Survey of MRI Usefulness for the Clinical Assessment of Bone Microstructure
Abstract
1. Introduction
1.1. Bone Disorders and Investigative Tools
1.2. Bone Microstructure
2. Bone Pathologies and Clinical Approach
2.1. Principal Bone Pathologies
2.2. Clinical Approach
3. MRI Based Approach
3.1. Technical Considerations for Clinical Usefulness
3.2. Microstructure Investigation
3.3. Microstructure vs. DXA
3.4. Voxel Size and Microstructure
3.5. Main Magnetic Field Strength Effect
3.6. Comparison with CT Measurements
3.6.1. Ex-Vivo
3.6.2. In-Vivo
3.7. Reported Limitations
4. Prospectives
4.1. Magnetic Resnance Spectroscopy vs. Chemical Shift Encoding-MRI
4.2. MR Susceptibility
4.3. Solid State MRI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| DXA | Dual-energy X-ray absorptiometry |
| qCT | Quantitative Computed Tomography |
| MSK | Musculoskeletal |
| SD | Standard Deviation |
| DALYs | Disability Adjusted Life Years |
| YLDs | Years Lived with Disability |
| OI | Osteoporosis Imperfecta |
| CKD | Chronic Kidney Disease |
| OS | Osteosarcoma |
| HR-pQCT | High-Resolution Peripheral Quantitative Computed Tomography |
| HF MRI | High Field MRI |
| UHF MRI | Ultra-High Field MRI |
| SNR | Signal to Noise Ratio |
| BMD | Bone Mineral Density |
| Tb.Th | Trabecular Thickness |
| Tb.Sp | Trabecular Spacing |
| Tb.N | Trabecular Number |
| BVF | Bone Volume Fraction |
| SE | Spin-Echo |
| GE | Gradient Echo |
| TSE | Turbo Spin Echo |
| GRE | Gradient Re-called Echo |
| 3D FLASE | 3D Fast Low Angle Spin Echo |
| 3D SSFP | 3D Steady-State Free Precession |
| 3D FIESTA | 3D Fast Imaging Employing Steady-state Acquisition |
| FIESTA-c | Fast Imaging Employing Steady-state Acquisition Cycled Phases |
| FSE | Fast Spin Echo |
| FLASH | Fast Low Angle Shot |
| 3D b-FFE | 3D Balanced Fast-Field Echo |
| SPGR | SPoiled Gradient-Recalled |
| µCT | Micro Computed Tomography |
| SWI | Susceptibility Weighted Imaging |
| QSM | Quantitative Susceptibility Mapping |
| NMR | Nuclear Magnetic Resonance |
| UTE | Ultrashort Echo-Time |
| ZTE | Zero Echo-Time |
| SWIFT | Sweep Imaging with Fourier Transformation |
| SAR | Specific Absorption Rate |
| TW | Total Bone Water |
| BW | Water Bound |
| PW | Pore Water |
| HR+ | Hormone Receptor Positive |
| BMFF | Bone Marrow Fat Fraction |
| PDFF | Proton Density Fat Fraction |
| MRS | Magnetic Resonance Spectroscopy |
| CSE-MRI | Chemical Shift Encoding MRI |
| VOI | Volume of Interest |
| ROI | Region of Interest |
| PRESS | Point-Resolved Spectroscopy |
| STEAM | Stimulated Echo Acquisition Mode |
| VCFs | Vertebral Compression Fractures |
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years Lived with Disability (YLDs) for 1160 Sequelae of 289 Diseases and Injuries 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A Comparative Risk Assessment of Burden of Disease and Injury Attributable to 67 Risk Factors and Risk Factor Clusters in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Murray, C.J.L. Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 27. [Google Scholar] [CrossRef]
- Woolf, A.D. Global Burden of Osteoarthritis and Musculoskeletal Diseases. BMC Musculoskelet. Disord. 2015, 16, S3. [Google Scholar] [CrossRef][Green Version]
- Johnell, O.; Kanis, J.A. An Estimate of the Worldwide Prevalence and Disability Associated with Osteoporotic Fractures. Osteoporos. Int. 2006, 8, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Odén, A.; McCloskey, E.V.; Kanis, J.A.; Harvey, N.C.; Johansson, H. Burden of High Fracture Probability Worldwide: Secular Increases 2010–2040. Osteoporos. Int. 2015, 26, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007, 22, 465–475. [Google Scholar] [CrossRef]
- Van Oostwaard, M. Osteoporosis and the Nature of Fragility Fracture: An Overview. In Fragility Fracture Nursing; Perspectives in Nursing Management and Care for Older Adults; Hertz, K., Santy-Tomlinson, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–13. ISBN 978-3-319-76680-5. [Google Scholar]
- Nayak, S.; Edwards, D.L.; Saleh, A.A.; Greenspan, S.L. Systematic Review and Meta-Analysis of the Performance of Clinical Risk Assessment Instruments for Screening for Osteoporosis or Low Bone Density. Osteoporos. Int. 2015, 26, 1543–1554. [Google Scholar] [CrossRef]
- Humadi, A.; Alhadithi, R.; Alkudiari, S. Validity of the DEXA Diagnosis of Involutional Osteoporosis in Patients with Femoral Neck Fractures. Indian J. Orthop. 2010, 44, 73. [Google Scholar] [CrossRef]
- Sharma, A.K.; Toussaint, N.D.; Elder, G.J.; Masterson, R.; Holt, S.G.; Robertson, P.L.; Ebeling, P.R.; Baldock, P.; Miller, R.C.; Rajapakse, C.S. Magnetic Resonance Imaging Based Assessment of Bone Microstructure as a Non-Invasive Alternative to Histomorphometry in Patients with Chronic Kidney Disease. Bone 2018, 114, 14–21. [Google Scholar] [CrossRef]
- Boutroy, S.; Bouxsein, M.L.; Munoz, F.; Delmas, P.D. In Vivo Assessment of Trabecular Bone Microarchitecture by High-Resolution Peripheral Quantitative Computed Tomography. J. Clin. Endocrinol. Metab. 2005, 90, 6508–6515. [Google Scholar] [CrossRef]
- Majumdar, S.; Newitt, D.; Mathur, A.; Osman, D.; Gies, A.; Chiu, E.; Lotz, J.; Kinney, J.; Genant, H. Magnetic Resonance Imaging of Trabecular Bone Structure in the Distal Radius: Relationship with X-Ray Tomographic Microscopy and Biomechanics. Osteoporos. Int. 1996, 6, 376–385. [Google Scholar] [CrossRef]
- Seifert, A.C.; Li, C.; Rajapakse, C.S.; Bashoor-Zadeh, M.; Bhagat, Y.A.; Wright, A.C.; Zemel, B.S.; Zavaliangos, A.; Wehrli, F.W. Bone Mineral 31P and Matrix-Bound Water Densities Measured by Solid-State 31P and 1H MRI: BONE DENSITY QUANTIFICATION BY MRI. NMR Biomed. 2014, 27, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Karamat, M.I.; Darvish-Molla, S.; Santos-Diaz, A. Opportunities and Challenges of 7 Tesla Magnetic Resonance Imaging: A Review. Crit. Rev. Biomed. Eng. 2016, 44, 73–89. [Google Scholar] [CrossRef]
- Majumdar, S.; Link, T.M.; Augat, P.; Lin, J.C.; Newitt, D.; Lane, N.E.; Genant, H.K. Trabecular Bone Architecture in the Distal Radius Using Magnetic Resonance Imaging in Subjects with Fractures of the Proximal Femur. Osteoporos. Int. 1999, 10, 231–239. [Google Scholar] [CrossRef]
- Krug, R.; Carballido-Gamio, J.; Banerjee, S.; Burghardt, A.J.; Link, T.M.; Majumdar, S. In Vivo Ultra-High-Field Magnetic Resonance Imaging of Trabecular Bone Microarchitecture at 7 T. J. Magn. Reson. Imaging 2008, 27, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Honig, S.; Liu, Y.; Chen, C.; Chu, K.K.; Rajapakse, C.S.; Egol, K.; Xia, D.; Saha, P.K.; Regatte, R.R. 7 Tesla MRI of Bone Microarchitecture Discriminates between Women without and with Fragility Fractures Who Do Not Differ by Bone Mineral Density. J. Bone Miner. Metab 2015, 33, 285–293. [Google Scholar] [CrossRef][Green Version]
