteogenesis Compared to Rigid Fracture Healing. Bone 2005, 37, 781–790. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, D.; Ma, X.; Zhao, H.; Nie, C.; Si, Z. Successful Repair of a Critical-Sized Bone Defect in the Rat Femur with a Newly Developed External Fixator. Tohoku J. Exp. Med. 2009, 219, 115–120. [Google Scholar] [CrossRef]
- Matsubara, H.; Hogan, D.E.; Morgan, E.F.; Mortlock, D.P.; Einhorn, T.A.; Gerstenfeld, L.C. Vascular Tissues Are a Primary Source of BMP2 Expression during Bone Formation Induced by Distraction Osteogenesis. Bone 2012, 51, 168–180. [Google Scholar] [CrossRef]
- de Lageneste, O.D.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum Contains Skeletal Stem Cells with High Bone Regenerative Potential Controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Gao, B.; Fan, J.; Cheng, P.; Hu, Y.; Jie, Q.; Luo, Z.; Yang, L. Mesenchymal Progenitors Derived from Different Locations in Long Bones Display Diverse Characteristics. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.; Hürzeler, M.B.; Quiñones, C.R.; Ohlms, A.; Caffesse, R.G. Contribution of the Periosteum to Bone Formation in Guided Bone Regeneration: A Study in Monkeys. Clin. Oral Implant. Res. 2000, 11, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, K.A.; Al-Aql, Z.S.; Wan, C.; Fitch, J.L.; Stapleton, S.N.; Mason, Z.D.; Cole, R.M.; Gilbert, S.R.; Clemens, T.L.; Morgan, E.F.; et al. Bone Formation During Distraction Osteogenesis Is Dependent on Both VEGFR1 and VEGFR2 Signaling. J. Bone Miner. Res. 2008, 23, 596–609. [Google Scholar] [CrossRef]
- Dhaliwal, K.; Kunchur, R.; Farhadieh, R. Review of the Cellular and Biological Principles of Distraction Osteogenesis: An in Vivo Bioreactor Tissue Engineering Model. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, e19–e26. [Google Scholar] [CrossRef]
- Dahl, A.; Almeida-Porada, G.; Porada, C.D.; Soker, S. Peripheral Blood Stem Cells. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–333. ISBN 978-0-12-809880-6. [Google Scholar]
- Wang, L.C.; Takahashi, I.; Sasano, Y.; Sugawara, J.; Mitani, H. Osteoclastogenic Activity during Mandibular Distraction Osteogenesis. J. Dent. Res. 2005, 84, 1010–1015. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; Du, Z.; Liu, P.; Zhang, Y.; Sui, J.; Liu, Y.; Lei, D. Recruitment of Exogenous Mesenchymal Stem Cells in Mandibular Distraction Osteogenesis by the Stromal Cell-Derived Factor-1/Chemokine Receptor-4 Pathway in Rats. Br. J. Oral Maxillofac. Surg. 2013, 51, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Dimmeler, S. Endothelial Progenitor Cells: Characterization and Role in Vascular Biology. Circ. Res. 2004, 95, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kalka, C.; Masuda, H.; Chen, D.; Silver, M.; Kearney, M.; Magner, M.; Isner, J.M.; Asahara, T. Ischemia- and Cytokine-Induced Mobilization of Bone Marrow-Derived Endothelial Progenitor Cells for Neovascularization. Nat. Med. 1999, 5, 434–438. [Google Scholar] [CrossRef]
- Lee, D.Y.; Cho, T.-J.; Kim, J.A.; Lee, H.R.; Yoo, W.J.; Chung, C.Y.; Choi, I.H. Mobilization of Endothelial Progenitor Cells in Fracture Healing and Distraction Osteogenesis. Bone 2008, 42, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Cetrulo, C.L.; Knox, K.R.; Brown, D.J.; Ashinoff, R.L.; Dobryansky, M.; Ceradini, D.J.; Capla, J.M.; Chang, E.I.; Bhatt, K.A.; McCarthy, J.G.; et al. Stem Cells and Distraction Osteogenesis: Endothelial Progenitor Cells Home to the Ischemic Generate in Activation and Consolidation. Plast. Reconstr. Surg. 2005, 116, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.D.; Salim, A.; Xia, W.; Nacamuli, R.P.; Guccione, S.; Song, H.M.; Carano, R.A.; Filvaroff, E.H.; Bednarski, M.D.; Giaccia, A.J.; et al. Angiogenesis Is Required for Successful Bone Induction During Distraction Osteogenesis. J. Bone Miner. Res. 2005, 20, 1114–1124. [Google Scholar] [CrossRef]
- Pacicca, D. Expression of Angiogenic Factors during Distraction Osteogenesis. Bone 2003, 33, 889–898. [Google Scholar] [CrossRef]
- Kumar, G.; Narayan, B. The Tension-Stress Effect on the Genesis and Growth of Tissues Part I. The Influence of Stability of Fixation and Soft-Tissue Preservation. In Classic Papers in Orthopaedics; Banaszkiewicz, P.A., Kader, D.F., Eds.; Springer: London, UK, 2014; pp. 519–522. ISBN 978-1-4471-5450-1. [Google Scholar]
- Bendall, L.J.; Bradstock, K.F. G-CSF: From Granulopoietic Stimulant to Bone Marrow Stem Cell Mobilizing Agent. Cytokine Growth Factor Rev. 2014, 25, 355–367. [Google Scholar] [CrossRef]
