pH-Responsive Self-Assembly of Designer Aromatic Peptide Amphiphiles and Enzymatic Post-Modification of Assembled Structures
Abstract
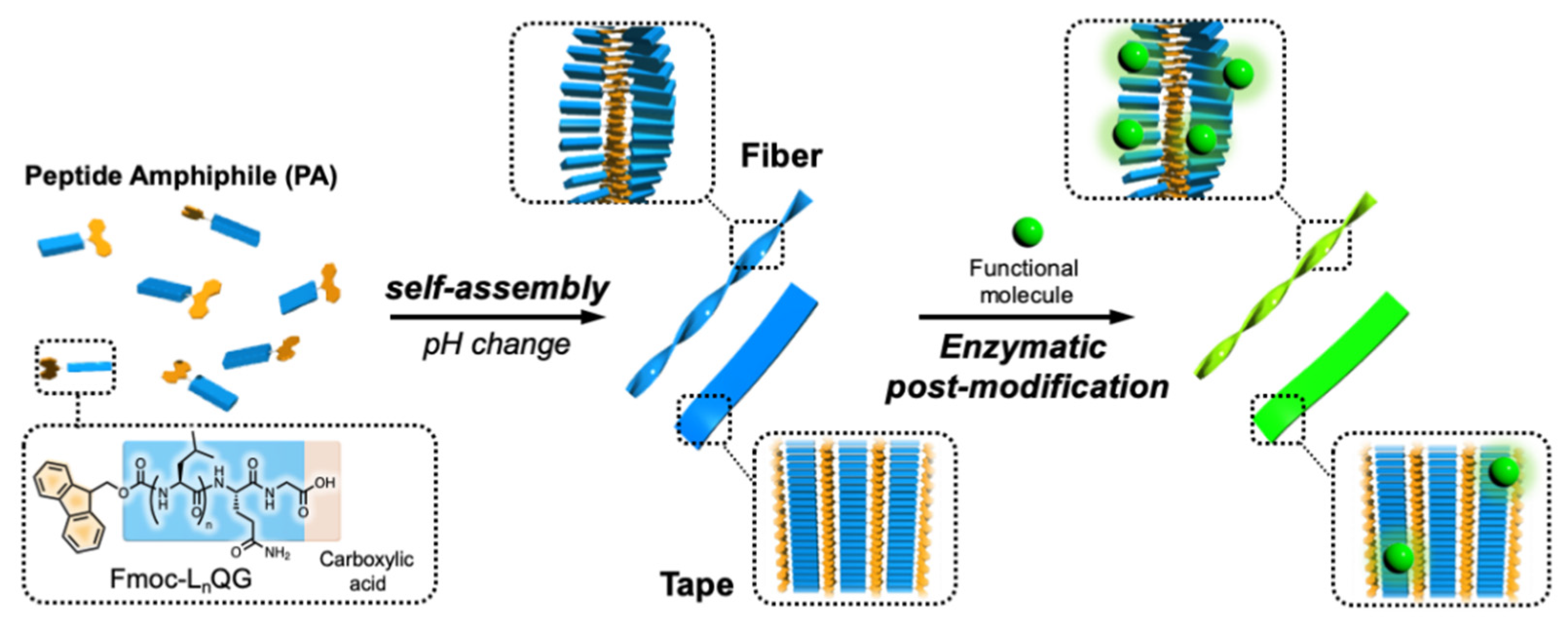
1. Introduction
2. Results and Discussion
2.1. Change in Self-Organization Behavior of Fmoc-LnQG (n = 2, 3) Depending on pH
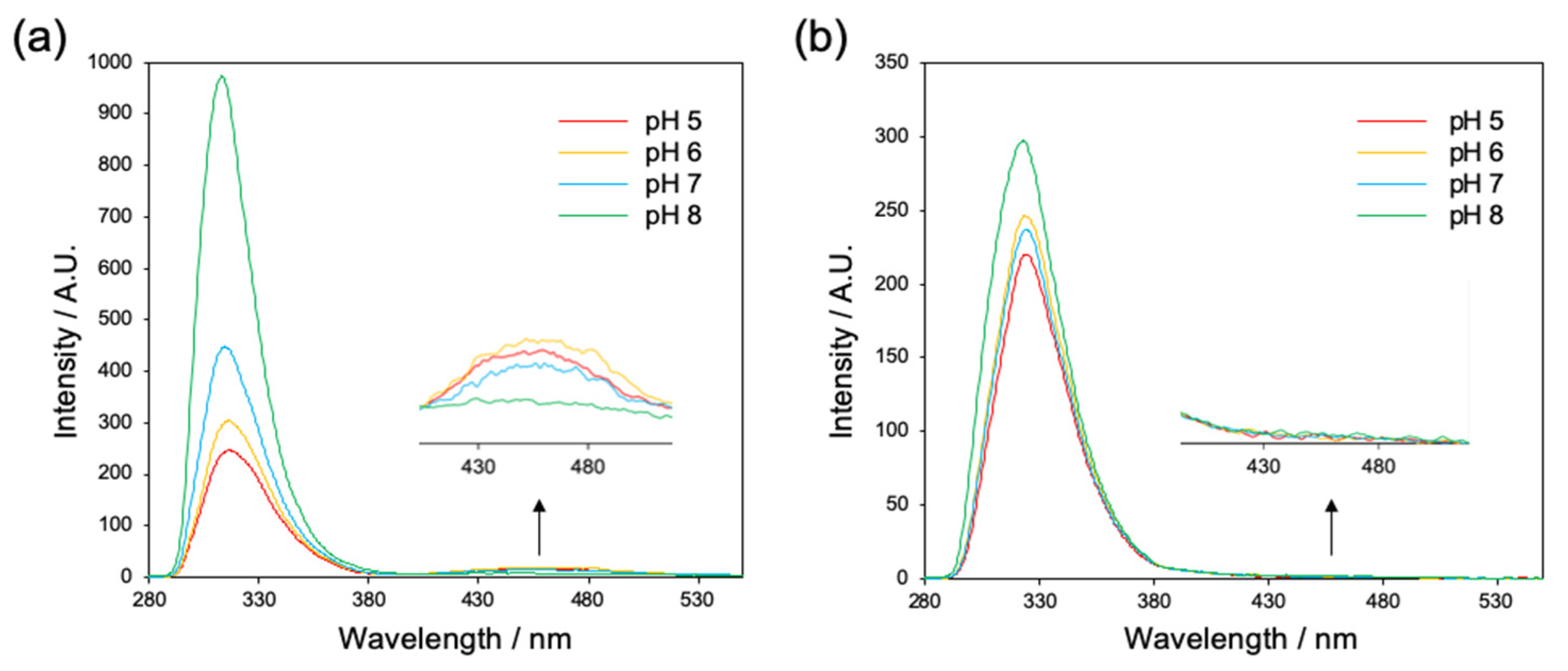
2.1.1. Evaluation of Interaction between Fmoc Groups by Fluorescence Spectroscopy
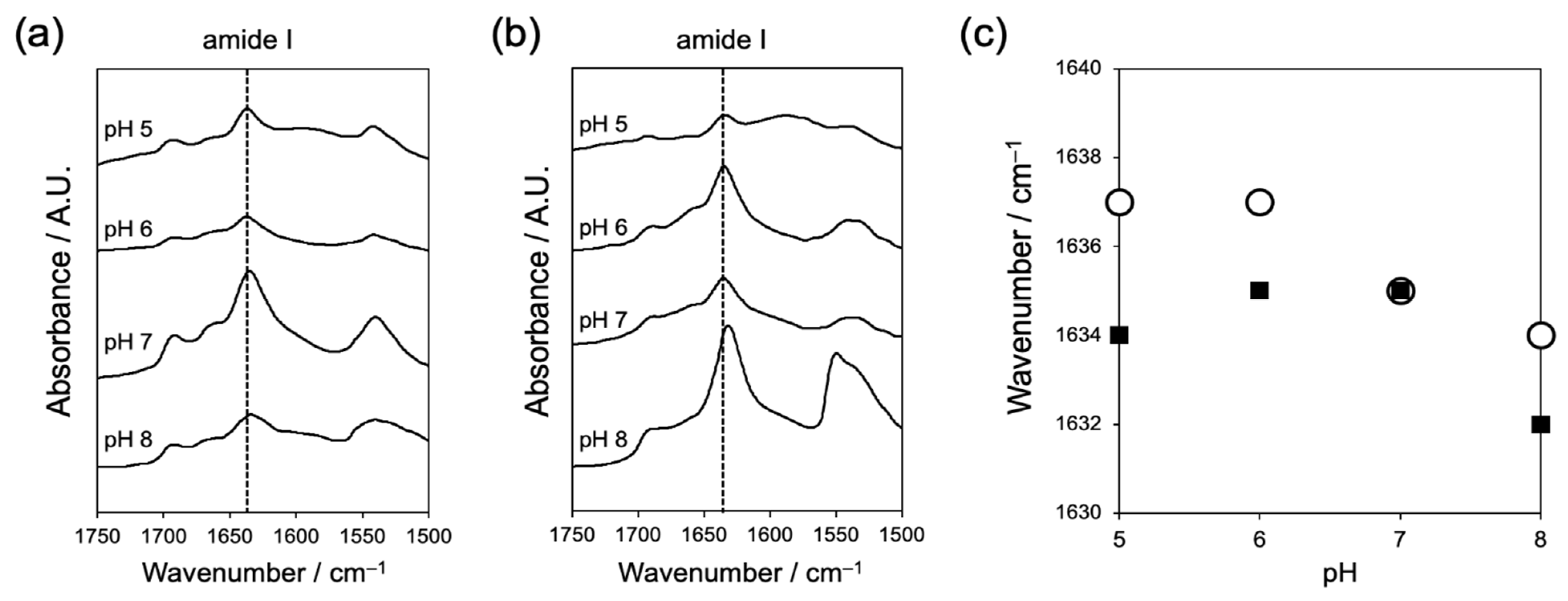
2.1.2. Evaluation of Interaction between Peptides by Fourier Transform-Infrared Spectroscopy (FT-IR)
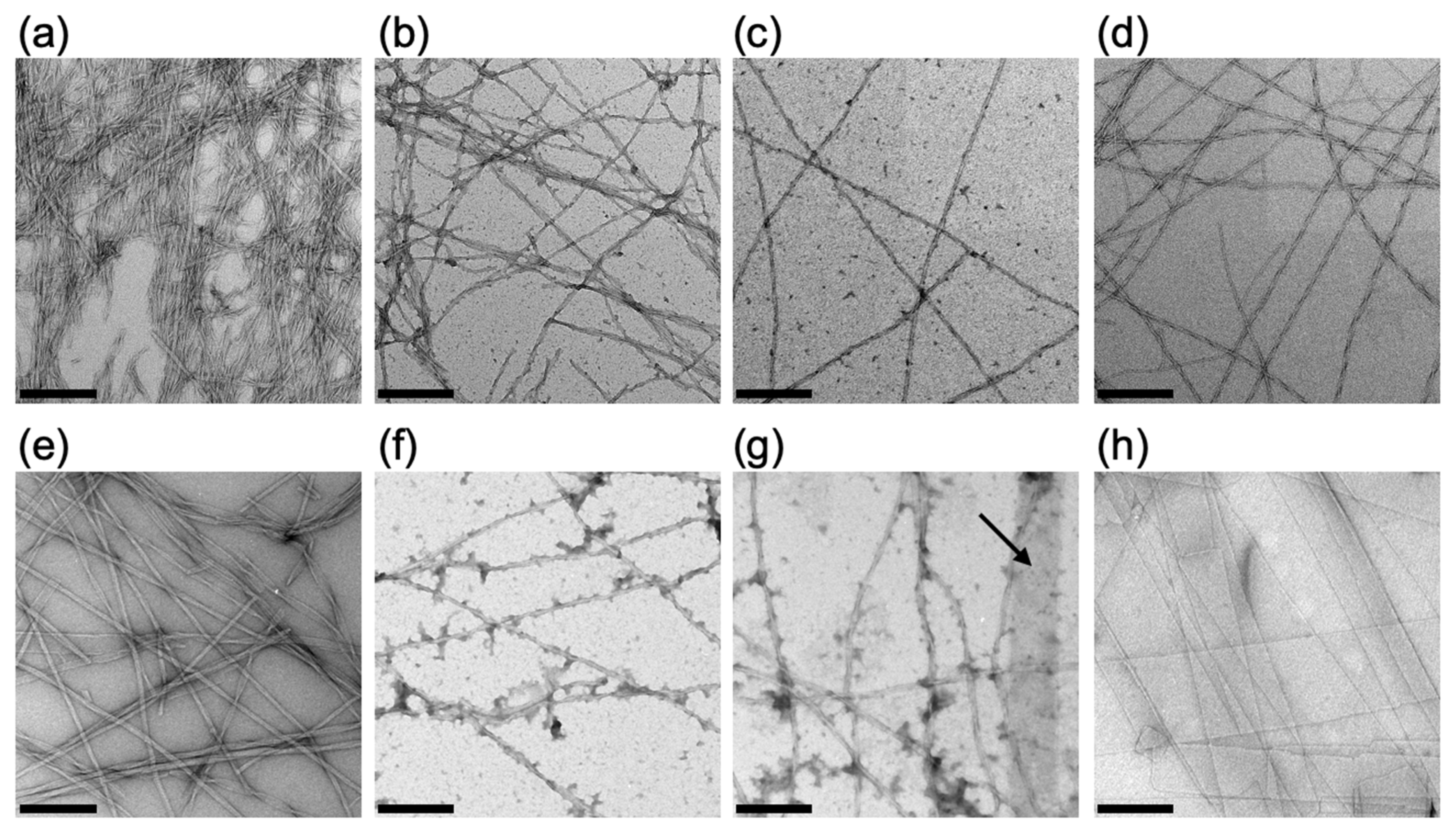
2.1.3. Evaluation of the Self-Assembled Structure of Fmoc-LnQG in Response to pH by Transmission Electron Microscopy (TEM)
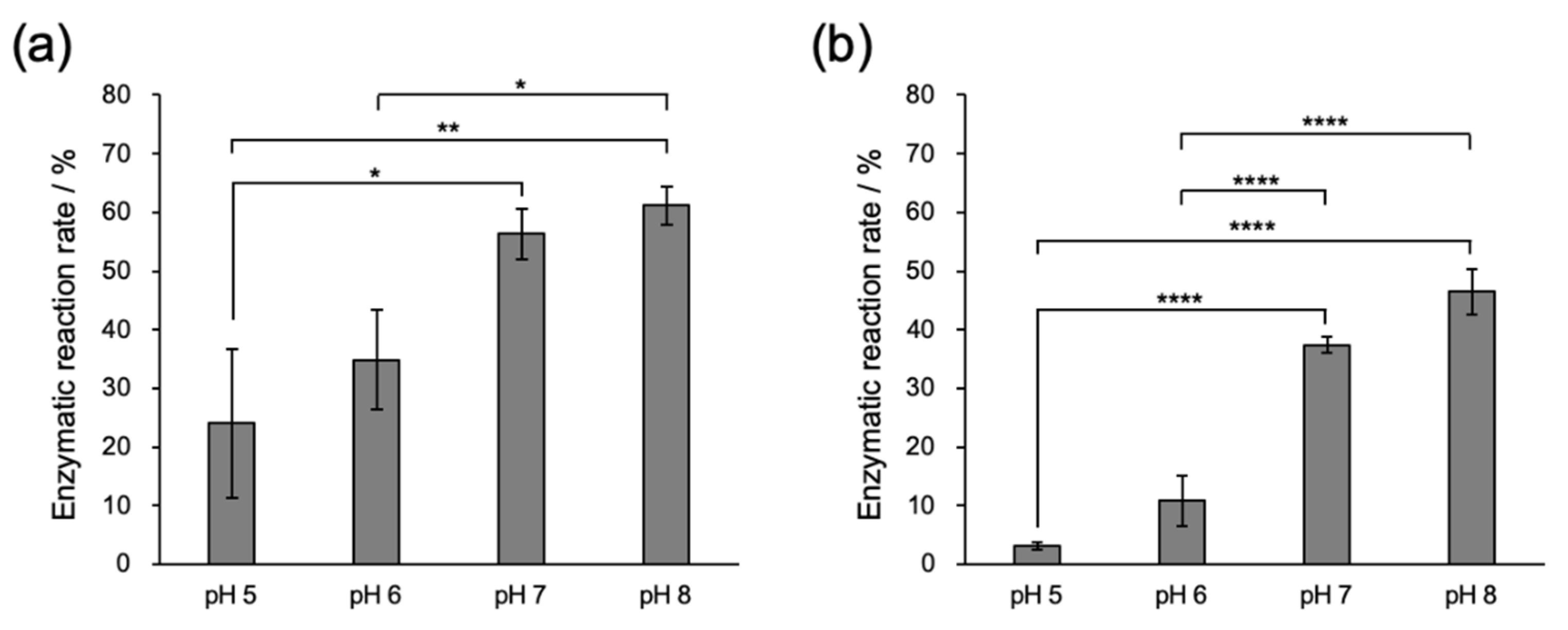
2.2. Enzymatic Modification of Fmoc-LnQG (n = 2, 3) Assemblies with Small Fluorescent Substrates
2.2.1. Conjugation of Fmoc-LnQG and Oregon Green 488 Cadaverine (OG) by MTG Catalysis
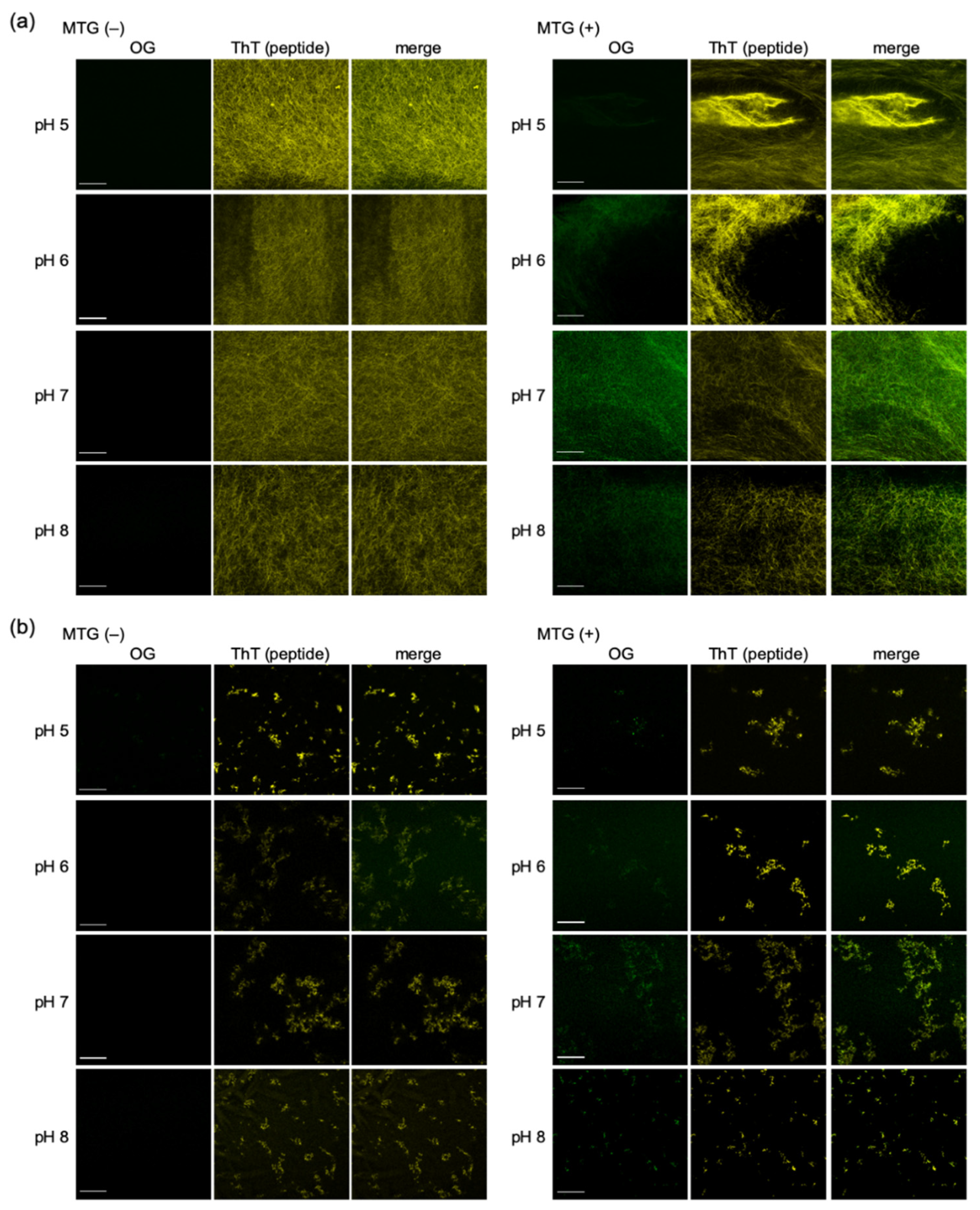
2.2.2. Confocal Fluorescence Microscope Images of OG on Fmoc-LnQG Peptide Assemblies
3. Experimental Section
3.1. General
3.2. Synthesis of Aromatic Peptide Amphiphiles
3.3. Preparation of MTG