- Rajapakse, C.S.; Kobe, E.A.; Batzdorf, A.S.; Hast, M.W.; Wehrli, F.W. Accuracy of MRI-Based Finite Element Assessment of Distal Tibia Compared to Mechanical Testing. Bone 2018, 108, 71–78. [Google Scholar] [CrossRef]
- Wang, X.; Nyman, J.S.; Dong, X.; Leng, H.; Reyes, M. Fundamental Biomechanics in Bone Tissue Engineering. Synth. Lect. Tissue Eng. 2010, 2, 1–225. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S. Nanoscale Mechanisms of Bone Deformation and Fracture. In Handbook of Biomineralization; Buerlein, E., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007; pp. 397–414. ISBN 978-3-527-61944-3. [Google Scholar]
- Nyman, J.S.; Roy, A.; Shen, X.; Acuna, R.L.; Tyler, J.H.; Wang, X. The Influence of Water Removal on the Strength and Toughness of Cortical Bone. J. Biomech. 2006, 39, 931–938. [Google Scholar] [CrossRef]
- Cowin, S.C. Bone Poroelasticity. J. Biomech. 1999, 32, 217–238. [Google Scholar] [CrossRef]
- Brage, S.; Nygard, J.F.; Tellnes, G. The Gender Gap in Musculoskeletal-Related Long Term Sickness Absence in Norway. Scand. J. Soc. Med. 1998, 26, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, E.; Felten, R.; Sagez, F.; Sibilia, J.; Devilliers, H.; Arnaud, L. The World-Wide Burden of Musculoskeletal Diseases: A Systematic Analysis of the World Health Organization Burden of Diseases Database. Ann. Rheum. Dis. 2019, 78, 844–848. [Google Scholar] [CrossRef] [PubMed]
- International Osteoporosis Foundation; Borgström, F.; Karlsson, L.; Ortsäter, G.; Norton, N.; Halbout, P.; Cooper, C.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; et al. Fragility Fractures in Europe: Burden, Management and Opportunities. Arch. Osteoporos. 2020, 15, 59. [Google Scholar] [CrossRef]
- Cheng, X.; Yuan, H.; Cheng, J.; Weng, X.; Xu, H.; Gao, J.; Huang, M.; Wáng, Y.X.J.; Wu, Y.; Xu, W.; et al. Chinese Expert Consensus on the Diagnosis of Osteoporosis by Imaging and Bone Mineral Density. Quant. Imaging Med. Surg. 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Kemmak, A.R.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic Burden of Osteoporosis in the World: A Systematic Review. Med. J. Islam Repub. Iran 2020. in review. [Google Scholar]
- Bartl, R.; Bartl, C. Corticosteroid-Induced Osteoporosis. In Bone Disorders; Springer International Publishing: Cham, Switzerland, 2017; pp. 431–434. ISBN 978-3-319-29180-2. [Google Scholar]
- Keenan, H.A.; Maddaloni, E. Bone Microarchitecture in Type 1 Diabetes: It Is Complicated. Curr. Osteoporos. Rep. 2016, 14, 351–358. [Google Scholar] [CrossRef]
- Chen, S.C.; Shepherd, S.; McMillan, M.; McNeilly, J.; Foster, J.; Wong, S.C.; Robertson, K.J.; Ahmed, S.F. Skeletal Fragility and Its Clinical Determinants in Children With Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 3585–3594. [Google Scholar] [CrossRef]
- Abdalrahaman, N.; McComb, C.; Foster, J.E.; Lindsay, R.S.; Drummond, R.; McKay, G.A.; Perry, C.G.; Ahmed, S.F. The Relationship between Adiposity, Bone Density and Microarchitecture Is Maintained in Young Women Irrespective of Diabetes Status. Clin. Endocrinol. 2017, 87, 327–335. [Google Scholar] [CrossRef]
- Singhal, V.; Tulsiani, S.; Campoverde, K.J.; Mitchell, D.M.; Slattery, M.; Schorr, M.; Miller, K.K.; Bredella, M.A.; Misra, M.; Klibanski, A. Impaired Bone Strength Estimates at the Distal Tibia and Its Determinants in Adolescents with Anorexia Nervosa. Bone 2018, 106, 61–68. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Klibanski, A. The Paradox of Marrow Adipose Tissue in Anorexia Nervosa. Bone 2019, 118, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Fintini, D.; Cianfarani, S.; Cofini, M.; Andreoletti, A.; Ubertini, G.M.; Cappa, M.; Manco, M. The Bones of Children With Obesity. Front. Endocrinol. 2020, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Cordes, C.; Baum, T.; Dieckmeyer, M.; Ruschke, S.; Diefenbach, M.N.; Hauner, H.; Kirschke, J.S.; Karampinos, D.C. MR-Based Assessment of Bone Marrow Fat in Osteoporosis, Diabetes, and Obesity. Front. Endocrinol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Cordes, C.; Dieckmeyer, M.; Ott, B.; Shen, J.; Ruschke, S.; Settles, M.; Eichhorn, C.; Bauer, J.S.; Kooijman, H.; Rummeny, E.J.; et al. MR-Detected Changes in Liver Fat, Abdominal Fat, and Vertebral Bone Marrow Fat after a Four-Week Calorie Restriction in Obese Women: MR-Detected Fat Changes After a Diet. J. Magn. Reson. Imaging 2015, 42, 1272–1280. [Google Scholar] [CrossRef]
- Ganie, M.A.; Raizada, N.; Chawla, H.; Singh, A.K.; Aggarwala, S.; Bal, C.S. Primary Hyperparathyroidism May Masquerade as Rickets-Osteomalacia in Vitamin D Replete Children. J. Pediatr. Endocrinol. Metab. 2016, 29. [Google Scholar] [CrossRef]
- Minisola, S.; Peacock, M.; Fukumoto, S.; Cipriani, C.; Pepe, J.; Tella, S.H.; Collins, M.T. Tumour-Induced Osteomalacia. Nat. Rev. Dis. Prim. 2017, 3, 17044. [Google Scholar] [CrossRef]
- Florenzano, P.; Hartley, I.R.; Jimenez, M.; Roszko, K.; Gafni, R.I.; Collins, M.T. Tumor-Induced Osteomalacia. Calcif. Tissue Int. 2021, 108, 128–142. [Google Scholar] [CrossRef]
- Ruderman, I.; Rajapakse, C.S.; Opperman, A.; Robertson, P.L.; Masterson, R.; Tiong, M.K.; Toussaint, N.D. Bone Microarchitecture in Patients Undergoing Parathyroidectomy for Management of Secondary Hyperparathyroidism. Bone Rep. 2020, 13, 100297. [Google Scholar] [CrossRef]
- Winn, N.; Lalam, R.; Cassar-Pullicino, V. Imaging of Paget’s Disease of Bone. Wien. Med. Wochenschr. 2017, 167, 9–17. [Google Scholar] [CrossRef]
- Gennari, L.; Rendina, D.; Falchetti, A.; Merlotti, D. Paget’s Disease of Bone. Calcif. Tissue Int. 2019, 104, 483–500. [Google Scholar] [CrossRef]
- Kravets, I. Paget’s Disease of Bone: Diagnosis and Treatment. Am. J. Med. 2018, 131, 1298–1303. [Google Scholar] [CrossRef]
- Cundy, T. Paget’s Disease of Bone. Metabolism 2018, 80, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Appelman-Dijkstra, N.M.; Papapoulos, S.E. Paget’s Disease of Bone. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Palomo, T.; Vilaça, T.; Lazaretti-Castro, M. Osteogenesis Imperfecta: Diagnosis and Treatment. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Lee, B.; Marom, R. Osteogenesis Imperfecta: Advancements in Genetics and Treatment. Curr. Opin. Pediatr. 2019, 31, 708–715. [Google Scholar] [CrossRef]
- Trejo, P.; Rauch, F. Osteogenesis Imperfecta in Children and Adolescents—New Developments in Diagnosis and Treatment. Osteoporos Int 2016, 27, 3427–3437. [Google Scholar] [CrossRef]
- Marini, J.C.; Forlino, A.; Bächinger, H.P.; Bishop, N.J.; Byers, P.H.; Paepe, A.D.; Fassier, F.; Fratzl-Zelman, N.; Kozloff, K.M.; Krakow, D.; et al. Osteogenesis Imperfecta. Nat. Rev. Dis. Prim. 2017, 3, 17052. [Google Scholar] [CrossRef]