- Lévesque, J.-P.; Hendy, J.; Takamatsu, Y.; Simmons, P.J.; Bendall, L.J. Disruption of the CXCR4/CXCL12 Chemotactic Interaction during Hematopoietic Stem Cell Mobilization Induced by GCSF or Cyclophosphamide. J. Clin. Invest. 2003, 111, 187–196. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, X.; Ou, L.; Zhou, X.; Liu, Y.; Jia, X.; Zhang, J.; Li, Y.; Kong, D. Mobilization of Mesenchymal Stem Cells by Granulocyte Colony-Stimulating Factor in Rats with Acute Myocardial Infarction. Cardiovasc. Drugs Ther. 2008, 22, 363–371. [Google Scholar] [CrossRef]
- Kim, J.; Kim, N.K.; Park, S.R.; Choi, B.H. GM-CSF Enhances Mobilization of Bone Marrow Mesenchymal Stem Cells via a CXCR4-Medicated Mechanism. Tissue Eng. Regen. Med. 2019, 16, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.M.; Paul, J.D.; Hill, J.M.; Thompson, M.; Benjamin, M.; Rodrigo, M.; McCoy, J.P.; Read, E.J.; Khuu, H.M.; Leitman, S.F.; et al. Granulocyte Colony-Stimulating Factor Mobilizes Functional Endothelial Progenitor Cells in Patients With Coronary Artery Disease. ATVB 2005, 25, 296–301. [Google Scholar] [CrossRef]
- Tigue, C.C.; McKoy, J.M.; Evens, A.M.; Trifilio, S.M.; Tallman, M.S.; Bennett, C.L. Granulocyte-Colony Stimulating Factor Administration to Healthy Individuals and Persons with Chronic Neutropenia or Cancer: An Overview of Safety Considerations from the Research on Adverse Drug Events and Reports Project. Bone Marrow Transplant. 2007, 40, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Nervi, B.; Link, D.C.; DiPersio, J.F. Cytokines and Hematopoietic Stem Cell Mobilization. J. Cell. Biochem. 2006, 99, 690–705. [Google Scholar] [CrossRef]
- Kuroda, R.; Matsumoto, T.; Niikura, T.; Kawakami, Y.; Fukui, T.; Lee, S.Y.; Mifune, Y.; Kawamata, S.; Fukushima, M.; Asahara, T.; et al. Local transplantation of granulocyte colony stimulating factor-mobilized CD34+ cells for patients with femoral and tibial nonunion: Pilot clinical trial. Stem Cells Transl. Med. 2013, 3, 128–134. [Google Scholar] [CrossRef]
- Bozlar, M.; Aslan, B.; Kalaci, A.; Baktiroglu, L.; Yanat, A.N.; Tasci, A. Effects of Human Granulocyte-Colony Stimulating Factor on Fracture Healing in Rats. Saudi Med. J. 2005, 26, 1250–1254. [Google Scholar] [PubMed]
- Moukoko, D.; Pourquier, D.; Genovesio, C.; Thezenas, S.; Chabrand, P.; Roffino, S.; Pithioux, M. Granulocyte-Colony Stimulating Factor Enhances Bone Fracture Healing. Clin. Biomech. 2018, 58, 62–68. [Google Scholar] [CrossRef]
- Herrmann, M.; Zeiter, S.; Eberli, U.; Hildebrand, M.; Camenisch, K.; Menzel, U.; Alini, M.; Verrier, S.; Stadelmann, V.A. Five Days Granulocyte Colony-Stimulating Factor Treatment Increases Bone Formation and Reduces Gap Size of a Rat Segmental Bone Defect: A Pilot Study. Front. Bioeng. Biotechnol. 2018, 6, 5. [Google Scholar] [CrossRef]
- Drum, M.G.; Marcellin-Little, D.J.; Davis, M.S. Principles and Applications of Therapeutic Exercises for Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.N.; Ejiri, S.; Kobayashi, T.; Anwar, R.B.; Oda, K.; Ohshima, H.; Saito, C. Histologic Study of the Cellular Events during Rat Mandibular Distraction Osteogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2009, 107, 325–335. [Google Scholar] [CrossRef]
- Yasui, N.; Sato, M.; Ochi, T.; Kimura, T.; Kawahata, H.; Kitamura, Y.; Nomura, S. Three Modes of Ossification during Distraction Osteogenesis in the Rat. Bone Joint J. 1997, 79, 824–830. [Google Scholar] [CrossRef]
- Sato, M.; Yasui, N.; Nakase, T.; Kawahata, H.; Sugimoto, M.; Hirota, S.; Kitamura, Y.; Nomura, S.; Ochi, T. Expression of Bone Matrix Proteins MRNA during Distraction Osteogenesis. J. Bone Miner. Res. 1998, 13, 1221–1231. [Google Scholar] [CrossRef]
- Aronson, J.; Shen, X.C.; Skinner, R.A.; Hogue, W.R.; Badger, T.M.; Lumpkin, C.K. Rat Model of Distraction Osteogenesis. J. Orthop. Res. 1997, 15, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Touaitahuata, H.; Cres, G.; de Rossi, S.; Vives, V.; Blangy, A. The Mineral Dissolution Function of Osteoclasts Is Dispensable for Hypertrophic Cartilage Degradation during Long Bone Development and Growth. Dev. Biol. 2014, 393, 57–70. [Google Scholar] [CrossRef]
- Liu, X.L.; Hu, X.; Cai, W.X.; Lu, W.W.; Zheng, L.W. Effect of Granulocyte-Colony Stimulating Factor on Endothelial Cells and Osteoblasts. BioMed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, H.; Wilson, W.; van Donkelaar, C.C.; Huiskes, R.; Ito, K. Comparison of Biophysical Stimuli for Mechano-Regulation of Tissue Differentiation during Fracture Healing. J. Biomech. 2006, 39, 1507–1516. [Google Scholar] [CrossRef]