- SGGGGSDSDDRVTPPAEPLDRMPDPYRPSYGRAETVVNNYIRWQQVYSHRDGRKQQ
- MTEEQREWLSYGCVGVTWVNSGQYPTNRLAFASFDEDRFKNELKNGRPRSGETRAE
- FEGRVAKESFDEEKGFQRAREVASVMNRALENAHDESAYLDNLKKELANGNDALR
- NEDARSPFYSALRNTPSFKERNGGNHDPSRMKAVIYSKHFWSGQDRSSSADKRKYGD
- PDAFRPAPGTGLVDMSRDRNIPRSPTSPGEGFVNFDYGWFGAQTEADADKTVWTHG
- NHYHAPNGSLGAMHVYESKFRNWSEGYSDFDRGAYVITFIPKSWNTAPDKVKQGW
- P
3.4. Critical Aggregation Concentration (CAC)
3.5. Fluorescence Spectra
3.6. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.7. Transmission Electron Microscopy (TEM)
3.8. Conjugation of Fmoc-LnQG and Oregon Green Cadaverine (OG) by MTG Catalysis
3.9. Confocal Fluorescence Microscope Images of OG on Fmoc-LnQG Peptide Assemblies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MTG | Microbial transglutaminase |
| CAC | Critical aggregation concentration |
| FT-IR | Fourier transform infrared spectroscopy |
| TEM | Transmission electron microscopy |
| OG | Oregon green 488 cadaverine |
| CLSM | Confocal laser scanning microscopy |
References
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2015, 15, 13–26. [Google Scholar] [CrossRef]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional supramolecular polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Hendricks, M.P.; Palmer, L.C.; Stupp, S.I. Peptide supramolecular materials for therapeutics. Chem. Soc. Rev. 2018, 47, 7539–7551. [Google Scholar] [CrossRef]
- Hauser, C.A.E.; Zhang, S. Designer self-assembling peptide nanofiber biological materials. Chem. Soc. Rev. 2010, 39, 2780–2790. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Self-assembly of amphiphilic peptides. Soft Matter 2011, 7, 4122–4138. [Google Scholar] [CrossRef]
- Cui, H.; Webber, M.J.; Stupp, S.I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Biopolymers 2010, 94, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Matsuura, K. Peptide Nanomaterials Designed from Natural Supramolecular Systems. Chem. Rec. 2019, 19, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Mart, R.J.; Osborne, R.D.; Stevens, M.M.; Ulijn, R.V. Peptide-based stimuli-responsive biomaterials. Soft Matter 2006, 2, 822. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Hamachi, I. Design Strategies of Stimuli-Responsive Supramolecular Hydrogels Relying on Structural Analyses and Cell-Mimicking Approaches. Acc. Chem. Res. 2017, 50, 740–750. [Google Scholar] [CrossRef]
- Ghosh, G.; Barman, R.; Sarkar, J.; Ghosh, S. pH-Responsive Biocompatible Supramolecular Peptide Hydrogel. J. Phys. Chem. B 2019, 123, 5909–5915. [Google Scholar] [CrossRef]
- Sarkar, A.; Kölsch, J.C.; Berač, C.M.; Venugopal, A.; Sasmal, R.; Otter, R.; Besenius, P.; George, S.J. Impact of NDI-Core Substitution on the pH-Responsive Nature of Peptide-Tethered Luminescent Supramolecular Polymers. Chem. Open 2020, 9, 346–350. [Google Scholar] [CrossRef]
- Gao, C.; Li, H.; Li, Y.; Kewalramani, S.; Palmer, L.C.; Dravid, V.P.; Stupp, S.I.; Olvera De La Cruz, M.; Bedzyk, M.J. Electrostatic Control of Polymorphism in Charged Amphiphile Assemblies. J. Phys. Chem. B 2017, 121, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. J. Am. Chem. Soc. 2019, 141, 4886–4899. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, M.; Wu, J.; Zhang, C.; Deng, X.; Dhinakar, A.; Huang, W.; Qian, H.; Ge, L. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017, 51, 294–303. [Google Scholar] [CrossRef]
- Ahlers, P.; Frisch, H.; Holm, R.; Spitzer, D.; Barz, M.; Besenius, P. Tuning the pH-Switch of Supramolecular Polymer Carriers for siRNA to Physiologically Relevant pH. Macromol. Biosci. 2017, 17, 1–7. [Google Scholar] [CrossRef]