- Hoyer-Kuhn, H.; Netzer, C.; Semler, O. Osteogenesis Imperfecta: Pathophysiology and Treatment. Wien. Med. Wochenschr. 2015, 165, 278–284. [Google Scholar] [CrossRef]
- Hermie, I.; Horvath, M.; Van Cauter, S. Temporal Bone Imaging Features in Osteogenesis Imperfecta. J. Belg. Soc. Radiol. 2017, 101, 27. [Google Scholar] [CrossRef] [PubMed]
- Ashinsky, B.G.; Fishbein, K.W.; Carter, E.M.; Lin, P.-C.; Pleshko, N.; Raggio, C.L.; Spencer, R.G. Multiparametric Classification of Skin from Osteogenesis Imperfecta Patients and Controls by Quantitative Magnetic Resonance Microimaging. PLoS ONE 2016, 11, e0157891. [Google Scholar] [CrossRef] [PubMed]
- Dwan, K.; Phillipi, C.A.; Steiner, R.D.; Basel, D. Bisphosphonate Therapy for Osteogenesis Imperfecta. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brennan, B.; et al. Bone Sarcomas: ESMO–PaedCan–EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2018, 29, iv79–iv95. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Geller, D.S.; Gill, J.D.; Lewis, V.O.; Gorlick, R. Current and Future Therapeutic Approaches for Osteosarcoma. Expert Rev. Anticancer Ther. 2018, 18, 39–50. [Google Scholar] [CrossRef]
- Hao, Y.; An, R.; Xue, Y.; Li, F.; Wang, H.; Zheng, J.; Fan, L.; Liu, J.; Fan, H.; Yin, H. Prognostic Value of Tumoral and Peritumoral Magnetic Resonance Parameters in Osteosarcoma Patients for Monitoring Chemotherapy Response. Eur. Radiol. 2020. [Google Scholar] [CrossRef]
- Saleh, M.M.; Abdelrahman, T.M.; Madney, Y.; Mohamed, G.; Shokry, A.M.; Moustafa, A.F. Multiparametric MRI with Diffusion-Weighted Imaging in Predicting Response to Chemotherapy in Cases of Osteosarcoma and Ewing’s Sarcoma. BJR 2020, 93, 20200257. [Google Scholar] [CrossRef] [PubMed]
- Damilakis, J.; Adams, J.E.; Guglielmi, G.; Link, T.M. Radiation Exposure in X-Ray-Based Imaging Techniques Used in Osteoporosis. Eur. Radiol. 2010, 20, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Deniz, C.M.; Honig, S.; Rajapakse, C.S.; Egol, K.; Regatte, R.R.; Brown, R. Feasibility of Three-Dimensional MRI of Proximal Femur Microarchitecture at 3 Tesla Using 26 Receive Elements without and with Parallel Imaging: 3D MRI of Proximal Femur Microarchitecture. J. Magn. Reson. Imaging 2014, 40, 229–238. [Google Scholar] [CrossRef]
- Agten, C.A.; Honig, S.; Saha, P.K.; Regatte, R.; Chang, G. Subchondral Bone Microarchitecture Analysis in the Proximal Tibia at 7-T MRI. Acta Radiol. 2018, 59, 716–722. [Google Scholar] [CrossRef]
- Guenoun, D.; Pithioux, M.; Souplet, J.-C.; Guis, S.; Le Corroller, T.; Fouré, A.; Pauly, V.; Mattei, J.-P.; Bernard, M.; Guye, M.; et al. Assessment of Proximal Femur Microarchitecture Using Ultra-High Field MRI at 7 Tesla. Diagn. Interv. Imaging 2020, 101, 45–53. [Google Scholar] [CrossRef]
- Rad, H.S.; Lam, S.C.B.; Magland, J.F.; Ong, H.; Li, C.; Song, H.K.; Love, J.; Wehrli, F.W. Quantifying Cortical Bone Water in Vivo by Three-Dimensional Ultra-Short Echo-Time MRI: Quantifying Cortical Bone Water In Vivo BY 3D UTE MRI. NMR Biomed. 2011, 24, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.E. Quantitative Computed Tomography. Eur. J. Radiol. 2009, 71, 415–424. [Google Scholar] [CrossRef]
- Majumdar, S.; Genant, H.K.; Grampp, S.; Newitt, D.C.; Truong, V.-H.; Lin, J.C.; Mathur, A. Correlation of Trabecular Bone Structure with Age, Bone Mineral Density, and Osteoporotic Status: In Vivo Studies in the Distal Radius Using High Resolution Magnetic Resonance Imaging. J. Bone Miner. Res. 1997, 12, 111–118. [Google Scholar] [CrossRef]
- Ladinsky, G.A.; Vasilic, B.; Popescu, A.M.; Wald, M.; Zemel, B.S.; Snyder, P.J.; Loh, L.; Song, H.K.; Saha, P.K.; Wright, A.C.; et al. Trabecular Structure Quantified With the MRI-Based Virtual Bone Biopsy in Postmenopausal Women Contributes to Vertebral Deformity Burden Independent of Areal Vertebral BMD. J. Bone Miner. Res. 2007, 23, 64–74. [Google Scholar] [CrossRef]
- Link, T.M.; Majumdar, S.; Augat, P.; Lin, J.C.; Newitt, D.; Lu, Y.; Lane, N.E.; Genant, H.K. In Vivo High Resolution MRI of the Calcaneus: Differences in Trabecular Structure in Osteoporosis Patients. J. Bone Miner Res. 1998, 13, 1175–1182. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, X.S.; Vasilic, B.; Wehrli, F.W.; Benito, M.; Rajapakse, C.S.; Snyder, P.J.; Guo, X.E. In Vivo ΜMRI-Based Finite Element and Morphological Analyses of Tibial Trabecular Bone in Eugonadal and Hypogonadal Men Before and After Testosterone Treatment. J. Bone Miner Res. 2008, 23, 1426–1434. [Google Scholar] [CrossRef]
- Zhang, N.; Magland, J.F.; Rajapakse, C.S.; Bhagat, Y.A.; Wehrli, F.W. Potential of in Vivo MRI-Based Nonlinear Finite-Element Analysis for the Assessment of Trabecular Bone Post-Yield Properties: Potential of in Vivo MRI-Based Nonlinear Finite-Element Analysis. Med. Phys. 2013, 40, 052303. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, C.S.; Leonard, M.B.; Bhagat, Y.A.; Sun, W.; Magland, J.F.; Wehrli, F.W. Micro–MR Imaging–Based Computational Biomechanics Demonstrates Reduction in Cortical and Trabecular Bone Strength after Renal Transplantation. Radiology 2012, 262, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Krug, R.; Banerjee, S.; Han, E.T.; Newitt, D.C.; Link, T.M.; Majumdar, S. Feasibility of in Vivo Structural Analysis of High-Resolution Magnetic Resonance Images of the Proximal Femur. Osteoporos. Int. 2005, 16, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Rajapakse, C.S.; Regatte, R.R.; Babb, J.; Saxena, A.; Belmont, H.M.; Honig, S. 3 Tesla MRI Detects Deterioration in Proximal Femur Microarchitecture and Strength in Long-Term Glucocorticoid Users Compared with Controls: Changes in Proximal Femur Microarchitecture in GIO. J. Magn. Reson. Imaging 2015, 42, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Wehrli, F.W. Structural and Functional Assessment of Trabecular and Cortical Bone by Micro Magnetic Resonance Imaging. J. Magn. Reson. Imaging 2007, 25, 390–409. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Cheng, Y.; Thompson, M.; Haacke, E.M.; Venkatesan, R. Magnetic Resonance Imaging: Physical Principles and Sequence Design; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 1-118-63397-0. [Google Scholar]
- Techawiboonwong, A.; Song, H.K.; Magland, J.F.; Saha, P.K.; Wehrli, F.W. Implications of Pulse Sequence in Structural Imaging of Trabecular Bone. J. Magn. Reson. Imaging 2005, 22, 647–655. [Google Scholar] [CrossRef]
- Chang, G.; Boone, S.; Martel, D.; Rajapakse, C.S.; Hallyburton, R.S.; Valko, M.; Honig, S.; Regatte, R.R. MRI Assessment of Bone Structure and Microarchitecture: Bone Structure and Microarchitecture. J. Magn. Reson. Imaging 2017, 46, 323–337. [Google Scholar] [CrossRef]