- Ishida, K.; Matsumoto, T.; Sasaki, K.; Mifune, Y.; Tei, K.; Kubo, S.; Matsushita, T.; Takayama, K.; Akisue, T.; Tabata, Y.; et al. Bone Regeneration Properties of Granulocyte Colony-Stimulating Factor via Neovascularization and Osteogenesis. Tissue Eng. Part A 2010, 16, 3271–3284. [Google Scholar] [CrossRef]
- Erdem, M.; Gulabi, D.; Sen, C.; Sahin, S.A.; Bozdag, E. Effects of Caffeic Acid Phenethyl Ester and Melatonin on Distraction Osteogenesis: An Experimental Study. SpringerPlus 2014, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, S.; Jiang, X.; Li, Y.; Song, D.; Hu, J. Systemic Administration of Lithium Improves Distracted Bone Regeneration in Rats. Calcif. Tissue Int. 2015, 96, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Meyer, T.; Vosshans, J.; Joos, U. Decreased Expression of Osteocalcin and Osteonectin in Relation to High Strains and Decreased Mineralization in Mandibular Distraction Osteogenesis. J. Cranio Maxillofac. Surg. 1999, 27, 222–227. [Google Scholar] [CrossRef]
- Lammens, J.; Liu, Z.; Aerssens, J.; Dequeker, J.; Fabry, G. Distraction Bone Healing versus Osteotomy Healing: A Comparative Biochemical Analysis. J. Bone Miner. Res. 1998, 13, 279–286. [Google Scholar] [CrossRef]
- Leung, K.S.; Fung, K.P.; Liu, P.P.L.; Lee, K.M. Bone-Specific Alkaline Phosphatase Activities in Plasma and Callus during Callotasis in Rabbits. Life Sci. 1995, 57, 637–643. [Google Scholar] [CrossRef]
- Fink, B.; Feldkamp, J.; Fox, F.; Hofmann, B.; Singer, J.; Krieger, M. Time Course of Osteocalcin, Bone-Specific Alkaline Phosphatase, and C-Terminal Procollagen Peptide During Callus Distraction. J. Pediatric Orthop. 2001, 21, 246–251. [Google Scholar] [CrossRef]
- Fink, B.; Fox, F.; Singer, J.; Skripitz, R.; Feldkamp, J. Monitoring of Bone Formation during Distraction Osteogenesis via Osteocalcin: A Time Sequence Study in Dogs. J. Orthop. Sci. 2002, 7, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Harrison, G.; Taylor, A.G.; Camper, S.; Shapiro, I.M. The Role of Alkaline Phosphatase in Cartilage Mineralization. Bone Miner. 1992, 17, 273–278. [Google Scholar] [CrossRef]
- Kassis, I.; Zangi, L.; Rivkin, R.; Levdansky, L.; Samuel, S.; Marx, G.; Gorodetsky, R. Isolation of Mesenchymal Stem Cells from G-CSF-Mobilized Human Peripheral Blood Using Fibrin Microbeads. Bone Marrow Transplant. 2006, 37, 967–976. [Google Scholar] [CrossRef]
- Ripa, R.S.; Haack-Sorensen, M.; Wang, Y.; Jorgensen, E.; Mortensen, S.; Bindslev, L.; Friis, T.; Kastrup, J. Bone Marrow Derived Mesenchymal Cell Mobilization by Granulocyte-Colony Stimulating Factor after Acute Myocardial Infarction: Results from the Stem Cells in Myocardial Infarction (STEMMI) Trial. Circulation 2007, 116. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, Q.; Zou, K.; Wang, H.; Zhang, X.; Yang, Z.; Lee, W.Y.W.; Wei, B.; Gu, W.; Yang, Y.P.; et al. Administration of Allogeneic Mesenchymal Stem Cells in Lengthening Phase Accelerates Early Bone Consolidation in Rat Distraction Osteogenesis Model. Stem Cell Res. Ther. 2020, 11, 129. [Google Scholar] [CrossRef]
- Kitoh, H.; Kitakoji, T.; Tsuchiya, H.; Mitsuyama, H.; Nakamura, H.; Katoh, M.; Ishiguro, N. Transplantation of Marrow-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma during Distraction Osteogenesis—A Preliminary Result of Three Cases. Bone 2004, 35, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Hu, J.; Zou, S.; Zhou, H.; Han, L. Mandibular Distraction Osteogenesis Enhanced by Bone Marrow Mesenchymal Stem Cells in Rats. J. Cranio Maxillofac. Surg. 2006, 34, 283–289. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, S.; Wang, B.; Gu, W.; Li, G. Stem Cell Therapy for Enhancement of Bone Consolidation in Distraction Osteogenesis: A Contemporary Review of Experimental Studies. Bone Joint Res. 2017, 6, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hermida-Gómez, T.; Fuentes-Boquete, I.; Gimeno-Longas, M.J.; Muiños-López, E.; Díaz-Prado, S.; de Toro, F.J.; Blanco, F.J. Bone Marrow Cells Immunomagnetically Selected For CD271+ Antigen Promote In Vitro the Repair of Articular Cartilage Defects. Tissue Eng. Part A 2011, 17, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shavit, Z. The Osteoclast: A Multinucleated, Hematopoietic-Origin, Bone-Resorbing Osteoimmune Cell. J. Cell. Biochem. 2007, 102, 1130–1139. [Google Scholar] [CrossRef]
- Minamino, K.; Adachi, Y.; Okigaki, M.; Ito, H.; Togawa, Y.; Fujita, K.; Tomita, M.; Suzuki, Y.; Zhang, Y.; Iwasaki, M.; et al. Macrophage Colony-Stimulating Factor (M-CSF), as Well as Granulocyte Colony-Stimulating Factor (G-CSF), Accelerates Neovascularization. Stem Cells. 2005, 23, 347–354. [Google Scholar] [CrossRef]