- Williams, R.J.; Mart, R.J.; Ulijn, R.V. Exploiting biocatalysis in peptide self-assembly. Biopolymers 2010, 94, 107–117. [Google Scholar] [CrossRef]
- He, H.; Tan, W.; Guo, J.; Yi, M.; Shy, A.N.; Xu, B. Enzymatic Noncovalent Synthesis. Chem. Rev. 2020, 120, 9994–10078. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.R.; Roy, S.; Arora, M.; Das, A.K.; Hodson, N.; Murray, P.; Marshall, S.; Javid, N.; Sefcik, J.; Boekhoven, J.; et al. Biocatalytic induction of supramolecular order. Nat. Chem. 2010, 2, 1089–1094. [Google Scholar] [CrossRef]
- He, H.; Guo, J.; Lin, X.; Xu, B. Enzyme-Instructed Assemblies Enable Mitochondria Localization of Histone H2B in Cancer Cells. Angew. Chem. Int. Ed. 2020, 59, 9330–9334. [Google Scholar] [CrossRef]
- Feng, Z.; Han, X.; Wang, H.; Tang, T.; Xu, B. Enzyme-Instructed Peptide Assemblies Selectively Inhibit Bone Tumors. Chem 2019, 5, 2442–2449. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, G.; Wang, L.; Xu, B. Using a kinase/phosphatase switch to regulate a supramolecular hydrogel and forming the supramolecular hydrogel in vivo. J. Am. Chem. Soc. 2006, 128, 3038–3043. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, W.; Kong, D.; Yang, Z. Dual enzymes regulate the molecular self-assembly of tetra-peptide derivatives. Soft Matter 2011, 7, 10443–10448. [Google Scholar] [CrossRef]
- Collier, J.H.; Messersmith, P.B. Enzymatic Modification of Self-Assembled Peptide Structures with Tissue Transglutaminase. Bioconjug. Chem. 2003, 14, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, R.; Suehiro, A.; Goto, M.; Kamiya, N. Designer aromatic peptide amphiphiles for self-assembly and enzymatic display of proteins with morphology control. Chem. Commun. 2019, 55, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Sakono, M. Enzymatic Installation of Functional Molecules on Amyloid-Based Polymers. Bioconjug. Chem. 2017, 28, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- Varland, S.; Vandekerckhove, J.; Drazic, A. Actin Post-translational Modifications: The Cinderella of Cytoskeletal Control. Trends Biochem. Sci. 2019, 44, 502–516. [Google Scholar] [CrossRef]
- Song, Y.; Brady, S.T. Post-translational modifications of tubulin: Pathways to functional diversity of microtubules. Trends Cell Biol. 2015, 25, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Strop, P. Versatility of Microbial Transglutaminase. Bioconjug. Chem. 2014, 25, 855–862. [Google Scholar] [CrossRef]