- Krug, R.; Carballido-Gamio, J.; Burghardt, A.J.; Kazakia, G.; Hyun, B.H.; Jobke, B.; Banerjee, S.; Huber, M.; Link, T.M.; Majumdar, S. Assessment of Trabecular Bone Structure Comparing Magnetic Resonance Imaging at 3 Tesla with High-Resolution Peripheral Quantitative Computed Tomography Ex Vivo and in Vivo. Osteoporos. Int. 2008, 19, 653–661. [Google Scholar] [CrossRef]
- Pritchard, J.M.; Giangregorio, L.M.; Atkinson, S.A.; Beattie, K.A.; Inglis, D.; Ioannidis, G.; Gerstein, H.; Punthakee, Z.; Adachi, J.D.; Papaioannou, A. Changes in Trabecular Bone Microarchitecture in Postmenopausal Women with and without Type 2 Diabetes: A Two Year Longitudinal Study. BMC Musculoskelet. Disord. 2013, 14, 114. [Google Scholar] [CrossRef]
- Link, T.M.; Vieth, V.; Stehling, C.; Lotter, A.; Beer, A.; Newitt, D.; Majumdar, S. High-Resolution MRI vs Multislice Spiral CT: Which Technique Depicts the Trabecular Bone Structure Best? Eur. Radiol. 2003, 13, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Link, T.M.; Vieth, V.; Langenberg, R.; Meier, N.; Lotter, A.; Newitt, D.; Majumdar, S. Structure Analysis of High Resolution Magnetic Resonance Imaging of the Proximal Femur: In Vitro Correlation with Biomechanical Strength and BMD. Calcif. Tissue Int. 2003, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, C.S.; Magland, J.F.; Wald, M.J.; Liu, X.S.; Zhang, X.H.; Guo, X.E.; Wehrli, F.W. Computational Biomechanics of the Distal Tibia from High-Resolution MR and Micro-CT Images. Bone 2010, 47, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Modlesky, C.M.; Subramanian, P.; Miller, F. Underdeveloped Trabecular Bone Microarchitecture Is Detected in Children with Cerebral Palsy Using High-Resolution Magnetic Resonance Imaging. Osteoporos. Int. 2008, 19, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, C.S.; Phillips, E.A.; Sun, W.; Wald, M.J.; Magland, J.F.; Snyder, P.J.; Wehrli, F.W. Vertebral Deformities and Fractures Are Associated with MRI and PQCT Measures Obtained at the Distal Tibia and Radius of Postmenopausal Women. Osteoporos. Int. 2014, 25, 973–982. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soldati, E.; Bendahan, D.; Pithioux, M.; Vicente, J. MRI Assessment of Bone Microarchitecture in Human Bone Samples: The Issue of Air Bubbles Artefacts. Bone Rep. 2020, 13, 100541. [Google Scholar] [CrossRef]
- Baum, T. Use of MR-Based Trabecular Bone Microstructure Analysis at the Distal Radius for Osteoporosis Diagnostics: A Study in Post-Menopausal Women with Breast Cancer and Treated with Aromatase Inhibitor. Clin. Cases Miner. Bone Metab. 2016. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Ren, X.; Si, L.; Shen, H.; Wang, Q.; Yao, W. Quantitative Evaluation of Subchondral Bone Microarchitecture in Knee Osteoarthritis Using 3T MRI. BMC Musculoskelet. Disord. 2017, 18, 496. [Google Scholar] [CrossRef]
- MacKay, J.W.; Murray, P.J.; Kasmai, B.; Johnson, G.; Donell, S.T.; Toms, A.P. Subchondral Bone in Osteoarthritis: Association between MRI Texture Analysis and Histomorphometry. Osteoarthr. Cartil. 2017, 25, 700–707. [Google Scholar] [CrossRef]
- Chiba, K.; Uetani, M.; Kido, Y.; Ito, M.; Okazaki, N.; Taguchi, K.; Shindo, H. Osteoporotic Changes of Subchondral Trabecular Bone in Osteoarthritis of the Knee: A 3-T MRI Study. Osteoporos. Int. 2012, 23, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Soldati, E.; Pithioux, M.; Vicente, J.; Bendahan, D. Trabecular Bone Microarchitecture: A Comparative Analysis between High Field, Ultra High Field MRI and X-Ray Micro CT in Humans Anatomical Samples. Bone Rep. 2020, 13, 100542. [Google Scholar] [CrossRef]
- Guenoun, D.; Fouré, A.; Pithioux, M.; Guis, S.; Le Corroller, T.; Mattei, J.-P.; Pauly, V.; Guye, M.; Bernard, M.; Chabrand, P.; et al. Correlative Analysis of Vertebral Trabecular Bone Microarchitecture and Mechanical Properties: A Combined Ultra-High Field (7 Tesla) MRI and Biomechanical Investigation. SPINE 2017, 42, E1165–E1172. [Google Scholar] [CrossRef]
- Rajapakse, C.S.; Magland, J.; Zhang, X.H.; Liu, X.S.; Wehrli, S.L.; Guo, X.E.; Wehrli, F.W. Implications of Noise and Resolution on Mechanical Properties of Trabecular Bone Estimated by Image-Based Finite-Element Analysis. J. Orthop. Res. 2009, 27, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Hipp, J.A.; Jansujwicz, A.; Simmons, C.A.; Snyder, B.D. Trabecular Bone Morphology from Micro-Magnetic Resonance Imaging. J. Bone Miner. Res. 2009, 11, 286–292. [Google Scholar] [CrossRef]
- Zaia, A.; Rossi, R.; Galeazzi, R.; Sallei, M.; Maponi, P.; Scendoni, P. Fractal Lacunarity of Trabecular Bone in Vertebral MRI to Predict Osteoporotic Fracture Risk in Over-Fifties Women. The LOTO Study. BMC Musculoskelet. Disord. 2021, 22, 108. [Google Scholar] [CrossRef]
- Kijowski, R.; Tuite, M.; Kruger, D.; Munoz Del Rio, A.; Kleerekoper, M.; Binkley, N. Evaluation of Trabecular Microarchitecture in Nonosteoporotic Postmenopausal Women with and without Fracture. J. Bone Miner. Res. 2012, 27, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Kazakia, G.J.; Carballido-Gamio, J.; Lai, A.; Nardo, L.; Facchetti, L.; Pasco, C.; Zhang, C.A.; Han, M.; Parrott, A.H.; Tien, P.; et al. Trabecular Bone Microstructure Is Impaired in the Proximal Femur of Human Immunodeficiency Virus-Infected Men with Normal Bone Mineral Density. Quant. Imaging Med. Surg. 2018, 8, 5–13. [Google Scholar] [CrossRef]
- Leonard, M.B.; Wehrli, F.W.; Ziolkowski, S.L.; Billig, E.; Long, J.; Nickolas, T.L.; Magland, J.F.; Nihtianova, S.; Zemel, B.S.; Herskovitz, R.; et al. A Multi-Imaging Modality Study of Bone Density, Bone Structure and the Muscle Bone Unit in End-Stage Renal Disease. Bone 2019, 127, 271–279. [Google Scholar] [CrossRef]
- Sharma, A.K.; Toussaint, N.D.; Elder, G.J.; Rajapakse, C.S.; Holt, S.G.; Baldock, P.; Robertson, P.L.; Ebeling, P.R.; Sorci, O.R.; Masterson, R. Changes in Bone Microarchitecture Following Kidney Transplantation-Beyond Bone Mineral Density. Clin. Transplant. 2018, 32, e13347. [Google Scholar] [CrossRef]
- Griffin, L.M.; Honig, S.; Chen, C.; Saha, P.K.; Regatte, R.; Chang, G. 7T MRI of Distal Radius Trabecular Bone Microarchitecture: How Trabecular Bone Quality Varies Depending on Distance from End-of-Bone: 7T MRI of Distal Radius. J. Magn. Reson. Imaging 2017, 45, 872–878. [Google Scholar] [CrossRef]
- Kang, C.; Paley, M.; Ordidge, R.; Speller, R. In Vivo MRI Measurements of Bone Quality in the Calcaneus: A Comparison with DXA and Ultrasound. Osteoporos. Int. 1999, 9, 65–74. [Google Scholar] [CrossRef]
- Guglielmi, G.; Selby, K.; Blunt, B.A.; Jergas, M.; Newitt, D.C.; Genant, H.K.; Majumdar, S. Magnetic Resonance Imaging of the Calcaneus: Preliminary Assessment of Trabecular Bone-Dependent Regional Variations in Marrow Relaxation Time Compared with Dual X-Ray Absorptiometry. Acad. Radiol. 1996, 3, 336–343. [Google Scholar] [CrossRef]