- Pithioux, M.; Roseren, F.; Jalain, C.; Launay, F.; Charpiot, P.; Chabrand, P.; Roffino, S.; Lamy, E. An Efficient and Reproducible Protocol for Distraction Osteogenesis in a Rat Model Leading to a Functional Regenerated Femur. JoVE 2017, 56433. [Google Scholar] [CrossRef]
- Cimato, T.R.; Palka, B.A.; Lang, J.K.; Young, R.F. LDL Cholesterol Modulates Human CD34+ HSPCs through Effects on Proliferation and the IL-17 G-CSF Axis. PLoS ONE 2013, 8, e73861. [Google Scholar] [CrossRef]
- Heyboer, M.; Milovanova, T.N.; Wojcik, S.; Grant, W.; Chin, M.; Hardy, K.R.; Lambert, D.S.; Logue, C.; Thom, S.R. CD34+/CD45-Dim Stem Cell Mobilization by Hyperbaric Oxygen—Changes with Oxygen Dosage. Stem Cell Res. 2014, 12, 638–645. [Google Scholar] [CrossRef]
- Thom, S.R.; Hampton, M.; Troiano, M.A.; Mirza, Z.; Malay, D.S.; Shannon, S.; Jennato, N.B.; Donohue, C.M.; Hoffstad, O.; Woltereck, D.; et al. Measurements of CD34+/CD45-Dim Stem Cells Predict Healing of Diabetic Neuropathic Wounds. Diabetes 2016, 65, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Kroepfl, J.M.; Pekovits, K.; Stelzer, I.; Fuchs, R.; Zelzer, S.; Hofmann, P.; Sedlmayr, P.; Dohr, G.; Wallner-Liebmann, S.; Domej, W.; et al. Exercise Increases the Frequency of Circulating Hematopoietic Progenitor Cells, But Reduces Hematopoietic Colony-Forming Capacity. Stem Cells Dev. 2012, 21, 2915–2925. [Google Scholar] [CrossRef]
- Harting, M.T.; Jimenez, F.; Pati, S.; Baumgartner, J.; Cox, C.S. Immunophenotype Characterization of Rat Mesenchymal Stromal Cells. Cytotherapy 2008, 10, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhang, Q.; Jia, L.Y.; Fang, N.; Chen, L.; Yu, L.M.; Liu, J.W.; Zhang, T. Isolation and Characterization of Rat Mesenchymal Stem Cells Derived from Granulocyte Colony-Stimulating Factor-Mobilized Peripheral Blood. Cells Tissues Organs 2016, 201, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Cesselli, D.; Beltrami, A.P.; Rigo, S.; Bergamin, N.; D’Aurizio, F.; Verardo, R.; Piazza, S.; Klaric, E.; Fanin, R.; Toffoletto, B.; et al. Multipotent Progenitor Cells Are Present in Human Peripheral Blood. Circ. Res. 2009, 104, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Kuci, S.; Kuci, Z.; Kreyenberg, H.; Deak, E.; Putsch, K.; Huenecke, S.; Amara, C.; Koller, S.; Rettinger, E.; Grez, M.; et al. CD271 Antigen Defines a Subset of Multipotent Stromal Cells with Immunosuppressive and Lymphohematopoietic Engraftment-Promoting Properties. Haematologica 2010, 95, 651–659. [Google Scholar] [CrossRef]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise Review: Evidence for CD34 as a Common Marker for Diverse Progenitors: CD34 as a Common Marker for Diverse Progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Giuffrida, R.; Furno, D.L.; Parrinello, N.; Forte, S.; Gulino, R.; Colarossi, C.; Schinocca, L.; Giuffrida, R.; Cardile, V.; et al. Potential Effect of CD271 on Human Mesenchymal Stromal Cell Proliferation and Differentiation. IJMS 2015, 16, 15609–15624. [Google Scholar] [CrossRef]
- Eguchi, M.; Masuda, H.; Asahara, T. Endothelial Progenitor Cells for Postnatal Vasculogenesis. Clin. Exp. Nephrol. 2007, 11, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ramcharan, K.S.; Lip, G.Y.H.; Stonelake, P.S.; Blann, A.D. Increased Pre-Surgical Numbers of Endothelial Progenitor Cells and Circulating Endothelial Cells in Colorectal Cancer Fail to Predict Outcome. Int J. Colorectal Dis. 2015, 30, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, F.; Lacroix, R.; Camoin-Jau, L.; Anfosso, F.; Sampol, J.; Dignat-George, F. Cellules endothéliales circulantes, microparticules et progéniteurs: Vers la définition de la «vasculocompétence. J. Intern. Med. 2011, 32, 54–63. [Google Scholar] [CrossRef]
- Drapeau, C.; Benson, K.F.; Jensen, G.S. Cell Types after Consumption of a Polyphenol-Rich Extract from Sea Buckthorn Berries (Hippophae) in Healthy Human Subjects. Clin. Interv. Aging 2019, 14, 253–263. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and Extensible Bone Image Analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A Machine Learning Tool for Microscopy Pixel Classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef] [PubMed]
 represents the latency period, the distraction phase is represented by
represents the latency period, the distraction phase is represented by  , and
, and  represents the consolidation phase. ** p < 0.05, *** p < 0.01. Cytometry assays were performed on 6 G-CSF animals and 4 control animals.