- Popp, M.W.L.; Ploegh, H.L. Making and breaking peptide bonds: Protein engineering using sortase. Angew. Chem. Int. Ed. 2011, 50, 5024–5032. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ulijn, R.V.; Saiani, A. Effect of glycine substitution on Fmoc-diphenylalanine self-assembly and gelation properties. Langmuir 2011, 27, 14438–14449. [Google Scholar] [CrossRef] [PubMed]
- Folk, J.E.; Cole, P.W. Mechanism of Action of Guinea Pig Liver Transglutaminase. J. Biol. Chem. 1966, 241, 5518–5525. [Google Scholar] [CrossRef]
- Kubota, R.; Nakamura, K.; Torigoe, S.; Hamachi, I. The Power of Confocal Laser Scanning Microscopy in Supramolecular Chemistry: In situ Real-time Imaging of Stimuli-Responsive Multicomponent Supramolecular Hydrogels. Chem. Open 2020, 9, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Minamihata, K.; Ariyoshi, R.; Taniguchi, H.; Kamiya, N. Recombinant production of active microbial transglutaminase in E. coli by using self-cleavable zymogen with mutated propeptide. Protein Expr. Purif. 2020, 176, 105730. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakabayashi, R.; Higuchi, A.; Obayashi, H.; Goto, M.; Kamiya, N. pH-Responsive Self-Assembly of Designer Aromatic Peptide Amphiphiles and Enzymatic Post-Modification of Assembled Structures. Int. J. Mol. Sci. 2021, 22, 3459. https://doi.org/10.3390/ijms22073459
Wakabayashi R, Higuchi A, Obayashi H, Goto M, Kamiya N. pH-Responsive Self-Assembly of Designer Aromatic Peptide Amphiphiles and Enzymatic Post-Modification of Assembled Structures. International Journal of Molecular Sciences. 2021; 22(7):3459. https://doi.org/10.3390/ijms22073459
Chicago/Turabian StyleWakabayashi, Rie, Ayato Higuchi, Hiroki Obayashi, Masahiro Goto, and Noriho Kamiya. 2021. "pH-Responsive Self-Assembly of Designer Aromatic Peptide Amphiphiles and Enzymatic Post-Modification of Assembled Structures" International Journal of Molecular Sciences 22, no. 7: 3459. https://doi.org/10.3390/ijms22073459
APA StyleWakabayashi, R., Higuchi, A., Obayashi, H., Goto, M., & Kamiya, N. (2021). pH-Responsive Self-Assembly of Designer Aromatic Peptide Amphiphiles and Enzymatic Post-Modification of Assembled Structures. International Journal of Molecular Sciences, 22(7), 3459. https://doi.org/10.3390/ijms22073459