- Arokoski, M.H.; Arokoski, J.P.A.; Vainio, P.; Niemitukia, L.H.; Kröger, H.; Jurvelin, J.S. Comparison of DXA and MRI Methods for Interpreting Femoral Neck Bone Mineral Density. J. Clin. Densitom. 2002, 5, 289–296. [Google Scholar] [CrossRef]
- Brismar, T.B. MR Relaxometry of Lumbar Spine, Hip, and Calcaneus in Healthy Premenopausal Women: Relationship with Dual Energy X-Ray Absorptiometry and Quantitative Ultrasound. Eur. Radiol. 2000, 10, 1215–1221. [Google Scholar] [CrossRef]
- Grampp, S.; Majumdar, S.; Jergas, M.; Newitt, D.; Lang, P.; Harry, K. Genant Distal Radius: In Vivo Assessment with Quantitative MR Imaging, Peripheral Quantitative CT, and Dual X-Ray Absorptiometry. Radiology 1996. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, F.C.; Luetkens, J.A.; Feißt, A.; Enkirch, S.J.; Endler, C.H.-J.; Wagenhäuser, P.J.; Schmeel, L.C.; Träber, F.; Schild, H.H.; Kukuk, G.M. Quantitative Evaluation of T2* Relaxation Times for the Differentiation of Acute Benign and Malignant Vertebral Body Fractures. Eur. J. Radiol. 2018, 108, 59–65. [Google Scholar] [CrossRef]
- Shen, W.; Chen, J.; Punyanitya, M.; Shapses, S.; Heshka, S.; Heymsfield, S.B. MRI-Measured Bone Marrow Adipose Tissue Is Inversely Related to DXA-Measured Bone Mineral in Caucasian Women. Osteoporos. Int. 2007, 18, 641–647. [Google Scholar] [CrossRef]
- Griffith, J.F.; Yeung, D.K.W.; Antonio, G.E.; Lee, F.K.H.; Hong, A.W.L.; Wong, S.Y.S.; Lau, E.M.C.; Leung, P.C. Vertebral Bone Mineral Density, Marrow Perfusion, and Fat Content in Healthy Men and Men with Osteoporosis: Dynamic Contrast-Enhanced MR Imaging and MR Spectroscopy. Radiology 2005, 236, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Woods, G.N.; Ewing, S.K.; Sigurdsson, S.; Kado, D.M.; Eiriksdottir, G.; Gudnason, V.; Hue, T.F.; Lang, T.F.; Vittinghoff, E.; Harris, T.B.; et al. Greater Bone Marrow Adiposity Predicts Bone Loss in Older Women. J. Bone Miner. Res. 2020, 35, 326–332. [Google Scholar] [CrossRef]
- Chang, G.; Rajapakse, C.S.; Chen, C.; Welbeck, A.; Egol, K.; Regatte, R.R.; Saha, P.K.; Honig, S. 3-T MR Imaging of Proximal Femur Microarchitecture in Subjects with and without Fragility Fracture and Nonosteoporotic Proximal Femur Bone Mineral Density. Radiology 2018, 287, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Kindler, J.M.; Pollock, N.K.; Ross, H.L.; Modlesky, C.M.; Singh, H.; Laing, E.M.; Lewis, R.D. Obese Versus Normal-Weight Late-Adolescent Females Have Inferior Trabecular Bone Microarchitecture: A Pilot Case-Control Study. Calcif. Tissue Int. 2017, 101, 479–488. [Google Scholar] [CrossRef]
- Koshi, R. Cunningham’s Manual of Practical Anatomy VOL 1 Upper and Lower Limbs, 16th ed.; OUP: Oxford, UK, 2017; Volume 1, ISBN 978-0-19-874936-3. [Google Scholar]
- Mulder, M.J.; Keuken, M.C.; Bazin, P.-L.; Alkemade, A.; Forstmann, B.U. Size and Shape Matter: The Impact of Voxel Geometry on the Identification of Small Nuclei. PLoS ONE 2019, 14, e0215382. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, C.; Si, L.; Shen, H.; Wang, Q.; Yao, W. Relationship between Subchondral Bone Microstructure and Articular Cartilage in the Osteoarthritic Knee Using 3T MRI: Interrelationships in the OA Knee. J. Magn. Reson. Imaging 2018, 48, 669–679. [Google Scholar] [CrossRef]
- Bolbos, R.I.; Zuo, J.; Banerjee, S.; Link, T.M.; Benjamin Ma, C.; Li, X.; Majumdar, S. Relationship between Trabecular Bone Structure and Articular Cartilage Morphology and Relaxation Times in Early OA of the Knee Joint Using Parallel MRI at 3T. Osteoarthr. Cartil. 2008, 16, 1150–1159. [Google Scholar] [CrossRef]
- Abdulaal, O.M. Evaluation of Optimised 3D Turbo Spin Echo and Gradient Echo MR Pulse Sequences of the Knee at 3T and 1.5T. 9. Radiography 2020. [Google Scholar] [CrossRef]
- Folkesson, J.; Goldenstein, J.; Carballido-Gamio, J.; Kazakia, G.; Burghardt, A.J.; Rodriguez, A.; Krug, R.; de Papp, A.E.; Link, T.M.; Majumdar, S. Longitudinal Evaluation of the Effects of Alendronate on MRI Bone Microarchitecture in Postmenopausal Osteopenic Women. Bone 2011, 48, 611–621. [Google Scholar] [CrossRef]
- Jarraya, M.; Heiss, R.; Duryea, J.; Nagel, A.M.; Lynch, J.A.; Guermazi, A.; Weber, M.-A.; Arkudas, A.; Horch, R.E.; Uder, M.; et al. Bone Structure Analysis of the Radius Using Ultrahigh Field (7T) MRI: Relevance of Technical Parameters and Comparison with 3T MRI and Radiography. Diagnostics 2021, 11, 110. [Google Scholar] [CrossRef]
- Weiger, M.; Stampanoni, M.; Pruessmann, K.P. Direct Depiction of Bone Microstructure Using MRI with Zero Echo Time. Bone 2013, 54, 44–47. [Google Scholar] [CrossRef]
- Kazakia, G.J.; Hyun, B.; Burghardt, A.J.; Krug, R.; Newitt, D.C.; de Papp, A.E.; Link, T.M.; Majumdar, S. In Vivo Determination of Bone Structure in Postmenopausal Women: A Comparison of HR-PQCT and High-Field MR Imaging. J. Bone Miner. Res. 2007, 23, 463–474. [Google Scholar] [CrossRef]
- Wu, H.-Z.; Zhang, X.-F.; Han, S.-M.; Cao, L.; Wen, J.-X.; Wu, W.-J.; Gao, B.-L. Correlation of Bone Mineral Density with MRI T2* Values in Quantitative Analysis of Lumbar Osteoporosis. Arch. Osteoporos 2020, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Bandirali, M.; Leo, G.D.; Papini, G.D.E.; Messina, C.; Sconfienza, L.M.; Ulivieri, F.M.; Sardanelli, F. A New Diagnostic Score to Detect Osteoporosis in Patients Undergoing Lumbar Spine MRI. Eur. Radiol. 2015, 25, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Horowitz, M.C.; MacDougald, O.A.; Scheller, E.L.; Rodeheffer, M.S.; Rosen, C.J.; Klibanski, A. Marrow Fat and Bone—New Perspectives. J. Clin. Endocrinol. Metab. 2013, 98, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Sollmann, N.; Löffler, M.T.; Kronthaler, S.; Böhm, C.; Dieckmeyer, M.; Ruschke, S.; Kirschke, J.S.; Carballido-Gamio, J.; Karampinos, D.C.; Krug, R.; et al. MRI-Based Quantitative Osteoporosis Imaging at the Spine and Femur. J. Magn. Reason. Imaging 2020, jmri.27260. [Google Scholar] [CrossRef]
- Reeder, S.B.; Hu, H.H.; Sirlin, C.B. Proton Density Fat-Fraction: A Standardized Mr-Based Biomarker of Tissue Fat Concentration. J. Magn. Reson. Imaging 2012, 36, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Ma, X.; Jiang, Y.; Huang, D.; Chen, X.; Zhang, M.; Hao, D. Percentage Fat Fraction in Magnetic Resonance Imaging: Upgrading the Osteoporosis-Detecting Parameter. BMC Med. Imaging 2020, 20, 30. [Google Scholar] [CrossRef]
- He, J.; Fang, H.; Li, X. Vertebral Bone Marrow Fat Content in Normal Adults with Varying Bone Densities at 3T Magnetic Resonance Imaging. Acta Radiol 2019, 60, 509–515. [Google Scholar] [CrossRef]