represents the consolidation phase. ** p < 0.05, *** p < 0.01. Cytometry assays were performed on 6 G-CSF animals and 4 control animals.
 represents the latency period, the distraction phase is represented by
represents the latency period, the distraction phase is represented by  , and
, and  represents the consolidation phase. ** p < 0.05, *** p < 0.01. Cytometry assays were performed on 6 G-CSF animals and 4 control animals.
represents the consolidation phase. ** p < 0.05, *** p < 0.01. Cytometry assays were performed on 6 G-CSF animals and 4 control animals.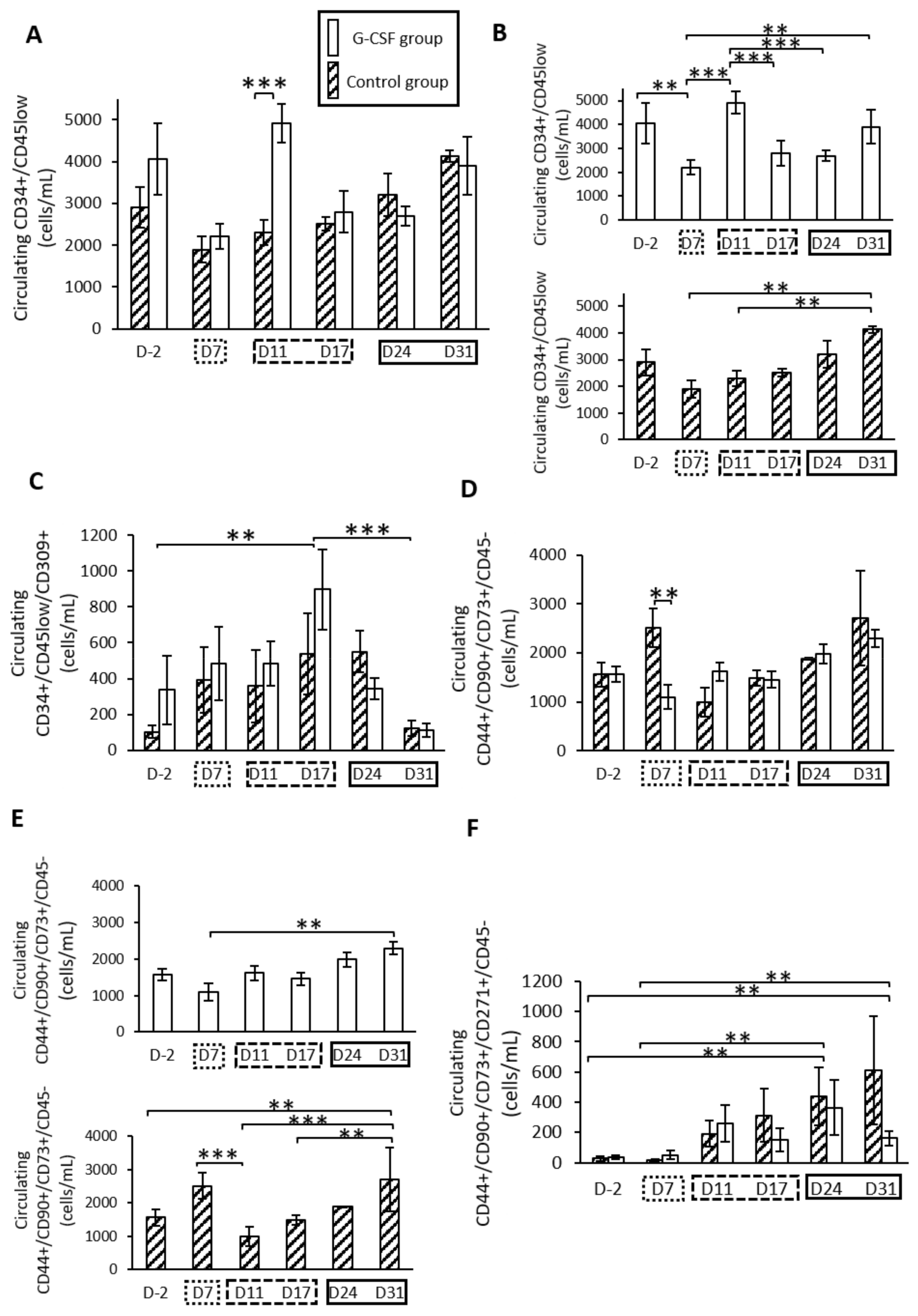
 represents the latency period,
represents the latency period,  represents the distraction phase and the consolidation phase is represented by
represents the distraction phase and the consolidation phase is represented by  . Planar scintigraphy was performed on 6 control animals and 4 G-CSF animals. ** p < 0.05, *** p < 0.01, **** p < 0.001, ***** p < 0.0001.