- Karampinos, D.C.; Ruschke, S.; Gordijenko, O.; Garcia, E.G.; Kooijman, H.; Burgkart, R.; Rummeny, E.J.; Bauer, J.S.; Baum, T. Association of MRS-Based Vertebral Bone Marrow Fat Fraction with Bone Strength in a Human In Vitro Model. J. Osteoporos. 2015, 2015, 152349. [Google Scholar] [CrossRef] [PubMed]
- Sheu, Y.; Amati, F.; Schwartz, A.V.; Danielson, M.E.; Li, X.; Boudreau, R.; Cauley, J.A. Vertebral Bone Marrow Fat, Bone Mineral Density and Diabetes: The Osteoporotic Fractures in Men (MrOS) Study. Bone 2017, 97, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Manenti, G.; Capuani, S.; Fanucci, E.; Assako, E.P.; Masala, S.; Sorge, R.; Iundusi, R.; Tarantino, U.; Simonetti, G. Diffusion Tensor Imaging and Magnetic Resonance Spectroscopy Assessment of Cancellous Bone Quality in Femoral Neck of Healthy, Osteopenic and Osteoporotic Subjects at 3T: Preliminary Experience. Bone 2013, 55, 7–15. [Google Scholar] [CrossRef][Green Version]
- Pietro, G.D.; Capuani, S.; Manenti, G.; Vinicola, V.; Fusco, A.; Baldi, J.; Scimeca, M.; Hagberg, G.; Bozzali, M.; Simonetti, G.; et al. Bone Marrow Lipid Profiles from Peripheral Skeleton as Potential Biomarkers for Osteoporosis: A 1H-MR Spectroscopy Study. Acad. Radiol. 2016, 23, 273–283. [Google Scholar] [CrossRef]
- Ismail, U.N.; Azlan, C.A.; Khairullah, S.; Azman, R.R.; Omar, N.F.; Md Shah, M.N.; Yeong, C.H.; Jackson, N.; Ng, K.H. Marrow Fat Content and Composition in Β-Thalassemia: A Study Using 1H-MRS. J. Magn. Reason. Imaging 2021, 53, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.W.; Greenblatt, L.; Eajazi, A.; Torriani, M.; Bredella, M.A. Marrow Adipose Tissue Composition in Adults with Morbid Obesity. Bone 2017, 97, 38–42. [Google Scholar] [CrossRef]
- Singhal, V.; Bredella, M.A. Marrow Adipose Tissue Imaging in Humans. Bone 2019, 118, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Karampinos, D.C.; Ruschke, S.; Dieckmeyer, M.; Diefenbach, M.; Franz, D.; Gersing, A.S.; Krug, R.; Baum, T. Quantitative MRI and Spectroscopy of Bone Marrow: Quantitative MR of Bone Marrow. J. Magn. Reson. Imaging 2018, 47, 332–353. [Google Scholar] [CrossRef]
- Ruschke, S.; Pokorney, A.; Baum, T.; Eggers, H.; Miller, J.H.; Hu, H.H.; Karampinos, D.C. Measurement of Vertebral Bone Marrow Proton Density Fat Fraction in Children Using Quantitative Water–Fat MRI. Magn. Reason. Mater. Phys. Biol. Med. 2017, 30, 449–460. [Google Scholar] [CrossRef]
- Li, G.; Xu, Z.; Gu, H.; Li, X.; Yuan, W.; Chang, S.; Fan, J.; Calimente, H.; Hu, J. Comparison of Chemical Shift-Encoded Water-Fat MRI and MR Spectroscopy in Quantification of Marrow Fat in Postmenopausal Females: Water-Fat Imaging Quantifies Marrow Fat. J. Magn. Reson. Imaging 2017, 45, 66–73. [Google Scholar] [CrossRef]
- Ruschke, S.; Eggers, H.; Kooijman, H.; Diefenbach, M.N.; Baum, T.; Haase, A.; Rummeny, E.J.; Hu, H.H.; Karampinos, D.C. Correction of Phase Errors in Quantitative Water-Fat Imaging Using a Monopolar Time-Interleaved Multi-Echo Gradient Echo Sequence: Phase Error Correction in Time-Interleaved Water-Fat Imaging. Magn. Reson. Med. 2017, 78, 984–996. [Google Scholar] [CrossRef]
- Martel, D.; Leporq, B.; Saxena, A.; Belmont, H.M.; Turyan, G.; Honig, S.; Regatte, R.R.; Chang, G. 3T Chemical Shift-encoded MRI: Detection of Altered Proximal Femur Marrow Adipose Tissue Composition in Glucocorticoid Users and Validation with Magnetic Resonance Spectroscopy. J. Magn. Reson. Imaging 2018, 50, 490–496. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, M.; Ding, J.; Zhang, X.; Spuhler, K.; Hu, S.; Li, M.; Fan, W.; Chen, L.; Zhang, X.; et al. Prediction of Abnormal Bone Density and Osteoporosis from Lumbar Spine MR Using Modified Dixon Quant in 257 Subjects with Quantitative Computed Tomography as Reference: Bone Density Prediction From MDixon MR. J. Magn. Reson. Imaging 2019, 49, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Martel, D.; Leporq, B.; Bruno, M.; Regatte, R.R.; Honig, S.; Chang, G. Chemical Shift-Encoded MRI for Assessment of Bone Marrow Adipose Tissue Fat Composition: Pilot Study in Premenopausal versus Postmenopausal Women. Magn. Reson. Imaging 2018, 53, 148–155. [Google Scholar] [CrossRef]
- Schmeel, F.C.; Luetkens, J.A.; Enkirch, S.J.; Feißt, A.; Endler, C.H.-J.; Schmeel, L.C.; Wagenhäuser, P.J.; Träber, F.; Schild, H.H.; Kukuk, G.M. Proton Density Fat Fraction (PDFF) MR Imaging for Differentiation of Acute Benign and Neoplastic Compression Fractures of the Spine. Eur. Radiol. 2018, 28, 5001–5009. [Google Scholar] [CrossRef]
- Dieckmeyer, M.; Junker, D.; Ruschke, S.; Mookiah, M.R.K.; Subburaj, K.; Burian, E.; Sollmann, N.; Kirschke, J.S.; Karampinos, D.C.; Baum, T. Vertebral Bone Marrow Heterogeneity Using Texture Analysis of Chemical Shift Encoding-Based MRI: Variations in Age, Sex, and Anatomical Location. Front. Endocrinol. 2020, 11, 555931. [Google Scholar] [CrossRef]
- Baum, T.; Yap, S.P.; Dieckmeyer, M.; Ruschke, S.; Eggers, H.; Kooijman, H.; Rummeny, E.J.; Bauer, J.S.; Karampinos, D.C. Assessment of Whole Spine Vertebral Bone Marrow Fat Using Chemical Shift-Encoding Based Water-Fat MRI: Whole Spine Water-Fat Imaging. J. Magn. Reson. Imaging 2015, 42, 1018–1023. [Google Scholar] [CrossRef]
- Gómez, M.P.A.; Benavent, C.A.; Simoni, P.; Aparisi, F.; Guglielmi, G.; Bazzocchi, A. Fat and Bone: The Multiperspective Analysis of a Close Relationship. Quant. Imaging Med. Surg. 2020, 10, 22. [Google Scholar]
- Haacke, E.M.; Xu, Y.; Cheng, Y.-C.N.; Reichenbach, J.R. Susceptibility Weighted Imaging (SWI). Magn. Reson. Med. 2004, 52, 612–618. [Google Scholar] [CrossRef]
- Rauscher, A.; Sedlacik, J.; Deistung, A.; Mentzel, H.-J.; Reichenbach, J.R. Susceptibility Weighted Imaging: Data Acquisition, Image Reconstruction and Clinical Applications. Zeitschrift für Medizinische Physik 2006, 16, 240–250. [Google Scholar] [CrossRef]
- Schweser, F.; Deistung, A.; Reichenbach, J.R. Foundations of MRI Phase Imaging and Processing for Quantitative Susceptibility Mapping (QSM). Z. Med. Phys. 2016, 26, 6–34. [Google Scholar] [CrossRef]
- Deistung, A.; Schweser, F.; Reichenbach, J.R. Overview of Quantitative Susceptibility Mapping: Overview of Quantitative Susceptibility Mapping. NMR Biomed. 2017, 30, e3569. [Google Scholar] [CrossRef]
- Dimov, A.V.; Liu, Z.; Spincemaille, P.; Prince, M.R.; Du, J.; Wang, Y. Bone Quantitative Susceptibility Mapping Using a Chemical Species-Specific R2* Signal Model with Ultrashort and Conventional Echo Data: Bone QSM Using a R2* Signal Model With UTE Conventional Echo Data. Magn. Reson. Med. 2018, 79, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, W.; Kovanlikaya, I.; Kovanlikaya, A.; Liu, T.; Wang, S.; Salustri, C.; Wang, Y. Intracranial Calcifications and Hemorrhages: Characterization with Quantitative Susceptibility Mapping. Radiology 2014, 270, 496–505. [Google Scholar] [CrossRef]
- Haacke, E.M.; Liu, S.; Buch, S.; Zheng, W.; Wu, D.; Ye, Y. Quantitative Susceptibility Mapping: Current Status and Future Directions. Magn. Reson. Imaging 2015, 33, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, B.; Liu, C. Quantitative Susceptibility Mapping of Human Brain Reflects Spatial Variation in Tissue Composition. NeuroImage 2011, 55, 1645–1656. [Google Scholar] [CrossRef]
- Du, J.; Hermida, J.C.; Diaz, E.; Corbeil, J.; Znamirowski, R.; D’Lima, D.D.; Bydder, G.M. Assessment of Cortical Bone with Clinical and Ultrashort Echo Time Sequences. Magn. Reson. Med. 2013, 70, 697–704. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; Zhang, X.; Mei, Y.; Feng, Y.; Zhang, X. Bone Susceptibility Mapping with MRI Is an Alternative and Reliable Biomarker of Osteoporosis in Postmenopausal Women. Eur Radiol. 2018, 28, 5027–5034. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, M.N.; Meineke, J.; Ruschke, S.; Baum, T.; Gersing, A.; Karampinos, D.C. On the Sensitivity of Quantitative Susceptibility Mapping for Measuring Trabecular Bone Density. Magn. Reson. Med. 2019, 81, 1739–1754. [Google Scholar] [CrossRef]
- Lu, X.; Jang, H.; Ma, Y.; Jerban, S.; Chang, E.; Du, J. Ultrashort Echo Time Quantitative Susceptibility Mapping (UTE-QSM) of Highly Concentrated Magnetic Nanoparticles: A Comparison Study about Different Sampling Strategies. Molecules 2019, 24, 1143. [Google Scholar] [CrossRef]
- Jerban, S.; Lu, X.; Jang, H.; Ma, Y.; Namiranian, B.; Le, N.; Li, Y.; Chang, E.Y.; Du, J. Significant Correlations between Human Cortical Bone Mineral Density and Quantitative Susceptibility Mapping (QSM) Obtained with 3D Cones Ultrashort Echo Time Magnetic Resonance Imaging (UTE-MRI). Magn. Reson. Imaging 2019, 62, 104–110. [Google Scholar] [CrossRef]
- Seifert, A.C.; Wehrli, F.W. Solid-State Quantitative 1H and 31P MRI of Cortical Bone in Humans. Curr. Osteoporos. Rep. 2016, 14, 77–86. [Google Scholar] [CrossRef]
- Gervais, C.; Bonhomme, C.; Laurencin, D. Recent Directions in the Solid-State NMR Study of Synthetic and Natural Calcium Phosphates. Solid State Nucl. Magn. Reson. 2020, 107, 101663. [Google Scholar] [CrossRef]
- Glover, G.H.; Pauly, J.M.; Bradshaw, K.M. Boron-11 Imaging with a Three-Dimensional Reconstruction Method. J. Magn. Reson. Imaging 1992, 2, 47–52. [Google Scholar] [CrossRef]
- Weiger, M.; Pruessmann, K.P.; Hennel, F. MRI with Zero Echo Time: Hard versus Sweep Pulse Excitation: MRI With Zero Echo Time. Magn. Reson. Med. 2011, 66, 379–389. [Google Scholar] [CrossRef]
- Jerban, S.; Chang, D.G.; Ma, Y.; Jang, H.; Chang, E.Y.; Du, J. An Update in Qualitative Imaging of Bone Using Ultrashort Echo Time Magnetic Resonance. Front. Endocrinol. 2020, 11, 555756. [Google Scholar] [CrossRef] [PubMed]
- Idiyatullin, D.; Corum, C.; Park, J.-Y.; Garwood, M. Fast and Quiet MRI Using a Swept Radiofrequency. J. Magn. Reson. 2006, 181, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Idiyatullin, D.; Suddarth, S.; Corum, C.A.; Adriany, G.; Garwood, M. Continuous SWIFT. J. Magn. Reson. 2012, 220, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, S.; Dou, W.; Jansen, J.A.; Walboomers, X.F. Magnetic Resonance Imaging of Hard Tissues and Hard Tissue Engineered Bio-Substitutes. Mol. Imaging Biol 2019, 21, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, M.; Hanada, H.; Oka, K.; Takahashi, T.; Yamamoto, E.; Fujii, M. A Robust Ultrashort TE (UTE) Imaging Method With Corrected k-Space Trajectory by Using Parametric Multiple Function Model of Gradient Waveform. IEEE Trans. Med Imaging 2013, 32, 11. [Google Scholar] [CrossRef]
- Kuethe, D.O.; Caprihan, A.; Lowe, I.J.; Madio, D.P.; Gach, H.M. Transforming NMR Data Despite Missing Points. J. Magn. Reson. 1999, 139, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ackerman, J.L.; Chesler, D.A.; Graham, L.; Wang, Y.; Glimcher, M.J. Density of Organic Matrix of Native Mineralized Bone Measured by Water- and Fat-Suppressed Proton Projection MRI. Magn. Reson. Med. 2003, 50, 59–68. [Google Scholar] [CrossRef]
- Grodzki, D.M.; Jakob, P.M.; Heismann, B. Ultrashort Echo Time Imaging Using Pointwise Encoding Time Reduction with Radial Acquisition (PETRA). Magn. Reson. Med. 2012, 67, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Idiyatullin, D.; Corum, C.A.; Nixdorf, D.R.; Garwood, M. Intraoral Approach for Imaging Teeth Using the Transverse B 1 Field Components of an Occlusally Oriented Loop Coil: Intraoral Approach for Imaging Teeth. Magn. Reson. Med. 2014, 72, 160–165. [Google Scholar] [CrossRef]
- Zhao, X.; Song, H.K.; Seifert, A.C.; Li, C.; Wehrli, F.W. Feasibility of Assessing Bone Matrix and Mineral Properties in Vivo by Combined Solid-State 1H and 31P MRI. PLoS ONE 2017, 12, e0173995. [Google Scholar] [CrossRef]
- Techawiboonwong, A.; Song, H.K.; Leonard, M.B.; Wehrli, F.W. Cortical Bone Water: In Vivo Quantification with Ultrashort Echo-Time MR Imaging. Radiology 2008, 248, 824–833. [Google Scholar] [CrossRef]
- Manhard, M.K.; Horch, R.A.; Gochberg, D.F.; Nyman, J.S.; Does, M.D. In Vivo Quantitative MR Imaging of Bound and Pore Water in Cortical Bone. Radiology 2015, 277, 221–229. [Google Scholar] [CrossRef]
- Chen, J.; Carl, M.; Ma, Y.; Shao, H.; Lu, X.; Chen, B.; Chang, E.Y.; Wu, Z.; Du, J. Fast Volumetric Imaging of Bound and Pore Water in Cortical Bone Using Three-Dimensional Ultrashort-TE (UTE) and Inversion Recovery UTE Sequences: Bound and Pore Water Imaging in Cortical Bone Using 3D UTE Sequences. NMR Biomed. 2016, 29, 1373–1380. [Google Scholar] [CrossRef]
- Jerban, S.; Ma, Y.; Li, L.; Jang, H.; Wan, L.; Guo, T.; Searleman, A.; Chang, E.Y.; Du, J. Volumetric Mapping of Bound and Pore Water as Well as Collagen Protons in Cortical Bone Using 3D Ultrashort Echo Time Cones MR Imaging Techniques. Bone 2019, 127, 120–128. [Google Scholar] [CrossRef]
- Kaflak, A.; Chmielewski, D.; Kolodziejski, W. Solid-State NMR Study of Discrete Environments of Bone Mineral Nanoparticles Using Phosphorus-31 Relaxation. J. Appl. Biomed. 2016, 14, 321–330. [Google Scholar] [CrossRef]
- Rajapakse, C.S.; Bashoor-Zadeh, M.; Li, C.; Sun, W.; Wright, A.C.; Wehrli, F.W. Volumetric Cortical Bone Porosity Assessment with MR Imaging: Validation and Clinical Feasibility. Radiology 2015, 276, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, I.; Cortes, A.R.G.; Sánchez-Siles, J.-M.; Ackerman, J.L.; González-Quevedo, D.; García, Á.; Yaghoubi, F.; Abdallah, M.-N.; Eimar, H.; Alsheghri, A.; et al. Composition and Characteristics of Trabecular Bone in Osteoporosis and Osteoarthritis. Bone 2020, 140, 115558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, H.K.; Wehrli, F.W. In Vivo Bone 31P Relaxation Times and Their Implications on Mineral Quantification. Magn. Reson. Med. 2018, 80, 2514–2524. [Google Scholar] [CrossRef]