. Planar scintigraphy was performed on 6 control animals and 4 G-CSF animals. ** p < 0.05, *** p < 0.01, **** p < 0.001, ***** p < 0.0001.
 represents the latency period,
represents the latency period,  represents the distraction phase and the consolidation phase is represented by
represents the distraction phase and the consolidation phase is represented by  . Planar scintigraphy was performed on 6 control animals and 4 G-CSF animals. ** p < 0.05, *** p < 0.01, **** p < 0.001, ***** p < 0.0001.
. Planar scintigraphy was performed on 6 control animals and 4 G-CSF animals. ** p < 0.05, *** p < 0.01, **** p < 0.001, ***** p < 0.0001.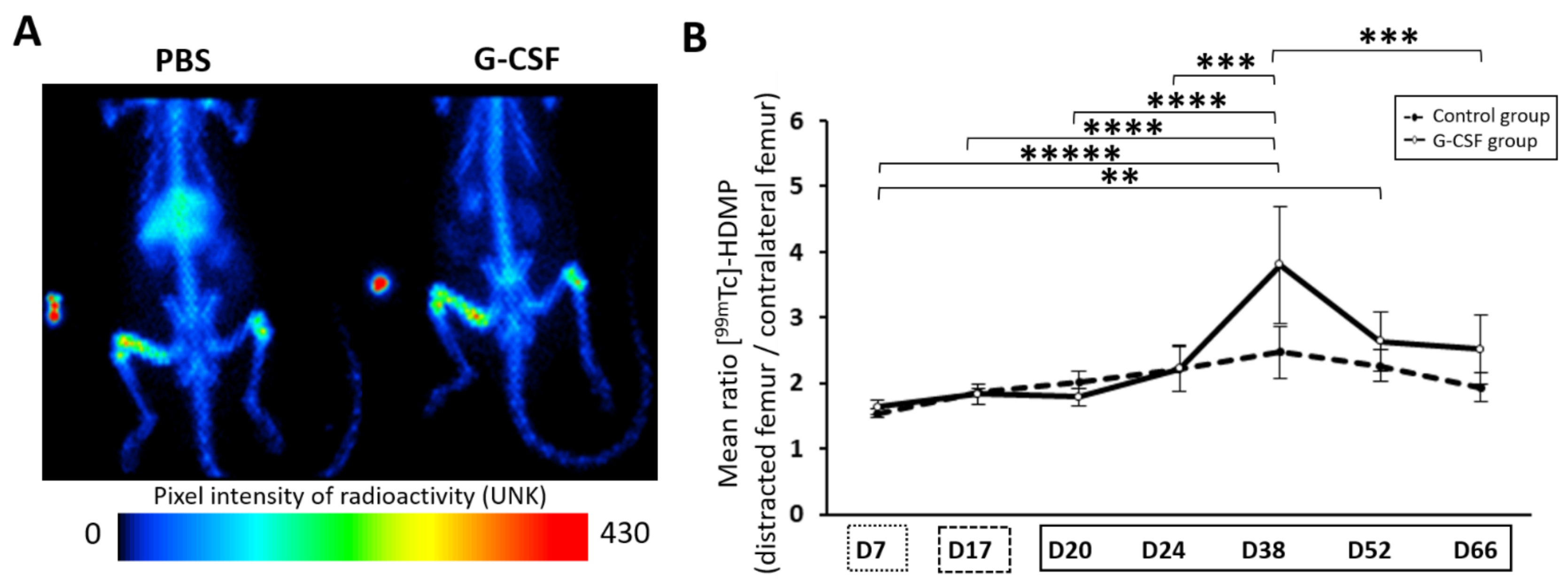
 represent the latency period, the distraction phase is represented by
represent the latency period, the distraction phase is represented by  , and
, and  the consolidation phase. *** p < 0.01, **** p < 0.001.
the consolidation phase. *** p < 0.01, **** p < 0.001.
 represent the latency period, the distraction phase is represented by
represent the latency period, the distraction phase is represented by  , and
, and  the consolidation phase. *** p < 0.01, **** p < 0.001.