- Li, C.; Seifert, A.C.; Rad, H.S.; Bhagat, Y.A.; Rajapakse, C.S.; Sun, W.; Lam, S.C.B.; Wehrli, F.W. Cortical Bone Water Concentration: Dependence of MR Imaging Measures on Age and Pore Volume Fraction. Radiology 2014, 272, 796–806. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yon, M.; Sarou-Kanian, V.; Scheler, U.; Bouler, J.-M.; Bujoli, B.; Massiot, D.; Fayon, F. Solid-State 31P and 1H Chemical MR Micro-Imaging of Hard Tissues and Biomaterials with Magic Angle Spinning at Very High Magnetic Field. Sci. Rep. 2017, 7, 8224. [Google Scholar] [CrossRef]
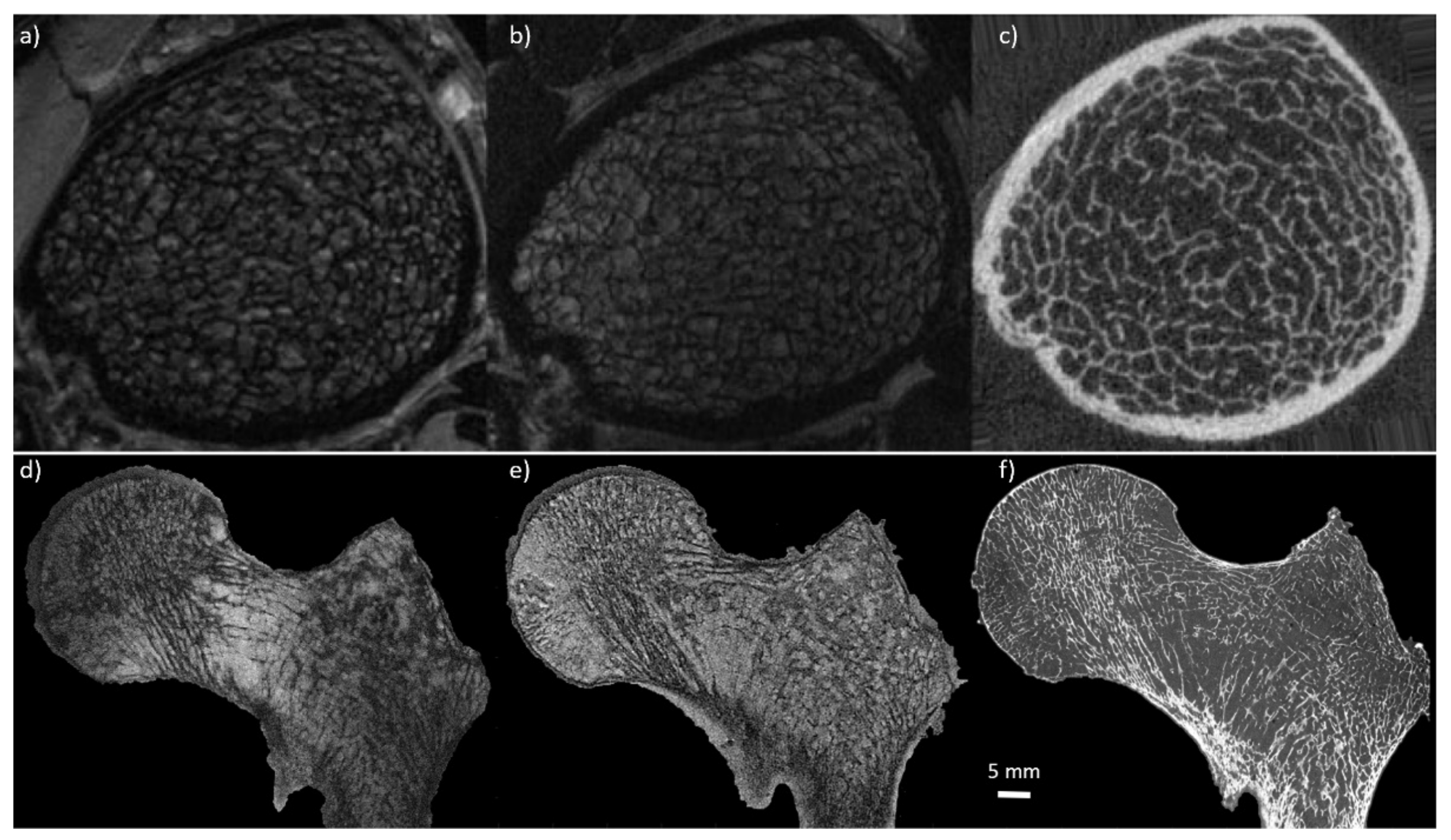
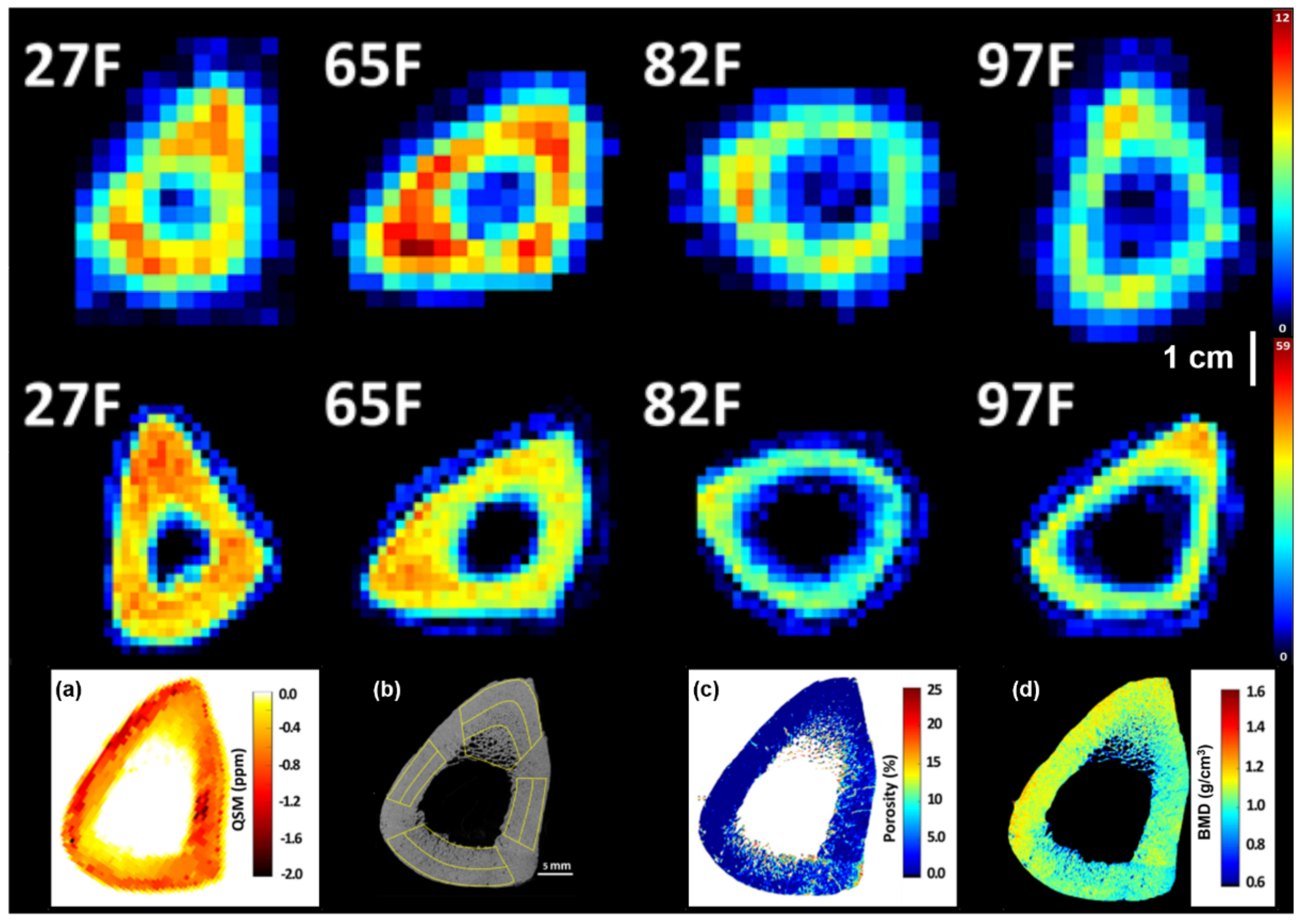
| Anatomical Site | Clinical History | Specimen /Patient | Acq. Time | Sl. Thickness [mm] [mm] | Pix. Size [mm] | FOV [mm] | Sequence | Main Field | N° | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| distal radii | type 2 diabetes | patient | 12 min 9 s | 1 | 0.195 × 0.195 | 100 × 100 | FSE | 1T | [78] | Pritchard et al. |
| calcaneus | osteoporotic hip fractures | patient | 15 min 15 s | 0.5 | 0.195 × 0.195 | 100 × 100 | GE | 1.5T | [67] | Link et al. |
| distal radii | healthy | patient | 16 min 25 s | 0.5 | 0.156 × 0.156 | 80 × 45 | 3D FLASE | 1.5T | [75] | Techawiboonwong et al. |
| distal radii | healthy | patient | 3 min 15 s | 0.5 | 0.156 × 0.156 | 80 × 45 | 3D SSFP | 1.5T | [75] | Techawiboonwong et al. |
| distal radii | NA | specimen | 15 min | 0.3 | 0.156 × 0.156 | 80 | GE | 1.5T | [13] | Majumdar et al. |
| lumbar spine | osteoporotic | patient | 16 min | 0.7 | 0.156 × 0.156 | 80 × 80 | GE | 1.5T | [65] | Majumdar et al. |
| distal radii | hip fractures | patient | NA | 0.5 | 0.156 × 0.156 | 80 × 80 | GE | 1.5T | [16] | Majumdar et al. |
| distal radii | NA | specimen | 58 min (1) 16 min (2) | 0.3 (1) 0.9 (2) | 0.153 × 0.153 | 49×78 | SE | 1.5T | [79] | Link et al. |
| prox. femur | NA | specimen | 74 min (1) 27 min (2) | 0.3 (1) 0.9 (2) | 0.195 × 0.195 | 75 × 100 | SE | 1.5T | [80] | Link et al. |
| prox. femur | healthy | patient | 6 min 12 s | 1.5 | 0.234 × 0.234 | NA | 3D FIESTA | 1.5T | [71] | Krug et al. |
| distal tibiae | NA | specimen | 40 min | 0.16 | 0.160 × 0.160 | 70 × 63 | 3D FLASE | 1.5T | [81] | Rajapakse et al. |
| lumbar spine | NA | specimen | 15 min 23 s | 0.41 | 0.137 × 0.137 | 70 × 64 × 13 | 3D FLASE | 1.5T | [70] | Rajapakse et al. |
| distal radii(1) distal tibiae(2) | osteopenic and osteoporotic | patient | 12 min (1) 16 min (2) | 0.4 | 0.137 × 0.137 | 70 × 40(1) 70 × 50(2) | 3D FLASE | 1.5T | [66] | Ladinsky et al. |
| distal femur | cerebral palsy (children) | patient | 9 min 52 s | 0.7 | 0.175 × 0.175 | 90 | 3D fast GE | 1.5T | [82] | Modlesky et al. |
| distal radii(1) distal tibi.ae(2) | osteoporotic | patient | 12 min (1) 16 min (2) | 0.41 | 0.137 × 0.137 | 70 × 40 × 13 (1) 70 × 50 × 13 (2) | 3D FLASE | 1.5T | [83] | Rajapakse et al. |
| prox. femur | NA | specimen | 16 min 55 s | 1.1 | 0.21 × 0.21 | 120 | TSE | 3T | [84] | Soldati et al. |
| prox. femur | healthy | patient | 12 min 43 s | 1.5 | 0.234 × 0.235 | NA | 3D FIESTA | 3T | [71] | Krug et al. |
| distal radii, distal tibiae | NA | specimen | <10 min | 0.5 | 0.156 × 0.156 | NA | GE | 3T | [77] | Krug et al. |
| distal radii, distal tibiae | NA | specimen | <10 min | 0.5 | 0.156 × 0.156 | NA | GRE | 3T | [77] | Krug et al. |
| distal radii, distal tibiae | NA | specimen | <10 min | 0.5 | 0.156 × 0.156 | NA | SE | 3T | [77] | Krug et al. |
| distal tibiae | osteoporotic | patient | 15 min | 0.41 | 0.137 × 0.137 | 70 × 64 × 13 | 3D FLASE | 3T | [69] | Zhang et al. |
| prox. femur | fragility fractured | patient | 25 min 30 s | 1.5 | 0.234 × 0.234 | 120 | FLASH | 3T | [60] | Chang et al. |
| prox. femur | long-term glucocorticoid | patient | 15 min 18 s | 1.5 | 0.234 × 0.234 | 100 | FLASH | 3T | [72] | Chang et al. |
| distal radii | HR+ breast cancer | patient | 7 min | 0.34 | 0.170 × 0.170 | 65 | GE | 3T | [85] | Baum et al. |
| distal femur | osteoarthritis | patient | 9 min 18 s | 1 | 0.180 × 0.180 | 100 | 3D B-FFE | 3T | [86] | Liu et al. |
| prox. tibia | osteoarthritis | patient | 3 min | 2.8 | 0.230 × 0.240 | 120 × 123 | SE | 3T | [87] | MacKey et al. |
| prox. tibia, distal femur | osteoarthritis | patient | NA | 1 | 0.195 × 0.195 | 100 | FIESTA-c | 3T | [88] | Chiba et al. |
| prox. tibia, distal femur | osteoarthritis | patient | NA | 1 | 0.195 × 0.195 | 160 | SPGR | 3T | [88] | Chiba et al. |
| distal tibiae | NA | specimen | 7 min | 0.41 | 0.137 × 0.137 | 70 × 53 × 13 | 3D FLASE | 3T | [19] | Rajapakse et al. |
| prox. femur | NA | specimen | 16 min 45 s | 1.5 | 0.13 × 0.13 | 130 | TSE | 7T | [89] | Soldati et al. |
| prox. femur | NA | specimen | 37 min 36 s | 0.5 | 0.170 × 0.170 | 140 × 140 | GRE | 7T | [62] | Guenoun et al. |
| distal tibiae | healthy | patient | 19 min 10 s | 0.5 | 0.156 × 0.156 | NA | SE | 7T | [17] | Krug et al. |
| distal tibiae | healthy | patient | 18 min 25 s | 0.5 | 0.156 × 0.157 | NA | FP | 7T | [17] | Krug et al. |
| vertebrae (1 axial, 2 sagittal) | NA | specimen | 34 min (1) 51 min (2) | 0.4 (1) 0.5 (2) | 0.170 × 0.170 | 140 × 140 | GRE | 7T | [90] | Guenoun et al. |
| distal femur | fragility fractured | patient | 7 min 9 s | 1 | 0.234 × 0.234 | 120 | FLASH | 7T | [18] | Chang et al. |
| femurs, tibiae, vertebrae | NA | specimen | 120 min | 0.05 | 0.05 × 0.05 | 6.4 × 6.4 × 25.6 | SE | 9.4T | [91] | Rajapakse et al. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldati, E.; Rossi, F.; Vicente, J.; Guenoun, D.; Pithioux, M.; Iotti, S.; Malucelli, E.; Bendahan, D. Survey of MRI Usefulness for the Clinical Assessment of Bone Microstructure. Int. J. Mol. Sci. 2021, 22, 2509. https://doi.org/10.3390/ijms22052509
Soldati E, Rossi F, Vicente J, Guenoun D, Pithioux M, Iotti S, Malucelli E, Bendahan D. Survey of MRI Usefulness for the Clinical Assessment of Bone Microstructure. International Journal of Molecular Sciences. 2021; 22(5):2509. https://doi.org/10.3390/ijms22052509
Chicago/Turabian StyleSoldati, Enrico, Francesca Rossi, Jerome Vicente, Daphne Guenoun, Martine Pithioux, Stefano Iotti, Emil Malucelli, and David Bendahan. 2021. "Survey of MRI Usefulness for the Clinical Assessment of Bone Microstructure" International Journal of Molecular Sciences 22, no. 5: 2509. https://doi.org/10.3390/ijms22052509
APA StyleSoldati, E., Rossi, F., Vicente, J., Guenoun, D., Pithioux, M., Iotti, S., Malucelli, E., & Bendahan, D. (2021). Survey of MRI Usefulness for the Clinical Assessment of Bone Microstructure. International Journal of Molecular Sciences, 22(5), 2509. https://doi.org/10.3390/ijms22052509