the consolidation phase. *** p < 0.01, **** p < 0.001.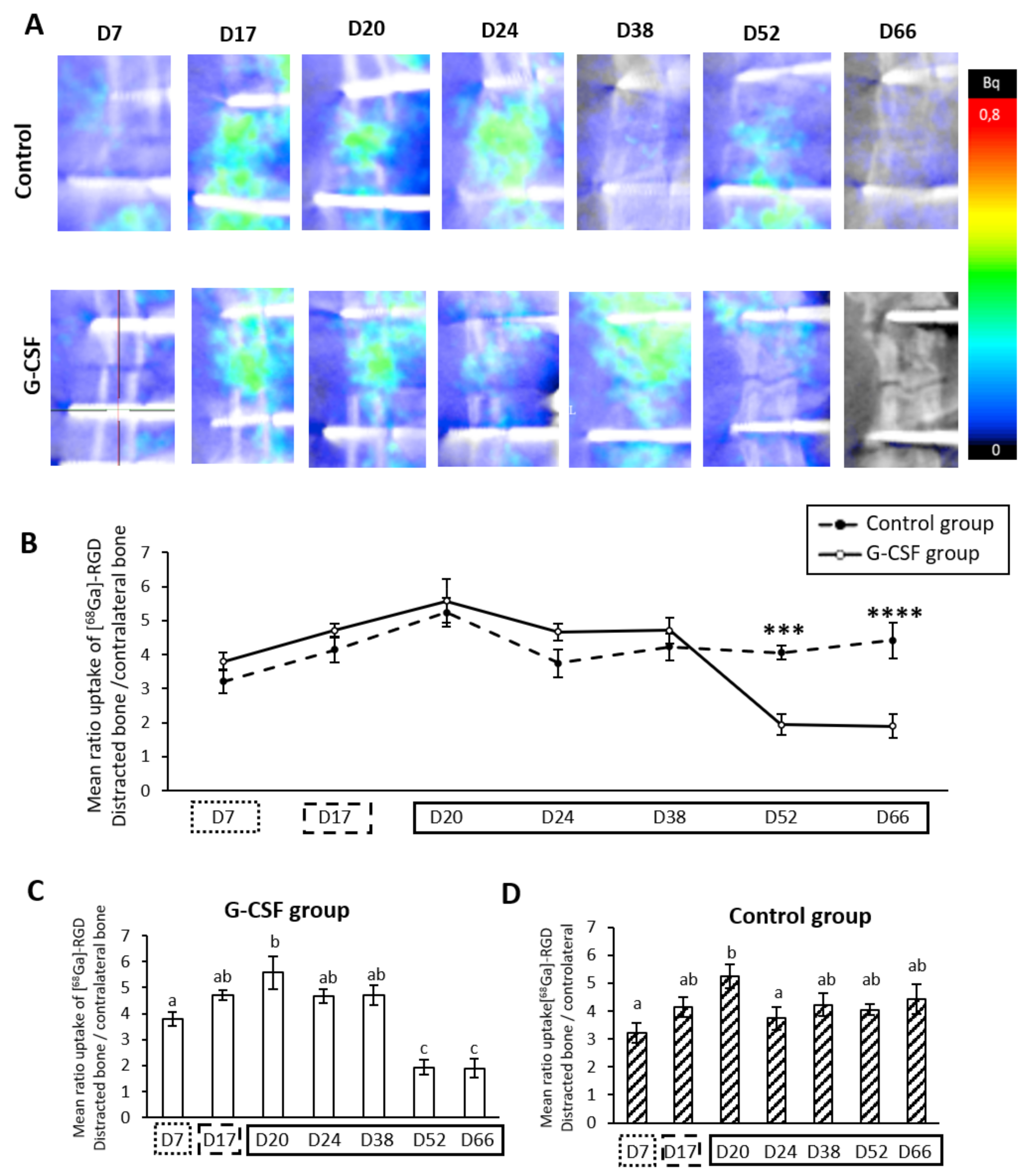
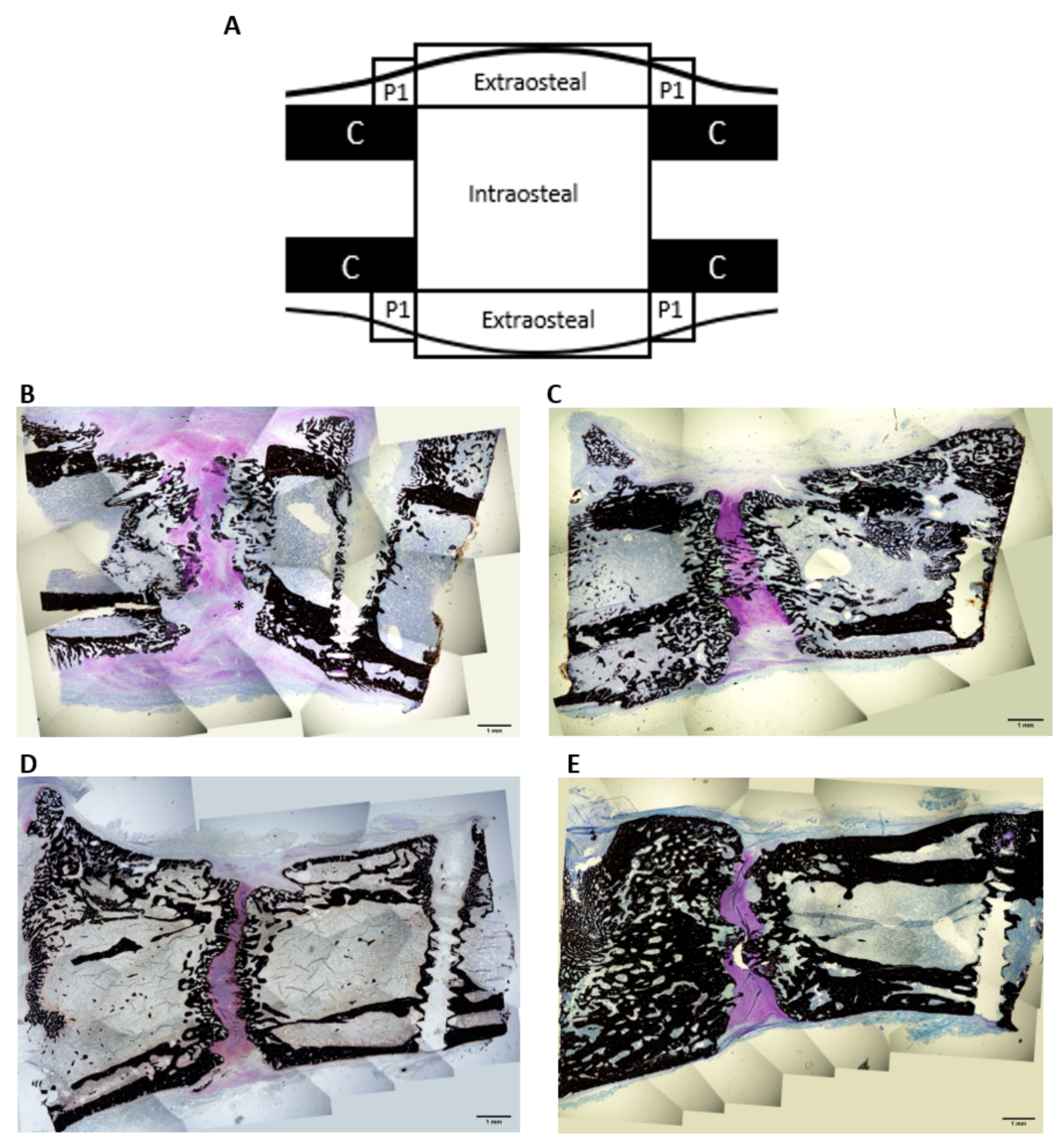
| ROIs | BA/TA (%) Mean ± SEM | Trabecular Thickness (mm) Mean ± SEM | |||
|---|---|---|---|---|---|
| Control Group (n = 2) | G-CSF Group (n = 2) | Control Group (n = 2) | G-CSF Group (n = 2) | ||
| D31 | Intraosteal | 0.260 ± 0.012 | 0.280 ± 0.027 | 0.061 ± 0.003 | 0.081 ± 0.003 a |
| Extraosteal | 0.095 ± 0.074 | 0.189 ± 0.018 | 0.049 ± 0.010 | 0.063 ± 0.005 | |
| Periosteal | 0.272 ± 0.03 | 0.333 ± 0.04 | 0.074 ± 0.006 | 0.073 ± 0.005 | |
| D66 | Intraosteal | 0.366 ± 0.027 b | 0.464 ± 0.031 a,b | 0.119 ± 0.002 b | 0.148 ± 0.10 a,b |
| Extraosteal | 0.304 ± 0.010 b | 0.344 ± 0.032 b | 0.127 ± 0.014 b | 0.123 ± 0.004 b | |
| Periosteal | 0.297 ± 0.018 | 0.506 ± 0.047 a,b | 0.126 ± 0.008 b | 0.145 ± 0.017 b | |
| ROIs | Area mm² Mean ± SEM | ||
|---|---|---|---|
| Control Group (n = 2) | G-CSF Group (n = 2) | ||
| D31 | Intraosteal | 0.593 ± 0.103 | 0.280 ± 0.047 a |
| Extraosteal | 0.089 ± 0.045 | 0.099 ± 0.028 | |
| Periosteal | 0.042 ± 0.004 | 0.044 ± 0.016 | |
| D66 | Intraosteal | 0.04 ± 0.009 | 0.068 ± 0.017 |
| Extraosteal | 0.059 ± 0.012 | 0.015 ± 0.006 a | |
| Periosteal | 0.002 ± 0.0005 | 0.017 ± 0.061 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roseren, F.; Pithioux, M.; Robert, S.; Balasse, L.; Guillet, B.; Lamy, E.; Roffino, S. Systemic Administration of G-CSF Accelerates Bone Regeneration and Modulates Mobilization of Progenitor Cells in a Rat Model of Distraction Osteogenesis. Int. J. Mol. Sci. 2021, 22, 3505. https://doi.org/10.3390/ijms22073505
Roseren F, Pithioux M, Robert S, Balasse L, Guillet B, Lamy E, Roffino S. Systemic Administration of G-CSF Accelerates Bone Regeneration and Modulates Mobilization of Progenitor Cells in a Rat Model of Distraction Osteogenesis. International Journal of Molecular Sciences. 2021; 22(7):3505. https://doi.org/10.3390/ijms22073505
Chicago/Turabian StyleRoseren, Flavy, Martine Pithioux, Stéphane Robert, Laure Balasse, Benjamin Guillet, Edouard Lamy, and Sandrine Roffino. 2021. "Systemic Administration of G-CSF Accelerates Bone Regeneration and Modulates Mobilization of Progenitor Cells in a Rat Model of Distraction Osteogenesis" International Journal of Molecular Sciences 22, no. 7: 3505. https://doi.org/10.3390/ijms22073505
APA StyleRoseren, F., Pithioux, M., Robert, S., Balasse, L., Guillet, B., Lamy, E., & Roffino, S. (2021). Systemic Administration of G-CSF Accelerates Bone Regeneration and Modulates Mobilization of Progenitor Cells in a Rat Model of Distraction Osteogenesis. International Journal of Molecular Sciences, 22(7), 3505. https://doi.org/10.3390/ijms22073505







